Abstract
Background: Exposure of the lungs to an antigen or pathogen elicits the formation of lymphoid satellite islands termed inducible bronchus-associated lymphoid tissue (iBALT). However, little is known about how the presence of iBALT, induced by a stimulus unrelated to the subsequent challenge agent, influences systemic immunity in distal locations, whether it be independently, antagonistically, or synergistically. Here, we determined the kinetics of the influenza-specific responses in the iBALT, tracheobronchial lymph node (TBLN), and spleen of mice with and without pre-formed iBALT.
Methods and Results: Mice with VLP-induced iBALT or no pre-formed iBALT were challenged with influenza. We found that, as we have previously described, those mice whose lungs contained pre-formed iBALT were protected from morbidity, and furthermore, that these mice had increased dendritic cell, and alveolar macrophage accumulation in both the iBALT and TBLNs. This translated to similarly accelerated kinetics and intensified influenza-specific CD4+, but not CD8+ T cell responses in the iBALT, TBLN, and spleen. This expansion was then followed by a more rapid T cell contraction in all lymphoid tissues in the mice with pre-formed iBALT.
Conclusions: Thus, iBALT itself may not be responsible for the accelerated primary immune response we observe in mice with pre-formed iBALT, but may contribute to an overall accelerated local and systemic primary CD4+, but not CD8+ T cell response. Furthermore, less damaging immune responses observed in mice with pre-formed iBALT may be due to a quicker contraction of CD4+ T cell responses in both local and systemic secondary lymphoid tissue.
Introduction
The immune response in the lung is dogmatically initiated when sentinel airway dendritic cells (DCs) sample and internalize inhaled antigens or pathogens and then migrate through the afferent lymphatics to the local lymph node.2–6 Within the lymph node, newly migrated DCs present their antigens to T cells which then migrate, as effectors, back to the site of infection. Interestingly however, recent evidence has revealed that an immune educated lung, like other mucosal tissues, may in fact be an effector site that is capable of clearing pathogens independently from the lymph node,7–10 thus suggesting that a second pathway for the initiation and execution of respiratory immunity exists. In this regard, the exposure of the lung to an antigen often elicits the formation of local lymphoid satellite islands termed inducible bronchus-associated lymphoid tissue (iBALT). Organized iBALT is usually found in areas around the bronchioles and adjacent to arteries, and has been reported to be induced in response to diverse respiratory stimuli, including viral infection,8 neonatal exposure to inhaled LPS,11 toll-like receptor ligands,12 small proteins,1,13, cigarette smoke,14,15 or as a result of certain autoimmune disorders.16,17 Importantly, we have previously shown that exposure of the lungs to nonreplicative virus-like particles (VLPs) also induces the formation of iBALT.1
Structurally, iBALT (and other tertiary lymphoid tissues18) are organized similarly to a lymph node, with antigen presenting cells,10,19 high endothelial venules (HEV's),20 and germinal center B cells surrounded by follicular dendritic cells, and T follicular helper cell zones.1,8,21 iBALT may also serve as a site of viral antigen persistence, providing a niche for resting memory lymphocytes that are important in subsequent pathogen exposures.7 Mice with pre-formed iBALT are known to mount accelerated primary immune responses which tend to be less damaging;1,8 however, it is not known whether those effects are strictly the result of a local response in the iBALT, or the iBALT affecting other local or systemic lymphoid tissues. Here, we have specifically microdissected areas of live iBALT from whole lung tissue to show that iBALT, which was induced to develop in response to the intranasal instillation of VLPs, modulated the local lung microenvironment to accelerate the clearance of the influenza virus. Furthermore, the kinetics of responses in the iBALT were similar to those observed in the tracheobronchial lymph node (TBLN) and spleen. These early responses were facilitated by the local accumulation of DCs and alveolar macrophages (AMs), specifically in iBALT areas, and were followed by an accelerated expansion and contraction of influenza-specific CD4+ T cells the iBALT, TBLN, and spleen.
Interestingly, CD8+ T cell responses did not appear to be altered by the presence of pre-formed iBALT. Thus, by utilizing VLPs to prime the lungs, we show that mice, which have not been previously exposed to influenza proteins, were highly efficient at clearing influenza virus, suggesting a role for synergistic effects of iBALT with systemic immunity. Importantly, it has been proposed by us,1,21 and others,13,22–25 that harnessing the function of iBALT may pose an important clinical approach to the augmentation of pathogen clearance.
