Abstract
Currently, root canal therapy is the only clinical treatment available to treat damaged or necrotic dental pulp tissue arising from caries. This treatment results in the loss of tooth vitality. Somatic dental stem cell-based tissue engineering approaches can alleviate this problem by preserving tooth vitality. Dental stem cells are multipotent and under appropriate conditions could be used for dental pulp tissue engineering. Successful use of these cells in pulp repair requires a combination of growth factors and appropriate scaffolds to induce cell differentiation. In this study, we demonstrate the odontogenic differentiation of human dental pulp stem cells (DPSCs) and the human periodontal ligament stem cells when cultured on a decellularized 3D extracellular matrix (ECM) scaffold without the need for exogenous addition of growth factors. Subcutaneous implantation of the ECM scaffolds containing DPSCs showed the formation of dental pulp-like tissue with cells expressing dentin sialoprotein (DSP) and dentin phosphophoryn (DPP). Additionally, we also show that the ECM scaffold can be exploited as a tool to study the extracellular function of multifunctional proteins. These promising results demonstrate the feasibility of developing these biomimetic scaffolds for treatment of dental caries.
Introduction
Dental caries is one of the most prevalent dental disease and a chronic disease in children aged 5–17.1 According to the World Health Organization (WHO) report from 2003, approximately 90% of the world's population has experienced dental caries. Apart from poor oral hygiene, treatments like chemotherapy and radiation therapy also contribute toward formation of caries. The conventional treatment for pulp inflammation due to dental caries is root canal therapy. Approximately 24 million root canals are performed annually in the United States alone. This therapy involves the complete removal of the infected pulp followed by disinfection and filling up of the chamber with a trioxide component such as mineral trioxide culminating in capping up with a crown. The problem with this approach lies in the removal of the pulp. The pulp tissue offers vitality, sensitivity, and regenerative ability to the tooth. All of these properties are lost as a result of root canal therapy, which in many cases leads to secondary infections. As tooth sensitivity is lost, the secondary infections go unnoticed, until the infection spreads to the surrounding tissues. This condition can, in some cases, lead to sepsis and other serious complications leading to a significant reduction in the quality of life of the patient.
Tissue engineering is a promising therapy to regenerate dental tissues and can provide an excellent replacement for root canal therapy. Two types of approaches exist in the current regenerative dental research to restore normal function to damaged tissue or missing teeth. One is the engineering of individual components such as the dental pulp,2,3 the periodontal ligament4 and alveolar bone5 and the other is engineering the entire functional tooth.6 In the current study, we have focused on dental pulp tissue engineering using somatic dental stem cells.
Several different dental stem cell sources have been investigated for regenerating the dental pulp. Of these, the most common ones are dental pulp stem cells (DPSCs), periodontal ligament stem cells (PDLSCs), and stem cells from human exfoliated deciduous teeth (SHED).7 Although SHED have shown promise for banking and to be useful for tissue engineering applications,8–12 in terms of immediate clinical relevance, the DPSCs and PDLSCs are well suited for engineering the dental pulp as they can be isolated from an adult patient. The multipotent ability of these stem cells has been well documented.13–15 However, to trigger the differentiation of these stem cells into the required lineage for tissue engineering purposes, a combination of growth factors and differentiation agents is required.
In vivo, the extracellular matrix (ECM) dictates cellular proliferation, migration, and differentiation.16 Mimicking the complexity of the ECM will result in cellular environments favorable for lineage-specific differentiation of stem cells.17–22 In the present study, the possibility of utilizing odontoblast ECM embedded type I collagen/chitosan scaffolds for dental pulp tissue engineering using DPSCs and PDLSCs was investigated. Type I collagen is the most abundant protein in the human body and it forms a major ECM component of the dental pulp tissue. Additionally, hydroxyapatite nucleation occurs on the type I collagen framework. For these reasons, we chose type I collagen as one of the components of the starting scaffold matrix. On the other hand, chitosan is a naturally occurring biocompatible biopolymer that has antimicrobial properties.23 We have discussed the use of type I collagen and chitosan as biomaterials in our published review article.24 We have shown that this blend is suitable for mesenchymal stem cell culture and for the generation of ECM integrated scaffolds.18,25 Finally, we have investigated the ability of odontogenic ECM scaffolds to be used as a tool to decipher the extracellular roles of multifunctional proteins.
Materials and Methods
Cell culture
The human DPSCs and the human PDLSCs were used in this study. Both cell types were kindly provided by Dr. Songtao Shi (University of Southern California). Both cell types were cultured in the αMEM (GIBCO) with 20% FBS (GIBCO), 1% L-glutamine (GIBCO), and 1% antibiotic and antimycotic solution (anti-anti; GIBCO).
Generation of ECM scaffolds
The ECM scaffolds were generated using previously published protocols.18 Briefly, DPSCs (2 million cells/mL) were cultured in a 1:1 collagen/chitosan hydrogel for 2 weeks in the presence of differentiation media. After this, the cells were lysed followed by DNA digestion resulting in an ECM embedded collagen/chitosan scaffold.
In vitro differentiation of DPSCs and PDLSCs
DPSCs and PDLSCs (1 million cells/scaffold) were cultured on the ECM scaffolds in the presence of growth media without any additional supplements for a period of 2 weeks or 4 weeks. RNA from the cells was isolated according to published protocol18 followed by cDNA synthesis. The osteogenesis real-time PCR array (Qiagen) was used to analyze changes in the expression levels involved in odontogenic differentiation of stem cells. When required, the ECM scaffolds were incubated overnight at 4°C in a 500 μL solution of DPP antibody (1/100 dilution). Before cell seeding and gene expression analysis, the scaffolds were washed extensively to remove nonspecifically bound antibodies. Scaffolds incubated similarly with preimmune serum served as controls.
Statistical analysis
The data were expressed as fold change in gene expression in cells cultured in the ECM scaffold with respect to the control scaffold. Statistical significance was calculated using the software package provided by the manufacturer and is expressed as a p value obtained from the Student's t-test.
