Abstract
HIV-1 envelope gp120 is the target for neutralizing antibodies (NAbs) against the virus. Various approaches have been explored to improve immunogenicity of broadly neutralizing epitopes on this antigen with limited success. We previously demonstrated that immunogenicity of gp120 and especially its V3 epitopes was enhanced when gp120 was co-administered as immune-complex vaccines with monoclonal antibodies (mAb) to the CD4-binding site (CD4bs). To define the mechanisms by which immune complexes influence V3 immunogenicity, we compared gp120 complexed with mAbs specific for the C2 region (1006-30), the V2 loop (2158), or the CD4bs (654), and found that the gp120/654 and gp120/2158 complexes elicited anti-V3 NAbs, but the gp120/654 complex was the most effective. gp120 complexed with 654 F(ab′)2 was as potent, indicating that V3 immunogenicity is determined by the specificity of the mAb’s Fab fragment used to form the complexes. Importantly, the gp120/654 complex not only induced anti-gp120 antibodies (Abs) to higher titers, but also of greater avidity. The Abs were cross-reactive with V3 peptides from most subtype B and some subtype C isolates. Neutralization was detected only against Tier-1 HIV-1 pseudoviruses, while Tier-2 viruses, including the homologous JRFL strain, were not neutralized. However, JRFL produced in the presence of a mannosidase inhibitor was sensitive to anti-V3 NAbs in the immune sera. These results demonstrate that the gp120/654 complex is a potent immunogen for eliciting cross-reactive functional NAbs against V3 epitopes, of which exposure is determined by the specific compositions of glycans shrouding the HIV-1 envelope glycoproteins.
Keywords: HIV-1 envelope gp120, immune complex, V3 loop, vaccine, antibody, neutralization, N-linked glycans
Introduction
HIV-1 envelope gp120 exhibits an extraordinary degree of variability that poses a major challenge for the development of vaccines against HIV/AIDS [1, 2]. This antigen mediates virus binding to CD4 and the chemokine receptors, and is the key target for virus-neutralizing antibodies (NAbs). However, eliciting broadly NAbs by vaccination remains an unrealized goal. In past trials, recombinant gp120 proteins do not generate cross-reactive NAbs [3–5], but gp120 complexed with anti-CD4-binding site (CD4bs) mAbs is more immunogenic for eliciting NAbs targeting the V3 loop of gp120 [6, 7]. While many HIV-1 isolates are resistant to anti-V3 neutralizing activity [8], anti-V3 NAbs remain a critical arsenal in the fight against HIV-1. Distinct epitopes are present on V3, many of which are highly conserved across subtypes [9]. While some are occluded [10, 11], others are accessible, as indicated by mAbs PGT 127 and PGT 128 that bind to a short beta-strand of the V3 loop and N-linked glycans at the base of this loop [12].
This study utilized the immune-complex strategy capable of inducing antibody (Ab) responses to V3 to understand the mechanisms by which V3 immunogenicity may be modulated to generate higher titers of NAbs. MAb binding to specific gp120 epitopes can alter recognition of distant epitopes by other mAbs [13]. Indeed, V3 antigenicity (in vitro recognition by Abs) is enhanced upon mAb binding to V2 and the CD4bs, but not to C2 [6, 13]. Here we evaluated modulation of V3 immunogenicity in vivo by immunizing mice with gp120 in the presence of the different mAbs and measuring induction of anti-V3 NAbs. The targeted V3 epitopes were characterized for conservation, accessibility, and glycan shielding. The data show that the immune-complex vaccine elicits broadly reactive anti-V3 NAbs, but V3 exposure is regulated by the sugar composition of N-linked glycans on the virus envelope.
Material and Methods
Immunization
Animal studies were approved by the NYU and VA IACUC. BALB/c mice (female, >6 weeks from Jackson lab, 5 animals/group) were injected with gp120/mAb, gp120 alone or no antigen, plus adjuvant. gp120JRFL and gp120LAI were produced in mammalian CHO cells [14, 15]. MAbs used to make gp120/mAb complexes were: 654 (CD4bs), 1006-30 (C2), 2158 (V2). For neutralization assays, anti-V3 mAbs 694, 447, 2424, and anti-CD4bs mAb NIH45-46 were tested along with a parvovirus B19-specific mAb 1418 as control.
Binding Ab assessment
ELISA to detect Abs binding to gp120 proteins or V3 peptides was performed as described [6]. V3 peptides were from commercial sources and the sequences listed in Supplementary Table S1; the majority of these V3 peptides were recognized by cross-reactive neutralizing human anti-V3 mAbs ([16, 17] and data not shown). To detect Abs to gp120 from pseudoviruses, a sandwich ELISA was performed using sheep anti-gp120 Abs (D7324; 5μg/ml; Aalto Bio Reagents, Dublin, Ireland) and 1%Triton X-treated viruses. Immune sera (1:500) were added for 2hrs, and bound Abs detected with alkaline phosphatase-conjugated goat anti-mouse IgG. Flow cytometry was used to detect Ab binding to HIV-1 envelope on 293T cells. The cells were transfected with HIV-1 env and rev for 48hrs with jetPEI (Polyplus, Illkirch, France).
Neutralization assay
Virus neutralization was measured with TZM-bl cells [18, 19]. Pseudoviruses were produced by co-transfecting 293T cells with env, rev, and pNL4-3.Luc.R-E- or pSG3 using ProFection Kit (Promega, Madison, WI) or polyethylenimine (PEI) MAX40,000, (Polysciences, Warrington, PA). Molecular clones (WITO and YU2) were also generated in 293T cells. Glycan-modified HIV-1JRFL was generated as above in the presence of 25μM kifunensine or 20μM swainsonine (Sigma St. Louis, MO). Sera were heat-inactivated before testing. In some experiments, sera were pre-treated with 40μg/ml V3 or scrambled peptides for 1hr before addition of virus.
Statistical analyses
Data were analyzed using a fixed effects two-way ANOVA followed by the Tukey’s post-hoc 95% confidence interval tests.
