Abstract
Biologic scaffolds composed of mammalian extracellular matrix (ECM) promote constructive remodeling of tissues via mechanisms that include the recruitment of endogenous stem/progenitor cells, modulation of the host innate immune response, and influence of cell fate differentiation. Such scaffold materials are typically prepared by decellularization of source tissues and are prepared as sheets, powder, or hydrogels. It is plausible that ECM derived from an anatomically distinct tissue would have unique or specific effects on cells that naturally reside in this same tissue. The present study investigated the in vitro effect of a soluble form of ECM derived from central nervous system (CNS) tissue, specifically the spinal cord or brain, versus ECM derived from a non-CNS tissue; specifically, the urinary bladder on the behavior of neural stem cells (NSCs) and perivascular stem cells. All forms of ECM induce positive, mitogenic, and chemotactic effects at concentrations of approximately 100 μg/mL without affecting stem cell viability. CNS-derived ECMs also showed the ability to differentiate NSCs into neurons as indicted by βIII-tubulin expression in two-dimensional culture and neurite extension on the millimeter scale after 24 days of three-dimensional cultures in an ECM hydrogel. These results suggest that solubilized forms of ECM scaffold materials may facilitate the postinjury healing response in CNS tissues.
Introduction
The human adult central nervous system (CNS) has limited regenerative capacity despite the presence of neural stem cells (NSCs) in both the brain1,2 and spinal cord,3 which suggest unrealized healing potential. Transplantation of NSCs in animal models of CNS pathology (e.g., stroke, Parkinson's disease, and congenital dysmyelination) have shown positive results,4–8 but there are concerns regarding the safety of a clinical NSC-based approach.9,10 In vivo maintenance and regulation of stem cell behavior in the CNS are partially attributable to the NSC niche, a complex microenvironment of extracellular cues.11–13 Therefore, prospective NSC therapies often include a biomimetic material component that is intended solely to support post-transplantation survival and integration of exogenously delivered NSCs.4,14,15 Alternatively, the creation of a favorable acellular niche microenvironment at a site of injury could theoretically induce and/or recruit endogenous stem cells. However, in situ CNS tissue engineering via recapitulation of the complex NSC niche would require both a comprehensive understanding of niche conditions and a multi-functional composite material that could interact with NSCs in an appropriately biomimetic fashion.
Extracellular matrix (ECM) bioscaffolds produced by decellularization and antigen depletion of mammalian tissues largely retain the structural and functional complexity of their tissues of origin13,16–20 and elicit desirable immune-mediated responses at sites of injury in multiple tissue types.21–25 Biologic scaffolds composed of ECM have been used in both preclinical studies and clinical applications to facilitate constructive remodeling rather than scar tissue formation after injury in several tissues21,26–29, including dura mater,30–32 skeletal and smooth muscles,33 and peripheral nervous system.34 There is evidence for enhanced cell differentiation when homologous, tissue-specific ECM scaffolds are used in contrast to ECM scaffolds that are prepared from heterologous tissues.18–20,35,36 This tissue specificity may be beneficial or even essential to the functional restoration of complex tissues and organs.37–40 Biologic scaffolds derived from CNS tissues have been recently described, 17–20 and such scaffolds may be appropriate for in situ CNS tissue engineering and repair.
The objectives of the present study were to determine the effects of CNS ECM scaffolds on stem cells that may be critical for constructive CNS remodeling, including human NSCs and perivascular stem cells which are capable of generating new CNS tissue and supporting vasculature. The postinjury formation and integration of new nervous tissue within a CNS defect may be achieved by amplification and recruitment of stem and progenitor cells and subsequent in situ terminal differentiation into a variety of neural cell types. The present study is focused on mitogenic, chemotactic, and differentiation properties of ECM scaffolds.
Materials and Methods
Preparation of ECM
Porcine spinal cord and brain tissues were decellularized as previously described.19 Briefly, tissues were processed as follows: a freeze-thaw cycle, dura mater removal, sectioning into small pieces, and treatment with a series of agitated baths that included water overnight, trypsin/EDTA, Triton X-100, sucrose, deoxycholate, and peracetic acid/ethanol. Water and phosphate-buffered saline (PBS) baths were interspersed and used at the end of processing to remove decellularization agent residues. Urinary bladder ECM was prepared as previously described41 and was used as a non-CNS ECM reference material to investigate tissue specificity of ECM-influenced cell behaviors. Decellularized tissues were lyophilized, comminuted (maximum dimension <1.0 mm), solubilized by digestion with 1.0 mg/mL pepsin (Sigma-Aldrich Corp., St. Louis, MO) in 0.01 N HCl (Fisher), neutralized to pH 7.4 with 0.1 N NaOH (Thermo Fisher Scientific, Inc., Waltham, MA), isotonically balanced with 10× PBS, diluted to desired concentrations with PBS, and stored at −20°C until use.
Characterization of ECM
To supplement the investigation of tissue-specific ECM-influenced cell behaviors, the solubilized protein profiles of spinal cord, brain, and urinary bladder ECMs were compared via gel electrophoresis after digestion with pepsin as described earlier. Non-neutralized ECM digest was diluted in 2× Laemmli sample buffer (Bio-rad, Hercules, CA) to a final concentration of 2.5 μg/μL and boiled for 5 min at 95°C. Once boiled, 15 μg of each ECM were loaded on 4–15% SDS PAGE gels (Bio-rad) and run at 140 V for 1 h. Gels were stained with Coomassie Blue R-250 stain (Fisher) and imaged using the Red Personal Gel Imaging System (Protein Simple, Santa Clara, CA).
Human stem cells
Human stem cells were obtained in accordance with IRB guidelines, and research was conducted following IRB study approval. Human NSCs were isolated from fetal cortical neuroepithelium.42,43 Cortical neuroepithelial stem cells were cultured in DMEM/F12 (Invitrogen Corp., Carlsbad, CA) containing 0.03% human albumin solution (Baxter Healthcare Corp., Deerfield, IL), 100 μg/mL human apo-transferrin (Sigma), 16.2 μg/mL putrescine dihydrochloride (Sigma), 5.0 μg/mL human recombinant insulin (Sigma), 60 ng/mL progesterone (Sigma), 40 ng/mL sodium selenite (Sigma), 10 ng/mL human basic fibroblast growth factor (Peprotech, Inc., Rocky Hill, NJ), 20 ng/mL human epithelial growth factor (Peprotech), 100 nM 4-hydroxytamoxifen (4-OHT; Sigma), 2.0 mM L-glutamine (Sigma), and 100 U/mL penicillin/100 mg/mL streptomycin at 37°C in 5% CO2. Cortical neuroepithelial stem cells (P10–16) were passaged at 30–80% confluence on flasks coated with laminin (Sigma).
Human perivascular stem cells were isolated from fetal muscle tissue by a combination of culture and flow cytometry methods.44 Perivascular stem cells (P10–12) were cultured in high-glucose DMEM (Invitrogen) containing 20% fetal bovine serum (FBS; Fisher) and 100 U/mL penicillin/100 mg/mL streptomycin (Sigma) at 37°C in 5% CO2.
