Summary
Objective
To determine whether patients with Systemic Lupus Erythematosus (SLE) and Mixed Connective Tissue Disease (MCTD) possess differential IgM-and IgG-specific reactivity against peptides from the U1 small nuclear ribonucleoprotein particle (U1 snRNP).
Methods
The IgM- and IgG-mediated responses against 15 peptides from subunits of the U1 snRNP were assessed by indirect ELISAs in sera from patients with SLE and MCTD and healthy individuals (n = 81, 41 and 31, respectively). Additionally, 42 laboratory tests and 40 clinical symptoms were evaluated to uncover potential differences. Binomial logistic regression analyses (BLR) were performed to construct models to support the independent nature of SLE and MCTD. Receiver Operating Characteristic (ROC) curves corroborated the classification power of the models.
Results
We analyzed IgM and IgG anti-U1 snRNP titers to classify SLE and MCTD patients. IgG anti-U1 snRNP reactivity segregates SLE and MCTD from non-disease controls with an accuracy of 94.1% while IgM-specific anti-U1 snRNP responses distinguish SLE from MCTD patients with an accuracy of 71.3%. Comparison of the IgG and IgM anti-U1 snRNP approach with clinical tests used for diagnosing SLE and MCTD revealed that our method is the best classification tool of those analyzed (p ≤ 0.0001).
Conclusions
Our IgM anti-U1 snRNP system along with lab tests and symptoms provide additional molecular and clinical evidence to support the hypothesis that SLE and MCTD may be distinct syndromes.
Keywords: Systemic Lupus Erythematosus (SLE), Mixed Connective Tissue Disease (MCTD), immunoglobulin M (IgM), U1 small nuclear ribonucleoprotein particle (U1 snRNP), auto-immune disorders, classification criteria
Introduction
Systemic Lupus Erythematosus (SLE) and Mixed Connective Tissue Disease (MCTD) are systemic autoimmune disorders with overlapping clinical manifestations that possess aberrant immune responses against common auto-antigens1, 2, 3–6. Despite its description as an independent auto-immune disease7, the classification of MCTD as distinct from SLE remains controversial due to the high number of common clinical features between SLE and MCTD patients7–13. Nevertheless, the concept of MCTD has been reported as a useful definition in clinical practice3, 11–13, and clinical and serological features segregate the two illnesses14–16. The American College of Rheumatology (ACR) has created universal classification parameters for SLE14; however, four different criteria sets exist for MCTD patients, with the Alarcόn-Segovia criteria being the most widely accepted17.
Currently, there is no single test with sufficient specificity and sensitivity to discriminate between SLE and MCTD2, 4, 15, 18–20, which has hampered the identification of MCTD as a separate syndrome. A positive diagnosis by any set of criteria requires a patient to exhibit at least four clinical symptoms and/or tests out of those included in each list, which can take years to develop2, 14. Moreover, traditional laboratory tests are performed with numerous commercially available kits that can vary in principle and cut-off values, which may alter the final results and diagnoses4, 15, 18, 21, 22. These and other factors complicate proper diagnosis of these two closely related and overlapping illnesses.
Previous investigations have demonstrated that SLE and MCTD patients often exhibit 1000-fold greater auto-reactivity to subunits of the U1 small nuclear ribonucleoprotein particle (snRNP) than to any other cellular component 23, 24. The U1 snRNP is an RNA-protein complex that is responsible for pre-mRNA processing and is composed of 10 proteins (U1–70K, U1A, U1C and seven Smith antigen (Sm) proteins)25, 26, 27. In general, previous studies aimed at finding biomarkers for SLE and MCTD have focused on IgG-specific responses to nuclear components, including the U1 snRNP; however, some studies have revealed differential IgM reactivity for nuclear components in SLE and MCTD patients24, 29–31. Yet, the potential use of the IgM response as a molecular tool to classify SLE and MCTD patients has not been fully explored.
To determine whether SLE and MCTD represented distinct disorders and test whether the two patient groups can be segregated, we evaluated the IgG- and IgM-specific responses of patients with SLE and MCTD and healthy individuals against 15 different U1 snRNP peptides (named P1–15) by indirect enzyme-linked immunosorbent assays (ELISAs). Interestingly, we observed higher IgG-based reactivity for U1 snRNP peptides in individuals with SLE or MCTD compared to healthy individuals, but elevated IgM responses in SLE patients compared to those with MCTD and healthy adults. The IgM response to two peptides, P4 and P10 (P4/P10), exhibited 71.3% accuracy in segregating between these two autoimmune disorders (p ≤ 0.05). In summary, these data support the notion that SLE and MCTD are, indeed, distinct disorders and highlight the potential clinical use of the IgM anti-U1 snRNP system as a molecular tool to assist in the classification of SLE and MCTD patients.
Methods
Collection and preparation of sample sera
Sera were obtained from whole blood of 122 patients previously diagnosed with SLE (n=81) or MCTD (n=41) and 31 healthy individuals. Samples were collected following the Institutional Review Board (IRB) accepted protocols of the University of Miami (IRB numbers: 200307-24 and 200402-86) and Florida International University (IRB number: 040308-00). SLE and MCTD patients (collectively referred to here as “ill” or “patient group”) were clinically diagnosed according to the American College of Rheumatology (ACR) criteria14 and the Alarcόn-Segovia criteria17, respectively, along with clinician judgment. The laboratory tests in this study were commercially performed by Quest Diagnostic Incorporated and their positive values are included in Table 2. Details of the flare or remission period in these SLE and MCTD patients were not recorded at the moment of whole blood collection and, therefore, disease activity for these SLE and MCTD patients has not been considered in this study.
Selection of U1 snRNP peptides
The U1 snRNP peptides included were previously reported in Somarelli et al.,32 and commercially synthesized by BioMatik Corporation (Wilmington, DE, USA) The observed IgM reactivity for each of the U1 snRNP peptides was ranked from most (1) to least (15) antigenic for each disease state (Table 1).
Table 1.
Overview of U1 snRNP peptides used in the study
| Peptide number |
U1 snRNP protein |
Peptide region (amino acid range) |
Peptide sequence |
Rank observed
IgM reactivity |
|
|---|---|---|---|---|---|
| SLE | MCTD | ||||
| 1†* | U1A | 196–203 | PPAQPLSE | 1 | 6 |
| 2†* | Sm E | 63–70 | EIHSKTKS | 3 | 2 |
| 3†* | Sm F | 46–53 | NTEEYIDG | 12 | 9 |
| 4†* | U1C | 90–97 | GMMPAPHM | 11 | 8 |
| 5* | U1-70K | 337–344 | PDGPDGPE | 6 | 4 |
| 6†* | Sm B | 83–90 | EGPPPKDT | 2 | 3 |
| 7†* | Sm G | 1–8 | MSKAHPPE | 5 | 5 |
| 8†* | Sm D3 | 20–27 | CETNTGEV | 4 | 1 |
| 9†* | Sm F | 77–84 | EEEEDGEM | 9 | 12 |
| 10†* | U1A | 112–119 | KPKSQETP | 13 | 13 |
| 11†* | Sm D2 | 14–21 | EELQKREE | 10 | 11 |
| 12†* | Sm D1 | 22–29 | GTQVHGTI | 8 | 10 |
| 13†* | U1A | 178–185 | GQIPPGAM | 14 | 14 |
| 14†* | U1-70K | 325–332 | APPDDGPP | 15 | 15 |
| 15†* | U1C | 66–73 | PFSAPPPA | 7 | 7 |
The peptide designation, region and sequences as well as the U1 snRNP protein column displayed in this table (first four columns) were previously published by Somarelli et al. (2011). The observed IgM antigenicity (columns 5 and 6, from left to right) was ranked from 1 to 15 where “1” represents the peptide with the highest IgM antigenicity and “15” indicates the peptide with the lowest IgM antigenicity.
