Abstract
An estimated 12% of women in the United States suffer from some form of infertility. In vitro fertilization (IVF) is the most common treatment for infertility encompassing over 99% of all assisted reproductive technologies. However, IVF has a low success rate. Live birth rates using IVF can range from 40% in women younger than 35 years to 4% in women older than 42 years. Costs for a successful IVF outcome can be upward of $61,000. The low success rate of IVF has been attributed to the inability of the blastocyst to implant to the uterus. Blastocyst implantation is initiated by L-selectin expressing cells, trophoblasts, binding to L-selectin ligands, primarily sialyl Lewis X (sLeX), on the uterine surface endometrium. Legal and ethical considerations have limited the research on human subjects and tissues, whereas animal models are costly or do not properly mimic human implantation biochemistry. In this work, we describe a cellular model system for quantifying L-selectin adhesion mechanics. L-selectin expression was confirmed in Jeg-3, JAR, and BeWo cell lines, with only Jeg-3 cells exhibiting surface expression. Jeg-3 cells were cultured into three-dimensional spheres, termed “trophospheres,” as a mimic to human blastocysts. Detachment assays using a custom-built parallel plate flow chamber show that trophospheres detach from sLeX functionalized slides with 2.75×10−3 dyn of force and 7.5×10−5 dyn-cm of torque. This work marks the first time a three-dimensional cell model has been utilized for quantifying L-selectin binding mechanics related to blastocyst implantation.
Introduction
Infertility is a major medical condition affecting an estimated 12% of women in the United States.1,2 The most common treatment is in vitro fertilization (IVF). Despite being the most common procedure for addressing infertility, IVF has a low success rate depending on the age and other health factors. The Society for Assisted Reproduction (SART) and the Center for Disease Control (CDC) report that live birth rates from IVF can range from 4% in those older than 40 years to 45% in those younger than 35 years, and these rates have remained relatively constant over the past 10 years1–3 (sart.org). Furthermore, a single IVF cycle may cost $24–38,000 with an average cost of $61,000 per successful cycle or pregnancy.4 The majority of this financial burden falls on those undergoing the treatment since an estimated 85% of all costs are paid out of the pocket by the patients.5 It has been suggested that the main reason for the low success rate of IVF is a failure of the blastocyst/embryo to properly attach to the endometrial surface of the uterus.6,7
Blastocyst implantation involves a complex, chemically and temporally synchronized dialogue between an implantation competent embryo and a receptive uterus. The steps between fertilization and the initiation of implantation generally follow a well-conserved autonomous process resulting in the blastocyst.8 In humans, endometrial receptivity is controlled by the ovarian steroids, estrogen (E2), and progesterone (P4).9
The cellular adhesion protein L-selectin mediates critical interactions in the earliest events of blastocyst attachment and implantation.10 Upregulation of oligosaccharide-based selectin ligands, specifically sialyl Lewis X (sLeX), during endometrial receptivity and L-selectin expression on human trophoblasts have been shown.11
Due to ethical and legal restrictions on obtaining and testing human tissue, animal models of implantation have been primarily investigated.12 However, animal models all have limitations as substitutes for human implantation. Primates and guinea pigs are most similar to humans; however, primates are costly and produce few offspring12–16 and guinea pig blastocyst implant with the zona pellucida intact.17,18 Mice, rats, and rabbits are the easiest to obtain and most well defined, but do not exhibit interstitial implantation.9,19,20 Pigs, sheep, and cows have prolonged implantation events, but also do not exhibit interstitial implantation.21–24 Furthermore, the role of L-selectin and L-selectin ligands has yet to be determined in these animals.
There remains a clear need for a human blastocyst implantation model. Few attempts have been made using trophoblast-like choriocarcinoma cells, but these models are limited in their scope. JAR,25 Jeg-3,26 and BeWo27 cells have been utilized since their protein expression profiles closely resemble the first trimester trophoblast cells.28–41 Whereas these studies have added knowledge to this complex biological behavior, their focus was not to specifically investigate the L-selectin binding process. Additionally, these single-cell studies do not capture the large three-dimensional structure of blastocysts. Few three-dimensional models exist,33,35,42,43 but they also do not quantify L-selectin binding.
The goal of this work is to evaluate L-selectin expression of Jeg-3, JAR, and BeWo cells to understand surface expression and appropriateness for a model trophoblast in the study of the role of L-selectin in blastocyst attachment. A three-dimensional blastocyst model, termed “trophosphere,” and a method for quantifying L-selectin/sLeX binding of trophospheres are also presented.
Materials and Methods
Cell and trophosphere culture
Jeg-3, BeWO, JAR, and U-937 (ATCC, Manassas, VA) cells were cultured in their recommended media supplemented with 5% fetal bovine serum (FBS; CellGro, Manassas, VA) in an incubator at 37°C with 5% CO2. Jeg-3 cells were passaged twice per week at a 1:5 ratio; U-937 cells were passaged weekly at a 1:10 ratio. Cells are typically cultured for 4–8 weeks before being replaced.
Trophospheres were cultured using Jeg-3 cells at passage number 136–145, in a manner similar to previous studies.33,42 Jeg-3 cells were seeded into PETG Erlenmeyer flasks at 106 cell/mL in 20 mL of MEM supplemented with 10% FBS. Seeded flasks were cultured on an orbital shaker at 50 rpm for 48–72 h.
