Abstract
Introduction
Both acute rejection (AR) and major infection events (MIE) can reduce long-term allograft survival. We assessed the simultaneous efficacy of serum and urine biomarker indoleamine 2,3 dioxygenase (IDO) enzyme activity and peripheral blood CD4-ATP levels for AR and MIE association, respectively.
Methods
We prospectively tested 217 blood and 167 urine serial samples, collected monthly for twelve months post-transplant from 29 consecutive children receiving a kidney transplant. The IDO activity was assessed by mass spectrometry assays using the ratio of product L-kynurenine (kyn) to substrate tryptophan (trp). Kyn/trp ratios and blood CD4 T-cell ATP levels were correlated with AR or MIE or stable group (no events) in the next 30 days.
Results
Using absolute cutoffs and allocating to samples to AR, MIE or stable group, mean serum kyn/trp ratios were significantly elevated in the group that experienced AR (p = 0.0007). Similarly, peripheral blood CD4-ATP levels were significantly lower in the group experiencing MIE (p = 0.0351). Urine kyn/trp ratios and blood tacrolimus levels were not different between AR and stable groups. Within-subject analyses, accounting for repeated measures in subjects, also showed that over time, serum kyn/trp ratios were higher prior to acute rejection (p = 0.031) and blood CD4-ATP levels were lower prior to MIE (p = 0.008).
Conclusions
These results from our pilot discovery group suggest that a panel of biomarkers together can predict over- or under-immunosuppression. Further independent validation in a multi-center cohort is suggested.
Keywords: Transplantation, immune monitoring, rejection, infection, pediatrics, kidney, IDO, Immuknow
Introduction
For the most part, organ transplantation remains the treatment of choice for most patients with end-stage organ disease of the kidney, liver, lung or heart. Such patients generally need to be maintained on lifelong extrinsic and multiple immunosuppressive medications to prevent acute rejection episodes (1). With newer more potent medications, the risk of acute rejection has been reduced, though still present. However, infections have emerged as a serious problem, which also affect graft survival (2, 3). Transplant professionals still do not have any good way to assess, at a given point in time, if a given patient is under- or over-immunosuppressed. Drug levels do not work well for a variety of reasons, such as poor correlation with immunosuppressive activity (mycophenolate mofetil), unavailability of levels commercially, (steroids, injectable induction agents) and lack of information on how to combine the effects of multiple drugs. Hence, the development of less invasive diagnostic methods that provide good prediction of acute rejection or infection risk remains a major need in transplantation and perhaps in all diseases where extrinsic immunosuppression is used.
Previous biomarker immune molecules such as FasL, Granzyme B, soluble CD30, FoxP3, IP-10 and fractalkine show strong associations for acute rejection prediction (4–7), but none has been tested adequately to predict both extremes of immunosuppression. The Immuknow® assay (CD4-ATP) (8–10) and serial viral PCR monitoring, such as peripheral blood CMV or EBV monitoring or urine BK virus testing, are used in some situations as markers of over-immunosuppression (11). Current efforts have been directed at large scale and multicenter validation of specific biomarkers such as in the CTOT studies, but primarily for detection of acute rejection. The complexity of the immune system may be such that no one molecule can adequately quantify the overall activity of the immune system. Therefore, a panel of tests, representing both extremes of immunosuppression and adjusting for confounding etiologies, may provide the best discrimination.
Indoleamine 2,3-dioxygenase (IDO) is an inducible enzyme that catabolizes tryptophan (trp) to multiple further intermediaries, the kynurenines (12–16). IDO activity has therefore conventionally been represented as a ratio of L-kynurenine (kyn) to trp. Immune cells and the semi-allogeneic fetus utilize the IDO pathway to establish immune unresponsiveness (17–22). In transplantation, Brandacher et al., using an HPLC platform, demonstrated elevated serum and urine kyn/trp ratio as a biomarker of acute rejection in the first 3 weeks days post-kidney transplant (23).
In this study, we hypothesized that a combination of serum kyn/trp ratios plus CD4-ATP levels, would provide better prediction of infection versus rejection risk than either test alone, out to 1 year post-transplant. We have previously published our proof of concept pilot results with our mass spectrometry platform (24), different from the HPLC platform used in prior studies, using a partial cohort. In this paper, we present the results of the full pilot study when all samples had been collected and assayed.
Results
Study population and outcome events
The study collected 217 blood samples and 167 urine samples from the enrolled 30 consecutive subjects, of whom 1 enrolled subject was subsequently excluded as this subject lived very far away and decided to be followed locally only, with no study samples provided. The demographics of the 29 subjects who comprised the final study group are presented in Table 1. At the initiation of the study, some patients were already in the later part of the first 12 month post-transplant window. All final study group subjects completed study follow up of 12 months post-transplant. There were 10 discrete episodes of acute rejection in 7 subjects and 21 discrete events of major infection in 18 subjects (8 BK viruria, 5 CM only viremia, 1 EB only viremia, 1 CM + EB viremia, 6 transplant pyelonephritis). One subject presented directly at 4 months post-transplant with simultaneous CM and EB viremia and PTLD. Four of the eight BK viruria subjects also developed BK viremia, one of whom also had evidence of BK virus associated nephropathy on biopsy. Four subjects had both acute rejection and major infection. No subject experienced fever + bacteremia in the study period. No subject had simultaneous acute rejection and acute infection.
Table 1.