In vivo implantation experiments
All experiments were performed according to approved UIC animal care protocols (Assurance No: A3460-01). Triplicate control (collagen/chitosan scaffolds without the ECM) and ECM scaffolds were implanted subcutaneously on the back of 1-month-old male nude mice (Charles River laboratories) for 2 weeks. Each mouse contained one control scaffold and one ECM scaffold on either side of the spine. After 2 weeks, the animals were euthanized followed by extraction of the scaffolds. The scaffolds were fixed in formalin, paraffin embedded, and sectioned into 5-μm-thick sections for histology and immunoshistochemistry according to published protocol.25
Histology
Tissue sections were deparaffinized in xylene, hydrated in graded ethanol solutions, and stained with hematoxylin and eosin (H&E) stains and Alizarin Red as per standard published protocols.25 The stained sections were imaged using a Zeiss Axiovert inverted microscope equipped with the axiovision imaging software. To observe the autofluorescence from red blood corpuscles (RBCs), the sections were imaged using TRITC red filter sets. Collagen autofluorescence was observed by imaging the samples using the green fluorescent protein (GFP) filter sets.
Immunoshistochemistry
All sections were deparaffinized in xylene, hydrated in graded ethanol solutions, and immunostained according to published protocols.18,25 The following antibodies were used: mouse anti-Tubulin antibody (1/1000; Sigma), rabbit anti-Fibronectin antibody (1/200; Sigma), mouse anti-bone morphogenetic protein 2 (BMP2) antibody (1/100; BD Biosciences), rabbit anti-transforming growth factor β (TGFβ) antibody (1/100; Santacruz biotechnology), rabbit anti-VEGF antibody (1/100; Santacruz biotechnology), mouse anti-PEDF antibody (1/100; Millipore), rabbit anti-MMP2 antibody (1/100; Santacruz), rabbit anti-MMP9 antibody (1/100; Santacruz), mouse anti-phospho serine and phosphor tyrosine antibodies (1/100; Sigma), mouse anti-C-DMP1 antibody (1/2000; gift from Dr. Qin, University of Texas), mouse anti-Thrombospondin antibody (1/100; Sigma), rabbit anti-dentin sialoprotein (DSP) and anti-dentin phosphophoryn (DPP) antibodies (1/250; characterized in our laboratory,26), and mouse anti-von Willebrand factor antibody (1/100; Santacruz biotechnology). For all immunohistochemistry experiments with fluorescent secondary antibody labeling, anti-mouse FITC (1/100; Sigma) and anti-rabbit TRITC (1/100; Sigma) antibodies were used. All fluorescently immunostained sections were imaged using a Zeiss LSM 710 confocal microscope equipped with Zen image analysis software.
In vitro nucleation
In vitro nucleation was performed as per previously published protocol.18 The nucleated samples were dehydrated, coated with osmium tetroxide, and imaged using a LEO Gemini 1525 scanning electron microscope. Energy dispersive X-ray (EDX) analysis was also performed using the same instrument to obtain the relative amounts of calcium and phosphorus in the samples. Transmission electron microscopy (TEM) was used to perform electron diffraction analysis on the nucleated samples. Briefly, the samples were dried, crushed, loaded onto TEM grids, and imaged using a Joel 3010EX transmission electron microscope.
Results
Characterization of the ECM scaffold
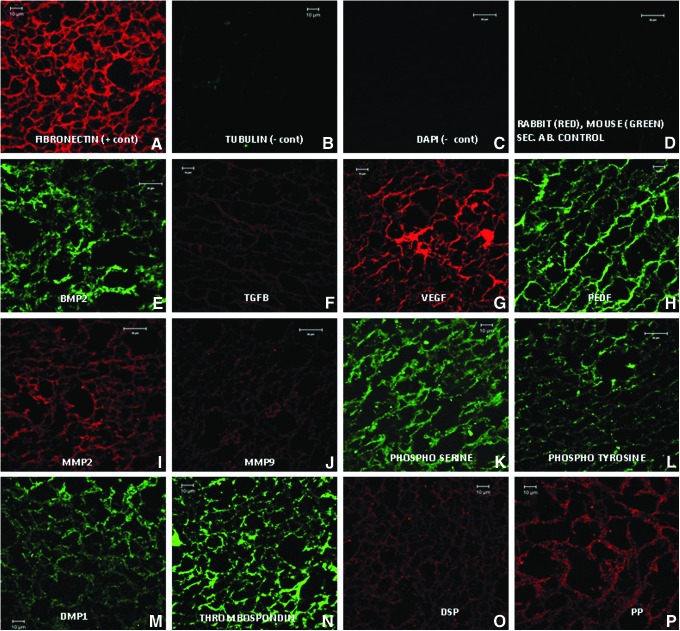
Fluorescent immunohistochemical analysis for several ECM proteins was performed to characterize the scaffold. Figure 1 shows representative confocal images of ECM scaffold sections stained with various antibodies. Tubulin served as a negative control for the intracellular protein (Fig. 1B) and fibronectin served as the positive control for the ECM protein (Fig. 1A). DAPI nuclear staining was performed to ensure the absence of residual DNA from lysed cells (Fig. 1C). Sections stained with the FITC-labeled mouse secondary antibody and TRITC-labeled rabbit secondary antibody alone served as controls for secondary antibody nonspecific binding (Fig. 1D). The ECM scaffold stained positive for growth factors such as BMP2, TGFβ, VEGF, and PEDF (Fig. 1E–H), metalloproteases such as MMP2 and MMP9 (Fig. 1I, J), phosphorylated proteins (Fig. 1K, L), noncollagenous proteins such as DMP1, thrombospondin (Fig. 1M, N), and pulp-specific noncollagenous proteins DSP and DPP (Fig. 1O, P).
FIG. 1.