Reagents
Immunoblot primary antibodies were LEAF purified anti-human CD62L DREG-56 (BioLegend, San Diego, CA); anti-actin rabbit antibody (Cell Signaling Technology, Danvers, MA). The immunoblot secondary antibody was the HRP goat anti-mouse IgG polyclonal antibody (BioLegend). Estradiol, progesterone, and dexamethasone (Sigma, St. Louis, MO) were used in western blot culture treatments. Flow cytometry primary antibodies were anti-human L-selectin (CD62L) monoclonal (Clone #4G8) antibody BBA24 (R&D Systems, Minneapolis, MN) or isotype-matched control (clone MOPC-1; BD Biosciences, San Jose, CA), and the secondary antibody was Alexa Fluor 488 goat anti-mouse IgG1 (Invitrogen, Carlsbad, CA). Immunoreagents for immunohistochemistry were mouse IgG1 (clone MOPC-21; BD Pharmingen), L-selectin antibody (Clone #4G8; R&D Systems), biotinylated goat anti-mouse IgG (H+L) (Vector Laboratories, Burlingame, CA), streptavidin/biotin blocking kit, and streptavidin-Texas Red (Vector Laboratories). The MECA-79 rat IgM antibody (BD Biosciences) and 3′-sLeX tetrasaccharide (Sigma) were used in flow detachment studies.
Western blot and immunoblot quantification
Western blotting was used to verify L-selectin expression of candidate cell lines. Jeg-3, BeWo, JAR, and U-937 cells were cultured normally, and total protein extracts were prepared using a lysis buffer described previously44 and quantified by the BCA protein assay (Pierce Biotechnology, Rockford, IL). The Drosophila line Deg-5r (gifted by Noreen Robertson, DMD), was used as a negative control for L-selectin, while the U-937 monocytic leukemia cell line was used as a positive control.45 For quantitative immunoblots, Jeg-3 cells were cultured normally, followed by a 2-day treatment of serum-free, phenol red-free medium and 10−8 M estradiol, 10−6 M progesterone, a combination of both estradiol and progesterone, or 10−7 M dexamethasone.
Sample lysates were separated on 4%–20% SDS-PAGE mini-gels (Bio-Rad, Hercules, CA), transferred onto nitrocellulose membranes (Amersham Biosciences, Piscataway, NJ), blocked with 5% powdered milk in phosphate-buffered saline (PBS)-T (0.05% Tween-20), incubated with primary and secondary antibodies in the blocking buffer, and washed with PBS-T. Signals were detected using enhanced chemiluminescent reagents (Pierce) and captured on an X-ray film (Amersham). Signals were inactivated by incubating blots in the SG reagent (Vector Laboratories) and blots were then probed for actin.
Flow cytometry
Jeg-3, JAR, BeWo, and U-937 cells were tested for surface L-selectin expression using flow cytometry. Trypsinized cells were stained in the staining buffer (PBS with 0.2% bovine serum albumin [BSA]) with primary and secondary antibodies (2 μg/106 cells). Washes were performed with PBS-0.2% Tween with 3% FBS. Samples were acquired using a FACSORT flow cytometer (BD Biosciences) with 488 nm excitation from an argon ion laser at 15 mW. Alexa Fluor 488 log fluorescence was captured on the FL1 channel equipped with a 530-nm wavelength filter with 30-nm bandwidth. Data were acquired with Lysis II software (version 2.0; BD Biosciences) and analyzed with WinMDI software (Joseph Trotter; The Scripps Research Institute, La Jolla, CA, available online from http://facs.scripps.edu).
Trophosphere dimensional analysis
Trophospheres used for binding mechanics testing were also subjected to dimensional analysis for average size and measures of sphericity using ImageJ (NIH, Bethesda, MD). Images of trophospheres were made into binary images, and then analyzed using the built-in particle analyzer. Mean diameters were determined by averaging both Feret's and minimum Feret's diameters. Both roundness (Major:Minor axes) and circularity (Perimeter2:Area) measurements were tabulated to determine sphericity. In total, 130 spheres over 6 testing days were analyzed.
Trophosphere-sLeX detachment mechanics
To determine binding the mechanics of L-selectin expressing trophospheres to sLeX, a custom-built parallel plate flow chamber was utilized. sLeX tetrasaccharides were immobilized to glass slides at 0.1 μg/mL (∼1012 molecules/cm2) using basic carbodiimide chemistry. Briefly, glass slides were cleaned using 1:1 vol:vol methanol:hydrochloric acid solution, sulfuric acid, and finally, boiling. Slides were then coated using aminopropyltriethoxysilane (APTS). sLeX was activated using EDC/s-NHS chemistry and allowed to bind to the silanized slides for 2 h. Coated slides were rinsed with sterile PBS before testing. To block binding, MECA-79 was incubated on selected sLeX-coated slides for 2 h before testing. Polycarbonate plastic and 10% BSA-coated slides were used as negative controls. Coatings were verified using contact angle and immunochemical staining (results not shown).
Trophospheres were injected into the parallel plate flow chamber (PPFC) and allowed to bind to the coated slides for 2–5 min. Individual spheres were imaged under bright field microscopy. Fluid flow, using PBS at 37°C was increased incrementally until trophospheres were removed from the surface. Shearing forces and torques were calculated using estimations previously described by Pozrikidis46 for spherical objects upon a plate in laminar flow.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software. For all data sets, one-way ANOVA followed by Tukey's post hoc tests at 95% significance levels were used. All results are displayed as mean±SEM.