Evaluation of human stem cell behavior by ECM
Effects of solubilized ECM on human stem cells were assayed using a range of concentrations (2.5, 10, 25, or 100 μg protein per mL as determined by bicinchoninic acid). PBS and/or neutralized pepsin-HCl lacking ECM served as controls in all assays. All surfaces used for NSC assays were coated with laminin before culture. All in vitro assays were conducted as three or more replicates in triplicate or quadruplicate per condition.
Viability
Cells were plated on sterile glass coverslips at 70,000 cells per well in 12-well plates. ECM or PBS was added to the medium at 100 μg/mL 24 h after plating, and cells were cultured for an additional 24 h. Cytocompatibility was determined using a Live/Dead Viability Kit (Invitrogen) as per manufacturer's instructions using 4.0 μM calcein AM and 4.0 μM ethidium homodimer-1. Fluorescence images allowed quantification of viability using ImageJ (NIH) to split red and green channels, threshold, and count cells, with binning to resolve cell clusters of various counts. All images were analyzed using the same ImageJ macro.
Mitogenesis
Cells were plated in 96-well plates at 4000 cells per well. After 24 h, ECM or PBS was added to each well and cells were cultured for another 20 h, followed by culture for 4.0 h with 5-bromo-2′-deoxyuridine (BrdU) and quantification of BrdU incorporation into DNA using a colorimetric BrdU cell proliferation ELISA (Roche Diagnostics Corp., Indianapolis, IN) as per manufacturer's instructions.
Chemotaxis
Human NSCs were harvested and resuspended in unsupplemented 50:50 DMEM/F12: PBS for ∼30 min before chemotaxis assays. Human perivascular stem cells were starved overnight (0.5% FBS), harvested, and resuspended in unsupplemented 50:50 DMEM:PBS for 60 min. Chemotaxis was tested by trans-membrane migration using blind-well chambers and polycarbonate filters with 8.0 μm pore size (AP48; Neuro Probe, Inc., Gaithersburg, MD). Filters were coated in 30 μg/mL laminin (Sigma) for 30 min for NSCs or 50 μg/mL rat tail collagen I (BD Biosciences, Bedford, MA) for 30 min for perivascular stem cells and allowed to dry completely. Each ECM was brought to twice its test concentration with PBS and diluted 50:50 with basal medium, with 10% FBS in basal medium used to verify chemotaxis through each filter. Wells were loaded with 30,000 cells per well, and after 6 h for NSCs or 3 h for perivascular stem cells each filter was removed, the upper (nonmigratory) surface was scraped, and the lower (migratory) surface was fixed with methanol, stained with 4′,6-diamidino-2-phenylindole (DAPI), and imaged. Migrated cells were quantified using ImageJ to threshold and count cells, with binning to resolve cell clusters of various counts. The same ImageJ macro was used to analyze all images. Fluorescence images allowed quantification of viability using ImageJ (NIH) to split red and green channels, threshold, and count cells, with binning to resolve cell clusters. All images were analyzed using the same ImageJ macro.
Differentiation
Differentiation was investigated for NSCs only. Terminal differentiation in two-dimensional culture was investigated via βIII-tubulin protein expression. Cells were plated in 96-well plates at 4000 cells per well. After 8 h, wells were rinsed with DMEM/F12 twice and received either 50:50 culture medium with ECM at 100 μg/mL, 50:50 culture medium with neutralized ECM-free pepsin-HCl (negative control), or 50:50 culture medium lacking growth factors with PBS (positive control). Cells were cultured for another 14 days and then fixed with 2% paraformaldehyde, permeabilized with 0.1% Triton X-100, rinsed twice with PBS, blocked with 2% normal goat serum (NGS), incubated with mouse monoclonal anti-βIII-tubulin (diluted 1:100; Abcam, Inc., Cambridge, MA) in 1% NGS, rinsed twice with PBS, incubated with goat anti-mouse IgG Alexa Fluor 488-conjugated secondary antibody (diluted 1:400; Sigma, F0257), rinsed twice with PBS, and incubated with a sufficient volume of DAPI to prevent drying before imaging (24–72 h). Three images per well were captured using a Zeiss AxioObserver Z1 microscope, and the quantity of neurons was determined by manual counting.
Terminal differentiation of NSCs was also investigated in three-dimensional culture via neurite extension within ECM hydrogels.20 Cells were suspended in 50:50 culture medium and solubilized ECM at 2.8 mg/mL at 500,000 cells per mL (final concentration of 1.4 mg/mL for either spinal cord ECM or urinary bladder ECM, the lowest concentration at which hydrogel integrity could be reasonably maintained). Suspensions were mixed thoroughly by gentle pipetting, divided evenly into 100 μL aliquots within 12-well plates, and allowed to form hydrogels at 37°C before addition of culture medium. Culture of NSCs in ECM hydrogels was continued for approximately 24 days, with half of each well's culture medium exchanged every sixth day. On termination of culture, the medium was gently removed from each well and hydrogel suspensions were rinsed with PBS and stained with Alexa Fluor 488-conjugated phalloidin to visualize actin within cell somas and processes. Images of neurite extension were captured using a Zeiss AxioObserver Z1 microscope.
Statistics
Graphical representation of all data show mean±standard deviation of at least three replicates, with each conducted in triplicate or quadruplicate. Experimental groups were compared using a one-way analysis of variance with Tukey–Kramer post hoc analysis. Groups were considered different when the Tukey–Kramer p-value was less than 0.05.
Results
Characterization of ECM
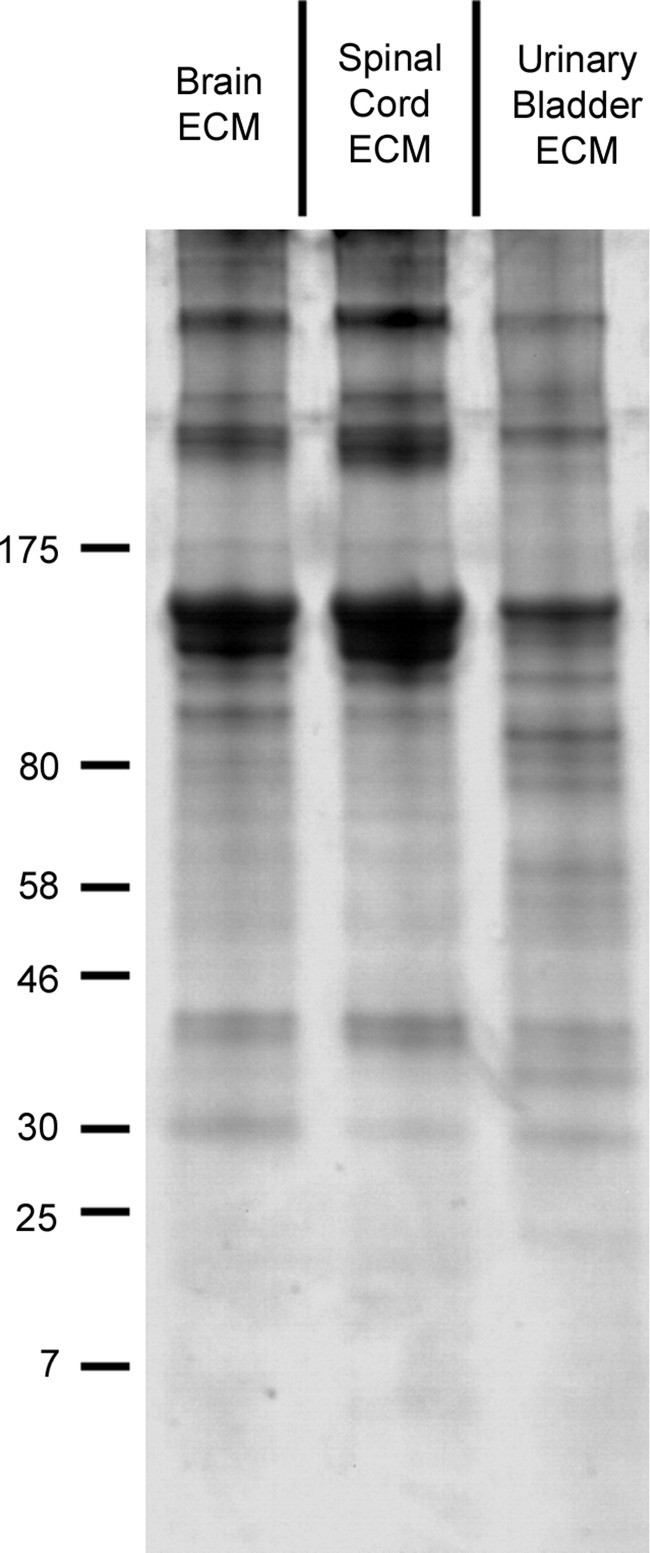
After enzymatic digestion of ECMs, solubilized protein profiles were compared visually via gel electrophoresis (Fig. 1). Distinct banding patterns, including both molecular weights and band intensities, were observed for each ECM, with a particular contrast between CNS and non-CNS ECMs. These differences complemented previous characterizations of the ECMs.19,20
FIG. 1.