Expression of extracellular matrix (ECM) proteins: (A–P) are representative confocal micrographs showing the expression of various ECM proteins in the ECM scaffold sections. Fibronectin (A) was used as a positive control and Tubulin (B) was used as a negative control for intracellular protein. DAPI staining (C) was performed to rule out the presence of DNA material in the ECM scaffold. (D) shows the absence of rabbit and mouse nonspecific secondary antibodies. BMP2, bone morphogenetic protein 2; TGFβ, transforming growth factor beta; VEGF, vascular endothelial growth factor; MMP, matrix metalloprotease. Color images available online at www.liebertpub.com/tea
In vitro hydroxyapatite nucleation
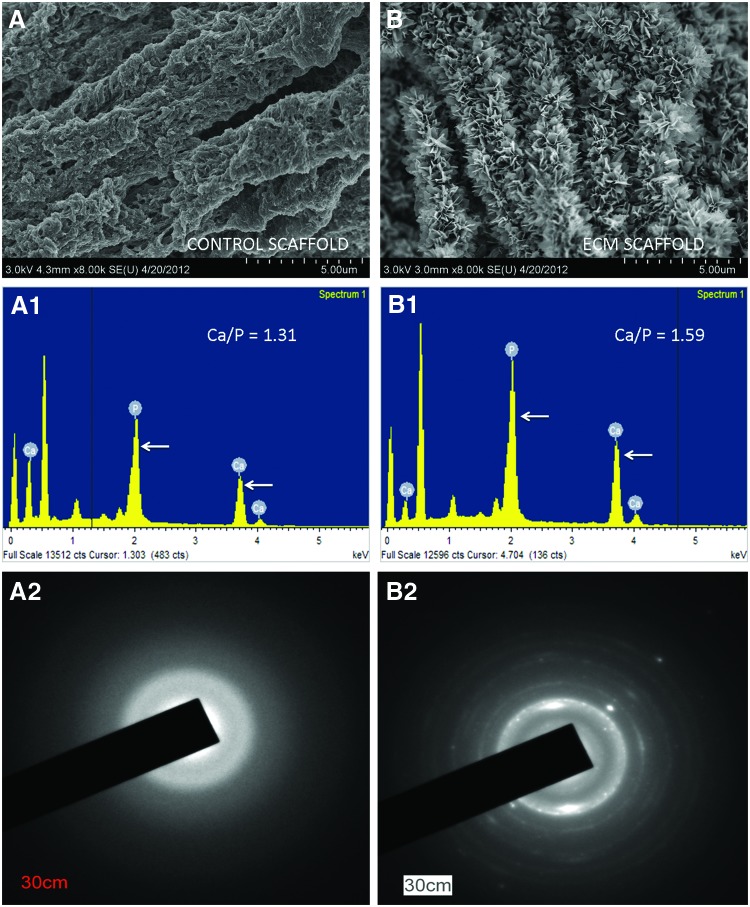
Dental pulp cells have the ability to differentiate into odontoblasts and form reparative dentin. This function implies that the secreted matrix possesses that ability to nucleate hydroxyapatite. Results from in vitro nucleation assays showed that the ECM was able to nucleate crystalline hydroxyapatite (Fig. 2B). The control scaffold (devoid of embedded ECM) only nucleated amorphous calcium phosphate (Fig. 2A). EDX analysis indicated that the ratio of calcium to phosphate in the nucleated ECM scaffolds was 1.59 (n=5, SEM=0.069) indicating the presence of crystalline hydroxyapatite (Fig. 2B1). This observation was confirmed using electron diffraction analysis that showed the presence of crystalline hydroxyapatite (Fig. 2B2). On the other hand, the ratio of calcium to phosphate in the nucleated control scaffolds was 1.31 (n=5, SEM 0.013) indicating the presence of amorphous calcium phosphate (Fig. 2A1, A2).
FIG. 2.
In vitro nucleation: (A) represents nucleation of amorphous calcium phosphate polymorphs in the control collagen/chitosan scaffold. (A1) shows the energy dispersive X-ray (EDX) analysis indicating the presence of calcium and phosphorus ions (arrows point to calcium and phosphorus peaks) along with the Ca to P ratio. (A2) shows electron diffraction data from the nucleated control scaffold indicating the presence of amorphous calcium phosphate. (B) represents nucleation of crystalline hydroxyapatite in the ECM scaffold. (B1) represents the corresponding EDX analysis showing the presence of calcium and phosphorus ions (arrows point to calcium and phosphorus peaks) and the Ca to P ratio. (B2) shows the electron diffraction data from the nucleated ECM scaffold indicating the presence of crystalline hydroxyapatite. Color images available online at www.liebertpub.com/tea
Differentiation of DPSCs and PDLSCs on the ECM scaffolds
DPSCs and PDLSCs were cultured on the ECM scaffold and the control scaffold for a period of 2 weeks in the presence of standard growth media. Real-time PCR was performed to analyze gene expression profiles of key genes involved in odontoblastic differentiation of stem cells. Table 1 provides a list of genes significantly regulated in DPSCs after 2 weeks of culture. They have been categorized according to functionality and the data show change in gene expression on the ECM scaffold compared to the control scaffold. It is evident from the table that several growth factors, transcription factors, ECM proteins, and receptors were significantly regulated as a result of culture on the ECM scaffold. Tables 2 and 3 provide a similar list of genes significantly altered with respect to the control scaffold when PDLSCs were cultured within the ECM scaffold for a period of 2 and 4 weeks, respectively. The PDLSCs showed significant positive regulation of odontoblast-specific genes.
Table 1.