Daggers (†) indicate IgM peptide antigenicities that significantly differ between SLE and MCTD patients while the asterisks (*) represent IgG reactivities for U1 snRNP peptides that significantly differ between ill (SLE and MCTD) and healthy individuals (p ≤ 0.05).
Monitoring IgM reactivity for U1 snRNP peptides by indirect ELISAs
The indirect ELISA protocol employed to assess IgM reactivity for each peptide and sample included was previously described32. The average IgM derived OD value for each peptide was normalized using the average OD value of the healthy group per peptide examined and was expressed as OD% based on the following formula33 (Supplementary data 1):
where “X̄ OD of sample in Px” is the average OD value of the sample group (SLE or MCTD) and “X̄ OD of control in Px” indicates the average OD of the control group (healthy group) from each of the peptides included in this study (P1–P15). To evaluate the relative reactivity contributed by IgM and IgG in SLE, MCTD and healthy populations, the average OD values from IgG-specific ELISAs previously reported by Somarelli et al.,32, which used the same samples and U1 snRNP peptides included in this study, were re-analyzed and converted to OD% using the equation described above33 (Supplementary data 2).
Statistical analyses
Significant differences in IgG and IgM reactivity between patients (SLE and MTCD) and healthy groups and between SLE and MCTD individuals for each of the peptides was assessed using independent sample t-tests. Clinical tests and symptoms were evaluated by independent sample t-tests (numerical data) or Chi (X) squared tests (nominal data). Receiver Operating Characteristic (ROC) curves were generated with the PASW software package (version 18). Forward binary logistic regression (BLR) analyses using the IgM and IgG anti-U1 snRNP titers in ill (SLE and MCTD) and healthy individuals as well as SLE and MCTD patients were performed with the PASW software package (version 18). P-values ≤ 0.05 were considered statistically significant for all tests. Correlations between the IgM anti-U1snRNP peptide reactivity and IgM anti-Rheumatoid Factor (RF) antigenicity were performed using the PASW software package (version 18). We found no significant correlation between the IgM-specific anti-U1 snRNP reactivity and IgM-mediated anti-RF activity. As a result, these analyses were not further considered in this study.
Results
IgM anti-U1 snRNP reactivity is elevated in SLE but not MCTD patients
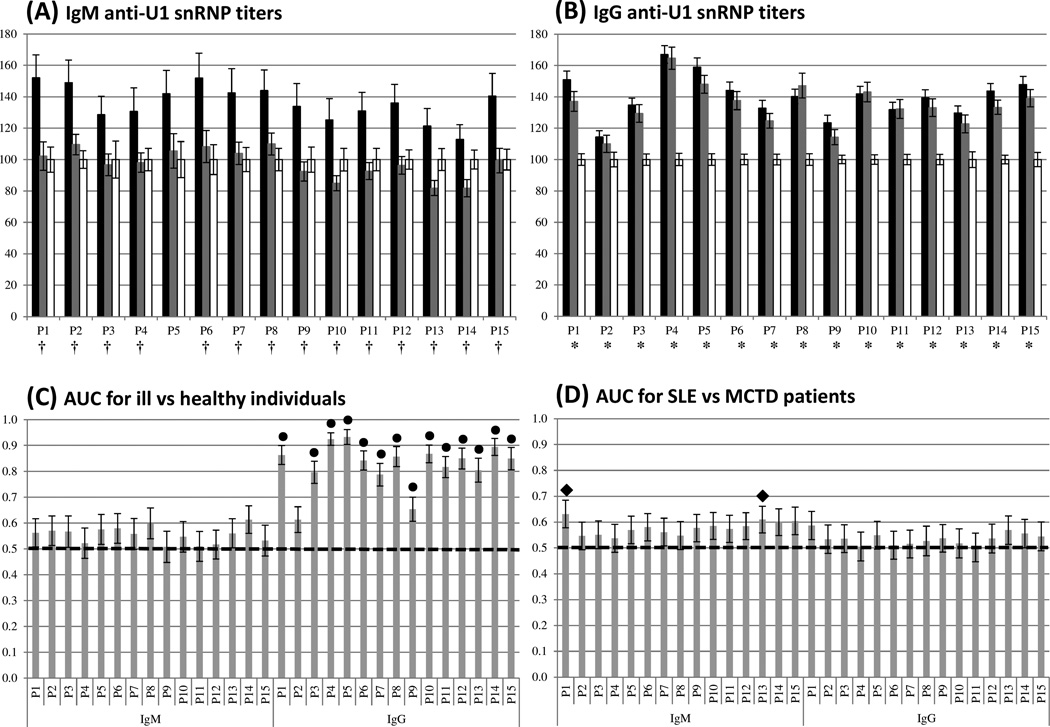
The IgM response to U1 snRNP peptides was monitored via indirect ELISAs and reported as OD% (Figure 1A and Supplementary data 1). IgM anti-U1 snRNP titers were significantly higher in the SLE group than either the MCTD population or healthy individuals (p ≤ 0.05). In fact, in many instances, IgM responses to U1 snRNP peptides in MCTD patients were equal to or below those exhibited by healthy individuals (P3, P4 and P9–P15 in Figure 1A). The discrimination capacity of IgM-anti-U1 snRNP peptide ELISAs was assessed by ROC curve analysis and indicates that IgM reactivity for P1 and P13 provides significant power to classify SLE and MCTD patients; however, none of the IgM responses were sufficient to discern SLE and MCTD from non-disease controls with statistical significance (Figures 1C–D and Supplementary data 3).
Figure 1. Contrasting IgM-specific anti-U1 snRNP peptide responses observed in SLE and MCTD patients.
(A) and (B) represent the average percent optical density (OD%) values for the IgM class and IgG classes, respectively. Peptide number and OD% are on the x and y axes, respectively. The black, gray and white bars symbolize the average OD% of SLE, MCTD and healthy groups, respectively. (†) and (*) indicate significantly different OD% between SLE and MCTD as well as patients and healthy populations, respectively (p ≤ 0.05). (C) and (D) correspond to the area under the curve (AUC), derived from ROC curves, for ill (SLE and MCTD) vs. healthy individuals as well as SLE vs. MCTD patients, respectively. Peptide number per Ig class and their AUC values are indicated on the x and y axes, respectively. (●) and (♦) symbolize significantly different AUC between patients and healthy individuals as well as SLE and MCTD patients, respectively (p ≤ 0.05). The dotted lines in C and D indicate the cut-off value (0.5). Black bars in all graphs represent standard error of the mean.