Results
L-selectin expression and modulation in choriocarcinoma cells
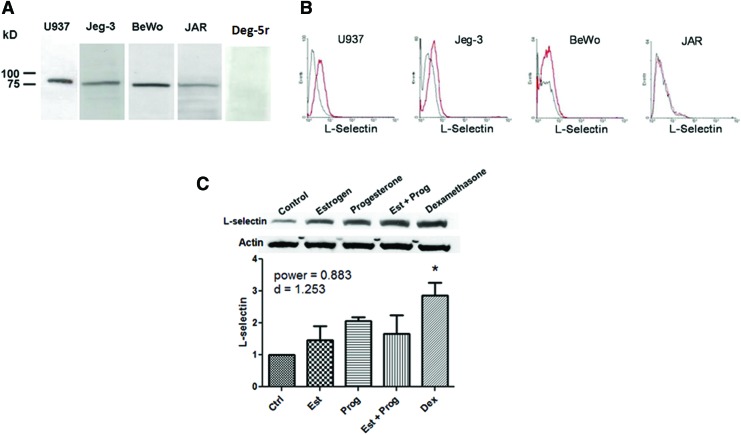
Jeg-3, BeWo, and JAR cell lines were evaluated for L-selectin expression using western blotting and surface expression was determined using flow cytometry with the DREG-56 antibody. U-937 cells, which constitutively express L-selectin, were used as positive controls (western blot and flow cytometry)45 and the Drosophila line Deg-5r was used as a negative control (western blot only). Western blots of total protein extracts show a specific band at ∼90 kDa, as seen in Figure 1A. Although all cell lines express L-selectin, flow cytometry studies, Figure 1B, show that only the Jeg-3 cell line exhibits surface L-selectin expression. For these reasons, Jeg-3 cells were chosen for further testing.
FIG. 1.
L-selectin expression and modulation. (A) Western blotting shows Jeg-3, BeWo, and JAR cells express L-selectin. U937 monocytes are a positive control, while the Drosophila line Deg-5r is a negative control. (B) Only Jeg-3 cells exhibit surface L-selectin expression through flow cytometry. (C) Jeg-3 expression of L-selectin is upregulated by estrogen (Est), progesterone (Prog), and dexamethasone (Dex). Statistical analysis using one-way ANOVA with p<0.05 and n=3 for each group, *p<0.05. Color images available online at www.liebertpub.com/tea
The effect of estrogen, progesterone, and dexamethasone on L-selectin expression in Jeg-3 cells was examined. To eliminate the effects of serum-derived growth factors and cytokines, cells were treated with the serum-free medium supplemented with physiological concentrations of hormones: 10−8 M estradiol (estrogen), 10−6 M progesterone, a combination of both estrogen and progesterone, or 10−7 M dexamethasone for 48 h. Control treatments were the serum-free media only. Figure 1C shows that L-selectin expression increased in response to all treatments: ∼2-fold with progesterone, 1.5 times with estrogen, or a combination of both, and 3-fold with dexamethasone. However, only the dexamethasone treatment was statistically significant using one-way ANOVA. Immunostaining of trophospheres, Figure 2B, shows expression of L-selectin, although quantification and modulation by hormones were not yet performed.
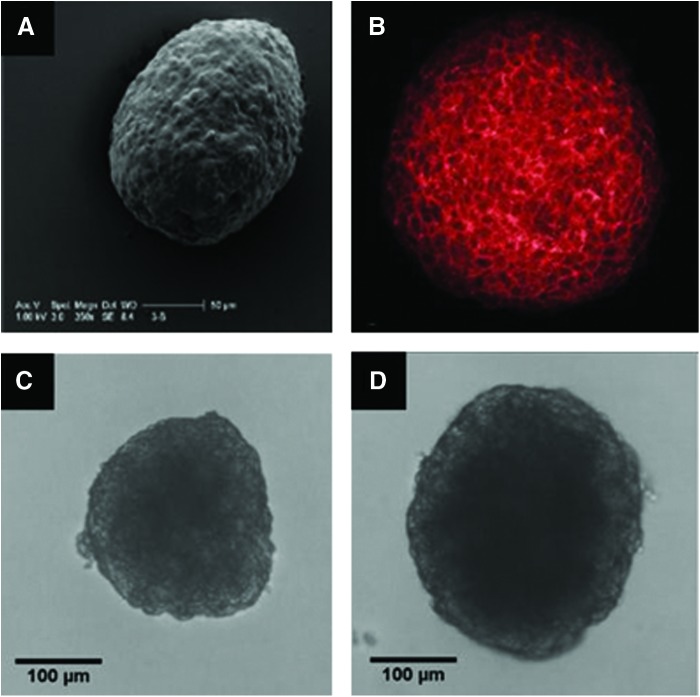
FIG. 2.