Showing a List of Genes Regulated When DPSCs Were Cultured for 2 Weeks in Growth Media in the ECM Scaffolds With Or Without DPP Blocking as Opposed to the Control Scaffold
| |
|
Fold change (p-value from t-test) |
|
|---|---|---|---|
| Genes | Description | ECM | ECM+pp block |
| |
Growth factors |
|
|
|
BMP2 |
Bone morphogenetic protein 2 |
3.2 (0.019) |
2.48 (0.065) |
|
BMP4 |
Bone morphogenetic protein 4 |
9.48 (0.001) |
5.24 (0.03) |
|
BMP5 |
Bone morphogenetic protein 5 |
19.89 (0.0002) |
9.86 (0.092) |
|
FGF1 |
Fibroblast growth factor 1 |
−3.84 (0.0001) |
−3.84 (0.0004) |
|
FGF2 |
Fibroblast growth factor 2 |
−1.96 (0.003) |
−1.63 (0.017) |
|
IGF2 |
Insulin-like growth factor 2 |
22.11 (0.017) |
9.90 (0.003) |
|
TGFB1 |
Transforming growth factor beta 1 |
1.57 (0.02) |
0.98 |
|
TGFB2 |
Transforming growth factor beta 2 |
−1.91 (0.001) |
−1.56 (0.001) |
|
VEGFA |
Vascular endothelial growth factor A |
5.45 (0.0007) |
5.63 (0.002) |
|
VEGFB |
Vascular endothelial growth factor B |
2.30 (0.002) |
1.67 (0.065) |
| |
Transcription factors |
|
|
|
RUNX2 |
Runt-related transcription factor 2 |
1.53 (0.07) |
1.08 |
|
NFKB1 |
Nuclear factor kappa of B-cells 1 |
−2.25 (0.001) |
−2.32 (0.001) |
|
SMAD2 |
SMAD family member 2 |
−5.5 (0.001) |
−4.9 (0.002) |
|
SMAD4 |
SMAD family member 4 |
−1.51 (0.04) |
−1.73 (0.01) |
|
TWIST1 |
Twist homolog 1 |
2.9 (0.003) |
2.24 (0.003) |
| |
ECM proteins |
|
|
|
BGN |
Biglycan |
10.75 (0.000023) |
3.53 (0.23) |
|
COL10A1 |
Type X collagen |
−1.85 (0.008) |
−1.02 (0.94) |
|
COL1A1 |
Type I collagen |
5.45 (0.05) |
2.6 (0.29) |
| |
Proteases |
|
|
|
BMP1 |
Bone morphogenetic protein 1 |
−3.83 (0.0007) |
−5.12 (0.0006) |
|
MMP10 |
Matrix metalloproteinase 10 |
30.65 (0.0001) |
20.68 (0.016) |
|
MMP2 |
Matrix metalloproteinase 2 |
4.34 (0.005) |
3.78 (0.018) |
| |
Adhesion molecules |
|
|
|
CDH11 |
Cadherin 11 |
1.71 (0.008) |
1.02 |
|
ITGA1 |
Integrin alpha 1 |
−2.78 (0.0003) |
−3.29 (0.0002) |
|
ITGA2 |
Integrin alpha 2 |
−16.23 (0.0001) |
−8.36 (0.0005) |
|
ITGB1 |
Integrin beta 1 |
−8.33 (0.0001) |
−7.57 (0.0002) |
|
VCAM1 |
Vascular cell adhesion molecule 1 |
−1.5 (0.05) |
−2.01 (0.03) |
| |
Receptors |
|
|
|
IGF1R |
Insulin-like growth factor 1 receptor |
−1.84 (0.006) |
−2.6 (0.005) |
| |
Other |
|
|
| ACTB | β Actin | 3.6 (0.00008) | 2.19 (0.09) |
Negative sign under fold change represents downregulation. p-Value was calculated using the Student's t-test. Underlined genes show differential expression with DPP blocking.
DPSCs, dental pulp stem cells; ECM, extracellular matrix.
Table 2.
Showing a List of Genes Regulated When PDLSCs Were Cultured for 2 Weeks in Growth Media in the ECM Scaffolds
| Gene symbol | Description | Fold change | p-Value (t-test) |
|---|---|---|---|
| |
Growth factors |
|
|
|
BMP2 |
Bone morphogenetic protein 2 |
10.11 |
0.064 |
|
BMP5 |
Bone morphogenetic protein 5 |
1.94 |
0.096 |
|
IGF1 |
Insulin-like growth factor 1 |
2.71 |
0.0146 |
|
PDGFA |
Platelet-derived growth factor A |
1.84 |
0.0728 |
|
TGFβ1 |
Transforming growth factor beta 1 |
2.15 |
0.0091 |
|
TGFβ2 |
Transforming growth factor beta 2 |
1.41 |
0.0674 |
|
TGFβ3 |
Transforming growth factor beta 3 |
1.97 |
0.0065 |
|
VEGFA |
Vascular endothelial growth factor A |
2.05 |
0.0115 |
| |
Transcription factors |
|
|
|
RUNX 2 |
Runt-related transcription factor 2 |
1.87 |
0.0575 |
|
SOX 9 |
(Sex determining region Y)-box 9 |
4.60 |
0.0123 |
|
VDR |
Vitamin D receptor |
2.96 |
0.0135 |
| |
ECM proteins |
|
|
|
AMELY |
Amelogenin |
3.11 |
0.0022 |
|
BGN |
Biglycan |
2.91 |
0.061 |
|
COL5A1 |
Type V collagen |
2.50 |
0.0407 |
|
COMP |
Cartilage oligomeric matrix protein |
3.92 |
0.0519 |
|
FN1 |
Fibronectin |
1.76 |
0.0665 |
| |
Adhesion molecules |
|
|
|
ICAM1 |
Intercellular adhesion molecule 1 |
4.02 |
0.0461 |
|
ITGA2 |
Integrin alpha 2 |
2.36 |
0.0010 |
|
ITGA3 |
Integrin alpha 3 |
1.97 |
0.0019 |
|
CDH11 |
Cadherin 11 |
2.06 |
0.0127 |
| |
Phosphatases |
|
|
|
MMP2 |
Matrix metalloprotease 2 |
2.89 |
0.0095 |
| |
Other |
|
|
| TFIP11 | Tuftelin interactin protein 11 | 1.72 | 0.0618 |
p-Value was calculated using the Student's t-test.
PDLSC, periodontal ligament stem cells.
Table 3.