SLE and MCTD patients exhibit an elevated IgG response for U1 snRNP peptides
As previous studies have reported 32, 34–38, the IgG-mediated reactivity for each of the U1 snRNP peptides was significantly higher in both SLE and MCTD populations than in the healthy group; however, IgG reactivity does not differ between the two autoimmune disorders (Figure 1B). ROC curve analyses on IgG anti-U1 snRNP titers per peptide ascertain their individual ability to discern between patients (SLE and MCTD) and healthy individuals and between SLE and MCTD patients (Figure 1C – D, respectively; and Supplementary data 4). As previously reported32, all but IgG anti-P2 responses were capable of significantly discriminating SLE and MCTD from healthy individuals with IgG anti-P4 being the best (p ≤ 0.05); however, none of the IgG anti-U1 snRNP titers had a statistically significant ability to classify SLE and MTCD patients (Figure 1D).
Differential auto-immune responses and symptoms are observed in SLE and MCTD patients
We found that SLE and MCTD patients exhibit significantly different IgM anti-U1 snRNP reactivity (p ≤ 0.05) despite similar IgG-mediated antigenicity for the same peptides (Figures 1A – B). To further support the idea that SLE and MCTD represent distinct auto-immune illnesses, statistical analysis of 42 standard laboratory tests were performed with blood samples from the SLE and MCTD patient cohort. These analyses revealed that 11 out of the 42 clinical tests were significantly different in SLE and MCTD patients (p ≤ 0.05) (Table 2). Specifically, differences were observed in tests designed to detect nuclear auto-antigens (RNP, Sm, SCL70, dsDNA, elevated DNA), renal function (creatine phosphokinase levels, renal proteinuria, renal hematuria) and immune system components (C3 and C4 complement levels) (p ≤ 0.05). These findings support the idea that SLE and MCTD represent distinct autoimmune manifestations, with specific antigenic targets and antibody class reactivities.
Table 2.
Clinical tests evaluated in SLE and MCTD patients
| Clinical test name | Definition of positive test |
SLE | MCTD |
P value |
|||
|---|---|---|---|---|---|---|---|
| Positive | Total | Positive | Total | ||||
| SLE/MCTD specific | Fluorescence antinuclear Abs titers |
> 1:320 IU/ml | 76.56% | 64 | 90.63% | 32 | 0.3860 |
| Fluorescence antinuclear Abs pattern |
Homogenous, mixed or speckled pattern |
86.30% | 73 | 85.71% | 35 | 0.4430 | |
| IgG anticardiolipin positive |
> 10 GPL U/ml | 23.53% | 68 | 23.81 | 21 | 0.8480 | |
| IgM anticardiolipin positive |
> 10 GPL U/ml | 6.06% | 66 | 18.18% | 22 | 0.0870 | |
| Rheumatoid factor titer by latex |
> 14 IU/ml | 8.33% | 12 | 20.00% | 5 | 0.0870 | |
| IgM anti-rheumatoid factor Abs by ELISA |
> 20 IU/ml | 41.86% | 43 | 38.10% | 21 | 0.8480 | |
| IgM anti-rheumatoid factor Abs titer |
> 20 IU/ml | 20.93% | 43 | 23.81% | 21 | 0.9040 | |
| RNP positive | > 20 EU/ml | 84.00% | 75 | 100% | 40 | 0.0080 | |
| Sm positive | > 20 EU/ml | 60.27% | 73 | 28.21% | 39 | 0.0010 | |
| SSA positive | > 20 EU/ml | 58.11% | 74 | 47.22% | 36 | 0.2820 | |
| SSB positive | > 20 EU/ml | 21.62% | 74 | 11.11% | 36 | 0.1800 | |
| SCL 70 positive | > 20 EU/ml | 1.45% | 69 | 16.13% | 31 | 0.0040 | |
| Elevated serum DNA titer | ≥ 10 IU/ml | 64.10% | 78 | 29.73% | 37 | 0.0010 | |
| Anti-dsDNA positive | 5–9 IU/ml >5 SD when |
49.35% | 77 | 27.03% | 37 | 0.0010 | |
| IgG anti U1-70K Abs* | compared to
healthy group >5 SD when |
37.84% | 37 | 55.00% | 20 | 0.2130 | |
| IgG anti SmB/B’ Abs* | compared to
healthy group >5 SD when |
44.12% | 34 | 50.00% | 20 | 0.7700 | |
| IgG anti SmD Abs* | compared to healthy group |
73.53% | 34 | 63.16% | 19 | 0.7260 | |
| Anemia | Hematocrit range < 35% |
25.00% | 80 | 22.50% | 40 | 0.7630 | |
| Hemolytic anemia | Positive for anemia and hemolysis |
3.23% | 62 | 8.33% | 12 | 0.4120 | |
| White blood count | > 10.8/mm | 8.77% | 57 | 6.06% | 33 | 0.2780 | |
| Leukopenia | White blood count <3.0/mm |
11.54% | 78 | 12.50% | 40 | 0.8780 | |
| Lymphopenia | Total lymphocyte count < 1500/mm |
36.71% | 79 | 42.5% | 40 | 0.5400 | |
| Thrombocytopenia | Platelet count < 100,000 |
10.13% | 79 | 2.50% | 40 | 0.1370 | |
| Thrombocytosis | Platelet count
> 600,000 |
5.13% | 78 | 2.56% | 39 | 0.5180 | |
| Creatine
phosphokinase positive |
<165 U/L | 91.07% | 56 | 74.29% | 35 | 0.4770 | |
| Creatine
phosphokinase elevated |
>165 U/L | 7.02% | 57 | 25.71% | 35 | 0.0120 | |
| Serum creatinine | > 1.07 mg/dL | 10.53% | 76 | 5.26% | 38 | 0.4500 | |
| Renal cellular cast | Abnormal cellular cast on urinalysis |
10.67% | 75 | 2.56% | 39 | 0.1280 | |
| Renal proteinuria | Defined by multiple creatine ratio criteria† |
40.85% | 71 | 13.51% | 37 | 0.0040 | |
| Renal hematuria | 5 red blood cells per high power field‡ |
18.92% | 74 | 2.70% | 37 | 0.0180 | |
| Creactive protein | > 1.0 mg/dl | 20.83% | 72 | 18.18% | 33 | 0.4850 | |
| Elevated C reactive protein | >10 mg/dl | 23.61% | 72 | 20.59% | 34 | 0.7290 | |
| Low C3 complement | < 90mg/dl | 41.77% | 79 | 15.00% | 40 | 0.0030 | |
| C3 complement level | 90 – 180 mg/dl | 56.96% | 79 | 82.50% | 40 | 0.0001 | |
| Low C4 complement | < 10 mg/dl | 48.10% | 79 | 30.77% | 39 | 0.0730 | |
| C4 complement level | 16 – 47 mg/dl | 44.30% | 79 | 69.23% | 39 | 0.0001 | |
| Erythrocyte
sedimentation rate |
0 – 20 mm/hr | 57.89% | 79 | 52.27% | 40 | 0.8530 | |
| Elevated erythrocyte sedimentation rate |
>20 mm/hr | 56.48% | 76 | 55.00% | 40 | 0.8710 | |
| General | IgG anti-rheumatoid
factor Abs |
> 20 IU/ml | 41.86% | 43 | 42.86% | 21 | 0.9400 |
| IgG anti-rheumatoid factor Abs titer |
> 20 IU/ml | 41.86% | 43 | 38.10% | 21 | 0.8480 | |
| Immunoglobulin isotypes for RF factor |
Presence of IgM and/or IgG antibodies | 79.07% | 43 | 76.19% | 21 | 0.5300 | |
| Lymphocyte absolute value |
850 – 3900 cells/uL | 74.14% | 58 | 75.86% | 29 | 0.3460 | |
Laboratory tests that significantly differ between SLE and MCTD patients are highlighted in gray (p ≤ 0.05). ‘Abs’, ‘IgG’ and ‘IgM’ stands for antibodies, immunoglobulin M and immunoglobulin G, respectively.