Methods
Virus-like particle production and purification
Virus-like particles (Methanocaldococcus jannaschii small heat-shock protein 16.5, G41C protein cage nanoparticles) were produced, purified, and characterized, as previously described.1,26
Influenza virus
The mouse-adapted influenza virus A/PR8/8/34 was produced at the Trudeau Institute (Saranac Lake, NY). Briefly, 10-day-old embryonated chicken eggs were infected for 55 hours, and resultant allantoic fluid was recovered and stored at −80°C until used.
Animals and in vivo procedures
Female or male Thy1.1, C57BL/6, or HNT27 mice were bred in-house at Montana State University, maintained in SPF conditions in HEPA-filtered cages, and fed sterile food and water ad libitum. All animal procedures were performed in accordance with protocols pre-approved by the Montana State University Institutional Animal Care and Use Committee (IACUC). Experimental results were confirmed by at least two independent repetitions of similar design with 5 animals per group.
Virus-like particles and subsequent challenges were delivered intranasally (i.n.) in 50 μL volumes to mice lightly anesthetized with 5% inhaled isoflurane. VLP-treatment was delivered in five doses of 100 μg spaced 3 days apart. Mice were then rested for 72 h before challenge with 1.5×103 plaque-forming units (pfu) PR8 influenza virus, delivered in 50 μL volumes i.n. At indicated timepoints per experiment, mice were sacrificed by an intraperitoneal (i.p.) injection of sodium pentobarbital (90 mg/kg) and exsanguinated after no pedal response could be elicited.
Bronchoalveolar lavage (BAL) samples were collected by instilling the lung with 2 mL DPBS with 3 mM EDTA. Resultant cells were spun at 209 g for 10 min at 4°C, and pellets were then resuspended in FcR block (clone 93) prior to staining for FACS. TBLNs and spleens were either homogenized through a wire mesh screen to collect lymphocytes or digested to collect macrophages and DCs. Total cells from each tissue were counted by hemocytometer to allow for total quantification by FACS.
For adoptive transfer studies, the spleens of naïve HNT mice27 (Thy1.2) were sterilely collected and homogenized. Red blood cells were lysed, and remaining cells were labeled with 5 μM CFSE (eBioscience; San Diego, CA). CD4+ T cells were then positively selected by CD4+ T cell columns (R and D Systems; Minneapolis, MN), washed, and resuspended in sterile DPBS. 1.2×107 CD4+ T cells were then adoptively transferred into VLP-treated or control mice (Thy1.1) in 200 μL intravenously (i.v.). Mice were rested for 24 h prior to infection with 1.5×103 pfu PR8.
iBALT microdissection
To specifically obtain areas of iBALT that were not contaminated with additional cellular components of the lung, whole lungs were instilled with 1% warm low-melt agarose (Fluka Analytical; St. Louis, MO) in DPBS and excised en bloc into ice-cold DPBS. 300 μM sections were then cut by vibratome (Leica Microsystems; Wetzlar, Germany) and resultant sections were floated in cold RPMI with 5% FBS (Atlas Biologicals; Fort Collins, CO). Each section was then gently arranged on a microscope slide and flooded with media. Areas of iBALT were identified by dissecting microscopy according to the characteristic density and location (areas of airway bifurcation and adjacency to bronchi and blood vessels), and excised with a scalpel. For control mice whose lungs did not contain areas of iBALT, areas of the lung where iBALT was observed to form in VLP-treated or influenza infected mice were selected, despite the lack of iBALT present. Areas of excised iBALT (or parallel control) were placed in cold RPMI with FBS and remaining parenchymal areas of the lung section were discarded. Total sections from the left lung were analyzed and all areas of iBALT present were excised. For controls, a similar amount of lung tissue was excised, although the cellularity was noticeably decreased. Combined iBALT areas per animal were then either digested in collagenase/DNase, or homogenized through a wire mesh screen. Single-cell suspensions were then filtered through 100 μM mesh, resuspended in FcR block, and subsequently stained for FACS.