Showing a List of Genes Regulated When PDLSCs Were Cultured for 4 Weeks in Growth Media in the ECM Scaffolds
| Gene symbol | Description | Fold change | p-Value (t-test) |
|---|---|---|---|
| |
Growth factors |
|
|
|
BMP2 |
Bone morphogenetic protein 2 |
2.64 |
0.102 |
|
BMP5 |
Bone morphogenetic protein 5 |
2.69 |
0.032 |
|
CSF3 |
Colony stimulating factor 3 |
2.03 |
0.073 |
|
FGF2 |
Fibroblast growth factor 2 |
−2.23 |
0.004 |
|
IGF2 |
Insulin-like growth factor 2 |
−3.4 |
0.043 |
|
TGFβ2 |
Transforming growth factor beta 2 |
1.72 |
0.001 |
|
TGFβ3 |
Transforming growth factor beta 3 |
2.09 |
0.051 |
|
VEGFA |
Vascular endothelial growth factor A |
1.65 |
0.162 |
| |
Transcription factors |
|
|
|
RUNX 2 |
Runt-related transcription factor 2 |
1.92 |
0.0051 |
|
SMAD1 |
SMAD family member 1 |
1.33 |
0.011 |
|
SMAD2 |
SMAD family member 2 |
1.22 |
0.0033 |
|
SMAD3 |
SMAD family member 3 |
1.43 |
0.075 |
|
SMAD4 |
SMAD family member 4 |
1.54 |
0.002 |
|
SOX9 |
Sex determining region Y)-box 9 |
1.87 |
0.25 |
|
VDR |
Vitamin D receptor |
1.7 |
0.0204 |
| |
ECM proteins |
|
|
|
COL12A1 |
Type X1I Collagen |
1.72 |
0.0065 |
|
COL14A1 |
Type X1V Collagen |
1.72 |
0.0005 |
|
COL1A1 |
Type I Collagen |
1.81 |
0.17 |
|
COL5A1 |
Type V Collagen |
1.62 |
0.090 |
|
COMP |
Cartilage oligomeric matrix protein |
2.27 |
0.14 |
|
DSPP |
Dentin sialo phosphoprotein |
3.32 |
0.011 |
|
FN1 |
Fibronectin |
1.55 |
0.043 |
| |
Adhesion molecules |
|
|
|
ITGA1 |
Integrin alpha 1 |
1.53 |
0.042 |
|
ITGA2 |
Integrin alpha 2 |
1.57 |
0.009 |
|
ITGAM |
Integrin alpha M |
3.32 |
0.034 |
|
CDH11 |
Cadherin 11 |
1.81 |
0.019 |
| |
Phosphatases |
|
|
|
MMP10 |
Matrix metalloprotease 10 |
3.14 |
0.005 |
|
MMP8 |
Matrix metalloprotease 8 |
2.37 |
0.2221 |
| |
Receptors |
|
|
| CD36 | CD36 molecule | −1.91 | 0.03 |
p-Value was calculated using the Student's t-test.
The ECM scaffold can be used to identify the extracellular function of multifunctional proteins
There are several proteins involved in the cellular machinery that perform multiple functions both intra- and extracellularly depending on the biological scenario. One such protein is dentin phosphophoryn. The extracellular role of DPP in nucleating hydroxyapatite is well established.27 However, the extracellular signaling function of DPP is not well defined. We attempted to isolate the extracellular signaling role of DPP by blocking DPP in the ECM scaffold by means of antibody treatment. The last column in Table 1 gives the list of genes that were regulated during differentiation of DPSCs on the ECM scaffolds that were blocked with the DPP antibody. The underlined genes in Table 1 were the ones that were significantly affected as a result of DPP blocking. It is evident from the table that DPP blocking resulted in reduced growth factor production, downregulation of Runx2, and reduced type I collagen synthesis. No significant change was observed when the scaffolds were blocked with rabbit IgG control.
ECM scaffold permits neovascularization in vivo
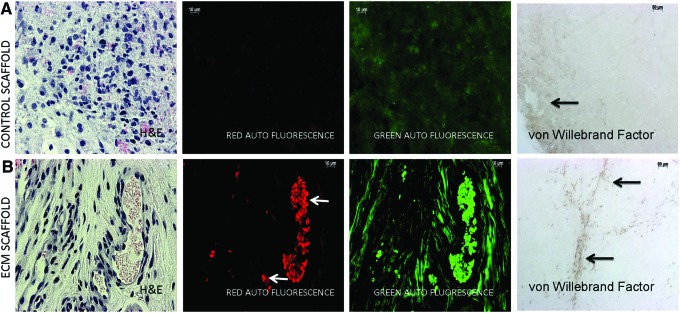
The control and ECM scaffolds were implanted subcutaneously in male nude mice for a period of 2 weeks. Neovascularization was evident only in the ECM scaffolds. The red fluorescent images of H&E stained sections showing non-nucleated RBCs (Fig. 3A, B) demonstrate this clearly. The green fluorescent images in Figure 3A and B are from collagen autofluorescence in the green channel. A stronger fluorescence signal from the ECM scaffold suggests increased collagen synthesis. Immunohistochemistry was performed with the anti-von Willebrand factor antibody to look for the presence of endothelial cells within the control and ECM scaffolds. Results presented in Figure 3A and B show the presence of endothelial cells within the ECM scaffolds forming a capillary network (black arrows, last panel in Fig. 3B). However, in the control scaffolds, endothelial cells were observed only in the periphery (black arrow, last panel in Fig. 3A). No capillary-like structures were observed in these scaffolds.
FIG. 3.
H&E staining and von Willebrand factor immunohistochemistry (IHC) of subcutaneous implant sections: (A) is a representative micrograph of an H&E stained section from the control scaffold after 2 weeks of subcutaneous implantation in nude mice imaged under brightfield, red fluorescence (autofluorescence from the red blood corpuscles (RBCs) and eosin stained areas) and green fluorescence (autofluorescence from the collagen) channels. The last panel in (A) shows the presence of endothelial cells stained with the von Willebrand factor antibody present in the periphery of the scaffold (black arrow). (B) is a similar imaging of an H&E stained section from the ECM scaffold. The arrows in (B) represent RBCs from the blood vessels. Also, note the increased signal in the green channel indicating increased collagen presence. The last panel in (B) shows the von Willebrand factor stained endothelial cells within the ECM scaffold forming capillary-like structures. All imaging parameters were maintained constant. Color images available online at www.liebertpub.com/tea
ECM scaffold induces increased calcium deposition and polarization of collagen fibrils in vivo
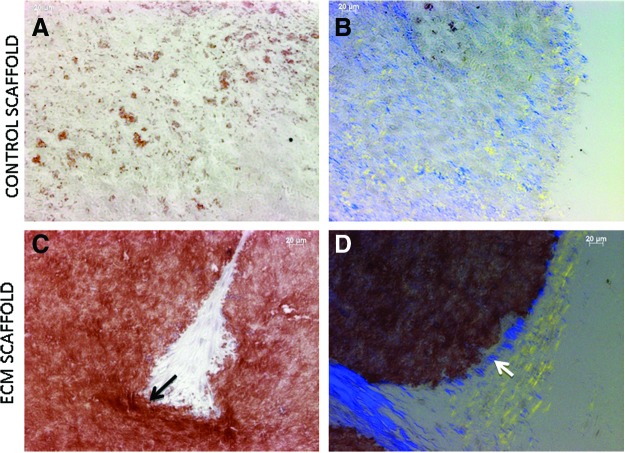
Sections from the implanted control and ECM scaffolds were stained with Alizarin Red to look for calcium deposition. Results indicated that the ECM scaffold triggered increased extracellular calcium deposition (Fig. 4C) when compared with the control scaffold (Fig. 4A). Collagen fibril orientation serves as a template for mineral deposition. When the sections were viewed under polarized light to visualize collagen fibril orientation, we could observe polarized collagen fibrils on the edges of the ECM scaffold (Fig. 4D). Only randomly oriented collagen fibrils were observed in the control scaffold (Fig. 4A). This result indicated that the differentiating DPSCs in the ECM scaffold triggered collagen synthesis and organization.