Protein profiles of solubilized ECMs. Digested brain ECM and spinal cord ECM displayed very similar protein profiles with a strong bias toward high-molecular-weight species (>130 kDa). Urinary bladder ECM had a more evenly distributed profile over a variety of molecular weights, particularly in the 30–100 kDa range. Equal quantities of ECM were loaded in each lane, resolved on 4–15% SDS gels, stained with Coomassie Blue R-250, and imaged. A representative image is shown. Markers at left have units of kDa. ECM, extracellular matrix.
Viability
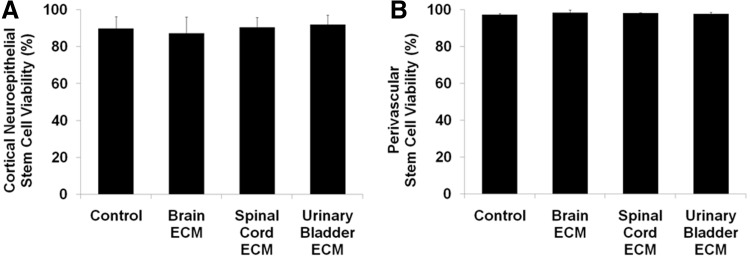
Human stem cells isolated from the cortical neuroepithelium had a viability of 90% during live/dead assays, and their viability did not vary when cultured with brain ECM, spinal cord ECM, or urinary bladder ECM at 100 μg/mL (Fig. 2A). Viability of human perivascular stem cells (control=97%) also did not vary during culture with any of the three bioscaffolds tested (Fig. 2B).
FIG. 2.
Viabilities of human stem cells cultured with ECM. Undifferentiated human neural and perivascular stem cells showed normal viability when cultured with brain ECM, spinal cord ECM, or urinary bladder ECM as determined by Live/Dead assay, including quantification of live and dead (A) cortical neuroepithelial stem cells and (B) perivascular stem cells. Cells were cultured in each ECM at a concentration of 100 μg protein per mL medium.
Mitogenesis
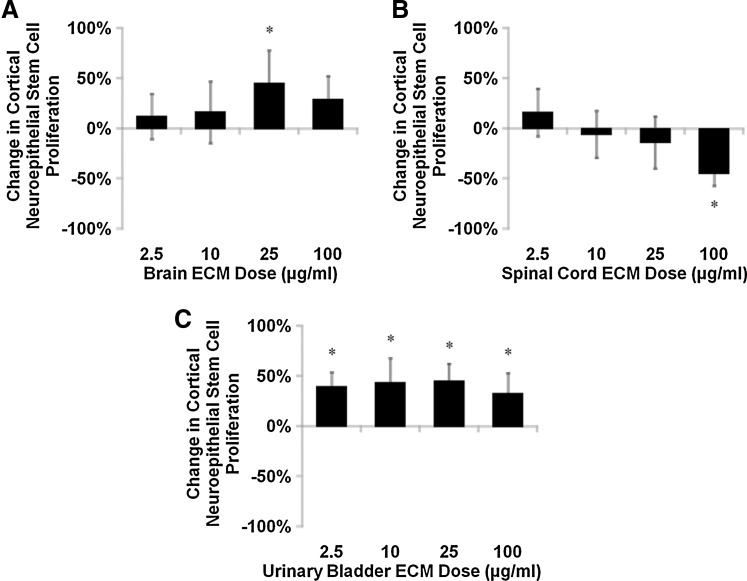
Mitogenic responses of human NSCs to ECM varied with matrix concentration, with unique response profiles for each cell–matrix combination. NSC mitogenesis differed from ECM-free control in as much as±45% within the 24-h assay period. The proliferation rate of cortical neuroepithelial stem cells increased in response to brain ECM (Fig. 3A), while spinal cord ECM decreased NSC proliferation rate (Fig. 3B). Interestingly, spinal cord ECM had a nonsignificant positive effect on cortical neuroepithelial stem cell mitogenesis at the lowest concentration tested (2.5 μg/mL) that contrasted with responses at higher concentrations. Urinary bladder ECM caused the most robust mitogenic response in NSCs, which showed an increase in proliferation rate at every concentration tested (Fig. 3C).
FIG. 3.
Mitogenesis of human neural stem cells cultured with ECM. Undifferentiated human cortical neuroepithelial stem cell proliferation was modulated by (A) brain ECM, (B) spinal cord ECM, and (C) urinary bladder ECM as determined by BrdU incorporation during mitosis. Changes in mitogenesis ranged from increases of 45% (brain ECM and urinary bladder ECM, each at 25 μg/mL) to decreases of 44% (spinal cord ECM, 100 μg/mL). Concentrations are μg protein per mL ECM solution. *p<0.05 by one-way ANOVA with Tukey–Kramer post hoc analysis. ANOVA, analysis of variance; BrdU, 5-bromo-2′-deoxyuridine.
