Abstract
Background: The mobile intercellular fluid flowing to and in the lymphatics contains filtered plasma products and substances synthesized and excreted by tissue cells. Among them are signaling proteins such as cytokines, chemokines, enzymes, and growth factors. They act locally in autocrine and paracrine systems regulating cell metabolism, proliferation, and formation of the ground matrix. They play an immunoregulatory role in infections, wound healing, and tumor cell growth.
Methods and Results: In this study we measured the concentration of selected cytokines, chemokines, tissue enzymes, and growth factors in tissue fluid/lymph drained from normal human leg soft tissues. Legs exposed to infections and trauma often result in development of lymphedema. Lymph was drained from superficial calf lymphatics using microsurgical techniques. Our studies showed generally higher concentrations of cytokines, chemokines, enzymes, and growth factors in lymph than in serum. The total protein L/S ratio was 0.22, whereas that of various lymph signaling proteins ranged between 1 and 10.
Conclusions: This indicates that in addition to proteins filtered from blood, local cells contribute to lymph concentration by own production, depending on the actual cell requirement. Moreover, there were major individual differences of lymph levels with simultaneous stable serum levels. This suggests existence of a local autonomous regulatory humoral mechanism in tissues, not reflected in serum.
Introduction
Tissue cell metabolic processes, proliferation, differentiation, senescence, and apoptosis are regulated by a plethora of low molecular proteins and peptides, among them cytokines, chemokines, growth factors, enzymes, and neurotransmitters. These signaling proteins are present in the tissue fluid.1–4 They easily diffuse in the liquid environment and get access to individual cells. Here they are absorbed by cell multimerized specific receptors subsequently activating the JAK/STAT signal transduction pathway. The concentration of signaling proteins in tissue fluid is not regulated solely by filtration from blood but also by local production by parenchymal and immigrating immune cells. In skin and subcutaneous tissue, these are keratinocytes, blood, and lymphatic endothelial cells, Langerhans' cells, fibroblasts, and recirculating lymphocytes. Tissue fluid flowing into lymphatics is called lymph. It is known from multiple studies that lymph composition changes continuously due to influx of plasma components, and consumption and production/secretion of various substances by the parenchyma cells. Consequently, lymph flow rate and its composition change from minute to minute.5 Knowledge of their concentration and activity can give insight into the cellular and interstitial processes of the studied tissue. Harvesting lymph from the cannulated lymphatic collectors provides volumes sufficient for biochemical studies.6 Blood samples from the studied tissue do not provide data reflecting intercellular events to such an extent as lymph.
The literature on the chemical composition of lymph is scarce and provides only limited knowledge on the chemical processes in the intercellular space.7–10 Lower limbs are exposed to infections and trauma and the signaling proteins here play a dominant role in regulating the immune homeostasis. This prompted us to study lymph cytokines, chemokines, and growth factors drained from normal skin, subcutaneous tissue, and joints of the foot and lower part of calf (Fig. 1).
FIG. 1.

The network of lymphatic vessels draining foot joints, subcutaneous tissues, and skin. Since superficial and deep vessels are interconnected, cannulated collecting trunks in the calf contain lymph from all foot tissues. Normal lymph pressure during lymphangion contractions reaches 10–40 mm Hg. Cannulation of lymphatics and external drainage of lymph to atmospheric pressure directs the lymph stream from most areas to the cannula. The 24-hour lymph output from a cannulated lymphatic ranges from 5 mL to 60 mL. Any event developing in the tissues drained by the cannulated lymphatics that changes local tissue fluid chemistry and cellularity is reflected in lymph composition. The box encloses the region of the extremity drained by the cannulated lymphatic vessel. 1 indicates lymphatic plexus of skin, 2 indicates lymphatics connecting the deep and superficial lymphatic systems, 3 indicates perivascular space where tissue fluid accumulates, and 4 indicates lymphatics draining bone and joints. Reproduced, with permission, from Kubik S. Atlas of the Lymphatics of the Lower Limbs. Paris: Servier; 1999: 15.
Materials and Methods
Clinical procedures
Twenty-five healthy male subjects were studied in the project on reverse cholesterol transport.11 A portion of the harvested lymph and blood samples was used for signaling protein studies. All subjects had been screened for cardiovascular, renal, hepatic, and endocrine as previously described.12 Blood samples were also examined for recreational drugs. The study had been approved by the appropriate institutional review boards, and the subjects gave informed written consent.