FACS staining and antibodies
Single-cell suspensions from indicated organs were filtered through 100 μM mesh, and incubated with FcR block for 10 min on ice. Antibody cocktails were then added and the cells were incubated on ice for an additional 20–30 min. Tetramers were allowed to incubate for 45 min at room temperature. Antibodies purchased from Biolegend (San Diego, CA) included CD11c-PerCP-Cy5.5 (clone N418); CD4-PerCP-Cy5.5 (clone GK1.5); and CD8-PE-Cy7 (clone IM7). Antibodies purchased from BD Biosciences (San Jose, CA) included CD44-PE (clone 515); and Siglec-F-PE (clone E50-2440). APC-conjugated MHC I influenza tetramers H-2Db NP366–374 (NP) and H-2Db PA224–233 (PA) were purchased from the Trudeau Institute Molecular Biology Core Facility (Saranac Lake, NY). Samples were acquired on a FACSCanto (BD Biosciences) and data was analyzed using FlowJo software (Treestar; Ashland, OR). Briefly, forward and side scatter plots were gated on the population of interest (lymphocytes or DCs/macrophages) as determined by size and granularity. Cells were then further analyzed for the expression of appropriate combinations of surface antigens based off negative staining controls. Each individual figure legend further elaborates on the gating strategy used. Total cell numbers were then calculated based off total hemocytometer cell counts for each tissue.
Statistics
Statistical significance was determined by one-way ANOVA with a Bonferroni post-test of multiple comparisons, or in some cases an unpaired t-test using GraphPad Prism (La Jolla, CA). Significance was indicated by *p<0.05, **p<0.01, ***p<0.001, or ****p<0.0001, and figure legends disclose the use of statistics in each scenario. Figures are representative of one independent experiment (n=5 mice per group), and error bars represent the standard error of the mean (SEM).
Results
Pre-formed iBALT protects mice from influenza-associated morbidity
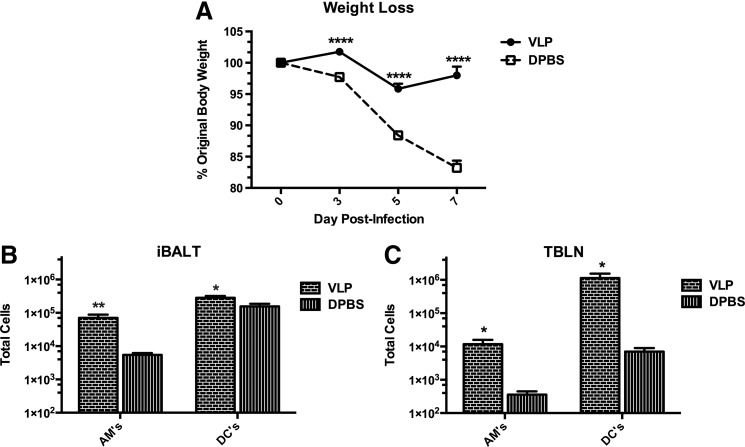
Wild-type mice were intranasally instilled with 100 μg doses of VLPs (or DPBS) five times to develop the formation of iBALT. Our VLPs of choice were derived from small heat shock protein cage nanoparticles,28–30 which as we have previously reported, do not replicate or induce toxic effects upon delivery to the lung.1,31 Mice were then rested for 72 h, and challenged with 1.5×103 pfu of A/PR/8/34 (PR8) influenza virus i.n. As we have previously reported,1 VLP-exposed mice are protected from weight loss due to influenza virus illness (Fig. 1A), indicating a contributory role for the function of iBALT in the clearance of influenza.
FIG. 1.
Pre-formed iBALT protects mice from influenza-associated morbidity and VLP-exposure enhances dendritic cell, and alveolar macrophage accumulation similarly in both the iBALT and TBLNs. Wild-type (WT) mice were i.n. instilled with 100 μg VLPs or 50 μL DPBS in 5 doses spaced evenly over 2 weeks. All mice were then rested for 72 h, and infected with 1.5×103 pfu PR8 influenza virus. Body weight was measured over a 7-day infection course in (A), whereas in (B and C) mice were euthanized at 12 h post-influenza infection and their lungs were inflated with warm agarose, sectioned by vibratome, and total iBALT areas from live lung tissue were specifically microdissected from the left lobe. Total DCs and AMs in iBALT (B) and TBLNs (C) were quantified by FACS and total cell counts. DCs, macrophages, and monocytes were gated on from forward and side scatter plots. DCs were then identified by their high expression of CD11c and lack of Siglec-F, while macrophages were CD11c+Siglec-F+. A t-test was utilized to determine statistical significance between groups (n=5 per group). *p<0.05, **p<0.01.