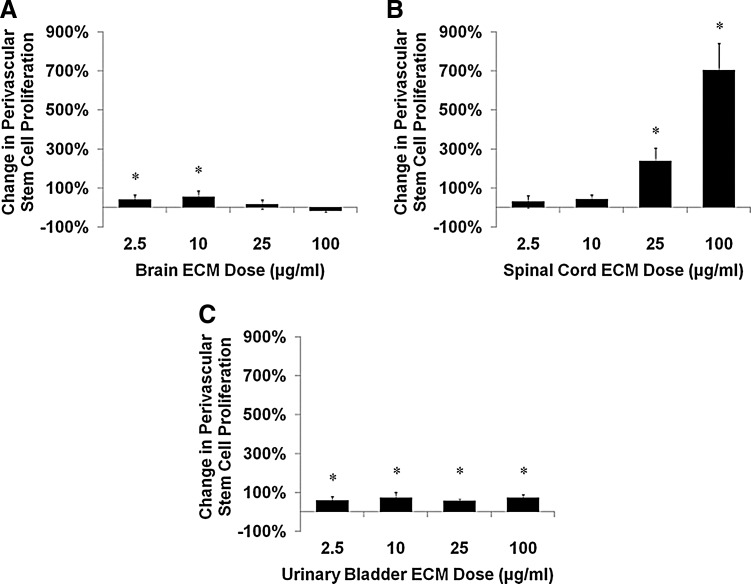
Mitogenic responses of human perivascular stem cells to ECM were predominantly positive and generally of a greater magnitude than NSC responses. Brain ECM increased the proliferation rate of perivascular stem cells at lower concentrations but not at higher concentrations (Fig. 4A). Spinal cord ECM induced increases in the rate of cell proliferation approximately seven-fold at higher concentrations at the assay's 24-h time point (Fig. 4B). Urinary bladder ECM caused consistent increases in perivascular stem cell proliferation rates across all concentrations tested (Fig. 4C).
FIG. 4.
Mitogenesis of human perivascular stem cells cultured with ECM. Undifferentiated human perivascular stem cell proliferation was modulated by (A) brain ECM, (B) spinal cord ECM, and (C) urinary bladder ECM as determined by BrdU incorporation during mitosis. After 24 h of exposure, mitogenesis increased as much as 700% over control for spinal cord ECM (100 μg/mL), 73% for urinary bladder ECM (10 μg/mL), and 54% for brain ECM (10 μg/mL). Concentrations are μg protein per mL ECM solution. *p<0.05 by one-way ANOVA with Tukey–Kramer post hoc analysis.
Chemotaxis
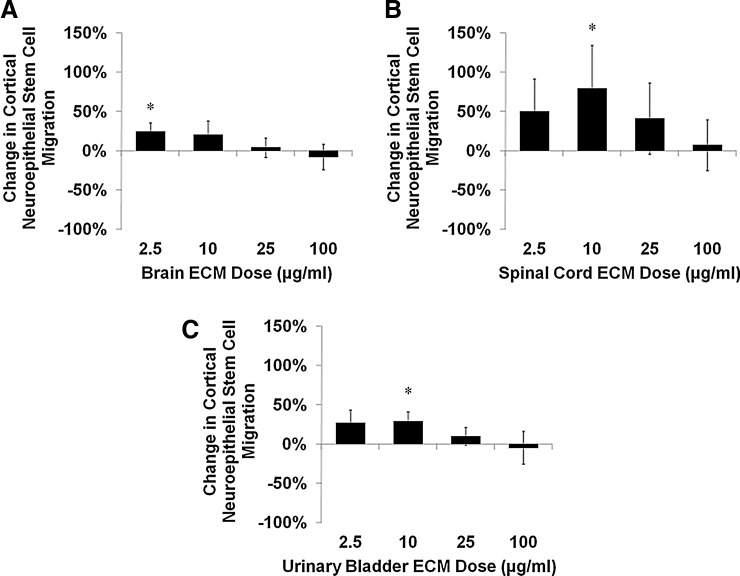
Similar to mitogenic results, chemotactic responses of human NSCs to ECM varied with matrix concentration and showed unique response profiles for each combination of cell and matrix origins. Cortical neuroepithelial stem cell migration increased in response to brain ECM at low concentrations (Fig. 5A). Spinal cord ECM induced the greatest chemotactic response by NSCs within the tested concentration range (Fig. 5B). Urinary bladder ECM induced a cortical neuroepithelial stem cell migration profile similar to that of brain ECM (Fig. 5C).
FIG. 5.
Chemotaxis of human neural stem cells cultured with ECM. Undifferentiated human cortical neuroepithelial stem cell motility was modulated by (A) brain ECM, (B) spinal cord ECM, and (C) urinary bladder ECM as determined by quantification of migrated cells in a transwell assay. After 6 h, cell migration increased as much as 80% over control for spinal cord ECM (10 μg/mL), 29% for urinary bladder ECM (10 μg/mL), and 25% for brain ECM (2.5 μg/mL). Concentrations are μg protein per mL ECM solution. *p<0.05 by one-way ANOVA with Tukey–Kramer post hoc analysis.
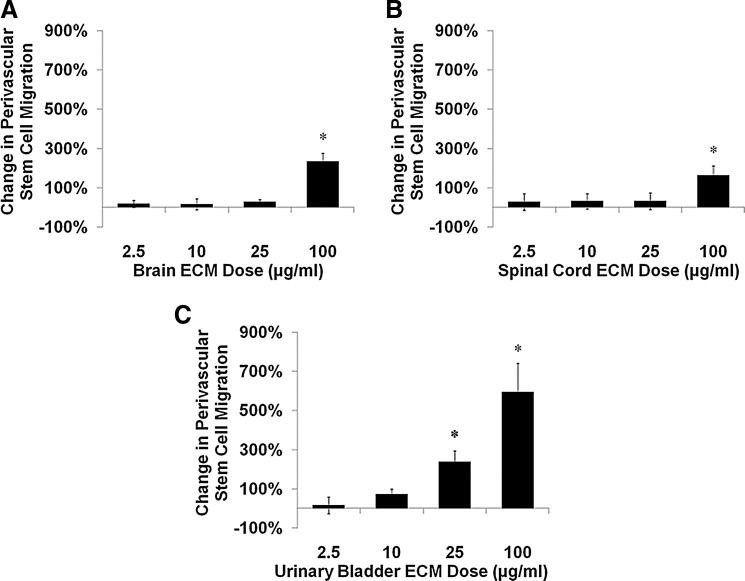
Chemotactic responses of human perivascular stem cells to ECM were solely positive and of multi-fold magnitude at the highest tested concentration of each ECM (100 μg/mL). Brain ECM increased perivascular stem cell migration approximately 2.3-fold (Fig. 6A), while spinal cord ECM induced increases approximately 1.6-fold (Fig. 6B). Urinary bladder ECM increased perivascular stem cell migration approximately six-fold at higher concentrations at the assay's 3-h time point (Fig. 6C).
FIG. 6.
Chemotaxis of human perivascular stem cells cultured with ECM. Undifferentiated human perivascular stem cell motility was modulated by (A) brain ECM, (B) spinal cord ECM, and (C) urinary bladder ECM as determined by quantification of migrated cells in a transwell assay. After 3 h, cell migration increased as much as 600% over control for urinary bladder ECM (100 μg/mL), 230% for brain ECM (100 μg/mL), and 160% for spinal cord ECM (100 μg/mL). Concentrations are μg protein per mL ECM solution. *p<0.05 by one-way ANOVA with Tukey–Kramer post hoc analysis.