Collection of lymph
Lymph vessel cannulations were performed under sterile conditions.6 Briefly, an area of skin 6–10 cm above the ankle was anesthetized, and an incision 15–20 mm wide made in the center. Under an operating microscope, a suitable subcutaneous lymph vessel was identified. A second smaller incision was made above the first, through which a tapered sterile siliconized polyethylene cannula was passed. The vessel was opened, and the first distal valve destroyed. The cannula was passed into the vessel, secured with a ligature, and the other end passed through the cap of a plastic vial. For some assays, the vial contained no additives, for others it contained 2 mg disodium EDTA powder. The vial was kept in crushed ice, contained in an expanded polystyrene holder strapped to the leg. The collection tubes were changed every 2 or 3 hours, and centrifuged at 4°C. Venous blood (10 mL) was collected during each 2–3 h collection period, and treated identically to the lymph samples. All samples were stored at −80°C prior to analysis.
Lymph and serum cytokines, chemokines, and growth factors
The investigated proinflammatory cytokines were interleukins IL1β, IL6, IL8, IL12, IL15, and TNFα (tumor necrosis factor), anti-inflammatory IL1Rα (anti-IL1-receptor), IL10, and TGFβ (transforming growth factor). The chemokines were MIP1α (macrophage inflammatory protein), MCP1 (macrophage chemotactic protein), enzymes MMP9 (metallopeptidase), TIMP1 and 2 (tissue inhibitor of metallopeptidase), CCL21 and CCL27. The growth factors were SCF (stem cell factor), BCL (B cell chemoattractant), M-CSF (monocyte colony stimulating factor), G-CSF (granulocytes colony stimulating factor), KGF (keratinocyte growth factor) and INFγ (interferon). Moreover, there were VEGF (vascular endothelial growth factor), VEGF-c, leptin, and adiponectin. The ELISA method on commercially obtained R&D System (Minneapolis, MN) plates was used.
Concentration of lymph proteins was expressed in g/100 mL, that of cytokines, chemokines, tissue enzymes, and growth factors in pg/mL or ng/mL and presented as means±SD. The lymph:serum (L:S) ratio was calculated for each pair of lymph and serum samples. For statistical difference between lymph and serum concentration, classic and two-tail Student tests were used, with significance at p>0.05.
Results
The concentrations of investigated cytokines, chemokines, enzymes, and growth factors in ng/mL and pg/mL are presented in Figures 2–6. For demonstration of differences between lymph and serum, the lymph to serum ratio (L/S) was calculated. The obtained data compared with total protein L/S ratio that was 0.22±0.1 (concentration 1.66±0.14 g/dL and 7.30±0.1 g/dL, respectively). The cytokine L/S ratio was above 1.0 for all cytokines except that of IL12, IL10, and TGFβ. It was 3.1 for IL1β, 3.9 for IL6, 1.9 for TNFα, 5.0 for IL15, and 10.0 for IL8 (all p<0.05). It was 1.1 for IL1Rα (NS), 0.29 for IL12, 0.4 for IL10, and 0.004 for TGFβ (all p<0.05) (Fig. 2A).
FIG. 2.
Cytokine concentration in lymph and serum. (A) protein levels; (B) L/S ratio. N=25, mean±sd, *p<0.05.
FIG. 3.
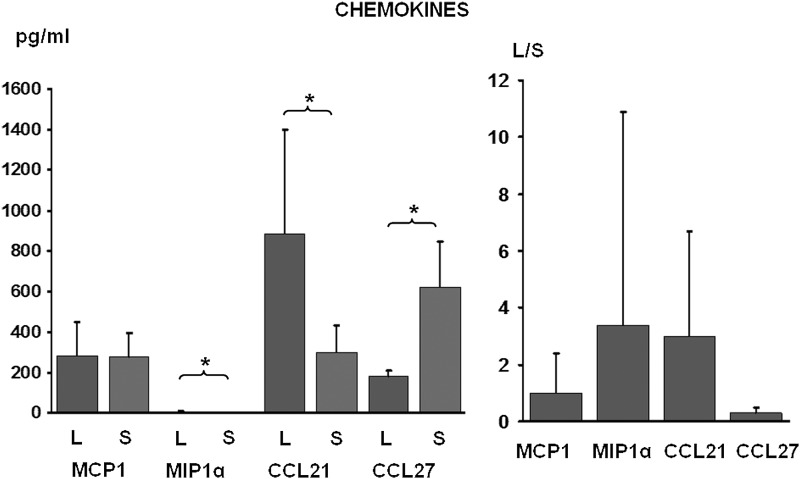
Chemokine concentration in lymph and serum. Protein levels and L/S ratio. N=25, mean±sd, *p<0.05.
