Abstract
Pneumatic compression of tissues with lymph stasis is, aside from the manual massage, a commonly used therapeutic modality in limb lymphedema. A number of pneumatic devices have been constructed. There is lack of reports of comparative studies determining inflation pressure levels, inflation/deflation cycle times, and total pumping times.
Aim: We tried to answer the question how high compression pressure and how long compression timing should be applied to the limb soft tissues to reach tissue fluid (TF) head pressure above 30 mmHg, necessary to initiate proximal flow.
Methods: TF pressures were measured subcutaneously during intermittent pneumatic compression in the lymphedematous limbs stage II to IV. Pressures of 50, 80, and 120 mmHg and timing 5, 20, and 50 sec were applied.
Results: a) the TF head pressures were lower than those in inflated chambers, b) inflation time of 5 and 20 sec was not long enough to generate TF head pressures above 30 mmHg, even if the compression pressures were as high as 120 mmHg, c) the 50 sec timing allowed to reach head pressures above 30 mmHg; however, they remained always lower than in the compression chamber, d) TF head pressures differed at various levels of the limb depending on the soft tissue mass, e) deflation of the inflated whole sleeve for 5 and 20 sec was followed by high end pressures, whereas that of 50 sec brought about pressure drop to 0, facilitating refilling with TF of the distal parts of the massaged limb.
Conclusions: Our observations point to the necessity of applying high pressures and compression times over 50 sec, to generate effective TF pressures and provide enough time for creating TF flow. Short inflation times generate TF pressures as in one-chamber devices that preclude its effectiveness compared to the multi-chamber devices.
Introduction
Pneumatic compression of tissues with limb edema is, aside from the manual massage, a commonly used therapeutic modality. The prescription and use of intermittent pneumatic pumps has been the mainstay of lymphedema therapy for many years. Several controlled studies already from the 1980–1990s have documented their usefulness in the treatment of this condition, thereby supporting their continued use.1–8 The new publications further strengthen the efficacy of intermittent pneumatic compression (IPC) in therapy of limb edema.9–11 A recent systemic review from 2004–2011 on the use of IPC, found that the use this method in treating lymphedema is well-founded in the literature.12
A variety of pumps are available. Pneumatic compression device product classification list includes nonsegmental and segmental home models for half or full leg without or with calibrated gradient pressure.13–20 The devices differ with respect to the number of chambers, time of inflation, deflation, regulation of inflation pressure, and calibrated gradient pressure, as well as garment shapes. To design effective pneumatic sleeves and pumps, the physiological criteria should be known as topography of the accumulated edema fluid, parameters of tissue fluid, lymph hydromechanics, in particular skin and subcutis compliance, as well as tissue hydraulic conductivity. In our previous studies, we showed that the bulk of fluid accumulates in the subcutaneous tissue spaces.21 We also documented that the minimum tissue fluid pressure necessary for initiating flow should be above 30 mmHg. Subcutaneous infusion of saline in lymphedematous limbs started only when the level of bottle was placed at the level of 45–50 cm of water.22 Moreover, we noticed that tissue fluid pressures had the tendency to be lower than in the compression chamber.23 A number of other important issues remain to be determined, such as optimum pumping pressures, the length and frequency of pumping sessions, and the need for continuation of pumping after initial reduction has been attained.
In the present study, we tried to answer the question of how high external pressure and timing are necessary to generate TF pressure above 30 mmHg necessary for initiating proximal flow in the edematous tissue. For this purpose, we used compression devices with regulated chamber pressures and different timing of inflation (Biocompression, Moonachie, NJ). The TF pressures were recorded subcutaneously under the inflated sleeve chambers in the 5, 20, and 50 sec inflation and 50, 80, and 120 mmHg pressure groups of lower limb lymphedema patients stage II to IV.
Material and Methods
Patients
Study was carried out on 15 patients, ages 24–62, mean weight 69 kg (58–74), mean height of 166 cm (161–178), with diagnosis of lymphedema of one lower limb, stage II to IV, duration 2 to 12 years (Table 1). Eleven patients reported foot skin infection episodes in the past, followed by transient foot and calf edema. Larger edema developed 10 to 60 months later, and in 5 cases was complicated by 1 to 3 attacks of dermato-lymphangio-adenitis. In 4 patients, edema developed without any detectable reason. Cases with acute inflammation, chronic venous insufficiency, and systemic etiology of edema were excluded from the study. The consent of patients was obtained and the study was approved by the Warsaw Medical University Ethics Committee.