VLP-exposure enhances dendritic cell, and alveolar macrophage accumulation similarly in both the iBALT and TBLNs
DCs are known to be both essential for the development and maintenance of organized iBALT,10,19 as well as for their antigen processing and presentation function during respiratory virus infection. We thus hypothesized that an increased accumulation of DCs and AMs, specifically in iBALT areas, may beneficially impact the quality of the immune response to influenza infection. We thus determined the role for iBALT-resident AMs and DCs as compared to AMs and DCs in the TBLNs at early timepoints after influenza challenge. Mice were dosed with VLPs or DPBS to establish the formation of iBALT, and were then challenged with influenza virus. At 12 h post-influenza infection, mice were euthanized and cells isolated from live microdissected areas of iBALT, and whole TBLNs were analyzed by flow cytometry for the presence of DCs and AMs. Importantly, we found that in parallel to the local TBLNs, areas of developed iBALT contained a significantly increased accumulation of AMs and DCs (Fig. 1B-C). We thus measured the downstream consequences of DC and AM augmentation by determining the function and proliferation abilities of influenza-specific CD4+ T cells.
Exposure of the lungs to VLPs promotes the expansion, and alters the trafficking of influenza-specific CD4+ T cells
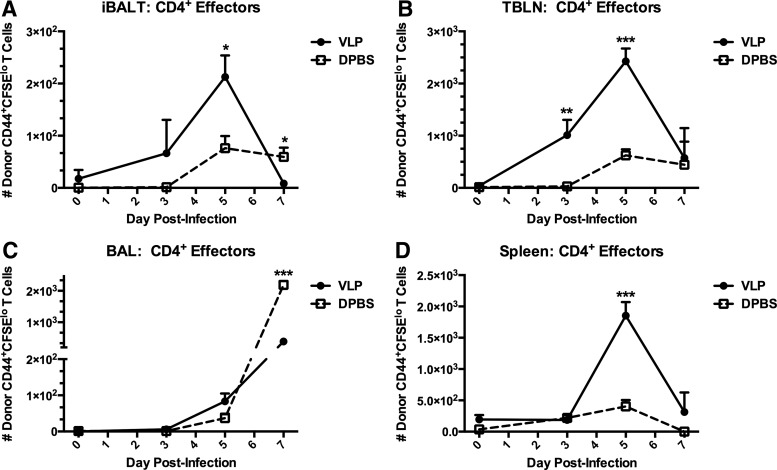
Mice with or without pre-formed iBALT were adoptively transferred with CFSE-labeled naïve transgenic CD4+ T cells specific to the influenza hemagglutinin (HA126–138) peptide from HNT mice.27 Twenty-four hours after the adoptive transfer, all mice were infected with PR8 influenza, and the transferred T cells were followed by FACS over the infection course to determine division and activation. We determined the CD4+ T cell responses specifically in microdissected iBALT, as well as in the TBLN, spleen, and BAL (Fig. 2). Influenza-specific CD4+ T cells migrated into areas of iBALT, and expanded to a peak response at day 5 of infection, as characterized by a loss of CFSE and upregulation of CD44 (Fig. 2A). This indicated that a highly potent and adept CD4+ T cell response was occurring, specifically within areas of iBALT in VLP-instilled mice, and was subdued in DPBS-instilled mice. Additionally, this CD4+ T cell expansion in the VLP-instilled mice was tightly regulated, and as the viral load was beginning to be controlled [as indicated by weight loss (Fig. 1A)], CD4+ T cells were significantly contracted. In the TBLN, donor CD4+ T cells in mice with pre-formed iBALT were considerably more rapid in division, and the upregulation of CD44, achieving a significantly more robust early response already by day 3 post-infection (Fig. 2B). As was seen in iBALT areas, total numbers of activated T cells peaked at day 5 and had been contracted significantly already by day 7 post-infection. CD4+ T cells present in the airway spaces (represented by BAL) continued to accumulate in both groups of mice. However, the potentially damaging accumulation of T cells in the lung was dampened at day 7 in VLP-exposed mice, indicating a likely decreased viral load and more closely regulated T cell responses (Fig. 2C). We also noted that the effects of pre-formed iBALT were not exclusive to the respiratory tract, and in fact promoted immunity in distal sites such as the spleen (Fig. 2D), which exhibited an almost identical pattern of expansion, activation, and contraction as we had observed in the local lymphoid sites of the lungs. Thus, the presence of pre-formed iBALT accelerated the expansion and contraction of influenza-specific CD4+ T cells in the iBALT, TBLN, and spleen, thus accelerating the rate of activation and resulting reduced morbidity.