Trophosphere imaging. (A) Scanning electron micrograph of a trophosphere. (B) L-selectin expression was analyzed by confocal microscopy with trophospheres stained with Dreg-56, biotinylated secondary antibody, and Texas Red-conjugated streptavidin (200×). (C, D) Representative light micrographs (100×) of trophospheres used for dimensional analysis. Diameter, circularity, and roundness measurements are (C, 238 μm, 0.77, 0.92) and (D, 299 μm, 0.84, 0.85). Color images available online at www.liebertpub.com/tea
Dimensional analysis of trophospheres
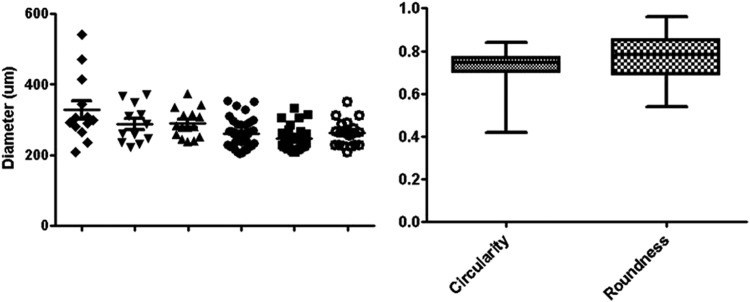
One hundred and thirty trophospheres over 6 days of testing were analyzed for size and sphericity, and results are displayed in Figure 3. Trophospheres were cultured with diameters of 278.4±4.6 μm. Two different sphericity measurements were taken. Average circularity, or the ratio between the area and squared perimeter, measured 0.73±0.006. Roundness, or the ratio between the major and minor axes, measured 0.77±0.009.
FIG. 3.
Dimensional analysis of trophospheres. Using ImageJ software, light micrographs (100×) are processed and analyzed for sphere diameter, circularity (Area:Perimeter2), and roundness (Major:Minor). Diameter measurements of 130 spheres over six trials are 278.4±4.6 μm. Trophospheres are highly circular (0.73±0.006) and round (0.77±0.009). All values are mean±standard error calculated using GraphPad Prism software.
Trophosphere-sLeX detachment mechanics
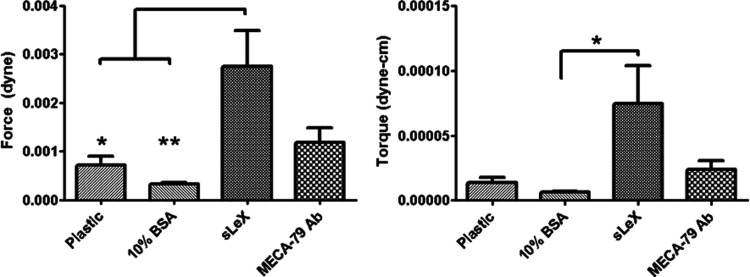
The binding strength of trophospheres to sLeX was determined by detaching trophospheres from sLeX-coated slides in a custom-built parallel plate flow chamber. Binding was blocked using MECA-79 antibodies, while polycarbonate plastic and 10% FBS-coated slides were used as controls. Average applied force and torque measurements are plotted in Figure 4. Trophospheres bound to sLeX were detached under 2.74×10−3 dyn of force and 7.5×10−5 dyn-cm of torque. MECA-79 blocking reduced detachment force and torque by 56% and 68%, respectively, however, these reductions were not significant. Binding to controls was virtually nonexistent.
FIG. 4.
Trophosphere detachment mechanics. Trophospheres are detached from sialyl Lewis X (sLeX)-coated slides (n=16) under 2.74×10−3 dyn of force and 7.5×10−5 dyn-cm of torque. Blocking with MECA-79 (n=17) decreases necessary detachment force and torque by 56% and 68%, respectively. Bare polycarbonate (n=10) plastic and 10% bovine serum albumin (n=12)-coated slides are used as controls. Statistical analysis performed using one-way ANOVA, with Tukey post hoc tests of significance signified by stars, *p<0.05 and **p<0.01.
Discussion
Millions of women worldwide suffer from infertility and rely on various medical treatments.1,2 IVF is the most commonly used treatment for infertility; however, IVF leads to pregnancy ∼30% of the time and costs upward of $10,000 per trial.4,47 Low success rates of IVF have been attributed to limitations in the ability of blastocysts to properly implant to the uterus.6,48–50 Due to cost, legal and ethical considerations, and insufficient supply of donor tissue, few studies of human blastocyst implantation exist. Animal studies, although important, are inadequate models for human implantation.12–15,17,23,24,51,52 For these reasons, development of an in vivo implantation model is necessary.
L-selectin has been implicated as a primary molecule responsible for blastocyst implantation by promoting initial blastocyst attachment to the uterine endometrial epithelium.11 It is believed that L-selectin is responsible for slowing and arresting the blastocyst during its travel from the Fallopian tube to the fundus of the uterus.6,53,54 We hypothesize that a certain amount of bonding strength is necessary to achieve this initial attachment and that in the absence of L-selectin, there may be a reduction in blastocyst attachment to the uterine endometrial epithelium or that the attachment would have to go through a different pathway. In vivo implantation models have been previously reported,35,42,43,55 however, these models are limited in scope in that they do not aim to quantify L-selectin binding mechanics.
Jeg-3, JAR, and BeWo cell lines, derived from first trimester trophoblasts, were evaluated for their L-selectin expression. This is crucial since protein expression profiles of trophoblast cells in vivo change significantly as pregnancy progresses, to the extent that, by 17 weeks of gestation, trophoblast staining for L-selectin is virtually absent.11 Comparing the L-selectin expression profile by immunoblot, Figure 1A, and flow cytometry analysis, Figure 1B, L-selectin expression was verified in all three cell lines, but only on the surface of Jeg-3 cells. Therefore, Jeg-3 cells were selected for further studies as surface expression of L-selectin is critical for the attachment function.