Results
gp120 complexed with anti-CD4bs mAb 654 elicited high-avidity V3-specific NAbs
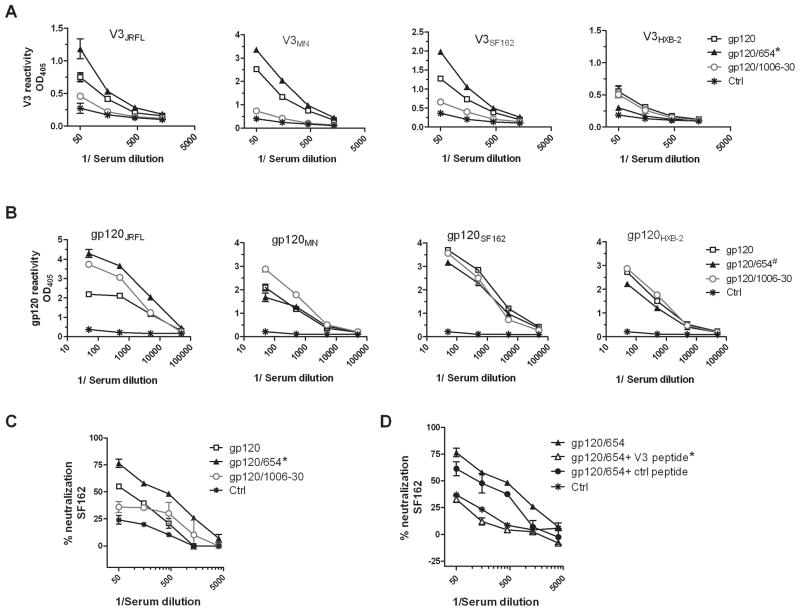
Previous studies have shown that immune complexes of gp120 and anti-CD4bs mAbs were potent immunogens for eliciting anti-V3 NAbs [6]. However, complexes made with other anti-gp120 mAbs had not been evaluated. This study compared gp120 complexed with anti-CD4bs mAb 654 (gp120/654) to that complexed with anti-C2 mAb 1006-30 (gp120/1006-30). gp120JRFL was used in this experiment. Mice immunized with gp120/654 had a high level of serum Abs binding to the homologous V3JRFL, whereas mice immunized with gp120/1006-30 did not produce anti-V3 Abs, similar to the control mice receiving only the adjuvant monophosphoryl lipid A (MPL) and dimethyldioctadecylammonium bromide (DDA) (Fig. 1A). The levels of anti-V3JRFL Abs induced by gp120/654 were also higher than that in uncomplexed gp120-immunized mice, but did not reach statistical significance. The Abs were cross-reactive with V3SF162 and V3MN, but not V3LAI. Interestingly, the anti-gp120 Ab levels were comparably high in mice immunized with gp120/654, gp120/1006-30, and gp120 (Fig. 1B). These Abs were cross-reactive with gp120 from four HIV-1 strains tested. Hence, the gp120/mAb complexes are as immunogenic as uncomplexed gp120 in eliciting gp120-binding Abs, but the complexes elicit anti-gp120 Abs with distinct fine specificities. The gp120/654 complex skews Ab response toward V3, whereas the gp120/1006-30 complex directs Abs to gp120 epitopes other than V3.
Figure 1. Comparison of Ab responses induced by immunization with gp120/654 (CD4bs) versus gp120/1006-30 (C2).
(A) BALB/c mice were immunized i.p. four times every two weeks with gp120JRFL alone or in complex with the anti-CD4bs mAb 654 or the anti-C2 mAb 1006-30. The complexes were prepared by incubating 3 μg gp120 and 9 μg mAb in 50 μl of PBS per animal and mixing them with adjuvant MPL/DDA. Sera were collected two weeks after the last immunization, pooled, diluted serially, and tested in ELISA for binding to V3 peptides from JRFL, MN, SF162, and HXB-2. Sera from control mice injected only with the adjuvant (ctrl) were tested in parallel. *, p<0.05 as compared to gp120/1006-30 and control. OD405, optical density at 405 nm. (B) Sera were also tested in ELISA for binding to recombinant gp120 proteins of JRFL, MN, SF162, and HXB-2. #, p<0.05 as compared to control, but not significantly different from gp120 and gp120/1006-30. (C) Virus-neutralizing activity was assessed against HIV-1 SF162 pseudovirus. Virus was incubated for 1 hr at 37°C with diluted sera and added to TZM-bl cells in the presence of diethylaminoethyl and indinavir. Virus infection was determined after 48 hrs using the Bright-Glo Luciferase Assay System (Promega, Madison, WI) *, p<0.05 as compared to gp120, gp120/1006-30, and control. (D) Sera from mice immunized with the gp120JRFL/654 complex were pre-treated with reactive V3 peptide or non-reactive control peptide (40 μg/ml), and tested for the capacity to neutralize SF162. *, p<0.05 as compared to untreated sera from gp120/654-immunized mice and sera treated with control peptide. Means and standard deviations from one representative experiment are shown. All experiments were repeated at least two times with consistent results.
Immune sera were tested for the capacity to neutralize HIV-1 SF162 pseudovirus, which is highly sensitive to anti-V3 Abs [16]. Sera from gp120/654-immunized mice showed ≥75% neutralization at 1:50 dilution (Fig. 1C and Supplemental Table S2). In contrast, sera from gp120-immunized mice were less potent. Sera from mice immunized with gp120/1006-30 also had poor neutralizing activity (<50%), similar to control sera from mice receiving only adjuvant and pre-bleed sera (Fig. 1C and Supplemental Table S2). Pretreatment with the V3 peptide, but not the scrambled control, reduced neutralization to background (Fig. 1D), demonstrating that the neutralizing activity was mainly by anti-V3 Abs.
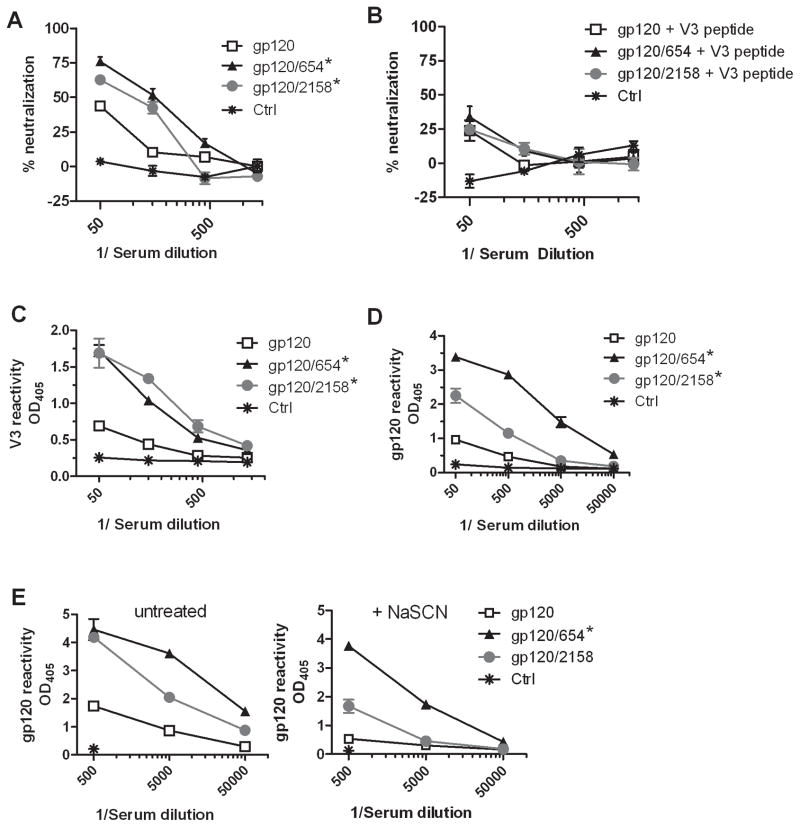
Another experiment was performed to compare gp120/654 with gp120 complexed with an anti-V2 mAb 2158 (gp120/2158). gp120/2158 induced Abs with neutralizing activity, which were significantly higher than control (Fig. 2A and Supplemental Table S2). The neutralizing activity was slightly lower, albeit not significantly different from that achieved with gp120/654. In contrast, gp120 induced weak neutralization, which did not reach significant difference from control. This experiment was done with gp120LAI and neutralization was tested with HIV-1 HXB-2, a LAI molecular clone sensitive to anti-V3 Abs [16]. Indeed, V3 peptide treatment abrogated neutralization induced by gp120/654, gp120/2158, and gp120 (Fig. 2B), confirming the elicitation of V3-specific NAbs by these immunogens. However, because V3 sequence of HIV-1 LAI has unique insertion and deletion at the crown of the loop, neutralization was restricted to the homologous virus (Supplemental Table S2 and [6]). ELISA data further show that gp120/654 and gp120/2158 induced comparable levels of V3-binding Abs (Fig. 2C). Nevertheless, there was a trend that gp120/654 elicited a higher level of gp120-binding Abs than gp120/2158 (Fig. 2D). Moreover, the Abs elicited by gp120/654 displayed higher functional avidity as indicated by the ability of these Abs to better withstand sodium thiocyanate (NaSCN) treatment (Fig. 2E). Ab avidity may be one of the critical immune parameters for anti-viral protection, as it inversely correlated with plasma viremia in SHIV and SIV infection of rhesus macaques [20, 21].