Differentiation
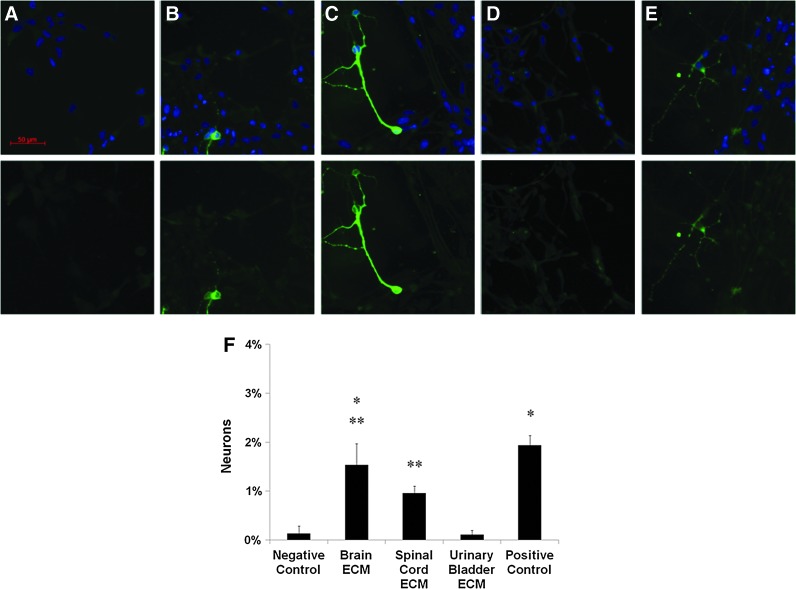
For two-dimensional NSC differentiation, the negative control showed cells with typical undifferentiated morphology (Fig. 7A). Brain ECM (Fig. 7B) and spinal cord ECM (Fig. 7C) each induced neuronal differentiation as evident from expression of βIII-tubulin and neurite extension. Urinary bladder ECM rarely induced neuronal differentiation (Fig. 7D), which contrasted with the presence of neurons in the positive control (Fig. 7E). Brain ECM induced the highest percentage of neuronal differentiation among experimental groups at 1.5%, which did not differ significantly from the positive control's rate of 1.9% (Fig. 7F). Spinal cord ECM induced neuronal differentiation at a rate of 1.0%, which was lower than the positive control but did not differ from brain ECM. Urinary bladder ECM and the negative control induced neuronal differentiation at lower rates (0.1%) than all other groups (Fig. 7F). Numerous cells that did not stain positive for βIII-tubulin but clearly showed long cell processes were observed in each ECM group and the positive control (Fig. 7B–E) and were likely differentiating into glial cell types.
FIG. 7.
Differentiation of human neural stem cells induced by ECM. Human cortical neuroepithelial stem cell two-dimensional neuronal differentiation induced by CNS and non-CNS ECM as indicated by immunofluorescent staining with βIII-tubulin. Differentiation was compared in culture medium supplemented 50:50 with: (A) neutralized ECM-free pepsin-HCl as a negative control, (B) brain ECM at 100 μg/mL, (C) spinal cord ECM at 100 μg/mL, or (D) urinary bladder ECM at 100 μg/mL. (E) Normal culture medium without growth factors diluted 50:50 in phosphate-buffered saline served as a positive control. (F) Neuronal differentiation of human neural stem cells was greater when exposed to CNS ECM compared with non-CNS ECM. *Brain ECM induced neurons at a rate comparable to the positive control and greater than urinary bladder ECM and the negative control. **Spinal cord ECM induced neurons at a lower rate compared with the positive control that did not differ significantly from brain ECM but was greater than urinary bladder ECM and the negative control. Urinary bladder ECM did not induce neurons at a greater rate than the negative control. Concentrations are μg protein per mL ECM solution. Negative control contrast increased to improve visualization of background immunofluorescence at 488 nm. Scale bar is 50 μm. Significant differences were determined between all groups shown by one-way ANOVA with Tukey–Kramer post hoc analysis (p<0.05). CNS, central nervous system. Color images available online at www.liebertpub.com/tea
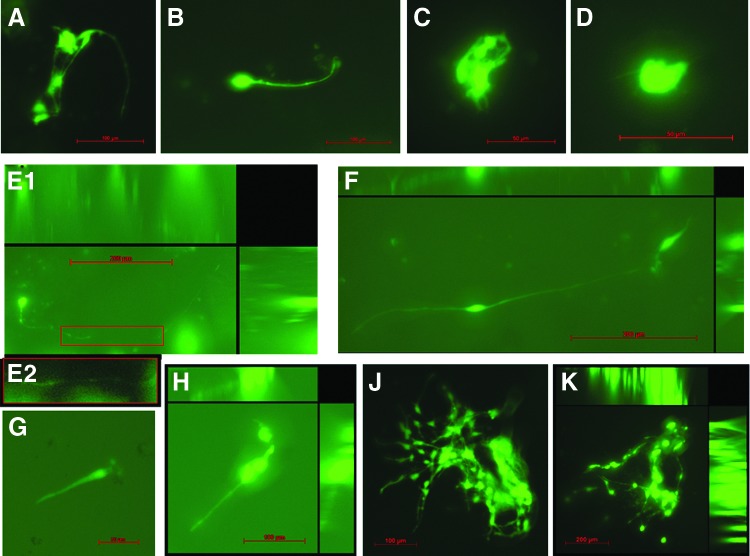
For three-dimensional differentiation within ECM hydrogel scaffolds,20 neurite extension was clearly visible by day 6 in spinal cord ECM, and contrasting lengths were visible by day 12 in spinal cord ECM (>100 μm; Fig. 8A, B) versus urinary bladder ECM (<20 μm; Fig. 8C, D). After 24 days of culture in hydrogels, neurite lengths in spinal cord ECM had reached the millimeter scale (∼0.50 mm; Fig. 8E, F), while neurites were relatively shorter in the urinary bladder ECM (∼100 μm; Fig. 8G, H). Small clusters of cells were visible within each ECM hydrogel soon after starting culture, but only spinal cord ECM showed extensive clusters of cells with neurite-soma and neurite-neurite contacts spanning considerable distances during the culture period (Fig. 8J, K).
FIG. 8.