Table 1.
Demographic Data of Patients with Lymphedema of Lower Limb
| M/F | Age | Group/stage | Level of edema | Skin changes | Lymphoscintigraphy |
|---|---|---|---|---|---|
| 2M,3F |
24–45 |
II |
Mid calf |
None |
Foot & lower calf spread, few collectors and inguinal nodes |
| 3M, 2F |
25–52 |
III |
Knee |
Foot keratosis |
Foot & calf spread, single collector, inguinal node remnants |
| 3M,2F | 26–62 | IV | Whole limb | Foot,calf keratosis | Foot & calf spread, no lymphatics and inguinal nodes |
Compression device
We used a device produced for us by Biocompression (Moonachie, NJ) (Fig. 1).23 The sleeve was composed of 8 chambers each 9 cm long; it was sequentially inflated, inflation pressures were regulated from 50 to 120 mmHg, gradient pressures were decreasing proximally by 20%.
FIG. 1.
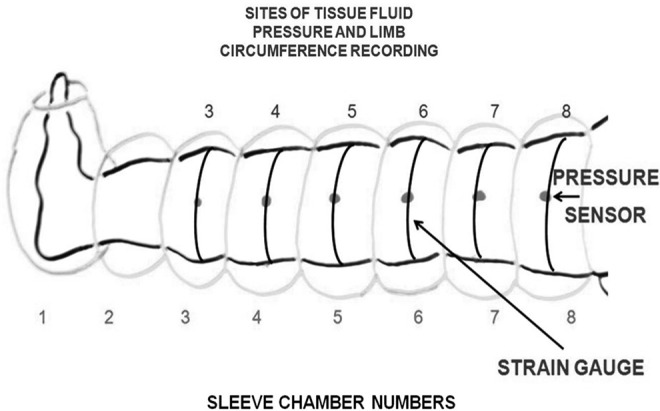
Schematic presentation of lower limb in a pneumatic sleeve with 8 chambers. TF pressure was measured at 6 points indicated by dots (pressure sensor, arrow): 1) above ankle joint at chamber 3 level, 2) in the mid of calf at chamber 4, and below the knee at chamber 5; in the thigh above the knee at chamber 6, in the mid-thigh at chamber 7, and in the groin at chamber 8.
Study setting
The pneumatic devices were set at three different inflation times 5, 20, or 50 sec in each chamber, and 50, 80, and 120 mmHg for each timing. There was no deflation of distal chambers. Total inflation time of the sleeve was either 40, 160, or 400 sec. Deflation time of all chambers at the end of each cycle lasted 5, 20, and 50 sec. The sleeve embraced the whole limb up to the inguinal crease
Tissue fluid pressure measurement
The wick-in-needle technique was used as previously described.23 Calf and thigh skin were disinfected with isopropyl alcohol. One mL of 2% xylocaine with 5 μg/mL adrenaline was injected intradermally and subcutaneously at six points of calf and thigh (Fig. 1). Adrenaline constricted arterioles and small veins to prevent blood leakage at the tip of the wick. An 8-gauge injection needle with a polyethylene tubing (OD 1.34 mm) containing glass-wool wick protruding 5 mm from the tubing tip was introduced under the skin at the depth of 5–10 mm. The needle was withdrawn, while the wick-in-tubing remained in situ. A drop of antibiotic ointment was placed at the site of tubing entry to seal off the channel made by the needle. The outer part of tubing was fixed to the skin by adhesive tape. It was led out through an opening in the compression sleeve, then connected to the pressure transducer (Honeywell, Elblinger, Poland). Recording was done using a 3-channel device, pressure range −20 to +150 mmHg (Telsoft, Warsaw, Poland) and LabView software (National Instruments, Austin, TX, USA). Position of the transducer was zeroed, placing it exactly at the level of the subcutaneously located wick. Pressure recording was started 1 min before inflation of the sleeve and continued over the entire sequential inflation of 8 chambers. The data were collected using Microsoft Excel program and were presented graphically on a pressure/time scale.
Interpretation of tissue fluid pressure curves
During sequential pneumatic compression, TF pressure gradient generated under the chamber moves fluid in the proximal direction of the limb. The efficacy of sequential compression for creating TF flow depends on the pressure generated in the tissue and time to build up the threshold level of 30 mmHg, a border value for mobilization of TF. The TF pressure under distal chambers should remain high to prevent backflow.