Figure 2. Comparison of Ab responses induced by immunization with gp120/654 (CD4bs) versus gp120/2158 (V2).
(A) BALB/c mice were immunized i.p. four times every two weeks with gp120LAI alone or in complex with the anti-CD4bs mAb 654 or the anti-V2 mAb 2158. Sera from immunized mice were collected two weeks after the last immunization, pooled, diluted serially, and tested for neutralization with TZM-bl target cells and HIV-1HXB-2 (a molecular clone of LAI). Sera from control mice receiving only MPL/DDA were also tested. Means and standard deviations from one representative experiment are shown. *, p<0.05 as compared to ctrl sera. (B) Sera from immunized mice were pre-treated with reactive V3HXB-2 peptide (40μg/ml) and tested to neutralize HIV-1HXB-2. The averages and standard deviations from one representative of at least two repeat experiments are shown. (C–D) Sera from the different groups of mice were also tested in ELISA for IgG reactivity to V3HXB-2 peptide (C) and gp120LAI (D). Results from one of two repeat experiments are shown. * p<0.05 as compared to gp120 and ctrl for (C). * p<0.05 as compared to ctrl for (D). (E) Relative avidity of anti-gp120 Abs generated in the different groups of mice was assessed by NaSCN treatment. ELISA plates were coated overnight at 4°C with gp120 (1μg/ml). Diluted immune sera were reacted with gp120 for 1.5 h and treated with 1.5 M NaSCN or PBS for 10 min. The levels of IgG that remained bound onto gp120 were detected by alkaline phosphatase-conjugated secondary anti-mouse IgG Abs. Means and standard deviations from one of two independent experiments are shown. *, p<0.05 as compared to gp120 and ctrl. OD405, optical density at 405 nm.
Given the enhanced NAb response induced by gp120/654, we evaluated this vaccine for i.p. or s.c. delivery with different adjuvants. High neutralization titers were induced whether gp120/654 was delivered in MPL/DDA i.p., MPL/DDA s.c., Sigma Adjuvant System i.p., or QS- 21/MPL s.c. (Supplemental Fig. S1 and Supplemental Table S2). V3-specific Ab responses were detected in all groups, with significantly higher titers in animals receiving gp120/654 in QS- 21/MPL and MPL/DDA via the s.c. route (Supplemental Fig. S1).
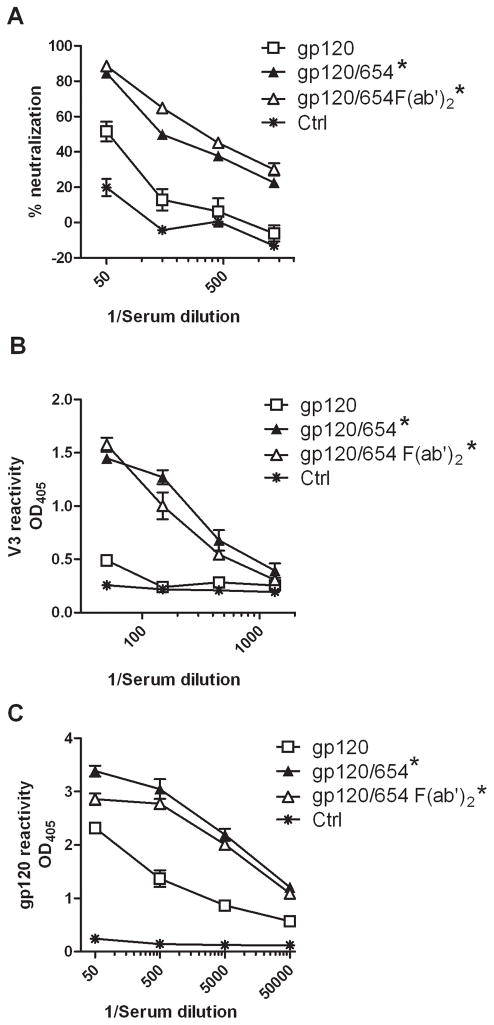
Comparison of the gp120/mAb complexes shows the importance of antigen-binding site fragment (Fab), rather than Fc fragment, for V3 immunogenicity. To confirm this, we immunized mice with gp120 complexed with 654 as intact IgG or F(ab′)2 fragment. Sera from mice immunized with gp120 in complex with 654 IgG or F(ab′)2 had comparable neutralization (Fig. 3A). Similarly high levels of Abs to V3 and gp120 were also elicited by gp120/654 IgG and gp120/654 F(ab′)2, but not gp120 alone (Fig. 3B–C). Hence, V3 immunogenicity was enhanced by 654 Fab activity.
Figure 3. Comparison of Ab responses induced by immunization with gp120/654 made with whole IgG or F(ab′)2.
BALB/c mice were immunized with gp120LAI alone or in complex with intact IgG or F(ab′)2 fragment of mAb 654. F(ab′)2 was produced by pepsin digestion in 20 mM acetate buffer (pH 4.0) at 37°C for 4 hrs and purified by size exclusion chromatography. Sera from mice immunized with PBS and the MPL/DDA adjuvant were tested for control. (A) Sera were collected two weeks after the last immunization, pooled, diluted serially, and tested for neutralization against HIV-1 HXB-2 with the TZM-bl target cells. (B–C) Sera were also tested in ELISA for IgG reactivity to V3HXB-2 peptide (B) and gp120LAI (C). Data from one of two independent experiments are shown. OD405, optical density at 405 nm. * p<0.05 as compared to gp120 and ctrl.