Study subject demographic characteristics (n= 29)
| Parameter | Mean (SE) or n (%) |
|---|---|
| Recipient age | 12.14 (0.82) |
| Recipient sex male | 18 (62%) |
| Recipient race | |
| Caucasian | 16 (55%) |
| African-American | 9 (31%) |
| Other | 4 (14%) |
| Deceased donor source | 22 (76%) |
| Delayed graft function | 8 (28%) |
| HLA mismatch | 4.58 (median 4.5) |
| Percent PRA > 10 | 3 (10%) |
| First transplant | 26 (90%) |
| Primary renal disease | |
| Hypoplasia/dysplasia | 7 |
| Obstructive uropathy | 8 |
| Glomerulonephritides | 9 |
| Other | 5 |
From these 29 subjects, 217 blood and 167 urine samples were available for analysis of kyn/trp ratios. Blood CD4 ATP level results were available from 180 samples. Tacrolimus level and mycophenolic acid level results were available from 218 and 113 samples, respectively, taken at or within one week of the kyn/trp and CD4-ATP blood samples. The 29 subjects contributed a range of 2–13 samples to the study, median = 8 per subject and mean = 8.07 for serum samples. The earlier subjects already in the later part of the first 12 months were predominantly the subjects contributing less samples.
Sample to event correlations showed elevated serum kyn/trp ratio in AR group
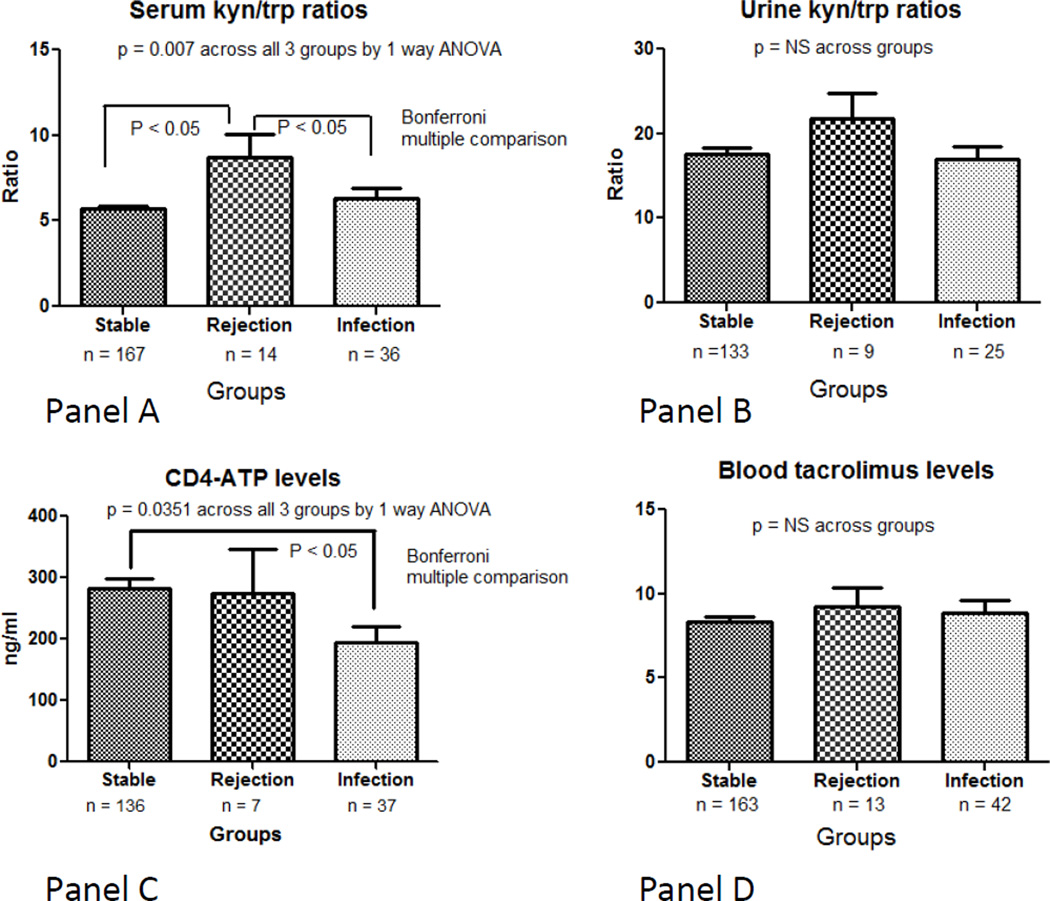
Serum kyn/trp ratios were significantly elevated in the group that experienced acute rejection within the next 30 days (mean ratio 8.690 ± SE 1.344; Figure 1A) compared to the other two groups (ratio 5.688 ± 0.1780 in stable group and 6.302 ± 0.6386 in major infection event group, p value = 0.0007). Bonferroni post-test showed that the AR group had significantly higher kyn/trp ratios compared to BOTH the stable or the infection groups. The stable and infection groups were not different from each other. In contrast, urine kyn/trp ratios were not significantly different among groups (mean 15.12 ± 0.93 stable group versus 17.67 ± 2.60 rejection group versus 15.12 ± 1.31 infection group, p value = 0.64; Figure 1B).
Figure 1.
A. Serum kyn/trp ratios across the three groups (sample size was 167 in stable group, 14 rejection group, 36 infection group). P value = 0.007 by ANOVA across all three groups, significant differences at p < 0.05 between rejection versus stable group and rejection versus infection group by Bonferroni post-test. (In many cases, more than 1 sample was collected for serum/urine IDO, CD4-ATP level or tacrolimus level around an event (eg, a sample within past 30 days, then another sample at time of event).
B. Urine kyn/trp ratios across the three groups (sample size was 133 in stable group, 9 rejection, 25 infection group). P value = 0.30 by ANOVA across all three groups (NS)
C. Blood CD4 ATP levels (Immuknow assay) across the three groups (sample size was 136 in stable group, 7 rejection group, 37 infection group). P value = 0.0351 by ANOVA across all three groups, significant difference at p < 0.05 is between stable and infection group by Bonferroni post-test
D. Blood trough tacrolimus levels in ng/ml across the three groups (sample size was 163 in stable group, 13 rejection group, 42 infection group). P value = 0.53 by ANOVA across all three groups (NS).