Asterisks (*) denote non-commercial laboratory tests for which positive values are recorded. All other laboratory tests listed were performed by Quest Diagnostic Incorporated, and each of their reported positive values are included. A positive value is indicated if the sample(s) exhibit values five times above the standard deviation obtained by the average value of the healthy group.
The dagger (†) defines the criteria for Renal proteinuria, which is >0.5g/24hr or >24mg/dl in random samples >2+ on urinalysis or spot urine/creatine ratio >0.2.
The double dagger (‡) indicates that a test for renal hematuria was considered positive when there was no other reason for hematuria, such as infection.
Similarly, statistical assessment of 40 clinical symptoms from patients in our SLE and MCTD cohort indicated that 16 out of the 40 clinical characteristics evaluated were significantly different between SLE and MCTD patients (Table 3). Most of the significantly different clinical manifestations involved the skin and joints of these patients; however, our data also confirmed that neuropsychiatric disorders and problems in the circulatory system were also significantly different between the two groups. Once again, the fact that clinical symptoms differ in SLE and MCTD populations supports the hypothesis that these maladies may be clinically distinct.
Table 3.
List of clinical symptoms observed in SLE and MCTD patients
| Clinical symptom name | Definition | SLE |
MCTD |
p- value |
||
|---|---|---|---|---|---|---|
| Positive | Total | Positive | Total | |||
| Skin telangiectasia | Vascular lesions formed by dilation of
small blood vessels |
5% | 79 | 15% | 40 | 0.0650 |
| Skin nasal/oral ulcers | Shallow and painful open sores that appear
as necrotic or eroded areas on the oral mucosa |
29% | 79 | 23% | 39 | 0.4880 |
| Raynaud’s syndrome | Vascular disorder causing periods of
severely restricted blood flow to the fingers and toes |
53% | 80 | 85% | 40 | 0.0001 |
| History of hand swelling | Hand swelling by history | 41% | 81 | 61% | 41 | 0.0120 |
| Observed hand swelling | Hand swelling observed on physical exam at
the date of visit |
19% | 81 | 39% | 41 | 0.0140 |
| Acrosclerosis | Scleroderma of the distal extremities,
sometimes extending to the neck and face |
4% | 80 | 26% | 38 | 0.0001 |
| Skin digital pitting | Loss of skin on the tips of the fingers
caused by scars and ulcers |
8% | 80 | 8% | 39 | 0.9700 |
| Proximal scleroderma | Skin fibrosis of the extremities proximal
to the elbows or knees, or of the thorax. |
3% | 79 | 0% | 39 | 0.3160 |
| Skin alopecia | Hair loss condition that usually affects the scalp | 58% | 80 | 72% | 39 | 0.1310 |
| Malar rash | Skin rash of both cheeks joined by an
extension across the bridges of the nose |
46% | 78 | 13% | 39 | 0.0001 |
| Discoid rash | Chronic skin problem resulting from lupus disease | 10% | 78 | 0% | 39 | 0.0380 |
| Skin rash | Red and swollen area on the skin | 33% | 78 | 38% | 39 | 0.5840 |
| Skin photosensitivity | Skin rash resulting from reaction to sunlight | 57% | 76 | 58% | 38 | 0.8940 |
| Skin calcinosis | Abnormal deposition of calcium salts in tissues | 1% | 79 | 5% | 38 | 0.2000 |
| Sicca, xerophthalmia
and xerostomia |
Characterized by dry eyes (xerophthalmia)
and dry mouth (xerostomia) |
49% | 81 | 66% | 41 | 0.1330 |
| Erosive inflammatory arthritis |
Synovitis with joint erosions | 43% | 23 | 45% | 20 | 0.9200 |
| Lymphadenopathy | Swollen or enlarged lymph nodes | 24% | 79 | 20% | 40 | 0.6180 |
| Fever | Increase in body temperature above the
normal range (98–100°F) |
22% | 78 | 15% | 40 | 0.7780 |
| Proximal muscle weakness | Malfunction of muscle fibers resulting in weakness | 29% | 76 | 49% | 39 | 0.0360 |
| Myositis | Inflammation of muscle tissue | 6% | 79 | 27% | 33 | 0.0020 |
| Myalgia | Tenderness or pain in the muscles | 54% | 80 | 48% | 40 | 0.8970 |
| Morning stiffness | Joint and muscle stiffness present upon awakening | 53% | 73 | 64% | 36 | 0.3000 |
| Swelling of three or
more joints |
Multiple joints swelling | 42% | 78 | 63% | 40 | 0.0380 |
| Joint tenderness | Sensitivity to touch or pressure on fat
pad, tendon attachment, ligament, muscle and/or skin |
35% | 79 | 69% | 39 | 0.0010 |
| Joint swelling | Intra-articular effusion, synovial
thickening, and periarticular soft tissue inflammation |
29% | 78 | 63% | 40 | 0.0010 |
| Symmetric swelling | Swelling occurs in the same joint on both
sides of the body |
27% | 78 | 64% | 39 | 0.0001 |
| Rheumatoid nodule | Includes subcutaneous nodules over bony
prominences, extensor surface or in juxta articular regions |
6% | 77 | 10% | 40 | 0.5000 |
| Arthralgia | Joint pain | 82% | 79 | 84% | 38 | 0.7950 |
| Neuropathy | Any type of nerve disorders | 31% | 77 | 28% | 40 | 0.6810 |
| Seizure | A sudden attack of pain, of a disease, or
of certain symptoms |
4% | 80 | 0% | 40 | 0.2150 |
| Psychosis | Any mental disorder characterized by
personality disintegration and loss of contact with reality |
3% | 79 | 0% | 39 | 0.3160 |
| Neuropsychiatric disorder | Evidence of mental illness | 19% | 78 | 51% | 39 | 0.0001 |
| Hypomotility in cine deglutition esophageal |
Decreased motility of the esophagus
(usually results in difficulty swallowing or increased acid reflux) |
41% | 78 | 58% | 40 | 0.0890 |
| Pulmonary fibrosis | Development of excess fibrous connective
tissue in the lungs |
22% | 45 | 23% | 26 | 0.6330 |
| Pleuritic pain or rubbing heard |
Inflammation of membrane that enfolds both lungs | 37% | 78 | 23% | 39 | 0.1250 |
| Pericarditis | Inflammation of the pericardium | 30% | 73 | 18% | 39 | 0.1610 |
| Avascular necrosis | Cellular death of bone components due to
interruption of the blood supply |
3% | 74 | 0% | 33 | 0.3040 |
| Clotting | Thick, viscous, or coagulated mass in blood stream. | 1% | 75 | 12% | 34 | 0.0160 |
| Myocardial infarction | Partial or complete occlusion of one or
more of the coronary arteries resulting in myocardial injury |
0% | 73 | 11% | 35 | 0.0030 |
| Stroke | Brain hemorrhage or lack of blood flow | 6% | 81 | 2% | 41 | 0.3570 |
Clinical manifestations that differ significantly between SLE and MCTD patients are highlighted in gray (p ≤ 0.05). Neuropathy, seizure and psychosis symptoms were diagnosed in the absence of offending drugs or known metabolic derangements.