FIG. 2.
Exposure of the lungs to VLPs promotes the expansion, and alters the trafficking of influenza-specific CD4+ T cells. 1.2×107 naïve CFSE-labeled influenza-transgenic CD4+ T cells from Thy1.2+ HNT mice27 were transferred i.v. into Thy1.1+ recipients who had been exposed to VLPs or DPBS i.n. Recipient mice were rested for 24 h post-transfer, then infected with 1.5×103 pfu PR8. At days 0, 3, 5, and 7 post-infection, one group was killed and CD4+Thy1.2+CD44hiCFSElo donor T cells were quantified by FACS and total cell counts from microdissected iBALT (A), TBLNs (B), BAL (C), and spleen (D). Lymphocytes were gated by forward and side scatter plots. CD4+Thy1.2+ T cells were then examined for their expression of CFSE and CD44. Total CD4+Thy1.2+CD44+CFSElo T cells are shown. Each experiment was independently repeated at least twice with n=5 per group. A t-test was utilized to determine statistical significance at each timepoint. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
CD8+ T cell responses are not altered by VLP-exposure
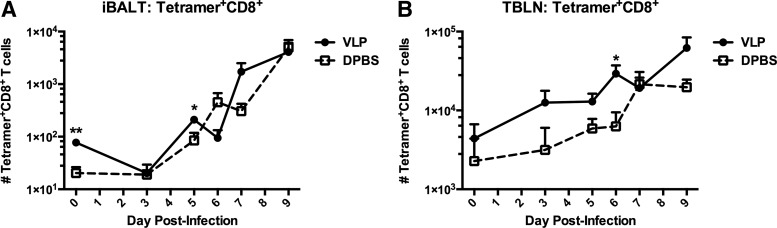
In a similar study, performed without adoptive transfer, we determined the kinetics of influenza-specific CD8+ T cells specifically within iBALT areas and TBLNs through combined H-2Db NP366–374 (NP) and H-2Db PA224–233 (PA) MHC I influenza tetramer staining and FACS analysis. While CD8+ T cells have historically been shown to be important in viral clearance of influenza, neither their function1 nor their associated kinetics (Fig. 3) seemed to be altered by the presence of pre-formed iBALT. Thus, it appeared that enhanced antiviral T cell responses after VLP-exposure were a contribution of the accelerated CD4+ T cell trafficking and division only, and were not dependent upon an enhancement of CD8+ T cell activity.
FIG. 3.
CD8+ T cell responses are not altered by VLP-exposure. Mice were exposed to VLPs or vehicle i.n, rested for 72 h, and then challenged with 1.5×103 pfu PR8. Total MHC I influenza NP and PA tetramer+CD8+ T cells were quantified from microdissected iBALT (A), and TBLNs (B) over 9 days of infection. Lymphocytes were gated by forward and side scatter plots. CD8+Tetramer+ T cells were then quantified. Each experiment was independently repeated at least twice with n=5 per group. A t-test was utilized to determine statistical significance at each timepoint. *p<0.05, **p<0.01.