When analyzed separately, the mean levels of serum kyn or serum trp, not combined into a ratio, did not significantly differ among the 3 groups. Similarly, mean levels of urine kyn or trp, not combined into a ratio, did not differ significantly between the 3 groups (data not shown).
Blood CD4 ATP levels were significantly lower in the infection group versus the stable group (mean 282.5 ± 16.42 stable group versus 275.1 ± 70.46 rejection group versus 194.2 ± 26.49 infection group, p value 0.0351 across all three groups; Figure 1C).
We also assessed for differences in immunosuppressive drug levels. Trough tacrolimus levels were not significantly different between the three groups (mean value 8.54 ± 0.34 stable group versus 7.85 ± 1.56 rejection group versus 8.95 ± 1.98 infection group, p value 0.78; Figure 1D). Trough mycophenolate levels, collected primarily to identify undetectable values (indicating possible non-compliance), were also not significantly different between groups (data not shown).
We present these ANOVA results because of the established traditions in transplant biomarker analyses in prior studies. However, caution is needed about interpreting these ANOVA, as our previously published diagnostic analysis showed strong associations within subjects, making the assumption of non-association doubtful (24). In that analysis, within- subject association explained at least 40% of the variation in the data.
Within subject analyses showed strong associations between rise in serum kyn/trp ratio to AR, and between drop in CD4-ATP level to MIE
We therefore proceeded with two different types of within-subject analyses. Using the mixed model repeated measures analysis, where all data samples are counted, serum kyn/trp ratios were higher prior to a rejection event than in non-rejection state (+2.2 ± 0.67; p = 0.031; Table 2). The effect sizes represent estimated mean differences before the event versus after the event occurs and were compared to the average of the other values for that subject over the first 12 months. In contrast, urine kyn/trp ratios and CD4-ATP levels did not show any significant differences