Antibody class reactivities for U1 snRNP peptides segregate among SLE, MCTD and healthy individuals
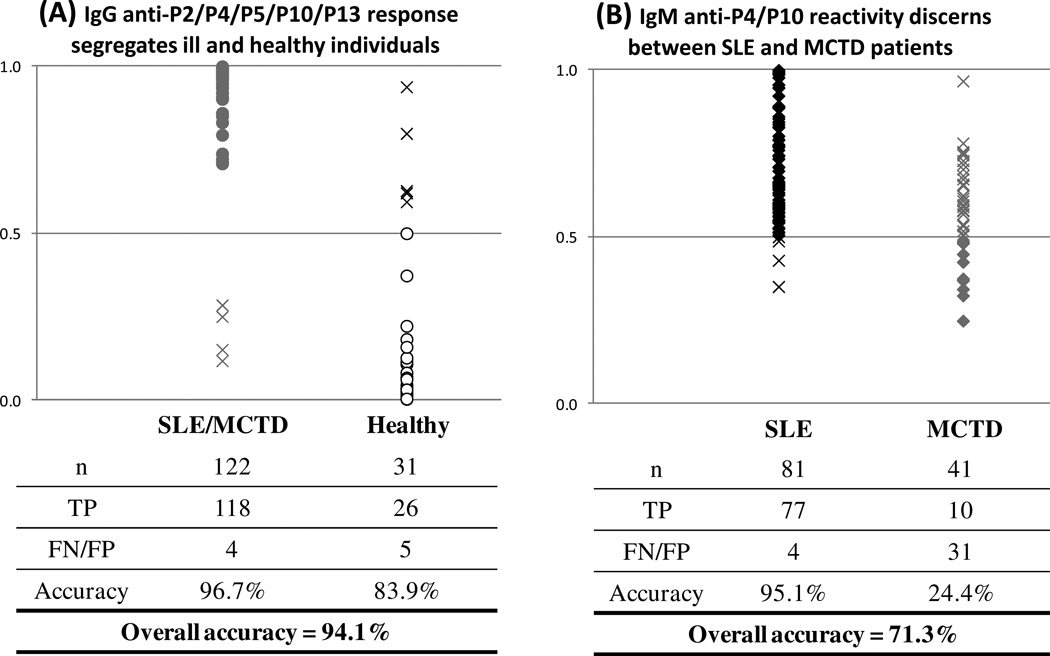
The IgM and IgG responses for all U1 snRNP peptides were combined in a BLR to determine which peptide and auto-antibody combinations might provide the highest segregation between patient (SLE and MCTD) and healthy populations. These analyses revealed that the combined IgG-specific response for P2, P4, P5, P10 and P13 has the greatest capacity to discern between sick and healthy individuals with an overall accuracy of 94% (p ≤ 0.05) (Figure 2A) where the probability of correctly predicting a patient with either SLE or MCTD is higher than that for correctly predicting a healthy individual (96.7% and 83.9%, respectively).
Figure 2. Identification of a two-step ELISA system for classification of SLE, MCTD and healthy individuals.
(A) The combination of IgG-mediated anti-P2/P4/P5/P10/P13 provides the best segregation between SLE and MCTD and non-disease controls. The distribution of Ill (SLE and MCTD) and healthy individuals and the predicted combined IgG-mediated reactivity are represented on the x and y axes, respectively. Gray and white circles indicate true positives (TP). (B) Combined IgM-anti-P4/P10 can classify SLE and MCTD patients. The distribution of SLE and MCTD patients’ combined IgM anti-P4/P10 predicted values are on the x and y axes, respectively. Black and gray diamonds indicate true positive (TP) samples for SLE and MCTD patients, respectively. The crosses represent false negatives (FN) or false positives (FP). Predicted values were obtained using binomial logistic regression (BLR) with a cut-off of 0.5 (p ≤ 0.05).
Additional BLRs were performed with the individual IgG and IgM reactivities for each U1 snRNP peptide to assess which peptide and Ig class combination significantly discriminates between SLE and MCTD patients. These analyses indicated that only the combined IgM response for P4 (U1C) and P10 (U1A) significantly discriminate between SLE and MCTD patients, with an overall accuracy of 71.3% (p ≤ 0.05) (Figure 2B). Remarkably, most of the classification power derives from the proper classification of SLE patients (95.1%) rather than proper grouping of MCTD patients (24.4%) (Figure 2B). Consequently, our data demonstrate that by first combining the IgG reactivity for P2, P4, P5 and P10 and then the titers for IgM anti-P4/P10, we can achieve an overall accuracy of 73.9% at discriminating among SLE, MCTD and healthy groups.