Conclusions
Dendritic cells are known to be essential to both influenza virus clearance32 and the maintenance of iBALT.10,19 Additionally, viral infection is known to increase the number and rate of DC trafficking from the lungs to the lymph node, which is especially dynamic at early stages of infection.33,34 Consistent with the literature, we found that in the lungs of VLP-primed mice, DCs preferentially accumulated in areas of organized iBALT, as well as in the TBLNs. Moreover, we additionally found that AMs followed the same pattern. Therefore, our data suggest an important role for the antigen-presenting abilities of both DCs and AMs in terms migration to the iBALT, as well as to the TBLN. Importantly, such AM antigen translocation is well documented, although often ignored.35,36
Proficient influenza virus clearance is dependent on both innate and adaptive immunity. Thus, while APC antigen uptake, processing, and trafficking are important in the initiation of immunity, T cell presentation and co-stimulation also represent essential APC functions. The established enhanced innate immunity in the lungs of VLP-primed mice likely facilitated a jump-start in CD4+ T cell expansion. Indeed, in VLP-exposed mice, influenza-specific naïve CD4+, but not CD8+ T cells, proliferated, upregulated activation markers, and then contracted in the iBALT, TBLN, and spleen, at an accelerated rate. We and others have previously shown that CD4+ T cells, and especially T follicular helper cells (TFH) are essential components to iBALT, as they are capable of T cell priming directly in iBALT areas, and mediate GC reactions, including the differentiation of plasma cells, and the promotion of clearance of virus.1,8,10,21 We have additionally shown previously that VLP-exposure augments and accelerates the production of both local and systemic antibody.1,21 Furthermore, recent evidence suggests that CD4+ T cells are capable of antiviral functions that have been historically associated with CD8+ T cells, including cytolytic activity and the production of perforin, granzyme B, and IFN-γ.37–39 Thus, we find that in our model CD4+ T cell responses, and not CD8+ T cell responses, can be enhanced during iBALT-mediated influenza clearance to great benefit.
Some debate remains about the benefits and drawbacks of the presence of pre-formed iBALT in the lung given an individual's current immune status.16,17,40–42 However, we and many others would argue for the benefits of iBALT-mediated local immunity during infection. Moyron-Quiroz et al. have demonstrated that mice who lack secondary lymphoid organs, but are able to develop iBALT in response to influenza infection, clear influenza virus (albeit delayed as compared to fully immunocompetent controls) and exhibit reduced collateral damage to great benefit.8 Van Panhuys et al. further argue that effector lymphoid tissue at the site of pathogen entry is of crucial benefit, as its location at sites of repeated pathogen entry may allow for the local sequestration of antigens and pathogens, thereby limiting the ability of the invader to access the lymph node where it may inspire unnecessary inflammation.43 These and other studies would indicate that iBALT may serve as a general priming site by which T cells may be activated by DCs, regardless of the antigen specificity which had induced the formation of iBALT.10,19 In this regard, it has been suggested by many groups that iBALT holds potential for exploitation as a site for the delivery of drugs and vaccines,24,44 and furthermore, that a locally-regulated response to infection is correlative with decreased collateral damage.1,25
In agreement with the literature, our results suggest that the presence of pre-formed iBALT provides augmented, yet highly controlled immunity to antigenic challenge, despite distinct disparities in the antigenic identity that elicited the initial formation of iBALT. Moreover, we speculate that the more rapid clearance of virus and protection from morbidity was not solely due to the presence of pre-formed iBALT, but also incorporated systemic responses in the lymph nodes and spleen. Indeed, while such sites are locationally disparate, they are certainly immunologically integrated via vasculature and lymphatic connections.
We thus illustrate a mechanism by which we can harness the pulmonary mucosa to provide accelerated site-specific immunity to a primary challenge with a distinct pathogen similarly to a lymph node. Furthermore, we suggest that the utilization of noninfectious and nonpathogenic VLPs, sharing no cross-reactive epitopes to influenza, may provide a novel model by which we may begin to further understand the complex interplay between iBALT, the local and systemic secondary lymphoid tissues, heterologous immunity, and memory responses.
Acknowledgments
We thank all Harmsen lab staff for technical assistance in experiments, and the staff of Montana State University's Animal Resource Center for animal care and technical aid.
This work was supported by NIH/NIAID R01 AI104905; NIH/NIAID R56AI089458; the Rocky Mountain Research Center of Excellence (RMRCE) U54AI065357; NIH/NIAID R21AI083520; the IDeA Network for Biomedical Research Excellence (INBRE) P20GM103474; the Center for Zoonotic and Emerging Infectious Diseases (COBRE) P20GM103500; the M.J. Murdock Charitable Trust, and the Montana State University Agricultural Experimental Station.