gp120JRFL/654 elicited Abs broadly reactive against V3 of B and non-B subtypes
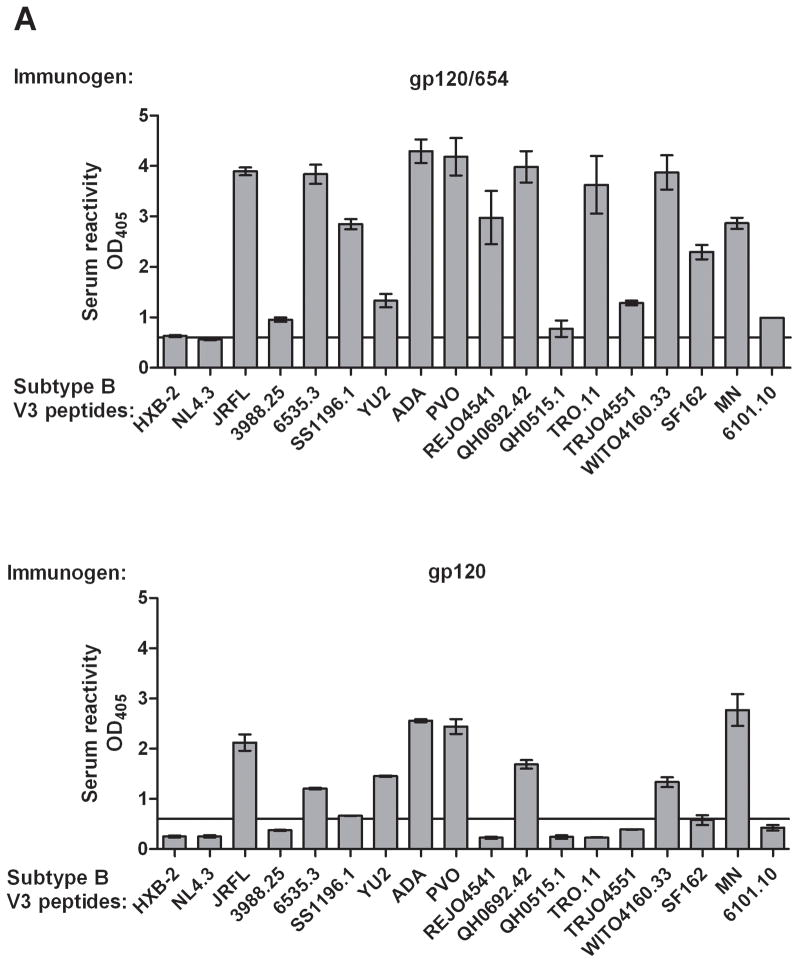
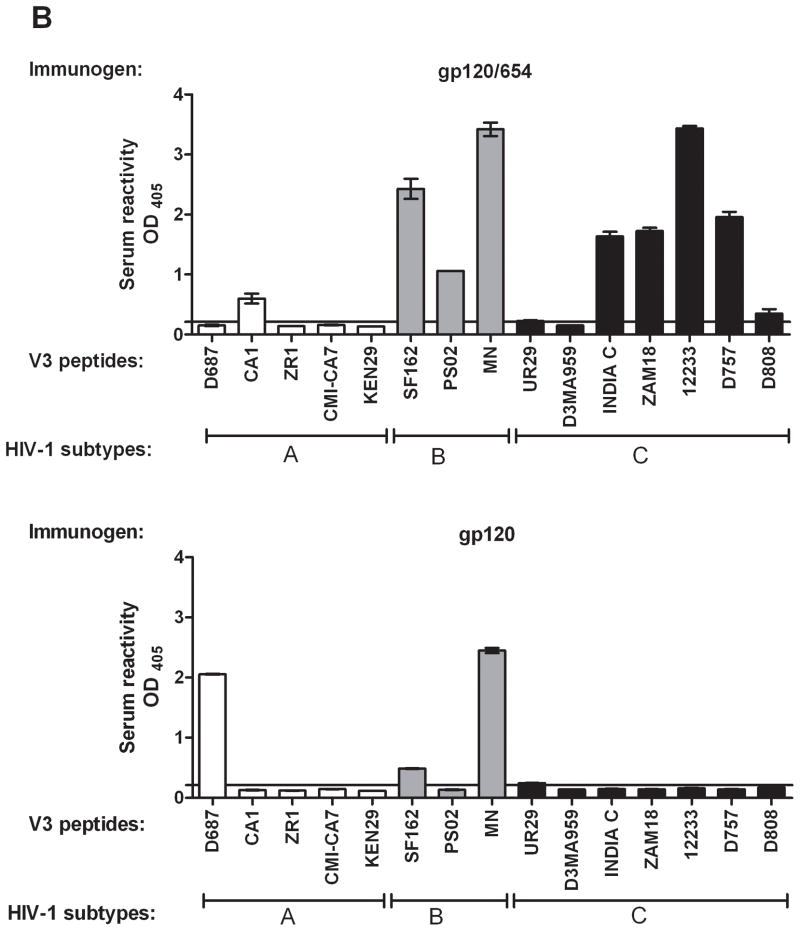
To investigate the breadth of anti-V3 Abs elicited by gp120/654, sera from mice immunized s.c. with gp120JRFL/654 in MPL/QS-21 were first evaluated for IgG reactivity against 18 subtype-B V3 peptides from representative Tier 1, Tier 2 and Tier 3 HIV-1 isolates [14, 22, 23]. Serum IgG from these mice reacted with 15 peptides tested (Fig. 4A top). Only peptides from two molecular clones of HIV-1LAI that have unusual V3 sequences (HXB-2 and NL4.3) were not recognized. Sera from gp120JRFL-immunized mice recognized ten peptides at lower levels (Fig. 4A bottom). Analyses with additional V3 peptides from B and non-B subtypes demonstrate cross-reactivity of serum IgG from gp120JRFL/654-immunized mice against one more subtype-B V3, four subtype-C V3, and one subtype-A V3 (Fig. 4B top). Sera from gp120JRFL-immunized mice were not as cross-reactive against non-B subtypes, recognizing only one subtype-A V3 (Fig. 4B bottom). Control sera showed no reactivity against these peptides. Most of these subtype-C and subtype-A V3 peptides were recognized by the neutralizing human anti-V3 mAb 447 [17]. These data demonstrate the ability of the gp120JRFL/654 complex to induce anti-V3 Abs broadly reactive across B and C subtypes.
Figure 4. Induction of serum Abs cross-reactive with V3 peptides of B and non-B subtypes by the gp120JRFL/654 complex.
(A) Sera from mice immunized with the gp120JRFL/654 complex (upper panel) or gp120JRFL (bottom panel) was tested for reactivity against a panel of subtype B V3 peptides. (B) Reactivity of sera from mice immunized with the gp120JRFL/654 complex (upper panel) or gp120JRFL alone (lower panel) against V3 peptides of B and non-B subtypes. For control, sera of animals injected with adjuvant only were tested in parallel and used to calculate the cut-off for positive reactivity (mean + 3 standard deviation, depicted as horizontal lines). Peptides were either covalently linked to Nunc plates (panel A) or coated using the standard protocol onto the 96-microwell plates (panel B) and reacted with sera at a 1:100 dilution. Alkaline phosphatase-conjugated anti-mouse IgG was used as the secondary Ab. Means and standard deviations from one of two independent experiments are shown. OD405, optical density at 405 nm.
Anti-V3 NAbs elicited by gp120JRFL/654 had restricted activity due to V3 masking
Sera from mice immunized with the gp120JRFL/654 complex or gp120JRFL were tested for neutralization breadth against ten HIV-1 pseudoviruses (Table 1). Sera from gp120JRFL- immunized mice had low levels of neutralization against two viruses, MN and SF162 (Table 1). By contrast, sera from gp120JRFL/654-immunized mice displayed potent neutralization against Tier 1 viruses SF162, MN, 3988.25, and SS196.1. Nevertheless, poor or no neutralization was detected against Tier 2 viruses, including the homologous JRFL, even though high titers of Abs binding to V3 of JRFL and many other viruses were induced by gp120JRFL/654 (Table 1 and Fig. 4). These results suggest the poor accessibility of neutralizing V3 epitopes on the native envelope of JRFL and other resistant viruses by gp120/654-induced Abs.
Table 1.