in the rejection state. In the mixed model analyses, peripheral blood CD4-ATP levels were significantly lower prior to a major infection event than in non-infection state (−82.2 ± 32.0, p = 0.021). Neither serum nor urine kyn/trp ratios differentiated the impending major infection state from the non-infection state. In these analyses, AR and MIE were treated as independent variables and kyn/trp ratios and CD4-ATP levels treated as continuous measures.
Table 2.
Within-subject analyses
| Analysis Type | Parameter | Acute rejection group Amount change (SE) P value |
Major infection group Amount change (SE) p value |
|---|---|---|---|
| Mixed model | Serum kyn/trp ratio | + 2.2 (0.67) P value 0.031 |
+0.62 (0.43) P value 0.16 |
| Urine kyn/trp ratio | +4.22 (2.61) P value 0.25 |
+3.86 (3.31) P value 0.27 |
|
| Serum CD4-ATP level | −19.9 (64.8) P value 0.78 |
−82.2 (32.0) P value 0.021 |
|
| Mean of means | Serum kyn/trp ratio | + 1.68 (2.43) P value 0.20 |
+0.915 (3.35) P value 0.27 |
| Urine kyn/trp ratio | −1.485 (7.78) P value 0.78 |
+7.370 (26.639) P value 0.3584 |
|
| Serum CD4-ATP level | −90.567 (181.66) P value 0.39 |
−144.64 (138.3) P value 0.008 |
Amount change is stable group minus affected group
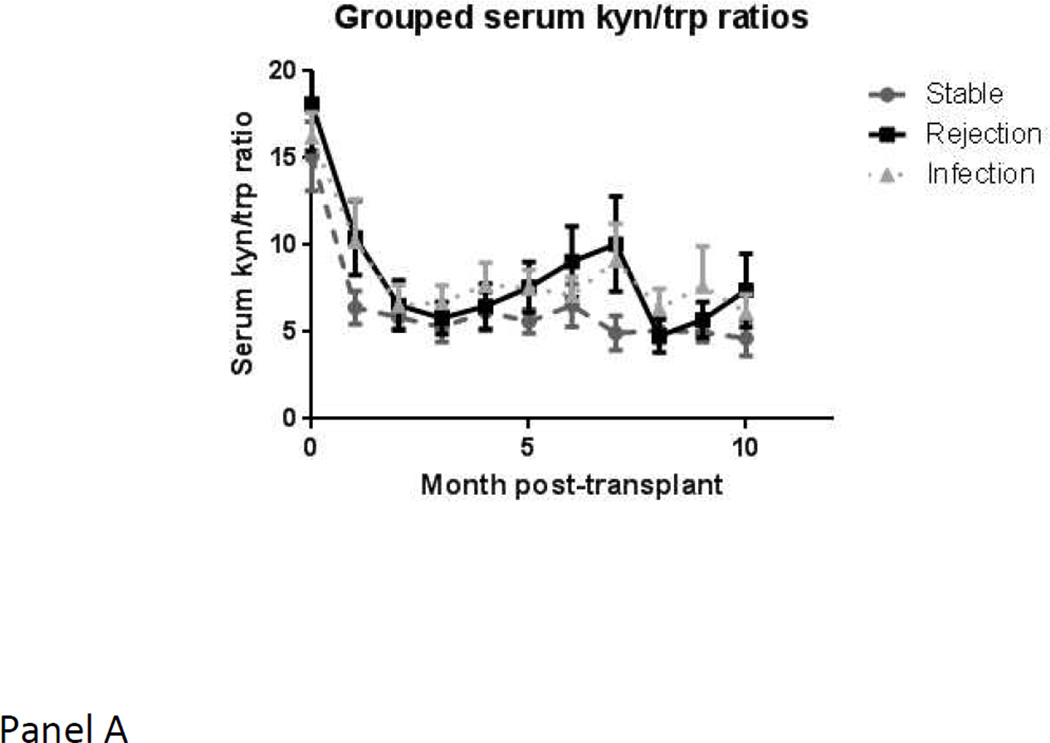
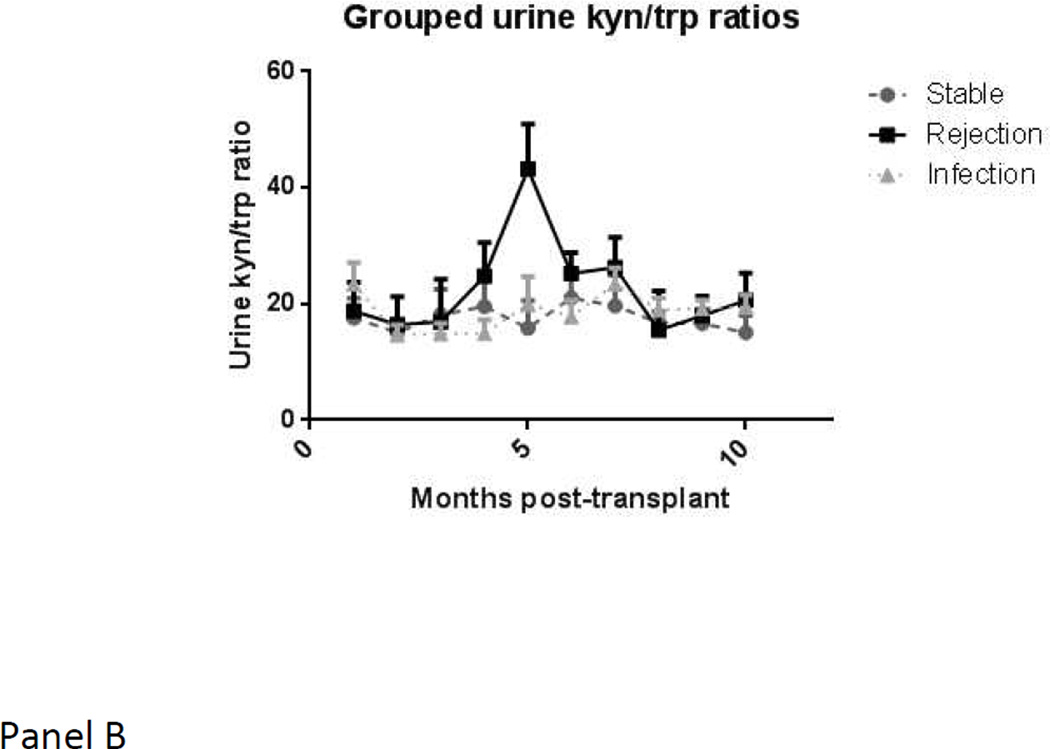
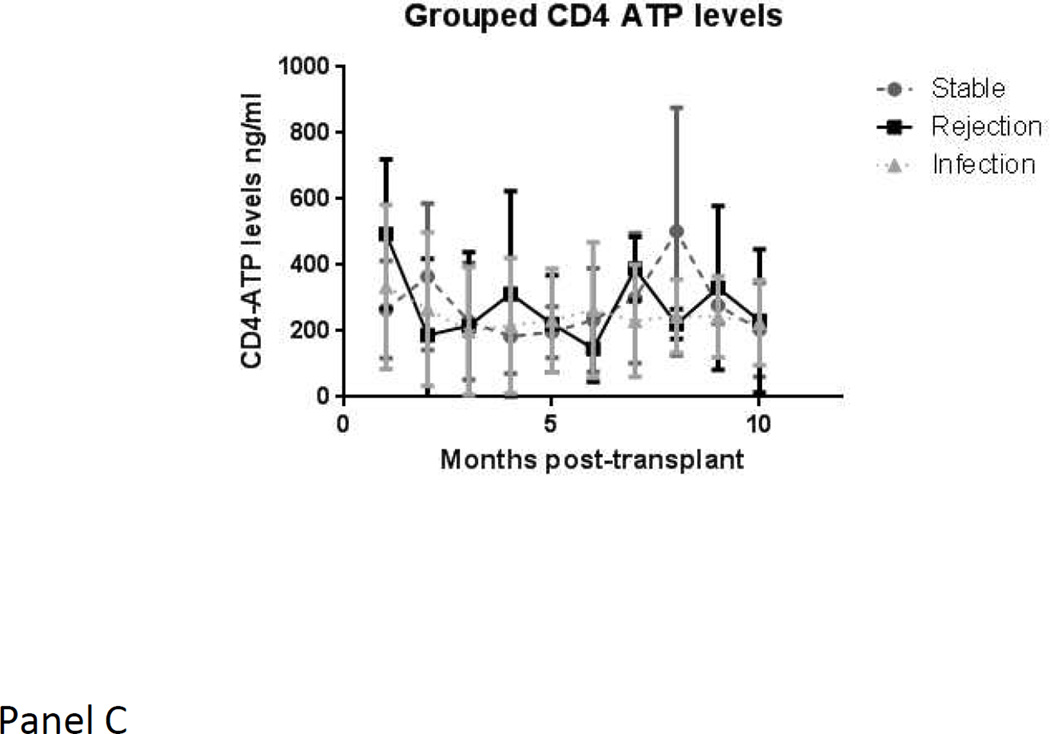
When using the mean of means procedure, which is restricted only to those subjects in whom values are available in more than one state, and therefore may provide different results from a mixed model, the blood CD4-ATP level showed a significant drop of −144.64 points (± 138.3; p value 0.008) from stable state to major infection. In contrast, serum or urine kyn/trp ratios levels did not show any significant changes within a given subject with respect to major infection. Serial mean values for serum and urine kyn/trp ratios and blood CD4-ATP levels, over time, are depicted in Figure 2. Serum kyn/trp ratios, in particular, showed a marked drop from pre-transplant levels in the first few months. The acute rejection group showed a consistently higher level than stable group over time.