Author Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Wiley JA, Richert LE, Swain SD, et al. . Inducible bronchus-associated lymphoid tissue elicited by a protein cage nanoparticle enhances protection in mice against diverse respiratory viruses. PLoS One 2009;4:e7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angeli V, Randolph GJ. Inflammation, lymphatic function, and dendritic cell migration. Lymphat Res Biol 2006;4:217–228 [DOI] [PubMed] [Google Scholar]
- 3.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol 2005;5:617–628 [DOI] [PubMed] [Google Scholar]
- 4.Randolph GJ, Sanchez-Schmitz G, Angeli V. Factors and signals that govern the migration of dendritic cells via lymphatics: Recent advances. Springer Semin Immunopathol 2005;26:273–287 [DOI] [PubMed] [Google Scholar]
- 5.Banchereau J, Briere F, Caux C, et al. . Immunobiology of dendritic cells. Annu Rev Immunol 2000;18:767–811 [DOI] [PubMed] [Google Scholar]
- 6.Forster R, Schubel A, Breitfeld D, et al. . CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 1999;99:23–33 [DOI] [PubMed] [Google Scholar]
- 7.Moyron-Quiroz JE, Rangel-Moreno J, Hartson L, et al. . Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organs. Immunity 2006;25:643–654 [DOI] [PubMed] [Google Scholar]
- 8.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, et al. . Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med 2004;10:927–934 [DOI] [PubMed] [Google Scholar]
- 9.Randall TD. Pulmonary dendritic cells: Thinking globally, acting locally. J Exp Med. 2010;207:451–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halle S, Dujardin HC, Bakocevic N, et al. . Induced bronchus-associated lymphoid tissue serves as a general priming site for T cells and is maintained by dendritic cells. J Exp Med 2009;206:2593–2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rangel-Moreno J, Carragher DM, de la Luz Garcia-Hernandez M, et al. . The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat Immunol 2011;12:639–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luhrmann A, Tschernig T, Pabst R. Stimulation of bronchus-associated lymphoid tissue in rats by repeated inhalation of aerosolized lipopeptide MALP-2. Pathobiology 2002;70:266–269 [DOI] [PubMed] [Google Scholar]
- 13.Foo SY, Phipps S. Regulation of inducible BALT formation and contribution to immunity and pathology. Mucosal Immunol 2010;3:537–544 [DOI] [PubMed] [Google Scholar]
- 14.Demoor T, Bracke KR, Maes T, et al. . Role of lymphotoxin-alpha in cigarette smoke-induced inflammation and lymphoid neogenesis. Eur Respir J 2009;34:405–416 [DOI] [PubMed] [Google Scholar]
- 15.van der Strate BW, Postma DS, Brandsma CA, et al. . Cigarette smoke-induced emphysema: A role for the B cell? Am J Respir Crit Care Med 2006;173:751–758 [DOI] [PubMed] [Google Scholar]
- 16.Rangel-Moreno J, Hartson L, Navarro C, Gaxiola M, Selman M, Randall TD. Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis. J Clin Invest 2006;116:3183–3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol 2006;6:205–217 [DOI] [PubMed] [Google Scholar]
- 18.Kirsh AL, Cushing SL, Chen EY, Schwartz SM, Perkins JA. Tertiary lymphoid organs in lymphatic malformations. Lymphat Res Biol 2011;9:85–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.GeurtsvanKessel CH, Willart MA, Bergen IM, et al. . Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice. J Exp Med 2009;206:2339–2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawamata N, Xu B, Nishijima H, et al. . Expression of endothelia and lymphocyte adhesion molecules in bronchus-associated lymphoid tissue (BALT) in adult human lung. Respir Res 2009;10:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richert LE, Servid AE, Harmsen AL, et al. . A virus-like particle vaccine platform elicits heightened and hastened local lung mucosal antibody production after a single dose. Vaccine 2012;30:3653–3665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holt PG. Development of bronchus associated lymphoid tissue (BALT) in human lung disease: A normal host defence mechanism awaiting therapeutic exploitation? Thorax 1993;48:1097–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holt PG, Strickland DH, Wikstrom ME, Jahnsen FL. Regulation of immunological homeostasis in the respiratory tract. Nat Rev Immunol 2008;8:142–152 [DOI] [PubMed] [Google Scholar]