Neutralizing and binding activities of serum Abs from mice immunized with gp120 versus gp120/654
| Mice immunized with | HIV-1 isolate | Tiera | % Neutralizationb | Ab binding to V3c | Ab binding to gp120d | Ab binding to native Envf |
|---|---|---|---|---|---|---|
| gp120JRFL | SF162 | 1A | 51.9 ± 4.4 | +e | +++ | Yes |
| MN | 1A | 51.0 ±3.8 | +++ | + | nt | |
| HXB-2 | 1B | ≤20 | − | ++ | nt | |
| SS1196.1 | 1B | ≤20 | + | ++ | nt | |
| 6535 | 1B | ≤20 | ++ | ++ | nt | |
| 3988.25 | 1B | ≤20 | − | ++ | nt | |
| JRFL | 2 | ≤20 | ++ | +++ | No | |
| YU2 | 2 | ≤20 | ++ | ++ | nt | |
| WITO | 2 | ≤20 | ++ | +++ | nt | |
| TRJO | 3 | ≤20 | − | ++ | nt | |
|
| ||||||
| gp120JRFL/654 | SF162 | 1A | 88.0 ±4.6 | +++ | +++ | Yes |
| MN | 1A | 78.0 ±2.8 | +++ | ++ | nt | |
| HXB-2 | 1B | 28.3 ±3.2 | + | ++ | nt | |
| SS1196.1 | 1B | 70.0 ±2.3 | +++ | ++ | nt | |
| 6535 | 1B | 42.0 ±4.0 | +++ | +++ | nt | |
| 3988.25 | 1B | 56.1 ±1.8 | + | ++ | nt | |
| JRFL | 2 | ≤20 | +++ | +++ | No | |
| YU2 | 2 | ≤20 | ++ | ++ | nt | |
| WITO | 2 | ≤20 | +++ | ++ | nt | |
| TRJO | 3 | 28.4 ±2.3 | ++ | ++ | nt | |
As previously defined in (36).
Neutralization of pseudoviruses with different HIV envelopes by sera (1:50 dilution) collected at two weeks after the last immunization in the standard TZM/bl assay. Neutralization above 50% is shown in bold.
ELISA reactivity against V3 peptides of the corresponding viruses at 1:100 serum dilution.
ELISA reactivity against gp120 proteins from the corresponding viruses at 1:500 serum dilution.
Average OD405 values are summarized as follows: (−) 0.2–0.5; (+) 0.5–1.0; (++) 1.0–2.5; (+++) 2.5–3.5.
Binding to membrane-bound HIV envelopes on transfected 293T cells as measured by flow cytometry; nt: not tested
To test the ability of gp120/654-induced Abs to bind to the membrane-bound envelope trimers, we transfected 293T cells with JRFL env, and measured the binding of serum IgG by flow cytometry. Sera from gp120/654- and gp120-immunize mice did not bind to cells expressing the JRFL envelope, similar to the control sera (Table 1 and Supplemental Fig. S2). In contrast, the same sera were reactive to soluble gp120 and V3 from JRFL and other isolates tested (Table 1). For comparison, 293T cells were transfected with neutralization-sensitive SF162 env, and dose-dependent binding was observed to this envelope. Both immune and control sera did not bind to mock-transfected cells (Supplemental Fig. S2). These results show that immunization with gp120/654 and gp120 elicits Abs that recognize epitopes on soluble gp120 and V3 from many HIV-1 isolates, but these epitopes are not accessible on the membrane-bound envelope of JRFL and other Tier 2 isolates.
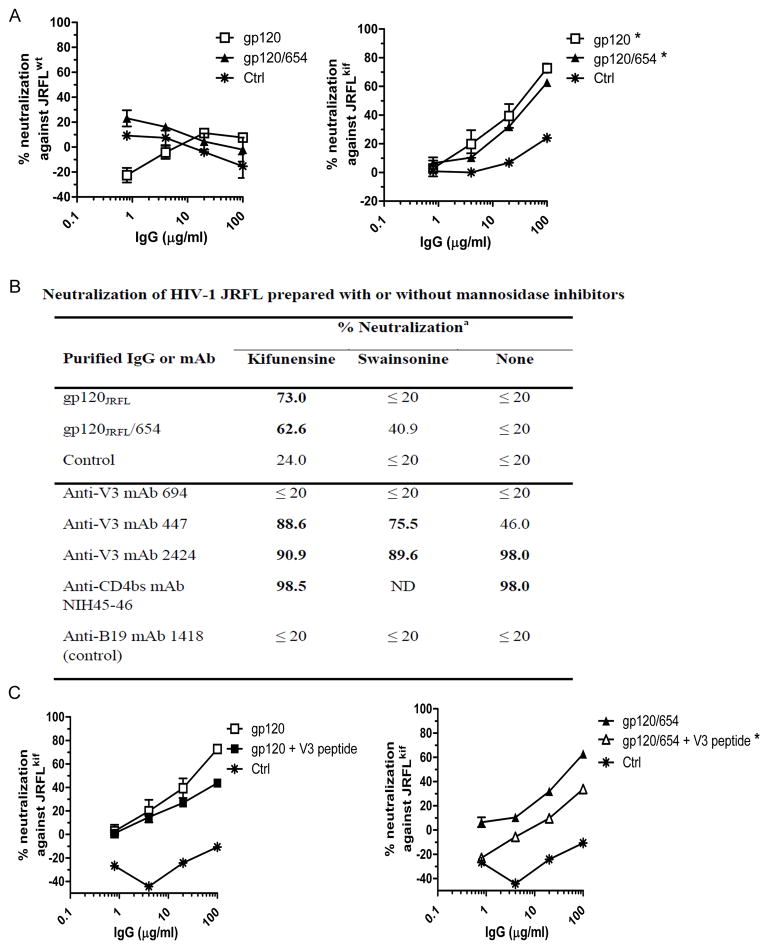
Sera from gp120JRFL/654-immunized mice neutralized JRFL produced with glycosidase inhibitors
Mutations affecting N-linked glycans on HIV-1 envelope are known to affect virus sensitivity to V3-specific NAbs [24, 25]. We next examined the role of glycan composition in occluding V3 epitopes targeted by gp120/654-induced NAbs. To this end, we produced JRFL pseudovirus in the presence or absence of a glycosidase inhibitor (kifunensine or swainsonine). By blocking mannosidases required for the generation of complex-type oligosaccharides, these inhibitors enrich for high mannose-type glycans and modulate HIV-1 neutralization by glycan-dependent mAbs 2G12, PG9, and PG16 [26, 27], but not the CD4bs mAbs b12 and VRC01 [23, 26]. No neutralizing activity was detected in serum IgG from mice immunized with gp120JRFL/654, gp120JRFL, or no antigen against JRFL produced without inhibitors (Fig. 5A and 5B). However, IgG from mice immunized with gp120JRFL and gp120JRFL/654 neutralized JRFL produced with kifunensine (JRFLkif) (Fig. 5A and 5B). IgG from the gp120JRFL/654 immune sera also showed low neutralization against the virus produced with swainsonine (Fig. 5B). IgG from control animals displayed no neutralization against these viruses.
Figure 5. Neutralization of JRFL produced in the presence of mannosidase inhibitors by serum IgG from mice immunized with gp120JRFL or the gp120JRFL/654 complex.
(A) JRFL pseudoviruses were produced in transfected 293T cells in the absence (left panel) or presence of the mannosidase inhibitor kifunensine (right panel), and incubated with protein A-purified IgG from immune or control sera. Neutralization activity was assessed in the TZM.bl target cells. *, p<0.05 as compared to control IgG. (B) Neutralization by serum IgG (100 μg/ml) from immunized mice or mAbs against HIV-1 JRFL pseudovirus generated in 293T cells in the presence or absence of mannosidase inhibitors (kifunensine or swainsonine). MAbs were also tested at 20 μg/ml (447 and 694) or 10 μg/ml (2424, NIH45-46, and 1418). Neutralization above 50% is shown in bold. ND, not done. (C) Serum IgG from mice immunized with gp120JRFL or the gp120JRFL/654 complex were pre-treated with V3 peptide (40 μg/ml) and then tested for neutralization against JRFL produced in the presence of kifunensine (JRFLkif) as described above. Means and standard deviations from one representative experiment are shown. * p<0.05 as compared to untreated gp120/654 IgG.