Results
Tissue fluid pressures during 5 sec/chamber pneumatic compression
Five seconds sequential inflation time did not allow discrimination of the TF head pressures, which were generated by inflation of consecutive chambers, on the recorded curve, irrespective of whether the inflation pressure was 50, 80, or 120 mmHg (Fig. 2). Inflation of chambers 3, 4, and 5 generated TF head pressures under each chamber as low as 10–20 mmHg only, although the chamber pressures were much higher (Fig. 2). Inflation of the whole sleeve to 50 mmHg created final pressures between 25 and 40 mmHg, to 80 mmHg 35–70 mmHg and to 120 mmHg 70–100 mmHg. Inflation time of the whole sleeve for 40 sec was too short to generate and maintain TF pressure at the level as high as that in the sleeve. Moreover, deflation period of 5 sec was not long enough to decrease pressure to 0 mmHg. It remained at 20–30 mmHg.
FIG. 2.
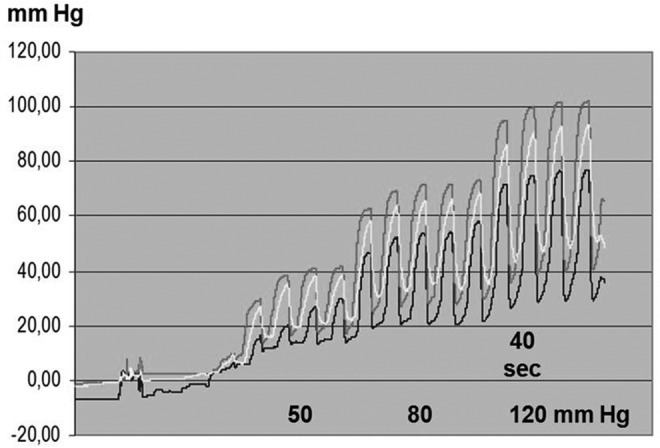
Chamber inflation time 5 seconds, of the entire sleeve 40 seconds. TF pressure recorded in the subcutaneous tissue in a lymphedematous calf, stage II, during sequential inflation. TF pressure above ankle under chamber 3 (upper curve), mid-calf chamber 4 (middle curve), and below knee chamber 5 (lower curve). Inflation pressure 50 mmHg (right four peaks), 80 mmHg (five middle peaks), and 120 mmHg (four left peaks). Short sequential chamber inflation time did not allow to discriminate on the recording curve the head pressures generated by first inflation of each of the three consecutive chambers. Note that inflation of the sleeve to 50 mmHg generated peak TF pressure of only 20–40 mmHg, to 80 mmHg of 50–70 mmHg, and to 120 mmHg of 70–100 mmHg. The total inflation time period of 8×5 sec (40 sec) seemed to be too short to reach and maintain TF pressures as in the sleeve. Moreover, 5 sec deflation period was too short for TF pressure to drop to 0 mmHg. Taken together, short inflation time of sequential chambers generated mean TF pressure similar to that of one chamber sleeve.
Tissue fluid pressures during 20 sec/chamber pneumatic compression
Twenty seconds sequential inflation time allowed only to roughly discriminate the TF head pressures generated by inflation of consecutive chambers (Fig. 3). These pressures ranged between 10 and 20 mmHg, irrespective whether the chamber pressures were 50, 80, or 120 mmHg (Fig. 4). Inflation of the whole sleeve to 50 mmHg generated TF pressures ranging from 50 mmHg in the calf to 10 mmHg in the groin; to 80 mmHg ranging from 80 mmHg in the calf to 20 mmHg in the groin; and inflation to 120 mmHg to 40 mmHg in the upper thigh (Fig. 5.) Upon deflation TF pressure decreased to 20–30 mmHg and did not reach the pre-inflation 0–5 mmHg.
FIG. 3.