L-selectin expression in Jeg-3 cells was investigated in response to hormones and corticosteroids: estrogen, which dominates in the follicular phase, progesterone, the primary hormone in the luteal phase of the menstrual cycle, and dexamethasone, a steroid used during oocyte retrieval in IVF. All treatments increased L-selectin expression in Jeg-3 cells with dexamethasone producing the most significant effect, Figure 1C. Post hoc power analysis confirms the significance of these results and suggests that a slight increase in the sample size (N=20, n=4 for each treatment) would result in significance for all treatments. Any effects on baseline expression in these experiments were avoided by using media free of steroid hormone mimetics and serum. These observations support that Jeg-3 cells have preserved responsiveness to endocrine regulators; nevertheless, caution should be used in trying to extend these results to primary human trophoblast cells.
To mimic the size and shape of a human blastocyst, Jeg-3 cells were cultured into three-dimensional structures, which we coined “trophospheres.” Trophospheres were cultured in a manner similar to Grümmer et al.42 We extended this technique by performing a parametric study of shaker speed, seeding density, and the serum level revealed which culture conditions were most suitable in achieving the desired trophosphere diameter and sphericity (data not shown). Trophospheres were cultured with diameters of 278.4±4.6 μm, which are slightly larger than the average diameter of a human blastocyst, 150–250 μm.56 Since the trophosphere shape is variable, both roundness and circularity measurements were taken. Figure 3C shows a trophosphere with average circularity, 0.77, and a high degree of roundness, 0.92. This difference is due to the slightly undulating surface of the trophosphere causing a larger perimeter and thus decreasing the circularity. Figure 3D shows a trophosphere with comparable degrees of circularity, 0.84, and roundness, 0.85. These differences can be attributed to the methods of calculating circularity, (Area/Perimeter2) and roundness (Major/Minor axis). Although roundness calculations are more robust than circularity, both average greater than 70% and show little variation suggesting that trophospheres can be cultured reproducibly. For comparison, we tested 20-μm latex microspheres, which resulted in average circularity, 0.91, and roundness, 0.95, to be only slightly higher compared with trophospheres. Immunostaining, Figure 3B, shows trophospheres express L-selectin. Quantification and modulation of L-selectin expression of trophospheres had not been performed at this time.
Few similar studies on three-dimensional trophoblast constructs binding to endometrial cells exist. However, these studies differ from ours and do not attempt to quantify L-selectin/sLeX binding. Spheroids have been cultured using JAR43,57,58 or BeWo35 cells and subsequently bound to monolayers of RL95-2 endometrial-like cells. However, we have shown that neither JAR nor BeWo cells exhibit surface L-selectin expression. Additionally, these studies incorporate binding times upward of 30 min and utilize centrifugation assays that impart large forces. Although these studies provide some insight into binding events in general, it is questioned as to what specific mechanisms are at work as well as what the binding mechanics are. Grümmer et al.42 use Jeg-3, JAR, and BeWo cells, but their spheroids range from 200 to 1000 in diameter. Our work eliminates nonspecific cell–cell interactions by binding directly to sLeX-coated slides and allows for quantifying cellular detachment force and torque.
Quantification of trophosphere detachment is modeled after the work by Prozirkidis,46 who made mathematical predictions of flow forces and torques on spherical objects in laminar flow. The shear force and torque are related to the particle radius and fluid shear rate under the assumptions that the particles are perfectly spherical, nondeforming, and subject to a fully developed laminar flow. In this study, a custom-built PPFC ensured laminar flow and trophospheres have been shown to be nearly spherical.
Trophospheres detached from sLeX functionalized slides under 2.74×10−3 dyn of force and 7.5×10−5 dyn-cm of torque. Blocking with MECA-7911,59,60 decreased detachment force and torque by 56% and 68%, respectively. The next logical step is to evaluate the effects that hormonal treatments have on trophosphere binding. However, effects of hormonal treatments are only detectable in cells cultured in the serum-free medium, based on our results with Jeg-3 monolayer cultures, while the serum-free medium does not support trophosphere culture. Decreasing serum levels below 10% results in sporadic and irregular spheres due to extensive cell death. There is currently much debate on the effects of hormone therapies, specifically progesterone, on IVF outcomes. Most recent evidence suggests that high progesterone serum levels results in lower IVF success and most likely lowered endometrial receptivity.61–63 Although our work does not address this body of evidence, the authors do have ongoing studies regarding trophospheres binding to model endometrial cell lines. Preliminary data suggest that hormone treatments to endometrial cell lines result in decreases to L-selectin ligand expression and trophosphere/endometrial detachment forces.
Although improper blastocyst implantation is thought to be the cause for the limited success rates of IVF, very little is known about the mechanics of implantation. It has been shown that during the secretory phase, uterine contractions occur at a rate of 1–5/min causing a uterine pressure of 5–25 mm Hg.64 These uterine contractions are directed from the cervix to the fundus, which may help explain why implantation generally occurs near the fundus. Eytan et al.65,66 have modeled uterine contractions showing directionality of fluid flow within the uterus. However, neither the forces imparted on the blastocyst by uterine contractions nor the uterine fluid flow is known. As our results show, L-selectin-mediated binding requires little force to be broken. Thus, it is within the realm of possibility that mechanical conditions within the uterus are sufficient to prevent the initial stages of implantation. Curiously, Eytan et al.,64–66 have also modeled instances of slow motion near the fluid–wall interface and trapping, where the blastocyst would become stuck within a regional vortex. These conditions may allow for blastocysts to initiate the implantation process and for enough time for stronger adhesions to occur.