Figure 2.
a. Serum kyn/trp ratios over time in the three groups (stable group in grey circles with dashed lines, rejection group in black squares with solid lines, infection group in grey triangles with dotted lines)
b. Urine kyn/trp ratios over time in the three groups (stable group in grey circles with dashed lines, rejection group in black squares with solid lines, infection group in grey triangles with dotted lines)
c. Blood CD4-ATP levels over time in the three groups (stable group in grey circles with dashed lines, rejection group in black squares with solid lines, infection group in grey triangles with dotted lines)
Discussion
This analysis presents data from the full pilot discovery study group of an initial set of subjects tested simultaneously for biomarkers that assess two opposing ends of the immune spectrum. Our results showed a strong association of 1) elevation in serum kyn/trp ratios with subsequent acute rejection events, and 2) decrease in blood CD4-ATP levels with subsequent major infection events.
Our results of serum kyn/trp ratios in relation to acute rejection are in accord with those of Brandacher et al. and Lahdou et al., who have previously demonstrated a rise in serum kyn/trp ratios in association with acute rejection events shortly thereafter, within 13 or 3 days, respectively (23, 25). The studies used differing units of expression for the kyn/trp ratio, but the results were very similar once units are converted to a common unit system, as we reported previously (10). However, ours is the first study, to our knowledge, to evaluate in detail the use of IDO activity as a biomarker out to a longer time frame of 1 year post-transplant. The Brandacher et al. study restricted to events within the first 3 weeks post-transplant only, while the study by Lahdou et al. tested retrospectively collected serum samples out to only 6 months post-transplant.
An increase in the ratio prior to acute rejection represents a paradox since trp depletion has been associated in vitro with a negative regulation of immune activation and T-regulatory cell generation (16). However, this paradox is similar to the results in human and mouse models of rejection analyzed for FOXP3, the signature gene of T-regulatory cells. Urinary FOXP3 expression was increased prior to human acute rejection (4). Intragraft FoxP3 and IDO were elevated in mouse allogeneic islet transplant models (26). This paradox may represent a host anti-donor immune repertoire during acute rejection that includes the activation of graft-destructive effector cells as well as graft-protective T-regulatory cells (4). An alternative explanation is that IDO and FoxP3 upregulation represent an insufficient downregulatory response on the part of the host immune system when alloactivation occurs.
In contrast to the study by Brandacher et al., we were unable to replicate a significant rise in urine kyn/trp ratios in subjects going on to experience an acute rejection event. The reasons for this contrasting result are not clear, but may relate to the longer time interval (out to 1 year post-transplant) studied by us, the potentially greater duration between sample collection to event, or the relatively low frequency of acute rejection events in our study. Of note, though we defined 30 days as upper limit of time from sample collection to event occurrence, in reality all events occurred within 14 days of sample collections.
Holmes et al. (27) postulated that infections would be another major cause of increased IDO enzyme activity, since the enzyme is induced by interferon-γ, which is stimulated by viruses such as cytomegalovirus and Epstein-Barr virus. Other viral infections such as HIV demonstrate increased IDO activity through interferon-γ mediated pathways (28–31). Our study also is unique in studying the association of IDO activity to the relevant MIEs in the first year post-transplant. The more recent era of transplantation is characterized by reduced frequency of acute rejection events and emergence of major infections such as BK virus. Consistent with this trend, our study group exhibited a greater number of major infection events than acute rejection episodes. Of note, the viral infection events detected by PCR monitoring did not lead to full blown disease in most cases, perhaps because of reductions in immunosuppression made in response to these standard of care tests. Holmes et al. found that serum kyn levels were markedly raised, on the day of infection, in 5 patients with Gram negative bacterial infection or viral infections, in a study that was restricted to the first 3 weeks post-transplant (27). However, kyn/trp ratios were not assessed in this study. The study by Brandacher et al. evaluated serum and urine kyn/trp ratios in a small group of 6 subjects with infection events (4 herpes simplex, 1 UTI, 1 sepsis). They were unable to detect any significant differences, probably due to a combination of small event number, time restriction to the first 21 days post-transplant only and 4 of the 6 infection events being relatively minor and localized.
Limitations of our study include the relatively small sample size of subjects and the need for a separate validation group in future. However, the repeated sampling performed greatly added to the power to detect a significant result. Neither we nor others have assessed changes in ratio with timing of meals, which may provide a trp load, or overall dietary intake of trp. The mass spectrometry technique that we employed is considered a more robust detection system than the HPLC platform used in prior studies. Conversely, mass spectrometry has in the past been associated with a higher cost and slower turnaround time. However, both these parameters are changing rapidly. Both costs and turnaround time have dropped enough that some assays for other molecules are already being run commercially in real-time on tandem MS platforms. Therefore a potential limitation of our study may now be a strength, given the greater precision of MS over HPLC. Though the ANOVA data correspond to the mixed model data, ANOVA analyses are not entirely appropriate for longitudinal sequential sampling, such that the p values may not be an accurate reflection of association. We believe that our mixed model results represent the most appropriate statistical tool for analyses of these data, accounting for within-subject variability and taking into account each data point. One could also interpret the similarity in results from the ANOVA and repeated measures techniques (higher kyn/trp ratio with AR, lower blood CD4-ATP with MIE) as indicative of a robust result that holds true irrespective of analytic technique employed.