FIG. 4.
Alizarin Red staining and collagen orientation in implant sections: (A, C) show representative images of Alizarin Red stained sections from control (A) and ECM scaffold (C) implant sections. Note the increased calcium deposition in (C). The arrow in (C) represents increased staining observed at the periphery. (B, D) show representative images of collagen fibril orientation in control (B) and ECM scaffold (D) implant sections. Note the presence of polarized collagen at the periphery of the scaffold in (D) (arrow in 4D) and its absence in (B). Color images available online at www.liebertpub.com/tea
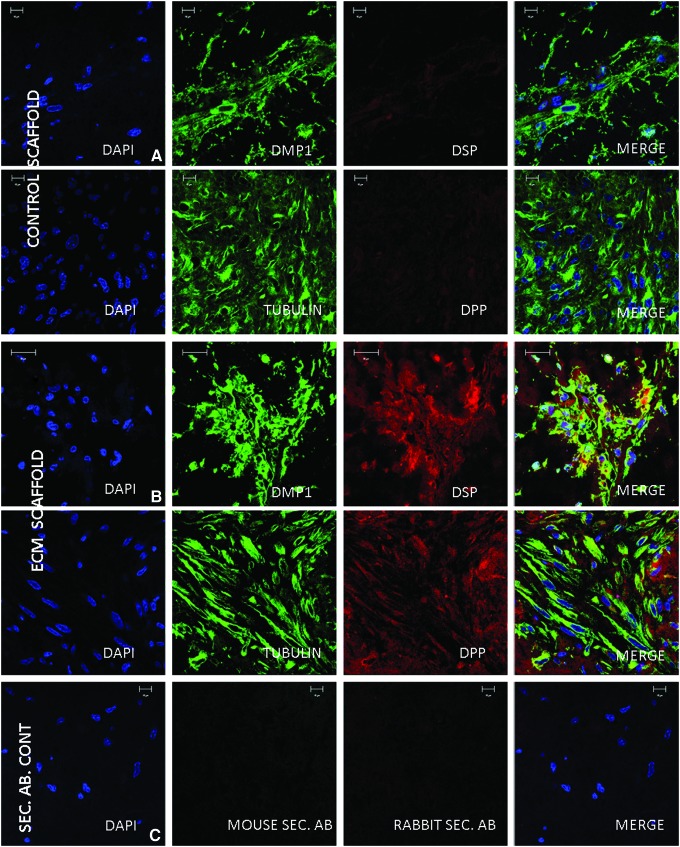
In vivo expression of DMP1, DSP, and DPP
We analyzed the expression pattern of DMP1, DSP, and DPP in the control and ECM implant sections. Immunohistochemical data showed a slight increase in the expression of DMP1 (Fig. 5A, B). However, a marked increase in the expression of DSP and DPP was observed in the ECM scaffold compared to the control scaffold (Fig. 5A, B). Tubulin levels remained constant in both scaffolds and served as a positive control (Fig. 4A, B). No staining was observed in the sections treated with the TRITC rabbit and FITC mouse secondary antibodies alone and served as a negative control (Fig. 4C). The laser intensities and imaging parameters were maintained constant.
FIG. 5.
Immunofluorescent staining of sections from subcutaneous implants: (A, B) are representative confocal micrographs showing the expression of DMP1, DSP, Tubulin, and DPP in the control (A) and ECM scaffolds (B) implanted with DPSCs subcutaneously in nude mice. Note the markedly increased expression of DSP and DPP in the ECM scaffold sections. (C) shows negative staining from the FITC-conjugated mouse and TRITC-conjugated rabbit secondary antibodies. All imaging parameters were maintained constant. DSP, dentin sialoprotein; DPP, dentin phosphophoryn. Color images available online at www.liebertpub.com/tea
Discussion
ECM-based scaffolds mimic the composition of the native tissue. In the present study, we have developed an odontoblast-specific ECM scaffold to promote odontogenic differentiation of two different multipotent adult dental stem cells, namely, DPSCs and PDLSCs.13,15 These cell-generated ECM-based scaffolds were first decellularized before analysis. Characterization of the ECM scaffolds by immunostaining showed that it contained a rich source of matrix proteins and matrix metalloproteases (MMPs), growth factors such as BMP2, TGF β, VEGF, and PEDF. Immunostaining with anti-p serine and p-tyrosine antibodies demonstrated the presence of several phosphorylated proteins in the ECM scaffold. It is well established that phosphorylated proteins play a key role in the mineralization process and serve as phosphorus donors for the nucleation of calcium phosphate polymorphs.28 Therefore, this scaffold could mimic the in vivo scenario of odontogenic cells and initiate calcium phosphate nucleation.
Three major noncollagenous proteins synthesized by the odontoblasts are DMP1, DSP, and DPP. The presence of these three proteins in the scaffold was essential to provide bioactive cues for early and terminal differentiation of the dental stem cells. Other matrix proteins that were identified were thrombospondin and fibronectin. Although this is not an exhaustive list of proteins present in the ECM, it provides glimpses into the rich bioactive composition of the ECM scaffold. We had published earlier on the composition of the osteogenic scaffold and shown that DPP and DSP were not present in detectable amounts.18 This observation confirmed published reports on the amount of DSP and DPP synthesized by osteoblasts.29 Other noted differences in the composition of the ECM scaffold were the levels of TGFβ. Low levels of TGFβ were present in the odontogenic matrix when compared with the osteogenic matrix. These differences show that although osteoblasts and odontoblasts synthesize a calcifying organic matrix, their compositions are markedly different. More detailed studies are required to quantitatively assess the difference in the levels of ECM proteins synthesized by the osteoblasts and odontoblasts and our future efforts will be dedicated to explore this possibility.