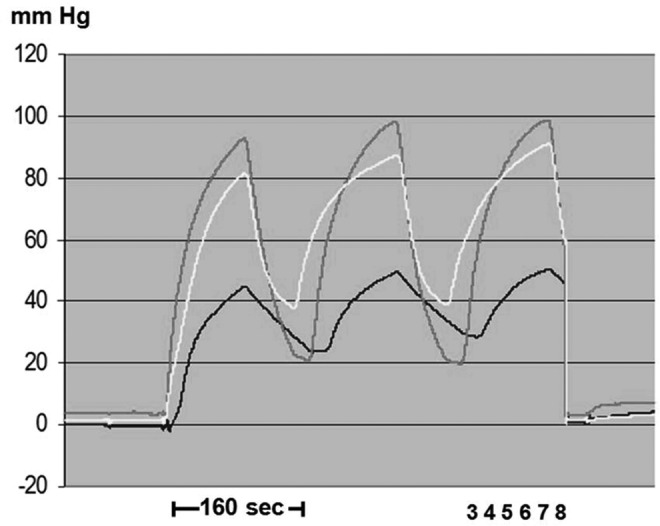
Chamber inflation time 20 seconds, of the entire sleeve 160 seconds. Numbers denote sleeve chambers. Inflation pressure was 120 mmHg. TF pressure recorded in the subcutaneous tissue of a lymphedematous calf, stage III, during sequential inflation above ankle under chamber 3 (upper curve), mid-calf chamber 4 (middle curve), and below knee chamber 5 (lower curve). Difficulties in discriminating TF head pressures on the ascending arm of the recorded curves. Note that the peak TF pressure level reached after inflation of the whole sleeve only 80–100 mmHg. Close to the knee TF pressure was only 50 mmHg (lower curve). Moreover, pressures after deflation did not drop to zero but remained at 20–40 mmHg.
FIG. 4.
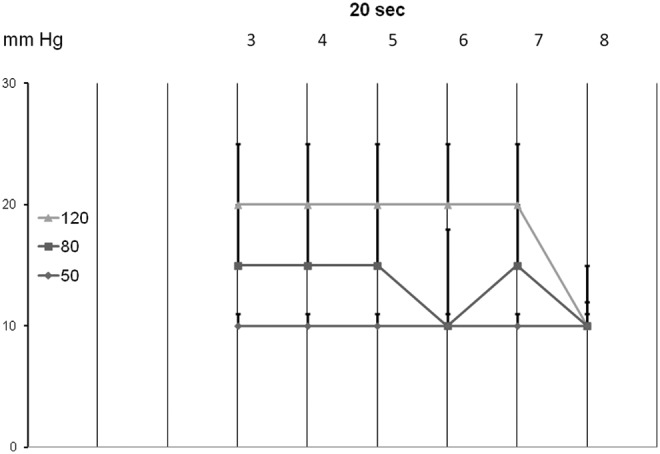
The TF head pressures generated by the first inflation of consecutive chambers 3 to 8 for 20 sec each to 50, 80, and 120 mmHg (mean±sd, n=15). Note that TF pressures were always lower than those in the chambers.
FIG. 5.
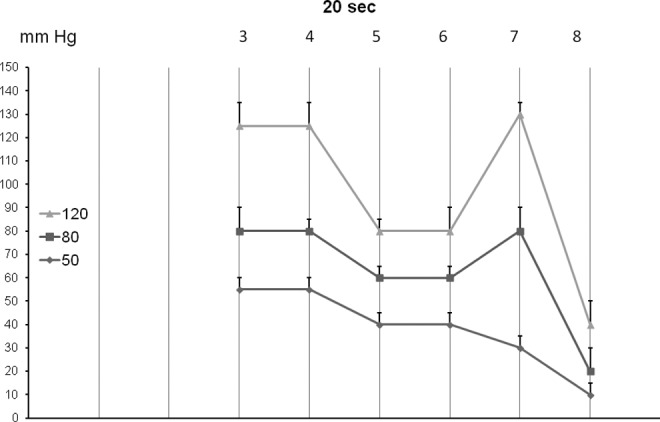
The TF pressures generated by inflation of all chambers 1 to 8 for 20 sec each to 50, 80, and 120 mmHg recorded after inflation of the last chamber (mean±sd, n=15). Note that TF pressures were different below and above knee and under chamber 8 in the groin.