Neurite extension by human neural stem cells induced within ECM hydrogel scaffolds. Human cortical neuroepithelial stem cell three-dimensional neuronal differentiation induced within CNS ECM hydrogel scaffolds20 as indicated by neurite extension shown with phalloidin staining of actin. Differentiation was compared in hydrogel scaffolds composed of spinal cord ECM or urinary bladder ECM immersed in culture medium. After 12 days, (A, B) neurites of cells cultured in spinal cord ECM hydrogel at 1.4 mg/mL exceeded 100 μm in length, whereas (C, D) neurites of cells cultured in urinary bladder ECM hydrogel at 1.4 mg/mL were less than 20 μm in length. After 24 days of culture, (E, F) neurite lengths within spinal cord ECM had increased to as much as ∼0.5 mm and were typically 100–200 μm, while (G, H) neurite lengths within urinary bladder ECM had increased to 50–100 μm. (J, K) Large cell clusters with neurite-soma and neurite-neurite contacts were visible within spinal cord ECM hydrogel scaffolds throughout the culture period (J: day 12; K: day 24). Images E1, F, H, J, and K are compressed z-stacks. Image E2 shows detail of neurite in image E1 with enhanced contrast. Some autofluorescence and/or background staining of gels is visible. Concentrations are μg protein per mL ECM solution. Scale bars are 50 μm (C, D, G), 100 μm (A, B, H, J), and 200 μm (E, F, K). Color images available online at www.liebertpub.com/tea
Discussion
The presence of stem cells in human CNS tissues1–3 suggests an unrealized inherent regenerative capacity. Treatment of mammalian CNS lesions using human cortical neuroepithelial stem cells has shown promising preclinical efficacy, including behavioral recovery5,6 and modifications of CNS tissue remodeling when transplanted alone6 or with tissue-derived ECM.4 In addition, an earlier study4 has shown that poststroke injection of a hydrogel form of ECM20 into a lesion not only supports transplantation of exogenous NSCs but also acts as a lesion filler and recruits an endogenous cell population into the gel-filled lesion. While development of exogenous cell-based treatments for CNS pathology continues to appear promising,45 clinical treatments using tissue-derived ECM scaffolds alone may be more acceptable based on potential tumorigenic complications of exogenous NSCs.9,10 Safety concerns aside, efficacy of NSCs in CNS therapies may be enhanced by or even require co-delivery with cell-supportive materials such as ECM, which appropriately direct cell behavior, thereby inducing graft-host integration and improving functional outcomes.4,46–48
With the exception of dura mater repair,30–32 the development and application of tissue-derived ECM scaffolds for CNS reconstruction has not been commonly described.17–20 Minimally invasive implantation of an ECM hydrogel scaffold4,18,20 may provide a niche-friendly microenvironment at a CNS injury site to encourage endogenous NSCs to migrate to the site, proliferate, and differentiate to replace lost neural cells and ultimately, produce functional tissue.11,12 Data from the present study show unique compositions for each ECM (Fig. 1) and further suggest that appropriately tuned concentrations of ECM bioscaffolds administered either in combination or in a staged approach might attract both NSCs and perivascular stem cells, thereby yielding CNS tissue engineered in situ through concomitant development of new neural tissue and supporting vasculature. Human perivascular stem cells showed robust positive mitogenic and chemotactic responses to ECM bioscaffolds in vitro (Figs. 4 and 6), and these results agree with earlier studies that showed mitogenic and chemotactic responses by perivascular stem cells to solubilized ECMs derived from the small intestine and urinary bladder.49,50
Human NSCs and perivascular stem cells responded in a similar positive fashion to all ECMs in this study (Figs. 3, 5, and 7) with regard to mitogenesis and chemotaxis. However, patterns of NSC mitogenic and chemotactic responses appeared to differ for spinal cord ECM versus brain and urinary bladder ECMs (Figs. 2B and 4B versus Figs. 2A, C and 4A, C). Earlier studies characterizing the three matrices found that spinal cord ECM contained relatively lower levels of sulfated glycosaminoglycans,20 and it has been shown that glycosaminoglycans such as heparan sulfate, chondroitin sulfate, and hyaluronan influence NSC proliferation.51–53 Differences in NSC and perivascular stem cell mitogenic and chemotactic responses to the ECMs might also differ based on their unique protein profiles (Fig. 1) and biomolecular constituents, including laminin, myelin, and collagen, all of which vary between the ECMs.19,20
NSC neuronal differentiation and neurite extension data (Figs. 7 and 8) suggest that CNS-derived ECM may evoke a tissue-specific response in human NSCs. Protein fragment profiles of solubilized CNS ECMs (i.e., spinal cord and brain ECMs) were similar and contrasted with the non-CNS ECM (i.e., urinary bladder ECM) used in this study (Fig. 1), suggesting tissue-specific bioactive peptides in the different ECMs as possible drivers of contrasting NSC neuronal differentiation and neurite extension (Figs. 7 and 8). Unique biomolecular constituents such as glycosaminoglycans probably also drove varying levels of neuronal NSC differentiation.52,53 Tissue-specific ECMs (i.e., originating from a tissue type homologous to that of a targeted repair site and/or a regenerative cell type) have previously shown the ability to support site-appropriate phenotype for cells from a variety of complex tissues.18–20,35,36,38 βIII-tubulin-negative NSCs exhibiting processes visible from background staining (Fig. 7B–E) as well as earlier studies showing differentiation of similar NSC lines42 indicate that NSCs in this study may also have differentiated into non-neuronal neural lineages (e.g., astrocytes, oligodendrocytes, etc.). While neurons as a percentage of total cells in this study were lower than those observed in many regions of the mammalian CNS,54–56 it is important to consider that only a few neurons representing a small percentage of an infiltrating cell population may be all that is necessary for penumbral functional regeneration postinfarct, particularly in CNS regions such as human cortical white matter where neurons compose similarly small fractions of the total cell population (Fig. 7F).57 In addition, other NSC responses (e.g., mitogenesis and chemotaxis) appear to dominate and likely peak at ECM concentrations less than 100 μg/mL (Figs. 3A, B and 5A, B). These data along with neurite extension results within ECM hydrogels at a concentration of 2.8 mg/mL (Fig. 8) suggest that exposure to solubilized ECM at relatively higher concentrations (e.g., those necessary for hydrogel formation and integrity20) may prompt terminal NSC differentiation (Fig. 7) rather than quiescence or inhibition of motility pathways.
When considered generally, responses of human stem cells to solubilized tissue-derived ECM in the present study were almost uniformly positive for the assays investigated rather than inhibitory. While the method of ECM solubilization was nonphysiologic, more physiologic methods have been shown to yield similar results in analogous studies.58 Cellular responses to each ECM represented the aggregate effect of all solubilized molecules; the individual effect of each specific molecule remains unknown, and isolation of molecule-specific effects will require extensive investigation. Each solubilized ECM showed a unique protein profile, with greater contrast between CNS and non-CNS ECMs, and further studies may provide additional insights into specific differences between the ECMs' biomolecular compositions. It is important to note that individual peptides with specific bioactivity in vitro and in vivo have previously been identified within the milieu of solubilized urinary bladder ECM.59,60 Given that cryptic peptide generation is a common mechanism of eliciting specific desirable cellular responses to injury or disease,61,62 it is likely that additional bioactive peptides are present in the solubilized CNS ECMs that were used in this study. The extensive three-dimensional nature of cell clusters and contacts within spinal cord ECM after 12 days and 24 days were apparent in z-stack series animations (Fig. S1–S5).
Conclusion
Human stem cells, such as cortical neuroepithelial stem cells and perivascular stem cells, show robust and dynamic positive responses to solubilized tissue-derived ECMs across a range of concentrations with no adverse effect on cell viability (Fig. 2). Stem cell responses include mitogenesis (Figs. 3 and 4) and chemotaxis (Figs. 5 and 6), and these responses are almost uniformly positive for ECM protein concentrations of 2.5–100 μg/mL. Perivascular stem cells exhibit particularly robust mitogenic and chemotactic responses, in some cases exceeding negative controls by as much as seven-fold (Fig. 4B) or six-fold (Fig. 6C), respectively. In the case of NSCs, responses to ECMs include neuronal differentiation (Fig. 7) and neurite extension within ECM hydrogel scaffolds (Fig. 8), and these responses are much stronger when the ECM is derived from CNS tissues. The results of the present study suggest that tissue-specific effects of ECM extend to the CNS and that CNS ECM scaffolds may facilitate constructive CNS remodeling and support in situ CNS tissue engineering through mechanisms such as activation of endogenous stem cells.
Supplementary Material
Acknowledgments
This work was supported in part by the NIH (5R01AR053603) and by an Ocular Tissue Engineering and Regenerative Ophthalmology (OTERO) Fellowship from the Louis J. Fox Center for Vision Restoration (a joint program of UPMC and the University of Pittsburgh). The authors also wish to thank Dr. Neill Turner for experimental assistance and Dr. Christopher J. Medberry for furnishing experimental materials.