In summary, our results suggest that less invasive immune monitoring may be able to detect a net state of immune suppression at either end of the spectrum. Further validation studies, including one that we have started, are needed to best define the combination of markers and time points that will provide the best prediction.
Methods
Patient populations and samples
From July 2008 till June 2010, we prospectively and longitudinally tested blood and urine samples from children monthly within the first 12 months post-kidney transplant. This study was approved by the University of Florida Institutional Review Board. All subjects provided full informed consent. The study investigators adhered to all principles outlined in the Declaration of Helsinki and Declaration of Istanbul. Clinical data collected included recipient and donor age/sex/race, donor source, delayed graft function presence or not, concomitant medications and clinical events. Data on serum and urine kyn/trp levels and ratios, blood CD4 ATP levels, trough tacrolimus and mycophenolate levels were correlated with occurrence of acute rejection event (rejection group) or major infection (infection group) event or no event (stable group) in the next 30 days from sample collection. Major infection event was defined as CM viremia, EB viremia, BK viruria (above our local lab cutoff), transplant pyelonephritis (fever > 38.5C + pyuria + positive urine culture for a single organism > 100,000 colonies/ml) or fever with culture proven bacteremia. All acute rejection events were biopsy proven.
Immunosuppression and viral studies
As part of our standard of care, we already perform Immuknow® assay (blood CD4-ATP level) testing and viral PCR monitoring (peripheral blood EBV and CMV and urinary BK virus) on a monthly basis in the first 12 months post-transplant. All PCR assays were performed at our local Shands Hospital Clinical Laboratories and used the same in-house methodology for each PCR amplification. Our current immunosuppression protocol, active since July 2007, includes a 3-day induction course of rabbit anti-thymocyte globulin and intravenous steroids, followed by oral maintenance tacrolimus and mycophenolate mofetil. Oral maintenance steroids beyond day 3 were reserved for specific situations. Standard anti-infective prophylaxis included trimethoprim-sulfamethoxazole three times a week for 6 months, anti-fungal prophylaxis (nystatin or clotrimazole) for 3 months and anti-viral prophylaxis with valganciclovir for 6 months.
L-kynurenine and tryptophan measurement
Serum and urine L-tryptophan and L-kynurenine (Sigma, St Louis, MO) were measured from batched samples stored at −80C by HPLC tandem mass spectrometry using a Thermo TSQ Quantum Ultra spectrometer (Thermo, San Jose, CA). IDO activity was expressed as the ratio of kyn/trp*100. Our detailed methods have been published in our proof-of-principle paper (24).
Statistical analyses
Statistical analyses were performed using GraphPad 5.0 (San Diego, CA, USA) or SAS 9.2 (Cary, NC, USA). Data and estimates for inferential purposes are expressed with mean and standard errors (SE) of estimation or proportions.
The major purpose of the analysis is to delineate potential prognostic factors for events (rejection or infection). Such factors, in combination with other clinical predictors could ultimately result in identifying subjects at greatest risk for events. The number of events in this study is insufficient to consider ROC curves.
Since the data are longitudinal, with repeated measures on subjects, the analysis should take serial correlation within subjects into account. We conducted two types of primary analyses (32, 33) that control in differing ways for within-subject variability: a) mixed model repeated measures analysis, using an autocorrelated structure to account for the timing of the repeated measures for a subject. (This analysis uses the assay as the sampling unit, so that patients contributing more observations are weighted higher); b) mean of means analysis (weights all subjects equally but restricts the analysis to those subjects with observations in more than one state, i.e. uses the subject as the sampling unit). Results are expected to be different since the mean of means test removes subjects whose data are only in one state, whereas the mixed model does not. To the best of our knowledge, these types of analyses have not been performed in prior biomarker longitudinal studies in transplantation. Results from each type of analyses are presented separately.
Some prior studies of immune biomarkers with longitudinal samples have treated each sample as independent, ignoring the clustering effect of repeated observations on the same subject, even if a given subject had samples that were in multiple groups such as the stable group or AR group at different time points (5, 23). This will tend to underestimate the true sampling errors. For example, a patient who had an acute rejection at month 1 and infection at 6 months could have his/her 1-month sample included in the acute rejection group and the 6-month sample included in the infection group. The rationale for such an approach appears to be that clinical decisions of adjustment of immunosuppression are made by the clinical team and not dictated by the biomarker, but may change the net state of immunosuppression in a subject from one time point to another. As such, a given patient may be under-suppressed in one month and develop an acute rejection, then over-suppressed in another month, developing an infection event, making each sample represent a new clinical situation.
We therefore first correlated each sample to events occurring in the next 30 days, irrespective of the subject origin of the sample. Mean values of the biomarkers between the three groups (stable, rejection or infection) were thus compared by ANOVA. These ANOVA methods presume that there is no within-subject association. However, we had previously run diagnostic tests and determined that such assumptions were likely not valid with these biomarkers (24). Past studies by others have often obtained multiple samples from the same subject, yet still used ANOVA and ROC techniques, but our study utilized a planned sequential sampling strategy at defined time points. We lack sufficient numbers of events to obtain meaningful ROC curves for this pilot study.
Finally, note that each P-value is presented without overall error control, such as a Bonferroni correction. This is consistent with pilot studies, where minimizing the overall false positive rate would undermine the false negative rate. Any significant result would need independent confirmation before treating it as conclusive evidence.
Acknowledgements
Funding:
This study was supported in part by the Children’s Miracle Network and the University of Florida Clinical and Translational Research Institute, through the National Institutes of Health (NIH) / National Clinical and Translational Science (NCATS) CTSA grant UL1TR000064.