Tissue fluid pressures during 50 sec/chamber pneumatic compression
In contrast to the 5 and 20 sec pump, the TF head pressures generated by each inflated chamber could be easily read off (Figs. 6 and 7. The mean head pressures under chamber 3 were 35, 45, and 60 mmHg at the inflation pressure of 50, 80, and 120 mmHg, respectively. The respective pressures in the thigh were evidently lower reaching in the groin 20 mmHg (Fig. 8). The head pressures at various limb levels depended on the shape of the limb. They were high at the mid-calf, lower below and above the knee, and again higher at the mid-thigh, to become low in the groin region (Fig. 8). Inflation of the whole sleeve to 50 mmHg created pressure gradient between calf 50–60 mmHg to 20 mmHg in the groin tissues; inflation to 80 and 120 mmHg generated pressures of 85 to 30 and 130 to 40 mmHg, respectively (Fig. 9). The total time of inflation was 400 sec. It was long enough to push TF to the proximal limb segments. During deflation, the TF pressure dropped to 0 mmHg, facilitating venous blood and TF inflow into the distal limb segments to be compressed during the consecutive cycle.
FIG. 6.
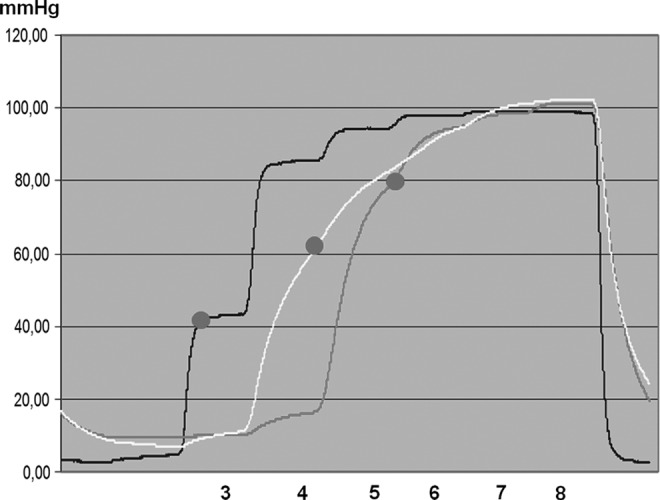
Chamber inflation time 50 seconds, of the entire sleeve 400 seconds. Numbers denote chamber levels. TF pressure recorded in the subcutaneous tissue of a lymphedematous calf, stage II, under chamber 3 (first curve), 4 (second curve), and 5 (third curve) during sequential inflation. The sleeve inflation pressure was 120 mmHg. Head pressure denoted by red dots. Note that the initial inflation of chamber 3 (above ankle) generated head pressure of 40 mmHg, of chamber 4 (mid-calf) 60 mmHg, and chamber 5 (below knee) also 80 mmHg. Note that increase of pressure under chamber 3 did not generate pressure increase at level 4 or 5. This can be interpreted as no transmission of pressure to the proximal segment because of low hydraulic conductivity of the lymphedematous tissue. After sleeve deflation, TF pressures dropped to 5 mmHg.
FIG. 7.
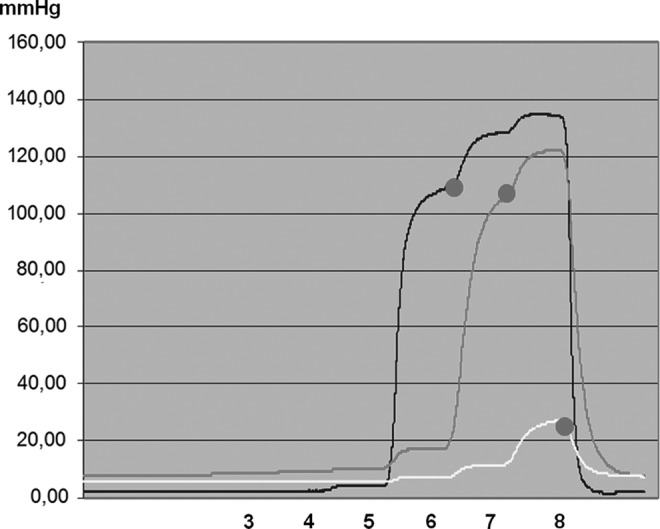
Chamber inflation time 50 seconds, of the entire sleeve 400 seconds. Numbers denote chamber levels. TF pressure recorded in the subcutaneous tissue of a lymphedematous thigh, stage II, under chamber 6 (first curve), 7 (second curve), and 8 (third curve) during sequential inflation. The sleeve inflation pressure was 120 mmHg. Head pressures denoted by red dot. Note that first inflation of chamber 6 (above knee) generated TF pressure of 110 mmHg (yellow), of chamber 7 100 mmHg, and of chamber 8 only 20 mmHg. This was due to dissipation of force to the groin with loose tissue. Note also that inflation of chambers 1 to 5 did not generate increase in pressure at levels 6 or 7 and 8. This can be interpreted as no transmission of pressure to the proximal segments because of low hydraulic conductivity of the lymphedematous tissue. After sleeve deflation, TF pressures dropped to 3–10 mmHg.