Conclusions and Future Works
To the authors' best knowledge, this work presents the first time Jeg-3 cells have been shown to exhibit surface L-selectin expression. Additionally, this is the first time that L-selectin/sLeX bonding in trophoblastic cells has been quantified by culturing Jeg-3 cells into three-dimensional blastocyst mimics or trophospheres. Quantification of this binding system is a crucial step in advancing the field of blastocyst implantation and provides groundwork for determining the forces experienced by blastocysts during the implantation process.
The work presented here may be useful in both the laboratory and clinic settings as either a research tool, or clinically, either to optimize implantation by pretreatment of blastocysts, or as part of a diagnostic test. In the short term, this model will be utilized in the laboratory environment. We have ongoing studies investigating the effect of various environmental factors on binding between trophospheres and a model uterine epithelium with hopes to transition into testing primary tissues. Patients undergoing IVF are introduced to extreme hormone therapies, yet the effects of these hormone therapies on the aspect of blastocyst implantation are not well known. Our model system may have direct impacts into understanding the role of hormonal effects on implantation mechanics. In the long term, model results may provide a metric for maximizing implantation capacity of blastocysts generated by IVF or provide diagnostic parameters to aid in the selection of implantation-competent blastocysts and, thus, increase the efficiency of IVF.
Acknowledgment
We would like to acknowledge the National Science Foundation Award 0853733 for funding.
Disclosure Statement
No competing financial interests exist.
References
- 1.Centers for Disease Control and Prevention ASfRM, Society for Assisted Reproductive Technology. Assisted Reproductive Technology Success Rates National Summary and Fertility Clinic Reports. 2006
- 2.Chandra A., Martinez G.M., Mosher W.D., Abma J.C., and Jones J.Fertility, family planning, and reproductive health of US women: data from the 2002 National Survey of Family Growth. Vital and Health Statistics Series 23. Data from the National Survey of Family Growth. 1, 2005 [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention ASfRM, Society for Assisted Reproductive Technology. 2010 Assisted Reproductive Technology Fertility Clinic Success Rates Report. 2010
- 4.Katz P., Showstack J., Smith J.F., Nachtigall R.D., Millstein S.G., Wing H., et al. . Costs of infertility treatment: results from an 18-month prospective cohort study. Fertil Steril 95, 915, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins J.A., Bustillo M., Visscher R.D., and Lawrence L.D.An estimate of the cost of in vitro fertilization services in the United States in 1995. Fertil Steril 64, 538, 1995 [DOI] [PubMed] [Google Scholar]
- 6.Carson D.D., Bagchi I., Dey S.K., Enders A.C., Fazleabas A.T., Lessey B.A., et al. . Embryo implantation. Dev Biol 223, 217, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Norwitz E., Schust D., and Fisher S.Implantation and the survival of early pregnancy. N Engl J Med 345, 1400, 2001 [DOI] [PubMed] [Google Scholar]
- 8.McLaren A.The Embryo. Cambridge, UK: Cambridge University Press, 1990 [Google Scholar]
- 9.Dey S., Lim H., Das S., Reese J., Paria B., Daikoku T., et al. . Molecular cues to implantation. Endocr Rev 25, 341, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Fukuda M., and Sugihara K.An integrated view of L selectin and trophinin function in human embryo implantation. J Obstet Gynaecol Res 34, 129, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Genbacev O.D., Prakobphol A., Foulk R.A., Krtolica A.R., Ilic D., Singer M.S., et al. . Trophoblast L-selectin-mediated adhesion at the maternal-fetal interface. Science 299, 405, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Lee K.Y., and DeMayo F.J.Animal models of implantation. Reproduction 128, 679, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Enders A.C., Hendrickx A.G., and Schlafke S.Implantation in the rhesus monkey: initial penetration of endometrium. Am J Anat 167, 275, 1983 [DOI] [PubMed] [Google Scholar]
- 14.Enders A.C., Lantz K.C., and Schlafke S.Differentiation of trophoblast of the baboon blastocyst. Anat Rec 225, 329, 1989 [DOI] [PubMed] [Google Scholar]
- 15.Enders A.C., and Lopata A.Implantation in the marmoset monkey: expansion of the early implantation site. Anat Rec 256, 279, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Tarantal A., and Hendrickx A.Use of ultrasound for early pregnancy detection in the rhesus and cynomolgus macaque (Macaca mulatta and Macaca fascicularis). J Med Primatol 17, 105, 1988 [PubMed] [Google Scholar]
- 17.Enders A.C.Trophoblast-uterine interactions in the first days of implantation: models for the study of implantation events in the human. Semin Reprod Med 18, 255, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Enders A.C., and Schlafke S.Cytological aspects of trophoblast-uterine interaction in early implantation. Am J Anat 125, 1, 1969 [DOI] [PubMed] [Google Scholar]