Abbreviations
- IDO
indoleamine 2,3 dioxygenase
- Trp
tryptophan
- Kyn
kynurenines
- CMV
cytomegalovirus
- EBV
Epstein-Barr virus
- BKV
BK virus
- FasL
Fas Ligand
- FoxP3
forkhead Box P3
- IP-10
Interferon gamma induced protein 10
- IFN-γ
interferon-gamma
- CTOT
Clinical Trials in Organ Transplantation
- HPLC
high performance liquid chromatography
- LC
liquid chromatography
- MS
mass spectrometry
- SRM
selected reaction monitoring
- CE
collision energy
- SE
standard error
- ANOVA
analysis of variance
- ROC
receiver operating characteristic
- AUC
area under the curve
- PTLD
post-transplant lymphoproliferative disorder
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Vikas Dharnidharka: participated in research design, performance of research data analysis, writing of paper
Eihab Al Khasawneh: participated in performance of research, writing of paper
Sushil Gupta: participated in performance of research, writing of paper
Jonathan Shuster: participated in research design, data analysis, writing of paper
Douglas Theriaque: participated in research design and data analysis
Amir Shahlaee: participated in research design, writing of paper
Timothy Garrett: participated in performance of research, writing of paper
Disclosure:
The authors of this manuscript have no relevant conflicts of interest to disclose.
References
- 1.Jackson JA, Kim EJ, Begley B, Cheeseman J, Harden T, Perez SD, et al. Urinary chemokines CXCL9 and CXCL10 are noninvasive markers of renal allograft rejection and BK viral infection. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(10):2228–2234. doi: 10.1111/j.1600-6143.2011.03680.x. Epub 2011/08/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dharnidharka VR, Caillard S, Agodoa LY, Abbott KC. Infection Frequency and Profile in Different Age Groups of Kidney Transplant Recipients. Transplantation. 2006;81(12):1662–1667. doi: 10.1097/01.tp.0000226068.66819.37. [DOI] [PubMed] [Google Scholar]
- 3.Dharnidharka VR, Stablein DM, Harmon WE. Post-transplant infections now exceed acute rejection as cause for hospitalization: a report of the NAPRTCS. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2004;4(3):384–389. doi: 10.1111/j.1600-6143.2004.00350.x. [DOI] [PubMed] [Google Scholar]
- 4.Muthukumar T, Dadhania D, Ding R, Snopkowski C, Naqvi R, Lee JB, et al. Messenger RNA for FOXP3 in the urine of renal-allograft recipients. N Engl J Med. 2005;353(22):2342–2351. doi: 10.1056/NEJMoa051907. [DOI] [PubMed] [Google Scholar]
- 5.Peng W, Chen J, Jiang Y, Wu J, Shou Z, He Q, et al. Urinary fractalkine is a marker of acute rejection. Kidney international. 2008;74(11):1454–1460. doi: 10.1038/ki.2008.459. [DOI] [PubMed] [Google Scholar]
- 6.Strehlau J, Pavlakis M, Lipman M, Shapiro M, Vasconcellos L, Harmon W, et al. Quantitative detection of immune activation transcripts as a diagnostic tool in kidney transplantation. Proc Natl Acad Sci U S A. 1997;94(2):695–700. doi: 10.1073/pnas.94.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Billing H, Sander A, Susal C, Ovens J, Feneberg R, Hocker B, et al. Soluble CD30 and ELISA-detected human leukocyte antigen antibodies for the prediction of acute rejection in pediatric renal transplant recipients. Transplant international : official journal of the European Society for Organ Transplantation. 2013;26(3):331–338. doi: 10.1111/tri.12049. Epub 2013/01/03. [DOI] [PubMed] [Google Scholar]
- 8.Kowalski R, Post D, Schneider MC, Britz J, Thomas J, Deierhoi M, et al. Immune cell function testing: an adjunct to therapeutic drug monitoring in transplant patient management. Clin Transplant. 2003;17(2):77–88. doi: 10.1034/j.1399-0012.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- 9.Kowalski RJ, Post DR, Mannon RB, Sebastian A, Wright HI, Sigle G, et al. Assessing relative risks of infection and rejection: a meta-analysis using an immune function assay. Transplantation. 2006;82(5):663–668. doi: 10.1097/01.tp.0000234837.02126.70. [DOI] [PubMed] [Google Scholar]
- 10.Batal I, Zeevi A, Heider A, Girnita A, Basu A, Tan H, et al. Measurements of global cell-mediated immunity in renal transplant recipients with BK virus reactivation. Am J Clin Pathol. 2008;129(4):587–591. doi: 10.1309/23YGPB1E758ECCFP. [DOI] [PubMed] [Google Scholar]
- 11.Dharnidharka V, Gupta S. Viral immune monitoring for post-transplant lymphoproliferative disorder. Pediatric transplantation. 2009;13(5):521–523. doi: 10.1111/j.1399-3046.2009.01144.x. Epub 2009/03/04. [DOI] [PubMed] [Google Scholar]
- 12.Lob S, Konigsrainer A. Role of IDO in organ transplantation: promises and difficulties. Int Rev Immunol. 2009;28(3–4):185–206. doi: 10.1080/08830180902989119. Epub 2009/10/09. [DOI] [PubMed] [Google Scholar]
- 13.Jia L, Tian P, Ding C. Immunoregulatory effects of indoleamine 2, 3-dioxygenase in transplantation. Transpl Immunol. 2009;21(1):18–22. doi: 10.1016/j.trim.2009.01.004. Epub 2009/07/01. [DOI] [PubMed] [Google Scholar]