Comparing the power of IgM anti-P4/P10 with conventional clinical tests
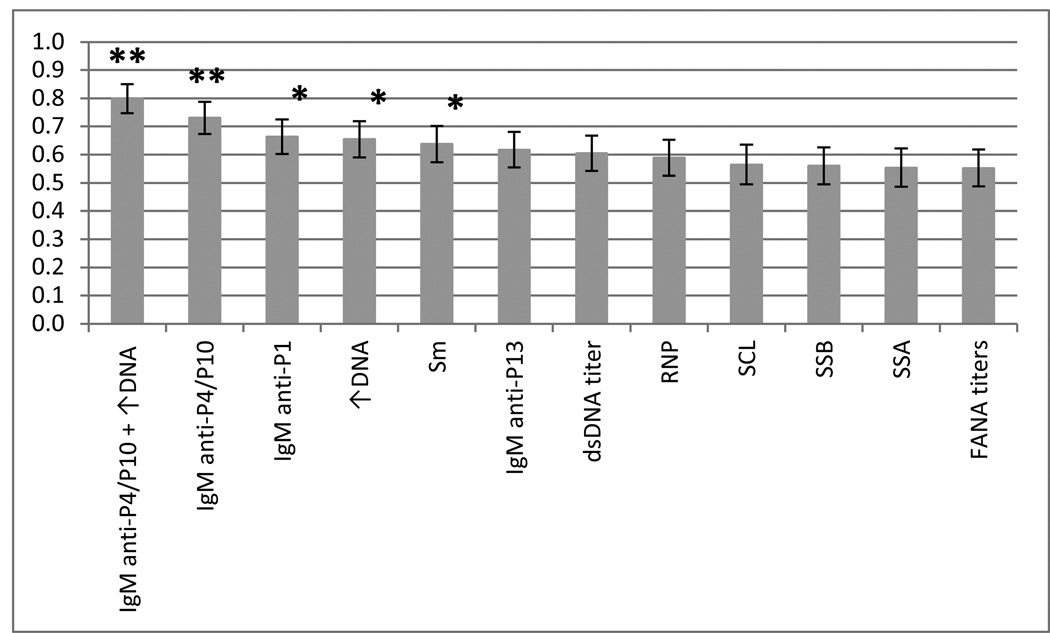
To determine the classification power of our proposed IgM-specific P4/P10 ELISA-based system, ROC curves were used to compare our system with eight conventional clinical tests. The individual IgM reactivities for P1 and P13 were also included in the ROC curves analyses because they discriminate between SLE and MCTD (Figure 1D). The 11 laboratory tests that significantly differ between SLE and MCTD patients were performed only in a small portion of each sub-population (Table 2). As a result, not all tests could be analyzed due to the reduced sample size. Instead, eight of the most frequently-used laboratory tests that are part of the classification criteria to diagnose SLE or MCTD were included in the ROC curve analysis (FANA titers, dsDNA ELISA, elevated serum DNA titers and positive results for RNP, Sm, SSA, SSB and SCL-70)4, 15–16, 21–22. When using the subset of individuals for whom clinical test results were available (SLE = 59 and MCTD = 24), the IgM anti-P4/P10 titers and IgM anti-P1 reactivity displayed the greatest discrimination capacity to classify SLE and MCTD patients (p ≤ 0.05) (Figure 3 and Supplementary data 3). ROC curves confirmed that among the conventional tests evaluated, elevated DNA and positive results for Sm are the third and fourth best at significantly segregate SLE and MCTD (p ≤ 0.05).
Figure 3. Area under the curve analysis reveals the classification power of IgM anti-P4/P10.
Receiver operating characteristic (ROC) curves were generated using peptide antigenicities or laboratory tests. The columns in the graph represent the area under the curve (AUC) on the y axis for each variable tested. The bars on top of each column indicate standard error of the mean. FANA titers, dsDNA, ↑DNA (elevated serum DNA) and positive results for RNP, Sm, SSA, SSB and SCL are clinical tests used during SLE and MCTD diagnosis. The “IgM anti-P4/P10” indicates the combined IgM anti-P4/P10 titer while “IgM anti-P4/P10 + ↑DNA” represents the combination of the IgM anti-P4/P10 ELISA and the elevated DNA assay. The “*” and “**” indicate significant differences in classifying SLE and MCTD with p values of ≤ 0.05 and ≤ 0.0001, respectively.
Improving the discriminatory capacity of IgM anti-P4/P10 titers
BLR analyses were performed to assess whether the combination of the IgM anti-P4/P10 system and any of the eight laboratory tests employed to diagnose SLE or MCTD (FANA titers, dsDNA ELISA, elevated serum DNA titers and positive results for RNP, Sm, SSA, SSB and SCL-70)4; 15–16; 21–22 might provide greater capacity to distinguish between these syndromes. The individual IgM reactivities for P1 and P13 were considered in this BLR analysis because they showed a significant ability to classify SLE and MCTD patients (p ≤ 0.05) (Figure 1D). BLR analyses indicated that the combination of the IgM-based reactivity for P4/P10 and an elevated DNA assay represent the best combination of variables to segregate SLE from MCTD when compared with IgM anti-P4/P10, -P1, or-P13 and any single laboratory test examined (p ≤ 0.0001) (Figure 3 and Supplementary data 3). None of the other clinical test combinations improved the power of discrimination between SLE and MCTD patients over that exhibited by the individual tests alone (p ≤ 0.05). Our analyses also suggest that, when combined with the standard elevated DNA test, the IgM response against P4/P10 may be useful in enhancing the current segregation of SLE from MCTD.
Discussion
Despite the fact that MCTD was described as a distinct rheumatic syndrome in 19727, placement of this disorder as a separate auto-immune illness remains controversial. Opinions are divided regarding classification of MCTD as a separate malady due to the number of auto-antigens and clinical symptoms that show overlap with SLE1–13. The immune responses of SLE and MCTD patients for overlapping ‘self’ antigens coupled with the diversity of commercially available clinical tests with differing protocols, reagents and cut-off values have impeded the development of standard and uniform assays to segregate these syndromes2, 4, 6, 21. With the exception of a few studies24, 29–31, most investigations have focused on IgG-mediated reactivity toward specific antigens as potential molecular tools to differentiate between SLE and MCTD patients34–38. Given that SLE and MCTD patients are characterized by elevated blood titers of multiple Ig classes, including IgM24, 39–40, we hypothesized that IgM responses to a number of U1 snRNP peptides may allow us to increase the present discrimination between SLE and MCTD and provide additional molecular evidence to claim the independent nature of these two disorders.
Our data indicate that the combined IgM reactivity for fragments of U1C (P4) and U1A (P10) is capable of classifying SLE and MCTD patients with an accuracy of 71.3% (Figure 2B), a value higher than previously reported peptide-based immunoassays that have been used to segregate these disorders21. These findings are in concordance with previous reports, which revealed a preponderance of IgM anti-U1 snRNP antibodies in SLE, but not MCTD patients24, 30. Therefore, our work is congruent with prior investigations and demonstrates the potential utility of differential Ig class responses as a classification tool for SLE and MCTD. The current work also provides molecular evidence to support the distinct etiology of these syndromes.
The binomial analyses identified combinations of laboratory tests and/or peptide reactivities that significantly discern between these maladies. Interestingly, the IgM anti-P4/P10 ELISA-based system provided the greatest capacity to segregate between SLE and MCTD disorders than eight other conventional laboratory tests (p ≤ 0.0001) (Figure 3). Additionally, we revealed that the combination of IgM anti-P4/P10 antigenicity with the elevated DNA test segregated 79.8% of SLE and MCTD patients, even in the smaller subset of patients for whom clinical test results were available (n = 59 for SLE and n = 24 for MCTD) (Figure 3). It is not surprising that the dsDNA test contributes to the differentiation of these diseases given that antibodies against DNA have been detected in approximately 70% of SLE patients and shows 95% specificity for this disorder (16; 18). Yet, the fact that the dsDNA test alone exhibits a lower ability to segregate SLE and MCTD patients (66.4%) than the IgM anti-P4/P10 system (73.1%), indicates the significant contribution of our ELISA-based system in discerning between these two maladies (Figure 3).