One of the important functions of the dental pulp is to provide regenerative potential to compensate for lost dentin by enabling the differentiation of pulp cells into odontoblasts capable of synthesizing a calcifying matrix. The odontogenic ECM scaffold used in this study had the potential to induce nucleation of hydroxyapatite crystals. Under the same conditions, the control collagen/chitosan scaffold could only induce deposition of amorphous calcium phosphate polymorphs. When implanted subcutaneously in mice, the proteins in the ECM scaffold could trigger the deposition of oriented collagen fibers. The capability of the ECM scaffolds to dictate deposition of oriented collagen fibers and hydroxyapatite suggests the possibility of using such scaffolds for the formation of a reparative dentin bridge in clinical settings.
To understand the mechanism by which the ECM scaffold facilitated the odontogenic differentiation of DPSCs, gene expression analysis was performed. Results from the osteogenic PCR array show that several growth factors, transcription factors, MMPs, and cell surface receptors, which are required for odontogenic differentiation, were upregulated, while others were downregulated when DPSCs were cultured within the 3D ECM scaffolds. Analysis of the scaffolds after subcutaneous implantation showed the formation of dental pulp-like tissue with cells expressing DSP and DPP. Additionally, the ECM scaffold promoted neovascularization and increased collagen deposition. Differentiation of PDLSCs on the odontogenic scaffold was also interesting. PDLSCs have to be potential to differentiate into osteogenic, odontogenic, or cementogenic lineage depending on the biological cues. Results from the PCR array showed that the PDLSCs could differentiate into odontogenic lineage when cultured on the 3D ECM scaffold. Two genes of interest that were upregulated and served as markers for odontogenic differentiation were amelogenin and DSPP. DSPP is a well-known marker of odontogenic differentiation and published reports show that amelogenin is expressed by odontoblasts and pulp cells.30–33 Expression of DSPP and amelogenin were not observed during osteogenic differentiation of mesenchymal stem cells.18 These results conclusively demonstrate the odontogenic potential of the ECM scaffold to drive lineage-specific differentiation of somatic dental stem cells.
Apart from its ability to induce lineage-specific differentiation and serve as a scaffold for tissue regeneration, the ECM scaffold can function as a biomimetic platform to analyze the extracellular function of multifunctional proteins. The cell produces and secretes several proteins that possess both intracellular and extracellular functions. Over the years, several such proteins have been identified. Many of the NCPs, such as DMP1 and DPP, are multifunctional.34–38 Recently, we identified that the endoplasmic reticulum chaperone protein GRP-78 plays a role in mineralized matrix formation.39,40 As proof of concept, we sought to identify the extracellular function of DPP during odontogenic differentiation of DPSCs. Thus far, all of the studies performed to analyze the role of DPP in odontoblast differentiation have been performed on 2D cultures and isolated systems where in there is minimal involvement from other ECM proteins. We used a loss of function approach by blocking the extracellular DPP in the ECM scaffold using the anti-DPP antibody followed by differentiating DPSCs within the ECM scaffold. Fold change in gene expression with respect to control was calculated for the unblocked and blocked ECM scaffolds. Results indicated that the expression of some genes was significantly influenced as a result of DPP blocking. Specifically, a significant reduction in the expression levels of growth factors BMP4, 5, IGF1, and TGFβ1 was observed. This could be attributed to the downregulation of RUNX2 and ECM protein biglycan and ultimately reduction in type I collagen synthesis all of which are evident in the gene expression data. Highlights from this analysis indicate that DPP influenced BMP and TGFβ signaling pathways and had minimum influence on FGF signaling and expression of VEGF and its downstream effectors. This data are novel, physiologically relevant, and cannot be obtained using conventional methods. Our future efforts will be directed toward understanding this process in a more detailed manner to better understand the functional complexities of ECM-mediated signaling enabling better biomimetic designs for tissue engineering purposes.
Acknowledgments
We would like to thank Dr. Songtao Shi (the University of Southern California) for generously providing us with the DPSCs and PDLSCs. We would like to thank the UIC Research Resources Center (RRC) electron microscopy facility for assistance with obtaining the electron diffraction data. This study was supported by NIH R01 DE19633 and the Brodie Endowment Fund.
Disclosure Statement
No competing financial interests exist.
References
- 1.Bagramian R.A., Garcia-Godoy F., and Volpe A.R.The global increase in dental caries. A pending public health crisis. Am J Dent 22,3, 2009 [PubMed] [Google Scholar]
- 2.Huang G.T., Yamaza T., Shea L.D., Djouad F., Kuhn N.Z., Tuan R.S., et al. Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng Part A 16,605, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang G.T.Pulp and dentin tissue engineering and regeneration: current progress. Regen Med 4,697, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park C.H., Rios H.F., Jin Q., Bland M.E., Flanagan C.L., Hollister S.J., et al. Biomimetic hybrid scaffolds for engineering human tooth-ligament interfaces. Biomaterials 31,5945, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim S.H., Kim K.H., Seo B.M., Koo K.T., Kim T.I., Seol Y.J., et al. Alveolar bone regeneration by transplantation of periodontal ligament stem cells and bone marrow stem cells in a canine peri-implant defect model: a pilot study. J Periodontol 80,1815, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Nakao K., Morita R., Saji Y., Ishida K., Tomita Y., Ogawa M., et al. The development of a bioengineered organ germ method. Nat Methods 4,227, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Miura M., Gronthos S., Zhao M., Lu B., Fisher L.W., Robey P.G., et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A 100,5807, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viale-Bouroncle S., Gosau M., Kupper K., Mohl C., Brockhoff G., Reichert T.E., et al. Rigid matrix supports osteogenic differentiation of stem cells from human exfoliated deciduous teeth (SHED). Differentiation 84,366, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Vakhrushev I.V., Antonov E.N., Popova A.V., Konstantinova E.V., Karalkin P.A., Kholodenko I.V., et al. Design of tissue engineering implants for bone tissue regeneration of the basis of new generation polylactoglycolide scaffolds and multipotent mesenchymal stem cells from human exfoliated deciduous teeth (SHED cells). Bull Exp Biol Med 153,143, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Nishino Y., Yamada Y., Ebisawa K., Nakamura S., Okabe K., Umemura E., et al. Stem cells from human exfoliated deciduous teeth (SHED) enhance wound healing and the possibility of novel cell therapy. Cytotherapy 13,598, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Arora V., Arora P., and Munshi A.K.Banking stem cells from human exfoliated deciduous teeth (SHED): saving for the future. J Clin Pediatr Dent 33,289, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Morsczeck C., Vollner F., Saugspier M., Brandl C., Reichert T.E., Driemel O., et al. Comparison of human dental follicle cells (DFCs) and stem cells from human exfoliated deciduous teeth (SHED) after neural differentiation in vitro. Clin Oral Investig 14,433, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Gronthos S., Mankani M., Brahim J., Robey P.G., and Shi S.Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A 97,13625, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.d'Aquino R., De Rosa A., Laino G., Caruso F., Guida L., Rullo R., et al. Human dental pulp stem cells: from biology to clinical applications. J Exp Zool B Mol Dev Evol 312B,408, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Seo B.M., Miura M., Gronthos S., Bartold P.M., Batouli S., Brahim J., et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364,149, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Clark R.A.Synergistic signaling from extracellular matrix-growth factor complexes. J Invest Dermatol 128,1354, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Thibault R.A., Scott Baggett L., Mikos A.G., and Kasper F.K.Osteogenic differentiation of mesenchymal stem cells on pregenerated extracellular matrix scaffolds in the absence of osteogenic cell culture supplements. Tissue Eng Part A 16,431, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravindran S., Gao Q., Kotecha M., Magin R.L., Karol S., Bedran-Russo A., et al. Biomimetic extracellular matrix-incorporated scaffold induces osteogenic gene expression in human marrow stromal cells. Tissue Eng Part A 18,295, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agrawal V., Brown B.N., Beattie A.J., Gilbert T.W., and Badylak S.F.Evidence of innervation following extracellular matrix scaffold-mediated remodelling of muscular tissues. J Tissue Eng Regen Med 3,590, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilbert T.W., Agrawal V., Gilbert M.R., Povirk K.M., Badylak S.F., and Rosen C.A.Liver-derived extracellular matrix as a biologic scaffold for acute vocal fold repair in a canine model. Laryngoscope 119,1856, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Badylak S.F., Freytes D.O., and Gilbert T.W.Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater 5,1, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Nieponice A., McGrath K., Qureshi I., Beckman E.J., Luketich J.D., Gilbert T.W., et al. An extracellular matrix scaffold for esophageal stricture prevention after circumferential EMR. Gastrointest Endosc 69,289, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Jarmila V., and Vavrikova E.Chitosan derivatives with antimicrobial, antitumour and antioxidant activities—a review. Curr Pharm Des 17,3596, 2011 [DOI] [PubMed] [Google Scholar]
- 24.George A., and Ravindran S.Protein templates in hard tissue engineering. Nano Today 5,254, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ravindran S., Song Y., and George A.Development of three-dimensional biomimetic scaffold to study epithelial-mesenchymal interactions. Tissue Eng Part A 16,327, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hao J., Ramachandran A., and George A.Temporal and spatial localization of the dentin matrix proteins during dentin biomineralization. J Histochem Cytochem 57,227, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.George A., and Hao J.Role of phosphophoryn in dentin mineralization. Cells Tissues Organs 181,232, 2005 [DOI] [PubMed] [Google Scholar]
- 28.George A., and Veis A.Phosphorylated proteins and control over apatite nucleation, crystal growth, and inhibition. Chem Rev 108,4670, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qin C., Brunn J.C., Cadena E., Ridall A., Tsujigiwa H., Nagatsuka H., et al. The expression of dentin sialophosphoprotein gene in bone. J Dent Res 81,392, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Biz M.T., Marques M.R., Crema V.O., Moriscot A.S., and dos Santos M.F.GTPases RhoA and Rac1 are important for amelogenin and DSPP expression during differentiation of ameloblasts and odontoblasts. Cell Tissue Res 340,459, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Papagerakis P., MacDougall M., Hotton D., Bailleul-Forestier I., Oboeuf M., and Berdal A.Expression of amelogenin in odontoblasts. Bone 32,228, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Oida S., Nagano T., Yamakoshi Y., Ando H., Yamada M., and Fukae M.Amelogenin gene expression in porcine odontoblasts. J Dent Res 81,103, 2002 [PubMed] [Google Scholar]
- 33.Nagano T., Oida S., Ando H., Gomi K., Arai T., and Fukae M.Relative levels of mRNA encoding enamel proteins in enamel organ epithelia and odontoblasts. J Dent Res 82,982, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Eapen A., Ramachandran A., and George A.Dentin phosphoprotein (DPP) activates integrin-mediated anchorage-dependent signals in undifferentiated mesenchymal cells. J Biol Chem 287,5211, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ravindran S., Narayanan K., Eapen A.S., Hao J., Ramachandran A., Blond S., et al. Endoplasmic reticulum chaperone protein GRP-78 mediates endocytosis of dentin matrix protein 1. J Biol Chem 283,29658, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narayanan K., Ramachandran A., Hao J., He G., Park K.W., Cho M., et al. Dual functional roles of dentin matrix protein 1. Implications in biomineralization and gene transcription by activation of intracellular Ca2+ store. J Biol Chem 278,17500, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Gajjeraman S., Narayanan K., Hao J., Qin C., and George A.Matrix macromolecules in hard tissues control the nucleation and hierarchical assembly of hydroxyapatite. J Biol Chem 282,1193, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Jadlowiec J.A., Zhang X., Li J., Campbell P.G., and Sfeir C.Extracellular matrix-mediated signaling by dentin phosphophoryn involves activation of the Smad pathway independent of bone morphogenetic protein. J Biol Chem 281,5341, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Ravindran S., Gao Q., Ramachandran A., Blond S., Predescu S.A., and George A.Stress chaperone GRP-78 functions in mineralized matrix formation. J Biol Chem 286,8729, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ravindran S., Gao Q., Ramachandran A., Sundivakkam P., Tiruppathi C., and George A.Expression and distribution of grp-78/bip in mineralizing tissues and mesenchymal cells. Histochem Cell Biol 138,113, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]