To verify that neutralization against JRFLkif was mediated by anti-V3 Abs, serum IgG were pre-treated with V3 peptide. Pretreatment with V3 peptide had little effect on JRFLkif neutralization by IgG from gp120-immunized mice (Fig. 5C left panel). However, it significantly reduced neutralizing activities of gp120/654-induced IgG (Fig. 5C right panel). We tested anti-V3 human mAbs for comparison and detected more potent neutralization by mAb 447 when JRFL was made with kifunensine or swainsonine (Fig. 5B). Neutralization activities of anti-V3 mAbs 694 and 2424 were not changed, indicating varying effects of these inhibitors on distinct V3 epitopes. The kifunensine inhibitor also had little effects on neutralization of the CD4bs mAb NIH45-46 (Fig. 5B). Altogether the data indicate that NAbs induced by gp120JFRL/654 target V3 epitopes of which Ab accessibility are determined by the composition of N-linked glycans on the virus envelope.
Discussion
This study demonstrates the capacity of gp120 complexed with anti-gp120 mAbs to modulate induction of Abs in an epitope-specific manner. Hence, gp120/654 (anti-CD4bs mAb) and gp120/2158 (anti-V2 mAb) complexes stimulated Ab responses skewed toward V3, whereas gp120/1006-30 (anti-C2 mAb) directed Abs to gp120 epitopes away from V3. gp120 complexed with anti-V3 mAbs (694 and 1006-15) also blocked Ab responses to V3 ([7] and unpublished data). Similarly, gp120/654 hindered induction of Abs against the CD4bs due to its occupancy by mAb 654 [7]. This study provides direct evidence that the ability of immune complex vaccines to direct Ab responses toward or away from specific epitopes is governed by the Fab fragment of the mAb used to form the complex. Previously immune complex vaccines have been shown to augment immune responses against other viral and bacterial pathogens [22, 28–33], but the activity has been attributed to Fc receptor targeting. Fc-mediated enhancement would likely increase the overall Ab response to gp120 but would not skew the response toward a specific epitope. Rather, our data demonstrates that the Fab fragment is the key component for the immuno-modulatory activity of the gp120/mAb complex. Each mAb due to its Fab’s specificity uniquely modulates V3 immunogenicity. For this reason, the activity would not be apparent with polyclonal Abs that bind different gp120 epitopes and have varying degrees of positive and negative effects on V3 immunogenicity. Interestingly, passive administration of SIV-specific mAbs, but not non-reactive mAbs, also enhanced the quantity and quality of Abs elicited after the subsequent virus challenge in the macaque model [18], although the contribution of immune complex formation was not evaluated.
The molecular basis for enhanced V3 immunogenicity as presented by the gp120/mAb complexes is not fully understood, but our past and present data support the notion that mAb binding to the CD4bs and V2 induces structural alterations in gp120 that affect V3 exposure and stability, resulting in enhanced B cell recognition. Firstly, both gp120/654 and gp120/2158 display enhanced V3 antigenicity, as shown by higher levels of anti-V3 mAb binding to the complexes than uncomplexed gp120. However, V3 antigenicity is greater on gp120/654 than gp120/2158, and the pattern is evident with other anti-CD4bs and anti-V2 mAbs [6]. Importantly, this corresponds with the ability of gp120/654 to elicit Abs with higher avidity and higher neutralizing titers than gp120/2158 (Fig. 2). gp120 complexed with another CD4bs mAb (559/64) was also as potent in eliciting anti-V3 neutralizing Abs [7], indicating the intrinsic activity of anti-CD4bs mAbs. Secondly, the high-affinity interaction of gp120/654 is stable under acid treatment and the complex is more resistant to proteases than uncomplexed gp120 [34]. Previously enhanced V3 immunogenicity was attained with the mutated gp120/654 complexes that also showed enhanced V3 antigenicity and proteolytic resistance [35]. Altogether, these studies indicate that V3 immunogenicity may be predicted by in vitro V3 antigenicity and stability.
Anti-V3 Abs induced by gp120/654 display broad reactivity across subtypes B and C. These data support previous studies showing the presence of conserved V3 epitopes that are immunogenic in humans and animals [36–38]. These epitopes are distinct from glycan-bearing V3 epitopes recognized by the PGT mAbs [12], as they are devoid of glycans and often masked in resistant Tier 2 isolates. Indeed, although gp120/654 induced cross-reactive anti-V3 Abs, the Abs were not effective against Tier 2 isolates, including the homologous JRFL strain. The Abs also did not bind to cell surface-expressed JRFL envelope. The masking mechanisms for these V3 epitopes remain unclear. Previous studies have demonstrated the contribution of the V1V2 loops [11, 39], and V1V2 is shown to converge with V3 on the apex of the envelope trimers [40, 41]. However, how Abs are restricted by V1V2 from accessing the V3 epitopes are not known. Glycans have also been implicated in shielding neutralizing epitopes [42–44]. In particular, removal of N197-linked glycan in the C terminus of V2 was reported to increase HIV-1 89.6 sensitivity to anti-CD4bs and anti-V3 mAbs, and improve elicitation of NAbs in immunized animals [15]. Here we demonstrate for the first time that sugar composition of the glycans influences exposure of V3 epitopes targeted by gp120/654-induced NAbs. When JRFL was grown in the presence of kifunensine, the virus was enriched with high mannose-type glycans and became sensitive to anti-V3 Abs from gp120/654-immunized mice and to human anti-V3 mAb 447. These results suggest that the N-glycan composition affects the packing of the trimeric envelope spike, and that the high mannose-type glycans may relax the intra-molecular interactions among gp120 subunits, rendering V3 more accessible for Ab binding. Nevertheless, the effect varies depending on the specific V3 epitopes. Kifunensine treatment also renders viruses more sensitive to mAb 2G12, although it has little effects on the CD4bs epitopes recognized by mAbs b12, VRC01, and NIH45-46 ([23, 26] and Fig. 5). In contrast, kifunensine disrupts PG9 and PG16 epitopes [26]. Of note, sugar composition of HIV envelope also influences whether HIV virions are taken by dendritic cells for transmission to neighboring CD4 target cells or for antigen degradation and presentation to virus-specific T cells [45]. Interestingly, high mannose-type N-glycans increased the envelope affinity for DC-SIGN and virus capture into the antigen processing pathway, reducing virus transmission. On the other hand, complex-type N-glycans reduced degradation and promotes virus transmission. Altogether, these studies show that sugar composition of N-glycans on the HIV envelope is a critical determinant for immune evasion.
Supplementary Material
Highlights.
HIV-1 gp120/mAb complexes are more potent vaccine immunogens than gp120 alone.
Immune complex made with the anti-CD4bs mAb 654 is the most effective vaccine.
Enhanced immunogenicity of gp120/mAb complexes is due to Fab-mediated activity.
The gp120/654 complexes elicit cross-reactive neutralizing V3-specific Abs.
Sugar moieties on HIV-1 envelope glycans modulate Ab accessibility of V3 epitopes.
Acknowledgments
This paper is dedicated to the memory of our colleague and friend Dr. Jianping Liu.