We delineated a total of 16 out of 40 clinical manifestations that differed significantly between SLE and MCTD patients (Table 3). On average, MCTD patients exhibited hand/joint swelling and muscle weakness with 25% higher frequency than SLE patients. Similarly, malar and discoid rashes were found to be more prevalent in the SLE than the MCTD group (46% and 10% versus 13% and 0%, respectively). These findings are in concordance with previous studies that reported these clinical manifestations as key features in SLE or MCTD patients19–20. Evidence of mental illness was also found to be 32% higher in MCTD than SLE patients. Although we cannot rule out selection bias of the clinicians diagnosing these disorders, our results obtained from a subset of SLE and MCTD patients suggest that the immune response of SLE patients seems to be directed to skin areas on the face while those suffering from MCTD appear to develop a more systemic immune response that attacks the skin, joints and muscles throughout various parts of the body. Furthermore, these findings highlight specific clinical manifestations that appear to differ between SLE and MCTD patients and should be considered as clinical evidence that they may be distinct diseases.
Overall, this study further highlights the current challenges in developing quantitative tests for the classification of SLE and MCTD and therefore the recognition of MCTD as a separate entity4,2,6,21. Here, we describe a novel approach based on differential antibody class (IgM and IgG) responses as a mechanism to discriminate between SLE and MCTD patients with better accuracy than conventional laboratory tests currently employed as part of the classification criteria to diagnose these syndromes. In addition, our data revealed contrasting frequencies of clinical symptoms characterizing these auto-immune syndromes whereby SLE patients showed a concentrated auto-immune manifestation directed to skin areas on the face while those suffering from MCTD developed more systemic immune responses that attack the skin, joints and muscles throughout various parts of the body. Consequently, our results provide further evidence to support the fact that there are molecular and clinical aspects of SLE and MCTD to indicate that these diseases are, indeed, two distinct autoimmune syndromes.
Supplementary Material
Acknowledgments
This study was supported by NIH/NIGMS R25 GM061347 (to AM), American Cancer Society PF-11-036-01-DDC (to JAS), the Department of Veterans Affairs, the Lupus Research Institute, NIH AR48805 (to ELG) and S06 GM08205 (to RJH). The authors kindly thank Dr. DeEtta Mills for her guidance and support. Additionally, we thank Mr. Alvaro Velandia, Ms. Mylene Rios and Ms. Shaina Henriquez for their participation.
References
- 1.Riemekasten G, Hahn BH. Key autoantigens in SLE. Rheumatol (Oxford) 2005;44:975–982. doi: 10.1093/rheumatology/keh688. [DOI] [PubMed] [Google Scholar]
- 2.Liu CC, Ahearn JM. The search for lupus biomarkers. Best Pract Res Clin Rheumatol. 2009;4:507–523. doi: 10.1016/j.berh.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zdrojewicz Z, Budzyń-Kozioł E, Puławska J. Mixed connective tissue disease--etiology, pathogenesis, clinical significance, treatment. Postepy Hig Med Dosw. 1999;53:751–766. [PubMed] [Google Scholar]
- 4.Egner W. The use of laboratory tests in the diagnosis of SLE. J Clinical Pathol. 2000;53:424–432. doi: 10.1136/jcp.53.6.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greidinger EL, Hoffman RW. The appearance of U1 RNP antibody specificities in sequential autoimmune human antisera follows a characteristic order that implicates the U1-70 kd and B'/B proteins as predominant U1 RNP immunogens. Arthritis Rheum. 2001;44:368–375. doi: 10.1002/1529-0131(200102)44:2<368::AID-ANR55>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 6.Neogi T, Gladman DD, Ibanez D, Urowitz M. Anti-dsDNA antibody testing by Farr and ELISA techniques is not equivalent. J Rheumatol. 2006;33:1785–1788. [PubMed] [Google Scholar]
- 7.Sharp GC, Irvin WS, Tan EM, Gould RG, Holman HR. Mixed connective tissue disease--an apparently distinct rheumatic disease syndrome associated with a specific antibody to an extractable nuclear antigen (ENA) Am J Med. 1972;52:148–159. doi: 10.1016/0002-9343(72)90064-2. [DOI] [PubMed] [Google Scholar]
- 8.López-Longo FJ, Fernández J, Monteagudo I, Rodríguez-Mahou M, Sánchez-Atrio AI, Pérez T, Escalona M, González CM, Lapointe N, Carreño L. Clinical and serologic course of patients with mixed connective tissue disease. Rev Clin Esp. 1994;194:682–688. [PubMed] [Google Scholar]
- 9.Aringer M, Steiner G, Smolen JS. Does mixed connective tissue disease exist? Yes. Rheum Dis Clin North Am. 2005;31:411–420. doi: 10.1016/j.rdc.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Swanton J, Isenberg D. Mixed connective tissue disease: still crazy after all these years. Rheum Dis Clin North Am. 2005;31:421–436. doi: 10.1016/j.rdc.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Venables PJ. Mixed connective tissue disease. Lupus. 2006;15(3):132–137. doi: 10.1191/0961203306lu2283rr. [DOI] [PubMed] [Google Scholar]
- 12.von Bierbrauer A, Willert J, Barth P. Histomorphometrical analysis of microvascular abnormalities in connective tissue diseases. Rheumatol Int. 2008;28:253–259. doi: 10.1007/s00296-007-0418-2. [DOI] [PubMed] [Google Scholar]
- 13.Nowicka-Sauer K, Czuszynska Z, Majkowicz M, Smolenska Z, Jarmoszewicz K, Olesinska M, Siebert J. Neuropsychological assessment in mixed connective tissue disease: comparison with systemic lupus erythematosus. Lupus. 2012;21:927–933. doi: 10.1177/0961203312441511. [DOI] [PubMed] [Google Scholar]
- 14.Hochberg MC. Updating the America College of Rheumatology revised criteria for the classification of systemic lupus Erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 15.Isenberg DA, Manson JJ, Ehrenstein MR, Rahman A. Fifty years of anti-dsDNA antibodies: are we approaching journey's end? Rheumatology (Oxford) 2007;46:1052–1056. doi: 10.1093/rheumatology/kem112. [DOI] [PubMed] [Google Scholar]
- 16.Breda L, Nozzi M, De Sanctis S, Chiarelli F. Laboratory tests in the diagnosis and follow-up of pediatric rheumatic diseases: an update. Semin Arthritis Rheum. 2010;4:53–72. doi: 10.1016/j.semarthrit.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Amigues JM, Cantagrel A, Abbal M, Mazieres B. Comparative study of 4 diagnosis criteria sets for MCTD in patients with anti-RNP antibodies. Autoimmunity group of the hospital of Toulouse. J Rheumatol. 1996;12:2055–2062. [PubMed] [Google Scholar]