- 19.Dey S.K.Visualizing early embryo implantation sites by dye injection. CSH Protoc 2006, DOI: 10.1101/pdb.prot4361 [DOI] [PubMed] [Google Scholar]
- 20.Hoffman L.H., Olson G.E., Carson D.D., and Chilton B.S.Progesterone and implanting blastocysts regulate muc1 expression in rabbit uterine epithelium. Endocrinology 139, 266, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Gray C., Burghardt R., Johnson G., Bazer F., and Spencer T.Evidence that absence of endometrial gland secretions in uterine gland knockout ewes compromises conceptus survival and elongation. Reproduction 124, 289, 2002 [PubMed] [Google Scholar]
- 22.Gray C.A., Bartol F.F., Tarleton B.J., Wiley A.A., Johnson G.A., Bazer F.W., et al. . Developmental biology of uterine glands. Biol Reprod 65, 1311, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Wooding F.Role of binucleate cells in fetomaternal cell fusion at implantation in the sheep. Am J Anat 170, 233, 1984 [DOI] [PubMed] [Google Scholar]
- 24.Wooding F.The synepitheliochorial placenta of ruminants: binucleate cell fusions and hormone production. Placenta 13, 101, 1992 [DOI] [PubMed] [Google Scholar]
- 25.Pattillo R., Ruckert A., Hussa R., Bernstein R., and Delfs E.The JAR cell line-continuous human multihormone production and controls. In Vitro 6, 30, 1971 [Google Scholar]
- 26.Kohler P.O., and Bridson W.E.Isolation of hormone-producing clonal lines of human choriocarcinoma. J Clin Endocrinol Metab 32, 683, 1971 [DOI] [PubMed] [Google Scholar]
- 27.Pattillo R.A., Gey G.O., Delfs E., and Mattingly R.F.Human hormone production in vitro. Science 159, 1467, 1968 [DOI] [PubMed] [Google Scholar]
- 28.Al-Shami R., Sorensen E.S., Ek-Rylander B., Andersson G., Carson D.D., and Farach-Carson M.C.Phosphorylated osteopontin promotes migration of human choriocarcinoma cells via a p70 S6 kinase-dependent pathway. J Cell Biochem 94, 1218, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Bahn R.S., Worsham A., Speeg K., Ascoli M., and Rabin D.Characterization of steroid production in cultured human choriocarcinoma cells. J Clin Endocrinol Metab 52, 447, 1981 [DOI] [PubMed] [Google Scholar]
- 30.Fitzgerald J.S., Tsareva S.A., Poehlmann T.G., Berod L., Meissner A., Corvinus F.M., et al. . Leukemia inhibitory factor triggers activation of signal transducer and activator of transcription 3, proliferation, invasiveness, and altered protease expression in choriocarcinoma cells. Int J Biochem Cell Biol 37, 2284, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Helige C., Hagendorfer G., Smolle J., and Dohr G.Uterine natural killer cells in a three-dimensional tissue culture model to study trophoblast invasion. Lab Invest 81, 1153, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Heneweer C., Kruse L.H., Kindhäuser F., Schmidt M., Jakobs K.H., Denker H.W., et al. . Adhesiveness of human uterine epithelial RL95-2 cells to trophoblast: rho protein regulation. Mol Hum Reprod 8, 1014, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Hohn H.P., Linke M., and Denker H.W.Adhesion of trophoblast to uterine epithelium as related to the state of trophoblast differentiation: in vitro studies using cell lines. Mol Reprod Dev 57, 135, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Lessey B.A.Adhesion molecules and implantation. J Reprod Immunol 55, 101, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Li H.Y., Chang S.P., Yuan C.C., Chao H.T., Ng H.T., and Sung Y.J.Establishment of an efficient method to quantify embryo attachment to endometrial epithelial cell monolayers. In Vitro Cell Dev Biol Anim 38, 505, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Li H.Y., Chang S.P., Yuan C.C., Chao H.T., Ng H.T., and Sung Y.J.Induction of p38 mitogen-activated protein kinase-mediated apoptosis is involved in outgrowth of trophoblast cells on endometrial epithelial cells in a model of human trophoblast-endometrial interactions. Biol Reprod 69, 1515, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Luo S., Yu H., Wu D., and Peng C.Transforming growth factor-β1 inhibits steroidogenesis in human trophoblast cells. Mol Hum Reprod 8, 318, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Nakatsuji Y., Nishio Y., Tani N., Adachi K., Ohmichi M., Hisamoto K., et al. . Epidermal growth factor enhances invasive activity of BeWo choriocarcinoma cells by inducing alpha2 integrin expression. Endocr J 50, 703, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Pongcharoen S., Niumsup P., Sanguansermsri D., Supalap K., and Butkhamchot P.The effect of interleukin-17 on the proliferation and invasion of JEG-3 human choriocarcinoma cells. Am J Reprod Immunol 55, 291, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Tinel H., Denker H.W., and Thie M.Calcium influx in human uterine epithelial RL95-2 cells triggers adhesiveness for trophoblast-like cells. Model studies on signalling events during embryo implantation. Mol Hum Reprod 6, 1119, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Zhang J., Cao Y.J., Li F.Y., Li J., Yao L.B., and Duan E.K.Effects of fibronectin, VEGF and angiostatin on the expression of MMPs through different signaling pathways in the JEG-3 cells. Am J Reprod Immunol 50, 273, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Grümmer R., Hohn H.P., Mareel M., and Denker H.W.Adhesion and invasion of three human choriocarcinoma cell lines into human endometrium in a three-dimensional organ culture system. Placenta 15, 411, 1994 [DOI] [PubMed] [Google Scholar]