We thank Sandra Cohen for assistance with serum IgG purification and Barbara Volsky for technical help with generation of HIV-1 pseudotyped viruses. The following reagent was obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: pWITO.c (cat#11739) from Dr. John Kappes; pTRJO4551 clone 58 (cat#11034) from Drs. B. H. Hahn, X. Wei and G. M. Shaw; pSG3Δenv (cat#11051) from Drs. John C. Kappes and Xiaoyun Wu; pYU-2 (cat# 1350) from Dr. Beatrice Hahn and Dr. George Shaw; pHXB2-env (cat#1069) from Dr. Kathleen Page and Dr. Dan Littman; 6535, clone 3 (SVPB5) (cat#11017) from Dr. David Montefiori and Dr. Feng Gao; anti-CD4bs mAb NIH45-46 G54W IgG (cat#12174) from Pamela Bjorkman. The plasmids encoding SS1196.1 and 3988.25 env genes were provided by Dr. D. Montefiori (Duke University). Human mAbs were provided by Drs. S. Zolla-Pazner and M. Gorny (New York University School of Medicine). Adjuvant QS21 was provided by Agenus Inc. gp120JRFL was obtained from Vaccine Research and Development Branch of Division of AIDS, NIAID, NIH.
This work is supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development, VA Merit Review, VA Research Career Scientist Award, and NIH Grant AI048371.
Abbreviations
- NAbs
neutralizing antibodies
- Abs
antibodies
- mAbs
monoclonal antibodies
- CD4bs
CD4-binding site of HIV-1 gp120
- MPL
monophosphoryl lipid A
- DDA
dimethyldioctadecylammonium bromide
- NaSCN
sodium thiocyanate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gaschen B, Taylor J, Yusim K, Foley B, Gao F, Lang D, et al. Diversity considerations in HIV-1 vaccine selection. Science. 2002 Jun 28;296(5577):2354–60. doi: 10.1126/science.1070441. [DOI] [PubMed] [Google Scholar]
- 2.Seaman MS, Janes H, Hawkins N, Grandpre LE, Devoy C, Giri A, et al. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. Journal of virology. 2010 Feb;84(3):1439–52. doi: 10.1128/JVI.02108-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, Martin M, van Griensven F, et al. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. The Journal of infectious diseases. 2006 Dec 15;194(12):1661–71. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- 4.Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. The Journal of infectious diseases. 2005 Mar 1;191(5):654–65. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 5.Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF, et al. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. The Journal of infectious diseases. 2005 Mar 1;191(5):654–65. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 6.Hioe CE, Visciano ML, Kumar R, Liu J, Mack EA, Simon RE, et al. The use of immune complex vaccines to enhance antibody responses against neutralizing epitopes on HIV-1 envelope gp120. Vaccine. 2009 Dec 11;28(2):352–60. doi: 10.1016/j.vaccine.2009.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Visciano ML, Tuen M, Gorny MK, Hioe CE. In vivo alteration of humoral responses to HIV-1 envelope glycoprotein gp120 by antibodies to the CD4-binding site of gp120. Virology. 2008 Mar 15;372(2):409–20. doi: 10.1016/j.virol.2007.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bou-Habib DC, Roderiquez G, Oravecz T, Berman PW, Lusso P, Norcross MA. Cryptic nature of envelope V3 region epitopes protects primary monocytotropic human immunodeficiency virus type 1 from antibody neutralization. Journal of virology. 1994 Sep;68(9):6006–13. doi: 10.1128/jvi.68.9.6006-6013.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang X, Burke V, Totrov M, Williams C, Cardozo T, Gorny MK, et al. Conserved structural elements in the V3 crown of HIV-1 gp120. Nature structural & molecular biology. 2010 Aug;17(8):955–61. doi: 10.1038/nsmb.1861. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal A, Hioe CE, Swetnam J, Zolla-Pazner S, Cardozo T. Quantitative assessment of masking of neutralization epitopes in HIV-1. Vaccine. 2011 Sep 9;29(39):6736–41. doi: 10.1016/j.vaccine.2010.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krachmarov CP, Honnen WJ, Kayman SC, Gorny MK, Zolla-Pazner S, Pinter A. Factors determining the breadth and potency of neutralization by V3-specific human monoclonal antibodies derived from subjects infected with clade A or clade B strains of human immunodeficiency virus type 1. Journal of virology. 2006 Jul;80(14):7127–35. doi: 10.1128/JVI.02619-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pejchal R, Doores KJ, Walker LM, Khayat R, Huang PS, Wang SK, et al. A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science. 2011 Nov 25;334(6059):1097–103. doi: 10.1126/science.1213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore JP, Sodroski J. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. Journal of virology. 1996 Mar;70(3):1863–72. doi: 10.1128/jvi.70.3.1863-1872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Xu CF, Blais S, Wan Q, Zhang HT, Landry SJ, et al. Proximal glycans outside of the epitopes regulate the presentation of HIV-1 envelope gp120 helper epitopes. Journal of immunology. 2009 May 15;182(10):6369–78. doi: 10.4049/jimmunol.0804287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Cleveland B, Klots I, Travis B, Richardson BA, Anderson D, et al. Removal of a single N-linked glycan in human immunodeficiency virus type 1 gp120 results in an enhanced ability to induce neutralizing antibody responses. Journal of virology. 2008 Jan;82(2):638–51. doi: 10.1128/JVI.01691-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hioe CE, Wrin T, Seaman MS, Yu X, Wood B, Self S, et al. Anti-V3 monoclonal antibodies display broad neutralizing activities against multiple HIV-1 subtypes. PloS one. 2010;5(4):e10254. doi: 10.1371/journal.pone.0010254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zolla-Pazner S, Gorny MK, Nyambi PN, VanCott TC, Nadas A. Immunotyping of human immunodeficiency virus type 1 (HIV): an approach to immunologic classification of HIV. Journal of virology. 1999 May;73(5):4042–51. doi: 10.1128/jvi.73.5.4042-4051.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haigwood NL, Montefiori DC, Sutton WF, McClure J, Watson AJ, Voss G, et al. Passive immunotherapy in simian immunodeficiency virus-infected macaques accelerates the development of neutralizing antibodies. Journal of virology. 2004 Jun;78(11):5983–95. doi: 10.1128/JVI.78.11.5983-5995.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herrera C, Spenlehauer C, Fung MS, Burton DR, Beddows S, Moore JP. Nonneutralizing antibodies to the CD4-binding site on the gp120 subunit of human immunodeficiency virus type 1 do not interfere with the activity of a neutralizing antibody against the same site. Journal of virology. 2003 Jan;77(2):1084–91. doi: 10.1128/JVI.77.2.1084-1091.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao J, Lai L, Amara RR, Montefiori DC, Villinger F, Chennareddi L, et al. Preclinical studies of human immunodeficiency virus/AIDS vaccines: inverse correlation between avidity of anti-Env antibodies and peak postchallenge viremia. Journal of virology. 2009 May;83(9):4102–11. doi: 10.1128/JVI.02173-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao P, Patterson LJ, Kuate S, Brocca-Cofano E, Thomas MA, Venzon D, et al. Replicating adenovirus-simian immunodeficiency virus (SIV) recombinant priming and envelope protein boosting elicits localized, mucosal IgA immunity in rhesus macaques correlated with delayed acquisition following a repeated low-dose rectal SIV(mac251) challenge. Journal of virology. 2012 Apr;86(8):4644–57. doi: 10.1128/JVI.06812-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rawool DB, Bitsaktsis C, Li Y, Gosselin DR, Lin Y, Kurkure NV, et al. Utilization of Fc receptors as a mucosal vaccine strategy against an intracellular bacterium, Francisella tularensis. Journal of immunology. 2008 Apr 15;180(8):5548–57. doi: 10.4049/jimmunol.180.8.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mikell I, Stamatatos L. Evolution of cross-neutralizing antibody specificities to the CD4-BS and the carbohydrate cloak of the HIV Env in an HIV-1-infected subject. PloS one. 2012;7(11):e49610. doi: 10.1371/journal.pone.0049610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schonning K, Jansson B, Olofsson S, Nielsen JO, Hansen JS. Resistance to V3-directed neutralization caused by an N-linked oligosaccharide depends on the quaternary structure of the HIV-1 envelope oligomer. Virology. 1996 Apr 1;218(1):134–40. doi: 10.1006/viro.1996.0173. [DOI] [PubMed] [Google Scholar]