Disclosure Statement
No competing financial interests exist.
References
- 1.Johansson C.B., Svensson M., Wallstedt L., Janson A.M., and Frisen J.Neural stem cells in the adult human brain. Exp Cell Res 253, 733, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Nunes M.C., Roy N.S., Keyoung H.M., Goodman R.R., McKhann G., 2nd, Jiang L., Kang J., Nedergaard M., and Goldman S.A.Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med 9, 439, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Dromard C., Guillon H., Rigau V., Ripoll C., Sabourin J.C., Perrin F.E., Scamps F., Bozza S., Sabatier P., Lonjon N., Duffau H., Vachiery-Lahaye F., Prieto M., Tran Van Ba C., Deleyrolle L., Boularan A., Langley K., Gaviria M., Privat A., Hugnot J.P., and Bauchet L.Adult human spinal cord harbors neural precursor cells that generate neurons and glial cells in vitro. J Neurosci Res 86, 1916, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Bible E., Dell'Acqua F., Solanky B., Balducci A., Crapo P.M., Badylak S.F., Ahrens E.T., and Modo M.Non-invasive imaging of transplanted human neural stem cells and ECM scaffold remodeling in the stroke-damaged rat brain by (19)F- and diffusion-MRI. Biomaterials 33, 2858, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith E.J., Stroemer R.P., Gorenkova N., Nakajima M., Crum W.R., Tang E., Stevanato L., Sinden J.D., and Modo M.Implantation site and lesion topology determine efficacy of a human neural stem cell line in a rat model of chronic stroke. Stem Cells 30, 785, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Stroemer P., Patel S., Hope A., Oliveira C., Pollock K., and Sinden J.The neural stem cell line CTX0E03 promotes behavioral recovery and endogenous neurogenesis after experimental stroke in a dose-dependent fashion. Neurorehabil Neural Repair 23, 895, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Miljan E.A., Hines S.J., Pande P., Corteling R.L., Hicks C., Zbarsky V., Umachandran M., Sowinski P., Richardson S., Tang E., Wieruszew M., Patel S., Stroemer P., and Sinden J.D.Implantation of c-mycER TAM immortalized human mesencephalic-derived clonal cell lines ameliorates behavior dysfunction in a rat model of Parkinson's disease. Stem Cells Dev 18, 307, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Windrem M.S., Nunes M.C., Rashbaum W.K., Schwartz T.H., Goodman R.A., McKhann G., 2nd, Roy N.S., and Goldman S.A.Fetal and adult human oligodendrocyte progenitor cell isolates myelinate the congenitally dysmyelinated brain. Nat Med 10, 93, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Amariglio N., Hirshberg A., Scheithauer B.W., Cohen Y., Loewenthal R., Trakhtenbrot L., Paz N., Koren-Michowitz M., Waldman D., Leider-Trejo L., Toren A., Constantini S., and Rechavi G.Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med 6, e1000029, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ben-David U., Mayshar Y., and Benvenisty N.Large-scale analysis reveals acquisition of lineage-specific chromosomal aberrations in human adult stem cells. Cell Stem Cell 9, 97, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Kazanis I., and ffrench-Constant C.Extracellular matrix and the neural stem cell niche. Dev Neurobiol 71, 1006, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massirer K.B., Carromeu C., Griesi-Oliveira K., and Muotri A.R.Maintenance and differentiation of neural stem cells. Wiley Interdiscip Rev Syst Biol Med 3, 107, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Discher D.E., Mooney D.J., and Zandstra P.W.Growth factors, matrices, and forces combine and control stem cells. Science 324, 1673, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Potter W., Kalil R.E., and Kao W.J.Biomimetic material systems for neural progenitor cell-based therapy. Front Biosci 13, 806, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Zurita M., Otero L., Aguayo C., Bonilla C., Ferreira E., Parajon A., and Vaquero J.Cell therapy for spinal cord repair: optimization of biologic scaffolds for survival and neural differentiation of human bone marrow stromal cells. Cytotherapy 12, 522, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Song J.J., and Ott H.C.Organ engineering based on decellularized matrix scaffolds. Trends Mol Med 17, 424, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Guo S.Z., Ren X.J., Wu B., and Jiang T.Preparation of the acellular scaffold of the spinal cord and the study of biocompatibility. Spinal Cord 48, 576, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Dequach J.A., Yuan S.H., Goldstein L.S., and Christman K.L.Decellularized porcine brain matrix for cell culture and tissue engineering scaffolds. Tissue Eng Part A 17, 2583, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crapo P.M., Medberry C.J., Reing J.E., Tottey S., van der Merwe Y., Jones K.E., and Badylak S.F.Biologic scaffolds composed of central nervous system extracellular matrix. Biomaterials 33, 3539, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medberry C.J., Crapo P.M., Siu B.F., Carruthers C.A., Wolf M.T., Nagarkar S.P., Agrawal V., Jones K.E., Kelly J., Johnson S.A., Velankar S.S., Watkins S.C., Modo M., and Badylak S.F.Hydrogels derived from central nervous system extracellular matrix. Biomaterials 34, 1033, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown B.N., Londono R., Tottey S., Zhang L., Kukla K.A., Wolf M.T., Daly K.A., Reing J.E., and Badylak S.F.Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater 8, 978, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keane T.J., Londono R., Turner N.J., and Badylak S.F.Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials 33, 1771, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Ariganello M.B., Simionescu D.T., Labow R.S., and Lee J.M.Macrophage differentiation and polarization on a decellularized pericardial biomaterial. Biomaterials 32, 439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong M.L., Leach J.K., Athanasiou K.A., and Griffiths L.G.The role of protein solubilization in antigen removal from xenogeneic tissue for heart valve tissue engineering. Biomaterials 32, 8129, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Sayk F., Bos I., Schubert U., Wedel T., and Sievers H.H.Histopathologic findings in a novel decellularized pulmonary homograft: an autopsy study. Ann Thorac Surg 79, 1755, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Ruzmetov M., Shah J.J., Geiss D.M., and Fortuna R.S.Decellularized versus standard cryopreserved valve allografts for right ventricular outflow tract reconstruction: a single-institution comparison. J Thorac Cardiovasc Surg 143, 543, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Badylak S.F., Hoppo T., Nieponice A., Gilbert T.W., Davison J.M., and Jobe B.A.Esophageal preservation in five male patients after endoscopic inner-layer circumferential resection in the setting of superficial cancer: a regenerative medicine approach with a biologic scaffold. Tissue Eng Part A 17, 1643, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mase V.J., Jr., Hsu J.R., Wolf S.E., Wenke J.C., Baer D.G., Owens J., Badylak S.F., and Walters T.J.Clinical application of an acellular biologic scaffold for surgical repair of a large, traumatic quadriceps femoris muscle defect. Orthopedics 33, 511, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Faleris J.A., Hernandez R.M., Wetzel D., Dodds R., and Greenspan D.C.In-vivo and in-vitro histological evaluation of two commercially available acellular dermal matrices. Hernia 15, 147, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Bejjani G.K., and Zabramski J.Safety and efficacy of the porcine small intestinal submucosa dural substitute: results of a prospective multicenter study and literature review. J Neurosurg 106, 1028, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Hoell T., Hohaus C., Huschak