- 18.Reveille JD. Predicted value of antibodies for activity of systemic lupus Erythematosus. Lupus. 2004;5:290–297. doi: 10.1191/0961203303lu1015oa. [DOI] [PubMed] [Google Scholar]
- 19.Perkins K, Hoffman RW, Bezruczko N. A Rasch analysis for classification of systemic lupus erythematosus and mixed connective tissue disease. J Appl Meas. 2008;9:136–150. [PubMed] [Google Scholar]
- 20.Hoffman RW, Bezruczko N, Perkins K. An external validation study of a classification of mixed connective tissue disease and systemic lupus erythematosus patients. J Appl Meas. 2012;13:205–216. [PubMed] [Google Scholar]
- 21.Mahler M, Stinton LM, Fritzler MJ. Improved serological differentiation between systemic lupus erythematosus and mixed connective tissue disease by use of an SmD3 peptide-based immunoassay. Clin Diagn Lab Immunol. 2005;1:107–113. doi: 10.1128/CDLI.12.1.107-113.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiaro TR, Davis KW, Wilson A, Suh-Lailam B, Tebo AE. Significant differences in the analytic concordance between anti-dsDNA IgG antibody assays for the diagnosis of systemic lupus erythematosus--implications for inter-laboratory testing. Clin Chim Acta. 2011;412:1076–1080. doi: 10.1016/j.cca.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 23.Hoet RM, Kastner B, Lϋhrmann R, Walther J, van V. Purification and characterization if human auto-antibodies directed to specific regions on U1RNA; recognition of native U1RNP complexes. Nucleic Acids Res. 1993;21:5130–5136. doi: 10.1093/nar/21.22.5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vlachoyiannopoulos PG, Guialis A, Tzioufas AG, Moutsopoulos HM. Predominance of IgM anti-U1RNP antibodies in patients with Systemic Lupus Erythematosus. British J of Rheum. 1996;35:534–541. doi: 10.1093/rheumatology/35.6.534. [DOI] [PubMed] [Google Scholar]
- 25.Mesa A, Somarelli JA, Herrera RJ. Spliceosomal Immunophilins. FEBS letters. 2008;582:2345–2351. doi: 10.1016/j.febslet.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buratti E, Baralle D. Novel roles of U1 snRNP in alternative splicing regulation. RNA Biol. 2010;7:412–419. doi: 10.4161/rna.7.4.12153. [DOI] [PubMed] [Google Scholar]
- 27.Somarelli JA, Mesa A, Herrera RJ. A three dimensional model of the U1 small nuclear ribonucleoprotein particle. Ent Res. 2010;40:104–112. [Google Scholar]
- 28.Luyckx A, Westhoven R, Oris E, Papisch W, Bossuyt X. Clinical relevance of measurement of antibodies to individual snU1 RNP proteins. Clinical Chemistry. 2005;51:1888–1890. doi: 10.1373/clinchem.2005.053652. [DOI] [PubMed] [Google Scholar]
- 29.Witte T, Hartung K, Sachse C, Matthias T, Fricke M, Deicher H, Kalden JR, Lakomek HJ, Peter HH, Schmidt RE. IgM anti-dsDNA antibodies in systemic negative association with nephritis. SLE Study Group. Rheumatol Int. 1998;18:85–91. doi: 10.1007/s002960050063. [DOI] [PubMed] [Google Scholar]
- 30.Zhang W, Reichlin M. IgM anti-A and D SnRNP proteins and IgM anti-dsDNA are closely associated in SLE sera. Clin Immunol Immunopathol. 1995;74:70–76. doi: 10.1006/clin.1995.1010. [DOI] [PubMed] [Google Scholar]
- 31.Palafox Sánchez CA, Satoh M, Chan EK, Carcamo WC, Muñoz Valle JF, Orozco Barocio G, Oregon Romero E, Navarro Hernández RE, Salazar Páramo M, Cabral Castañeda A, Vázquez Del Mercado M. Reduced IgG anti-small nuclear ribonucleoprotein autoantibody production in systemic lupus Erythematosus patients with positive IgM anti-cytomegalovirus antibodies. Arthritis Res Ther. 2009;11:R27. doi: 10.1186/ar2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Somarelli JA, Mesa A, Rodriguez R, Avellan R, Martinez L, Zang YJ, Greidinger EL, Herrera RJ. Epitope mapping of the U1 small nuclear ribonucleoprotein particle in patients with systemic lupus erythematosus and mixed connective tissue disease. Lupus. 2011;20:274–289. doi: 10.1177/0961203310387180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muñoz-Paredes JC, Oliveira LG, de Carvalho Braga B, Trevisol IM, Roehe PM. Development and standardization of an indirect ELISA for the serological diagnosis of classical swine fever. Pesq Vet Bra. 1999;19:123–127. [Google Scholar]
- 34.Fries LF, Mullins WW, Cho KR, Plotz PH, Frank MM. Monocyte receptors for the Fc portion of IgG are increased in systemic lupus erythematosus. J Immunol. 1984;132:695–700. [PubMed] [Google Scholar]
- 35.Nishimaki T, Aotsuka S, Kondo H, Yamamoto K, Takasaki Y, Sumiya M, Yokohari R. Immunological analysis of pulmonary hypertension in connective tissue diseases. J Rheumatol. 1999;26:2357–2362. [PubMed] [Google Scholar]
- 36.Lindorfer MA, Schuman TA, Craig ML, Martin EN, Taylor RP. A bispecific dsDNAxmonoclonal antibody construct for clearance of anti-dsDNA IgG in systemic lupus erythematosus. J Immunol Methods. 2001;248:125–138. doi: 10.1016/s0022-1759(00)00348-3. [DOI] [PubMed] [Google Scholar]
- 37.Routsias JG, Kyriakidis N, Latreille M, Tzioufas AG. RNA recognition motif (RRM) of La/SSB: the bridge for interparticle spreading of autoimmune response to U1-RNP. Mol Med. 2010;16:19–26. doi: 10.2119/molmed.2009.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryu HJ, Takeuchi F, Kuwata S, Kim YJ, Lee EY, Lee EB, Song YW. The diagnostic utilities of anti-agalactosyl IgG antibodies, anti-cyclic citrullinated peptide antibodies, and rheumatoid factors in rheumatoid arthritis. Rheumatol Int. 2011;31:315–319. doi: 10.1007/s00296-009-1260-5. [DOI] [PubMed] [Google Scholar]
- 39.Pollard KM, Tan EM. Purification of the Sm nuclear autoantigen. Detection and clinical significance of IgM antibody. Clin Exp Immunol. 1985;60:586–596. [PMC free article] [PubMed] [Google Scholar]
- 40.Kingsmore SF, Thompson JM, Crockard AD, Todd D, McKirgan J, Patterson C, Fay AC, McNeill TA. Measurement of circulating immune complexes containing IgG, IgM, IgA and IgE by flow cytometry: correlation with disease activity in patients with systemic lupus erythematosus. J Clin Lab Immunol. 1989;30:45–52. [PubMed] [Google Scholar]
- 41.Hopkinson ND, Doherty M, Powel RJ. Clinical features and race specific incident/prevalence rates of Systemic Lupus Erythematosus in geographically complete cohort of patients. Ann Rheum Dis. 1994;53:675–680. doi: 10.1136/ard.53.10.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weiss JE. Pediatric systemic lupus erythematosus: more than a positive antinuclear antibody. Pediatr Rev. 2012;33:62–73. doi: 10.1542/pir.33-2-62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.