- 14.Brandacher G, Margreiter R, Fuchs D. Clinical relevance of indoleamine 2,3-dioxygenase for alloimmunity and transplantation. Current opinion in organ transplantation. 2008;13(1):10–15. doi: 10.1097/MOT.0b013e3282f3df26. Epub 2008/07/29. [DOI] [PubMed] [Google Scholar]
- 15.Mulley WR, Nikolic-Paterson DJ. Indoleamine 2,3-dioxygenase in transplantation. Nephrology (Carlton) 2008;13(3):204–211. doi: 10.1111/j.1440-1797.2007.00921.x. [DOI] [PubMed] [Google Scholar]
- 16.King NJ, Thomas SR. Molecules in focus: indoleamine 2,3-dioxygenase. The international journal of biochemistry & cell biology. 2007;39(12):2167–2172. doi: 10.1016/j.biocel.2007.01.004. Epub 2007/02/27. [DOI] [PubMed] [Google Scholar]
- 17.Ozaki Y, Edelstein MP, Duch DS. The actions of interferon and antiinflammatory agents of induction of indoleamine 2,3-dioxygenase in human peripheral blood monocytes. Biochem Biophys Res Commun. 1987;144(3):1147–1153. doi: 10.1016/0006-291x(87)91431-8. Epub 1987/05/14. [DOI] [PubMed] [Google Scholar]
- 18.Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991;5(11):2516–2522. Epub 1991/08/01. [PubMed] [Google Scholar]
- 19.Beatty WL, Belanger TA, Desai AA, Morrison RP, Byrne GI. Tryptophan depletion as a mechanism of gamma interferon-mediated chlamydial persistence. Infect Immun. 1994;62(9):3705–3711. doi: 10.1128/iai.62.9.3705-3711.1994. Epub 1994/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee GK, Park HJ, Macleod M, Chandler P, Munn DH, Mellor AL. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology. 2002;107(4):452–460. doi: 10.1046/j.1365-2567.2002.01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189(9):1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281(5380):1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 23.Brandacher G, Cakar F, Winkler C, Schneeberger S, Obrist P, Bosmuller C, et al. Non-invasive monitoring of kidney allograft rejection through IDO metabolism evaluation. Kidney international. 2007;71(1):60–67. doi: 10.1038/sj.ki.5002023. [DOI] [PubMed] [Google Scholar]
- 24.Dharnidharka VR, Gupta S, Al Khasawneh E, Haafiz A, Shuster JJ, Theriaque DW, et al. Immune biomarker panel monitoring utilizing IDO enzyme activity and CD4 ATP levels: prediction of acute rejection versus viral replication events. Pediatric transplantation. 2011;15(3):321–328. doi: 10.1111/j.1399-3046.2011.01485.x. Epub February 24, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lahdou I, Sadeghi M, Daniel V, Schenk M, Renner F, Weimer R, et al. Increased pretransplantation plasma kynurenine levels do not protect from but predict acute kidney allograft rejection. Hum Immunol. 2010 doi: 10.1016/j.humimm.2010.08.013. Epub 2010/08/25. [DOI] [PubMed] [Google Scholar]
- 26.Yang H, Ding R, Sharma VK, Hilaire FS, Lagman M, Li B, et al. Hyperexpression of Foxp3 and IDO during acute rejection of islet allografts. Transplantation. 2007;83(12):1643–1647. doi: 10.1097/01.tp.0000263991.74052.46. Epub 2007/06/26. [DOI] [PubMed] [Google Scholar]
- 27.Holmes EW, Russell PM, Kinzler GJ, Reckard CR, Flanigan RC, Thompson KD, et al. Oxidative tryptophan metabolism in renal allograft recipients: increased kynurenine synthesis is associated with inflammation and OKT3 therapy. Cytokine. 1992;4(3):205–213. doi: 10.1016/1043-4666(92)90057-x. Epub 1992/05/11. [DOI] [PubMed] [Google Scholar]
- 28.Fuchs D, Moller AA, Reibnegger G, Werner ER, Werner-Felmayer G, Dierich MP, et al. Increased endogenous interferon-gamma and neopterin correlate with increased degradation of tryptophan in human immunodeficiency virus type 1 infection. Immunol Lett. 1991;28(3):207–211. doi: 10.1016/0165-2478(91)90005-u. Epub 1991/06/01. [DOI] [PubMed] [Google Scholar]
- 29.Schroecksnadel K, Winkler C, Werner ER, Sarcletti M, Romani N, Ebner S, et al. Interferon-gamma-mediated pathways and in vitro PBMC proliferation in HIV-infected patients. Biol Chem. 2009;390(2):115–123. doi: 10.1515/BC.2009.018. Epub 2008/12/02. [DOI] [PubMed] [Google Scholar]
- 30.Favre D, Mold J, Hunt PW, Kanwar B, Loke P, Seu L, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2010;2(32):32ra6. doi: 10.1126/scitranslmed.3000632. Epub 2010/05/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murray MF. Insights into therapy: tryptophan oxidation and HIV infection. Sci Transl Med. 2010;2(32):32ps23. doi: 10.1126/scitranslmed.3001082. Epub 2010/05/21. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan LM, Dukes KA, Losina E. Tutorial in biostatistics. An introduction to hierarchical linear modelling. Statistics in medicine. 1999;18(7):855–888. doi: 10.1002/(sici)1097-0258(19990415)18:7<855::aid-sim117>3.0.co;2-7. Epub 1999/05/18. [DOI] [PubMed] [Google Scholar]
- 33.Shuster JJ, Guo J, Skylar J. Meta-analysis of safety for low event-rate binomial trials. Research Synthesis Methods. 2012;3(1):20–30. doi: 10.1002/jrsm.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]