- 24.Pabst R, Tschernig T. Bronchus-associated lymphoid tissue: An entry site for antigens for successful mucosal vaccinations? Am J Respir Cell Mol Biol 2010;43:137–141 [DOI] [PubMed] [Google Scholar]
- 25.Hussell T, Goulding J. Structured regulation of inflammation during respiratory viral infection. Lancet Infect Dis 2010;10:360–366 [DOI] [PubMed] [Google Scholar]
- 26.Flenniken ML, Willits DA, Harmsen AL, et al. . Melanoma and lymphocyte cell-specific targeting incorporated into a heat shock protein cage architecture. Chem Biol 2006;13:161–170 [DOI] [PubMed] [Google Scholar]
- 27.Roman E, Miller E, Harmsen A, et al. . CD4 effector T cell subsets in the response to influenza: Heterogeneity, migration, and function. J Exp Med 2002;196:957–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim KK, Kim R, Kim SH. Crystal structure of a small heat-shock protein. Nature 1998;394:595–599 [DOI] [PubMed] [Google Scholar]
- 29.Kim R, Kim KK, Yokota H, Kim SH. Small heat shock protein of Methanococcus jannaschii, a hyperthermophile. Proc Natl Acad Sci USA 1998;95:9129–9133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flenniken ML, Willits DA, Brumfield S, Young MJ, Douglas T. The small heat shock protein cage from Methanococcus jannaschii is a versatile nanoscale platform for genetic and chemical modification. Nano Lett 2003;3:1573–1576 [Google Scholar]
- 31.Kaiser CR, Flenniken ML, Gillitzer E, et al. . Biodistribution studies of protein cage nanoparticles demonstrate broad tissue distribution and rapid clearance in vivo. Int J Nanomed 2007;2:715–733 [PMC free article] [PubMed] [Google Scholar]
- 32.McGill J, Van Rooijen N, Legge KL. Protective influenza-specific CD8 T cell responses require interactions with dendritic cells in the lungs. J Exp Med 2008;205:1635–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Legge KL, Braciale TJ. Accelerated migration of respiratory dendritic cells to the regional lymph nodes is limited to the early phase of pulmonary infection. Immunity 2003;18:265–277 [DOI] [PubMed] [Google Scholar]
- 34.Dahl ME, Dabbagh K, Liggitt D, Kim S, Lewis DB. Viral-induced T helper type 1 responses enhance allergic disease by effects on lung dendritic cells. Nat Immunol 2004;5:337–343 [DOI] [PubMed] [Google Scholar]
- 35.Harmsen AG, Muggenburg BA, Snipes MB, Bice DE. The role of macrophages in particle translocation from lungs to lymph nodes. Science 1985;230:1277–1280 [DOI] [PubMed] [Google Scholar]
- 36.Kirby AC, Coles MC, Kaye PM. Alveolar macrophages transport pathogens to lung draining lymph nodes. J Immunol 2009;183:1983–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown DM, Dilzer AM, Meents DL, Swain SL. CD4 T cell-mediated protection from lethal influenza: Perforin and antibody-mediated mechanisms give a one-two punch. J Immunol 2006;177:2888–2898 [DOI] [PubMed] [Google Scholar]
- 38.Swain SL, McKinstry KK, Strutt TM. Expanding roles for CD4(+) T cells in immunity to viruses. Nat Rev Immunol 2012;12:136–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown DM, Lee S, Garcia-Hernandez MD, Swain SL. Multi-functional CD4 cells expressing IFN-gamma and perforin mediate protection against lethal influenza infection. J Virol 2012;86:6792–6803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hogg JC, Chu F, Utokaparch S, et al. . The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004;350:2645–2653 [DOI] [PubMed] [Google Scholar]
- 41.Kocks JR, Adler H, Danzer H, et al. . Chemokine receptor CCR7 contributes to a rapid and efficient clearance of lytic murine gamma-herpes virus 68 from the lung, whereas bronchus-associated lymphoid tissue harbors virus during latency. J Immunol 2009;182:6861–6869 [DOI] [PubMed] [Google Scholar]
- 42.Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: From ontogeny to neogenesis. Nat Immunol 2006;7:344–353 [DOI] [PubMed] [Google Scholar]
- 43.van Panhuys N, Perret R, Prout M, Ronchese F, Le Gros G. Effector lymphoid tissue and its crucial role in protective immunity. Trends Immunol 2005;26:242–247 [DOI] [PubMed] [Google Scholar]
- 44.Tschernig T, Pabst R. What is the clinical relevance of different lung compartments? BMC Pulm Med 2009;9:39. [DOI] [PMC free article] [PubMed] [Google Scholar]