FIG. 8.
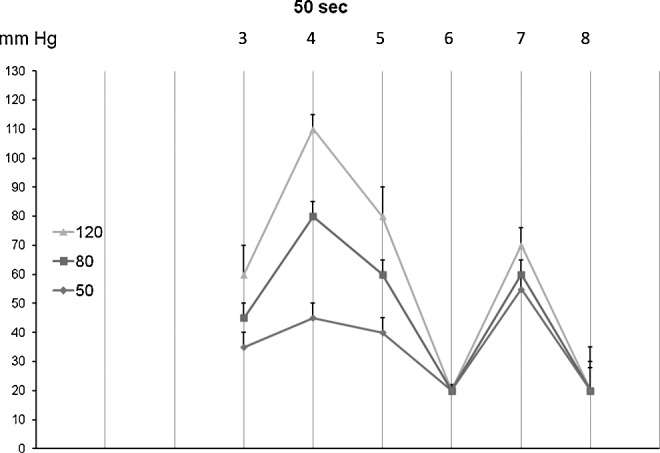
The TF head pressures generated by the first inflation of consecutive chambers 3 to 8 for 50 sec each to 50, 80, and 120 mmHg (mean±sd, n=15). Note that TF pressures were lower than those in the chambers, especially below and above knee and under chamber 8 in the groin.
FIG. 9.
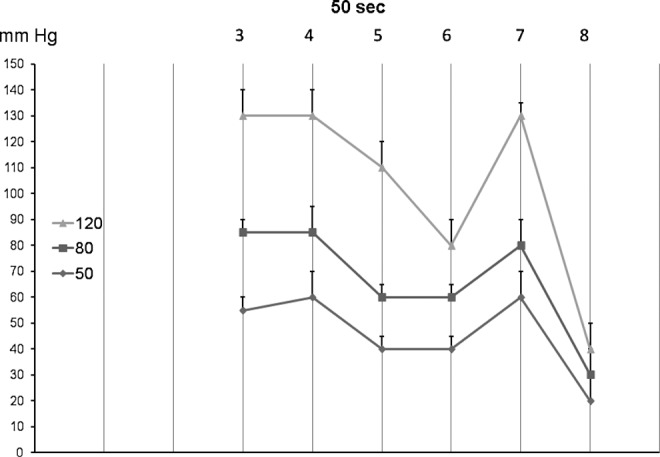
The TF pressures generated by inflation of all chambers 1 to 8 for 50 sec each to 50, 80, and 120 mmHg recorded after inflation of the last chamber (mean±sd, n=15). Note that TF pressures were low under chamber 8 in the groin.
Discussion
This study provided the following information: 1. low TF head pressure upon first inflation of consecutive chambers, below that in the chambers; 2. difficulty in identifying the head pressures generated by 5 and 20 sec inflated chambers due to short inflation times, 3. lack of transfer of TF pressure from compressed to the proximal segments; 4. uneven distribution of TF pressure along the limb with low levels below and above the knee and under the groin chamber.
Knowledge of the subcutaneous tissue hydraulic properties is scarce, whereas pneumatic compression therapy needs information on the hydraulic conditions in the massaged tissues. There have been some reports on pressures generated at the sleeve–skin interface; however, they gave no insight into the intra-tissue physical events.24 In the previously published preliminary studies, we showed that TF fluid pressures is lower than in the compression chambers due to dissipation of force at the chamber–skin interface. In the present study, we applied different sleeve inflation timings and pressures and estimated the TF head pressures. The most important finding was that head pressures were in all cases lower than in the inflated chambers. The high gradient across the skin and tissue fluid was most likely caused by skin rigidity (fibrosis) and dissipation of the applied force to the proximal noncompressed regions.