- 25.Polzer S, Dittmar MT, Schmitz H, Meyer B, Muller H, Krausslich HG, et al. Loss of N-linked glycans in the V3-loop region of gp120 is correlated to an enhanced infectivity of HIV-1. Glycobiology. 2001 Jan;11(1):11–9. doi: 10.1093/glycob/11.1.11. [DOI] [PubMed] [Google Scholar]
- 26.Doores KJ, Burton DR. Variable loop glycan dependency of the broad and potent HIV-1-neutralizing antibodies PG9 and PG16. Journal of virology. 2010 Oct;84(20):10510–21. doi: 10.1128/JVI.00552-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doores KJ, Fulton Z, Huber M, Wilson IA, Burton DR. Antibody 2G12 recognizes di-mannose equivalently in domain- and nondomain-exchanged forms but only binds the HIV-1 glycan shield if domain exchanged. Journal of virology. 2010 Oct;84(20):10690–9. doi: 10.1128/JVI.01110-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCluskie MJ, Wen YM, Di Q, Davis HL. Immunization against hepatitis B virus by mucosal administration of antigen-antibody complexes. Viral immunology. 1998;11(4):245–52. doi: 10.1089/vim.1998.11.245. [DOI] [PubMed] [Google Scholar]
- 29.Xu DZ, Zhao K, Guo LM, Li LJ, Xie Q, Ren H, et al. A randomized controlled phase IIb trial of antigen-antibody immunogenic complex therapeutic vaccine in chronic hepatitis B patients. PloS one. 2008;3(7):e2565. doi: 10.1371/journal.pone.0002565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wen YM. Antigen-antibody immunogenic complex: promising novel vaccines for microbial persistent infections. Expert opinion on biological therapy. 2009 Mar;9(3):285–91. doi: 10.1517/14712590802715749. [DOI] [PubMed] [Google Scholar]
- 31.Alber DG, Killington RA, Stokes A. Solid matrix-antibody-antigen complexes incorporating equine herpesvirus 1 glycoproteins C and D elicit anti-viral immune responses in BALB/c (H-2K(d)) and C3H (H-2K(k)) mice. Vaccine. 2000 Nov 22;19(7–8):895–901. doi: 10.1016/s0264-410x(00)00222-x. [DOI] [PubMed] [Google Scholar]
- 32.Roic B, Cajavec S, Ergotic N, Lipej Z, Madic J, Lojkic M, et al. Immune complex-based vaccine for pig protection against parvovirus. Journal of veterinary medicine B, Infectious diseases and veterinary public health. 2006 Feb;53(1):17–23. doi: 10.1111/j.1439-0450.2006.00907.x. [DOI] [PubMed] [Google Scholar]
- 33.Ivan J, Velhner M, Ursu K, German P, Mato T, Dren CN, et al. Delayed vaccine virus replication in chickens vaccinated subcutaneously with an immune complex infectious bursal disease vaccine: quantification of vaccine virus by real-time polymerase chain reaction. Canadian journal of veterinary research = Revue canadienne de recherche veterinaire. 2005 Apr;69(2):135–42. [PMC free article] [PubMed] [Google Scholar]
- 34.Tuen M, Visciano ML, Chien PC, Jr, Cohen S, Chen PD, Robinson J, et al. Characterization of antibodies that inhibit HIV gp120 antigen processing and presentation. European journal of immunology. 2005 Sep;35(9):2541–51. doi: 10.1002/eji.200425859. [DOI] [PubMed] [Google Scholar]
- 35.Kumar R, Tuen M, Li H, Tse DB, Hioe CE. Improving immunogenicity of HIV-1 envelope gp120 by glycan removal and immune complex formation. Vaccine. 2011 Nov 8;29(48):9064–74. doi: 10.1016/j.vaccine.2011.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zolla-Pazner S, Cohen SS, Krachmarov C, Wang S, Pinter A, Lu S. Focusing the immune response on the V3 loop, a neutralizing epitope of the HIV-1 gp120 envelope. Virology. 2008 Mar 15;372(2):233–46. doi: 10.1016/j.virol.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 37.Totrov M, Jiang X, Kong XP, Cohen S, Krachmarov C, Salomon A, et al. Structure-guided design and immunological characterization of immunogens presenting the HIV-1 gp120 V3 loop on a CTB scaffold. Virology. 2010 Sep 30;405(2):513–23. doi: 10.1016/j.virol.2010.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zolla-Pazner S, Kong XP, Jiang X, Cardozo T, Nadas A, Cohen S, et al. Cross-clade HIV-1 neutralizing antibodies induced with V3-scaffold protein immunogens following priming with gp120 DNA. Journal of virology. 2011 Oct;85(19):9887–98. doi: 10.1128/JVI.05086-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu L, Cimbro R, Lusso P, Berger EA. Intraprotomer masking of third variable loop (V3) epitopes by the first and second variable loops (V1V2) within the native HIV-1 envelope glycoprotein trimer. Proceedings of the National Academy of Sciences of the United States of America. 2011 Dec 13;108(50):20148–53. doi: 10.1073/pnas.1104840108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mao Y, Wang L, Gu C, Herschhorn A, Xiang SH, Haim H, et al. Subunit organization of the membrane-bound HIV-1 envelope glycoprotein trimer. Nature structural & molecular biology. 2012 Sep;19(9):893–9. doi: 10.1038/nsmb.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White TA, Bartesaghi A, Borgnia MJ, Meyerson JR, de la Cruz MJ, Bess JW, et al. Molecular architectures of trimeric SIV and HIV-1 envelope glycoproteins on intact viruses: strain-dependent variation in quaternary structure. PLoS pathogens. 2010;6(12):e1001249. doi: 10.1371/journal.ppat.1001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malenbaum SE, Yang D, Cavacini L, Posner M, Robinson J, Cheng-Mayer C. The N-terminal V3 loop glycan modulates the interaction of clade A and B human immunodeficiency virus type 1 envelopes with CD4 and chemokine receptors. Journal of virology. 2000 Dec;74(23):11008–16. doi: 10.1128/jvi.74.23.11008-11016.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Binley JM, Ban YE, Crooks ET, Eggink D, Osawa K, Schief WR, et al. Role of complex carbohydrates in human immunodeficiency virus type 1 infection and resistance to antibody neutralization. Journal of virology. 2010 Jun;84(11):5637–55. doi: 10.1128/JVI.00105-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kwong PD, Mascola JR, Nabel GJ. The changing face of HIV vaccine research. Journal of the International AIDS Society. 2012;15(2):17407. doi: 10.7448/IAS.15.2.17407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Montfort T, Eggink D, Boot M, Tuen M, Hioe CE, Berkhout B, et al. HIV-1 N-glycan composition governs a balance between dendritic cell-mediated viral transmission and antigen presentation. Journal of immunology. 2011 Nov 1;187(9):4676–85. doi: 10.4049/jimmunol.1101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.