- 43.Wang H., Pilla F., Anderson S., Martinez-Escribano S., Herrer I., Moreno-Moya J.M., et al. . A novel model of human implantation: 3D endometrium-like culture system to study attachment of human trophoblast (Jar) cell spheroids. Mol Hum Reprod 18, 33, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Lessey B., Damjanovich L., Coutifaris C., Castelbaum A., Albelda S., and Buck C.Integrin adhesion molecules in the human endometrium. Correlation with the normal and abnormal menstrual cycle. J Clin Invest 90, 188, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Coupade, C., Solito E., and Levine J.D.Dexamethasone enhances interaction of endogenous Annexin 1 with L-selectin and triggers shedding of L-selectin in the monocytic cell line U-937. Br J Pharmacol 140, 133, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pozrikidis C.Shear flow over a protuberance on a plane wall. J Eng Math 31, 29, 1997 [Google Scholar]
- 47.Trad F., Hornstein M., and Barbieri R.In vitro fertilization: a cost-effective alternative for infertile couples? J Assist Reprod Genet 12, 418, 1995 [DOI] [PubMed] [Google Scholar]
- 48.Busso C., Melo M., Fernandez M., Pellicer A., and Simon C.Implantation in IVF. Int Surg 91, 63, 2006 [PubMed] [Google Scholar]
- 49.Fazleabas A.T., and Kim J.J.Development. What makes an embryo stick? Science 299, 355, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Margarit L., Gonzalez D., Lewis P.D., Hopkins L., Davies C., Conlan R.S., et al. . L-selectin ligands in human endometrium: comparison of fertile and infertile subjects. Hum Reprod 24, 2767, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Teklenburg G., and Macklon N.S.Review: in vitro models for the study of early human embryo-endometrium interactions. Reprod Sci 16, 811, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Wang H., and Dey S.K.Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet 7, 185, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Dominguez F., Yanez-Mo M., Sanchez-Madrid F., and Simon C.Embryonic implantation and leukocyte transendothelial migration: different processes with similar players? FASEB J 19, 1056, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Kimber S.J., and Spanswick C.Blastocyst implantation: the adhesion cascade. Semin Cell Dev Biol 11, 77, 2000 [DOI] [PubMed] [Google Scholar]
- 55.Bischof P., and Campana A.A model for implantation of the human blastocyst and early placentation. Hum Reprod Update 2, 262, 1996 [DOI] [PubMed] [Google Scholar]
- 56.Richter K.S., Harris D.C., Daneshmand S.T., and Shapiro B.S.Quantitative grading of a human blastocyst: optimal inner cell mass size and shape. Fertil Steril 76, 1157, 2001 [DOI] [PubMed] [Google Scholar]
- 57.Liu S., Yang X., Liu Y., Wang X., and Yan Q.sLeX/L-selectin mediates adhesion in vitro implantation model. Mol Cell Biochem 350, 185, 2011 [DOI] [PubMed] [Google Scholar]
- 58.Thie M., Röspel R., Dettmann W., Benoit M., Ludwig M., Gaub H.E., et al. . Interactions between trophoblast and uterine epithelium: monitoring of adhesive forces. Hum Reprod 13, 3211, 1998 [DOI] [PubMed] [Google Scholar]
- 59.Foulk R.A., Zdravkovic T., Genbacev O., and Prakobphol A.Expression of L-selectin ligand MECA-79 as a predictive marker of human uterine receptivity. J Assist Reprod Genet 24, 316, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shamonki M.I., Kligman I., Shamonki J.M., Schattman G.L., Hyjek E., Spandorfer S.D., et al. . Immunohistochemical expression of endometrial L-selectin ligand is higher in donor egg recipients with embryonic implantation. Fertil Steril 86, 1365, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Labarta E., Martínez-Conejero J.A., Alamá P., Horcajadas J.A., Pellicer A., Simón C., et al. . Endometrial receptivity is affected in women with high circulating progesterone levels at the end of the follicular phase: a functional genomics analysis. Hum Reprod 26, 1813, 2011 [DOI] [PubMed] [Google Scholar]
- 62.Papanikolaou E.G., Pados G., Grimbizis G., Bili E., Kyriazi L., Polyzos N.P., et al. . GnRH-agonist versus GnRH-antagonist IVF cycles: is the reproductive outcome affected by the incidence of progesterone elevation on the day of HCG triggering? A randomized prospective study. Hum Reprod 27, 1822, 2012 [DOI] [PubMed] [Google Scholar]
- 63.Sundström P., Nilsson O., and Liedholm P.Scanning electron microscopy of human preimplantation endometrium in normal and clomiphene/human chorionic gonadotropin-stimulated cycles. Fertil Steril 40, 642, 1983 [DOI] [PubMed] [Google Scholar]
- 64.Eytan O., Jaffa A.J., Har-Toov J., Dalach E., and Elad D.Dynamics of the intrauterine fluid–wall interface. Ann Biomed Eng 27, 372, 1999 [DOI] [PubMed] [Google Scholar]
- 65.Eytan O., and Elad D.Analysis of intra-uterine fluid motion induced by uterine contractions. Bull Math Biol 61, 221, 1999 [DOI] [PubMed] [Google Scholar]
- 66.Eytan O., Jaffa A.J., and Elad D.Peristaltic flow in a tapered channel: application to embryo transport within the uterine cavity. Med Eng Phys 23, 475, 2001 [DOI] [PubMed] [Google Scholar]