G., Beier A., and Meisel H.J.Total dura substitute in the spine: double layer dural substitute made from polylactide layer and bovine pericardium. Acta Neurochir (Wien) 149, 1259, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Shah A.R., Pearlman A.N., O'Grady K.M., Bhattacharyya T.K., and Toriumi D.M.Combined use of fibrin tissue adhesive and acellular dermis in dural repair. Am J Rhinol 21, 619, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Agrawal V., Brown B.N., Beattie A.J., Gilbert T.W., and Badylak S.F.Evidence of innervation following extracellular matrix scaffold-mediated remodelling of muscular tissues. J Tissue Eng Regen Med 3, 590, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karabekmez F.E., Duymaz A., and Moran S.L.Early clinical outcomes with the use of decellularized nerve allograft for repair of sensory defects within the hand. Hand (NY) 4, 245, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y., He Y., Bharadwaj S., Hammam N., Carnagey K., Myers R., Atala A., and Van Dyke M.Tissue-specific extracellular matrix coatings for the promotion of cell proliferation and maintenance of cell phenotype. Biomaterials 30, 4021, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sellaro T.L., Ranade A., Faulk D.M., McCabe G.P., Dorko K., Badylak S.F., and Strom S.C.Maintenance of human hepatocyte function in vitro by liver-derived extracellular matrix gels. Tissue Eng Part A 16, 1075, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ott H.C., Clippinger B., Conrad C., Schuetz C., Pomerantseva I., Ikonomou L., Kotton D., and Vacanti J.P.Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med 16, 927, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Uygun B.E., Soto-Gutierrez A., Yagi H., Izamis M.L., Guzzardi M.A., Shulman C., Milwid J., Kobayashi N., Tilles A., Berthiaume F., Hertl M., Nahmias Y., Yarmush M.L., and Uygun K.Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med 16, 814, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petersen T.H., Calle E.A., Zhao L., Lee E.J., Gui L., Raredon M.B., Gavrilov K., Yi T., Zhuang Z.W., Breuer C., Herzog E., and Niklason L.E.Tissue-engineered lungs for in vivo implantation. Science 329, 538, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ott H.C., Matthiesen T.S., Goh S.K., Black L.D., Kren S.M., Netoff T.I., and Taylor D.A.Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med 14, 213, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Freytes D.O., Badylak S.F., Webster T.J., Geddes L.A., and Rundell A.E.Biaxial strength of multilaminated extracellular matrix scaffolds. Biomaterials 25, 2353, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Donato R., Miljan E.A., Hines S.J., Aouabdi S., Pollock K., Patel S., Edwards F.A., and Sinden J.D.Differential development of neuronal physiological responsiveness in two human neural stem cell lines. BMC Neurosci 8, 36, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pollock K., Stroemer P., Patel S., Stevanato L., Hope A., Miljan E., Dong Z., Hodges H., Price J., and Sinden J.D.A conditionally immortal clonal stem cell line from human cortical neuroepithelium for the treatment of ischemic stroke. Exp Neurol 199, 143, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Crisan M., Yap S., Casteilla L., Chen C.W., Corselli M., Park T.S., Andriolo G., Sun B., Zheng B., Zhang L., Norotte C., Teng P.N., Traas J., Schugar R., Deasy B.M., Badylak S., Buhring H.J., Giacobino J.P., Lazzari L., Huard J., and Peault B.A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3, 301, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Mack G.S.ReNeuron and StemCells get green light for neural stem cell trials. Nat Biotechnol 29, 95, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Hofstetter C.P., Holmstrom N.A., Lilja J.A., Schweinhardt P., Hao J., Spenger C., Wiesenfeld-Hallin Z., Kurpad S.N., Frisen J., and Olson L.Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat Neurosci 8, 346, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Sharp K.G., Dickson A.R., Marchenko S.A., Yee K.M., Emery P.N., Laidmae I., Uibo R., Sawyer E.S., Steward O., and Flanagan L.A.Salmon fibrin treatment of spinal cord injury promotes functional recovery and density of serotonergic innervation. Exp Neurol 235, 345, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adams R.A., Bauer J., Flick M.J., Sikorski S.L., Nuriel T., Lassmann H., Degen J.L., and Akassoglou K.The fibrin-derived gamma377–395 peptide inhibits microglia activation and suppresses relapsing paralysis in central nervous system autoimmune disease. J Exp Med 204, 571, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tottey S., Corselli M., Jeffries E.M., Londono R., Peault B., and Badylak S.F.Extracellular matrix degradation products and low-oxygen conditions enhance the regenerative potential of perivascular stem cells. Tissue Eng Part A 17, 37, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tottey S., Johnson S.A., Crapo P.M., Reing J.E., Zhang L., Jiang H., Medberry C.J., Reines B., and Badylak S.F.The effect of source animal age upon extracellular matrix scaffold properties. Biomaterials 32, 128, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Douet V., Kerever A., Arikawa-Hirasawa E., and Mercier F.Fractone-heparan sulphates mediate FGF-2 stimulation of cell proliferation in the adult subventricular zone. Cell Prolif 46, 137, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Preston M., and Sherman L.S.Neural stem cell niches: roles for the hyaluronan-based extracellular matrix. Front Biosci (Schol Ed) 3, 1165, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Purushothaman A., Sugahara K., and Faissner A.Chondroitin sulfate “wobble motifs” modulate maintenance and differentiation of neural stem cells and their progeny. J Biol Chem 287, 2935, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herculano-Houzel S., Collins C.E., Wong P., and Kaas J.H.Cellular scaling rules for primate brains. Proc Natl Acad Sci U S A 104, 3562, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Herculano-Houzel S., Mota B., and Lent R.Cellular scaling rules for rodent brains. Proc Natl Acad Sci U S A 103, 12138, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jehee J.F., and Murre J.M.The scalable mammalian brain: emergent distributions of glia and neurons. Biol Cybern 98, 439, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Azevedo F.A., Carvalho L.R., Grinberg L.T., Farfel J.M., Ferretti R.E., Leite R.E., Jacob Filho W., Lent R., and Herculano-Houzel S.Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol 513, 532, 2009 [DOI] [PubMed] [Google Scholar]
- 58.Beattie A.J., Gilbert T.W., Guyot J.P., Yates A.J., and Badylak S.F.Chemoattraction of progenitor cells by remodeling extracellular matrix scaffolds. Tissue Eng 5, 1119, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Agrawal V., Tottey S., Johnson S.A., Freund J.M., Siu B.F., and Badylak S.F.Recruitment of progenitor cells by an extracellular matrix cryptic peptide in a mouse model of digit amputation. Tissue Eng Part A 17, 2435, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Agrawal V., Kelly J., Tottey S., Daly K.A., Johnson S.A., Siu B.F., Reing J., and Badylak S.F.An isolated cryptic Peptide influences osteogenesis and bone remodeling in an adult Mammalian model of digit amputation. Tissue Eng Part A 17, 3033, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O'Reilly M.S., Holmgren L., Shing Y., Chen C., Rosenthal R.A., Moses M., Lane W.S., Cao Y., Sage E.H., and Folkman J.Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 79, 315, 1994 [DOI] [PubMed] [Google Scholar]
- 62.Harder J., Bartels J., Christophers E., and Schroder J.M.Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J Biol Chem 276, 5707, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.