In order to obtain high and stable TF pressures, a long time of compression seems to be necessary. Observations from debulking surgery of the lymphedematous limbs indicate that manual squeezing of fluid from edematous tissues requires 1–2 minutes (personal experience). Similar timing should then be recommended for the pneumatic massage. We showed that TF head pressures recorded during the 5 and 20 second/chamber inflation times were difficult for estimation. They were very low, usually below the required 30 mmHg. This was most likely caused by too short a time to generate pressures high enough to move mobile tissue fluid. Moreover, short inflation times of 5 and 20 sec and short deflation times resulted in maintaining TF pressures during deflation phase at levels above 20 mmHg. This means there was not enough long time lag between sequential inflations for TF pressure drop. Such high deflation pressures change the multi-chamber to a one-chamber sleeve. In contrast, inflation of chambers for 50 sec each allowed the TF head pressures to be estimated and to reach over 30 mmHg. The conclusion was that most favorable was the inflation time of 50 sec/chamber and 50 sec deflation.
An unexpected observation was lack of horizontal transmission of force from the compressed to the noncompressed proximal segments of the limb. This could be accounted for by low hydraulic conductivity of the subcutaneous tissue. The obtained data confirmed our previous results of lack of transmission of tissue fluid pressures in the advanced stages of lymphedema.25 Our computer simulation model of intra-tissue events during compression in a poroelastic material with linear flow also showed slow transfer of force and TF flow, and deformation of proximal tissue regions (unpublished data). The observed lack of transmission of tissue fluid pressure justifies using multi-chamber sleeves to generate a moving pressure gradient.
Although the inflation pressure was the same in all chambers, the TF pressures varied at different levels of the limb, being much lower below and above the knee than in mid-calf and mid-thigh. This was presumably due to dissipation of force to the popliteal fossa with loose connective tissue accumulating moving fluid and the groin rich in fat tissue. The question arises whether the compression pressures in the consecutive chambers should be adjusted to the different mass of soft tissues of the limb at various levels. This also questions the so far practiced calibrated pressure gradient of inflation pumps.
It was noteworthy that TF head pressures in the lymphedematous calf stage IV were lower than in stage II, however, the number of investigated cases was too small for statistical evaluation.
Taken together, this is the first detailed study presenting hydromechanic parameters of the massaged skin and subcutaneous tissue in lymphedema using various timings and pressures. Our observations point to the necessity of applying diversified pressures at different levels of the limb, and long compression times to generate effective transmural pressures moving fluid to the root of the limb. The obtained data should be useful for physiotherapy allowing proper parameters of compression devices to be set at levels corresponding to the in-tissue conditions.
Author Disclosure Statement
No competing financial interests exist.
This study was supported by grant No. 13002606 from the National Center for Research and Development, Poland.
References
- 1.Klein MJ, Alexander MA, Wright JM, Redmond CK, LaGasse AA.Treatment of adult lower extremity lymphedema with the Wright Linear pump: Statistical analysis of a clinical trial. Arch PMR 1988;69:202–206 [PubMed] [Google Scholar]
- 2.Kim-Sing C, Basco VE.Postmastectomy lymphedema treated with the Wright linear pump. Can J Surg 1987;5:368–370 [PubMed] [Google Scholar]
- 3.Pappas CJ, O'Donnell TF., Jr Long-term results of compression treatment for lymphedema. J Vasc Surg 1992;16:555–562 [DOI] [PubMed] [Google Scholar]
- 4.Richmand DM, O'Donnell TR, Jr, Zelikovski A.Sequential pneumatic compression for lymphedema. A controlled trial. Arch Surg 1985;120:1116–1119 [DOI] [PubMed] [Google Scholar]
- 5.Zanolla R, Monzeglio C, Balzarini A, Martino G.Evaluation of the results of three different methods of postmastectomy lymphedema treatment. J Surg Oncol 1984;26:210–213 [DOI] [PubMed] [Google Scholar]
- 6.Yamazaki Z, Idezuki Y, Nemoto T, Togawa T.Clinical experiences using pneumatic massage therapy for edematous limbs over the last 10 years. Angiology 1988;39:154–163 [DOI] [PubMed] [Google Scholar]
- 7.Rockson Stanley G.Accruing evidence for a beneficial role of pneumatic biocompression in lymphedema. Lymphat Res Biol 2010;8(4) [DOI] [PubMed] [Google Scholar]
- 8.Gurdal SO, Kostanoglu A, Cavdar I, et al. . Comparison of intermittent pneumatic compression with manual lymphatic drainage for treatment of breast cancer-related lymphedema. Lymphat Res Biol 2012;10:129–135 [DOI] [PubMed] [Google Scholar]
- 9.Adams K, Rasmussen J, et al. . Direct evidence of lymphatic function improvement after advanced pneumatic compression device treatment of lymphedema. Biomed Optics Express 2010;1:114–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pilch U, Wozniewski M, Szuba A.Influence of compression cycle time and number of sleeve chambers on upper extremity lymphedema volume reduction during intermittent pneumatic compression. Lymphology 2009;42:26–35 [PubMed] [Google Scholar]
- 11.Belgrado JP, Bourgeois P, Röh N, Moraine JJ.Intermittent pneumatic compression in the treatment of lymphedema: Current state of knowledge. Eur J Lymphology Relat Prob 2007;17:4–10 [Google Scholar]
- 12.Feldman JL, Stout NL, Wanchai A, Stewart BR, Cormier JN, Armer JM.Intermittent pneumatic compression therapy: A systemic review. Lymphology 2012;45:13–25 [PubMed] [Google Scholar]
- 13.Special report: Comparative efficacy of different types of pneumatic compression pumps for the treatment of lymphedema. Technologica MAP Suppl 1998;:42–3 [PubMed] [Google Scholar]
- 14.Centers for Medicare and Medicaid Services (CMS) National Coverage Determination for Pneumatic Compression Devices, Publication number 100-3, Manual section number 280.6
- 15.Rockson SG, Miller LT, Senie R, et al. . American Cancer Society lymphedema workshop. Workgroup III: Diagnosis and management of lymphedema. Cancer 1998;83:2882–2885 [DOI] [PubMed] [Google Scholar]
- 16.Blue Cross and Blue Shield Association Technology Assessment Program (TEC) Special report: Comparative efficacy of different types of pneumatic compression pumps for the treatment of lymphedema. 1998;13(2) [PubMed] [Google Scholar]
- 17.Centers for Medicare and Medicaid Services (CMS) National Coverage Determination for Pneumatic Compression Devices, Publication number 100-3, Manual section number 280.6. Retrieved from http://www.cms.hhs.gov/mcd/viewncd.asp?ncd_id=280.6&ncd_version=1&basket=ncd%3A280%2E6%3A1%3APneumatic+Compression+Devices
- 18.Palmetto GBA-Statistical Analysis Durable Medical Equipment Carrier (SADMERC) Durable Medical Equipment Coding System (DMECS) Product Search for HCPCS codes E0650, E0651, E0652. Retrieved from http://www3.palmettogba.com/dmecs/do/productsearch
- 19.National Lymphedema Network (NLN) Position statement of the national lymphedema network. Treatment. 2006August10 Retrieved from http://www.lymphnet.org/pdf Docs/nlntreatment
- 20.Mayrovitz HN.Interface pressures produced by two different types of lymphedema therapy devices. Phys Therapy 2007;87:1379–1388 [DOI] [PubMed] [Google Scholar]
- 21.Olszewski WL, Jain P, Ambujam G, Zaleska M, Cakala M.Where do lymph and tissue fluid accumulate in lymphedema of lower limbs caused by obliteration of lymphatic collectors. Lymphology 2009;42:105–111 [PubMed] [Google Scholar]
- 22.Olszewski WL, Zaleska M, Cakala M, Victor J, Tripathi M.Tissue fluid and lymph space pressure/volume relationship in obstructive lymphedema of lower limbs. Lymphology 2007;40:395 [Google Scholar]
- 23.Olszewski WL, Jain P, Ambujam G, Zaleska M, Cakala M, Gradalski T.Tissue fluid pressure and flow during pneumatic compression in lymphedema of lower limbs. Lymphat Res Biol 2011;9:77–83 [DOI] [PubMed] [Google Scholar]
- 24.Segers P, Belgrado JP, Leduc A, Leduc O, Verdonck P.Excessive pressure in multichambered cuffs used for sequential compression therapy. Phys Ther 2002;82:1000–108 [PubMed] [Google Scholar]
- 25.Olszewski WL.Contractility patterns of human leg lymphatic in various stages of obstructive lymphedema. Ann NY Acad Sci 2008;1131:110–118 [DOI] [PubMed] [Google Scholar]