FIG. 4.
Matrix enzyme concentration in lymph and serum. Protein levels and L/S ratio. N=25, mean±s d, *p<0.05.
FIG. 5.
Growth factor concentration in lymph and serum. (A) Protein levels and (B) L/S ratio. N=25, mean±sd, *p<0.05.
FIG. 6.
Angio- and adipogenic factor concentration in lymph and serum. Protein levels and L/S ratio. N=25, mean±sd, *p<0.05.
The chemokine and matrix enzyme L/S was above 1.0 for MIP 1α, CCL21, TIMP1 and TIMP2 (all p<0.05). It was 3.4 for MIP 1α, 3.0 for CCL21, 2.5 for TIMP1, 3.5 for TIMP2 and 1.0 for MCP1 (Fig. 3). It was below 1 for MMP9 (0.33) and CCL27 (0.28) (both p<0.05) (Fig. 4).
The growth factor lymph to serum ratio (L/S) was above 1.0 for SCF, M-CSF, and KGF (all p<0.05). It was 2.2 for SCF, 1.6 for M-CSF, 6.0 for KGF. It was 1.0 for BCL and 1.6 for G-CSF (NS). The values for INFγ were 0.15 (p<0.05) (Fig. 5A).
The angiogenic and adipocytogenic factor lymph to serum ratio (L/S) was above 1.0 for VEGF and leptin (both p<0.05) (Fig. 6). It was 5.3 for VEGF and 2.6 for leptin. The VEGF C L/S was 1.3 (NS) and of adiponectin 0.34 (p<0.05).
Discussion
Our studies have shown generally higher concentrations of cytokines, and growth factors and enzymes in lymph than in serum. This indicates that, in addition to proteins filtered from blood, local cells contribute to lymph concentration by their own production, depending on the actual cell requirement. The total protein L/S ratio was 0.22, whereas that of various lymph signaling proteins ranged between 1 and 10. Moreover, there were major individual differences of lymph levels, whereas those of serum remained stable. This suggests existence of a local autonomous regulatory humoral mechanism in tissues, not reflected in serum. Generally, the proinflammatory cytokine levels had the L/S ratios higher and the anti-inflammatory lower than in serum. The L/S ratio was also high for chemokines and tissue proteases inhibitors but low for metalloproatease 9. The values for growth factors were high except for interferon gamma. The L/S ratio for angiogenic factors and leptin was above 1, but low for adiponectin. Although not yet described in the literature, these results suggest a physiological balance of regulatory proteins in the intercellular space, which cannot be inferred from the serum studies. Low tissue fluid/lymph levels of IL10, IL12, TGFβ, MMP9, INFγ, and CCL27 suggest lack of local synthesis and secretion in normal tissues.
Cytokines and chemokines can be classified as proteins, peptides, or glycoproteins. The term "cytokine" encompasses a large and diverse family of regulators produced throughout the body by cells of diverse embryological origin. The term has also been used to refer to the immunomodulating agents, such as interleukins and interferons. Biochemists disagree as to which molecules should be termed cytokines and which hormones. As we learn more about each, anatomic and structural distinctions between the two are fading. Classic protein hormones circulate in nanomolar (10−9) concentrations that usually vary by less than one order of magnitude. In contrast, some cytokines (such as IL-6) circulate in picomolar concentrations.10–12
The original members of the IL-1 superfamily are IL-1α and IL-1β are produced by epithelial cells, macrophages, monocytes, fibroblasts, and dendritic cells. They form an important part of the inflammatory response of the body against infection. These cytokines increase the expression of adhesion factors on endothelial cells to enable transmigration of leukocytes. It is found in substantial amounts in normal human epidermis and is distributed in a 1:1 ratio between living epidermal cells and stratum corneum.7,13 In our studies the normal L/S ratio was 3.1.
Interleukin-6 (IL-6) is an interleukin that acts as both a pro-inflammatory and anti-inflammatory cytokine. It is secreted by T cells and macrophages to stimulate immune response. The role of IL-6 as an anti-inflammatory cytokine is mediated through its inhibitory effects on TNF-alpha and IL-1, and activation of IL-1ra and IL-10.14 In our studies, the normal L/S ratio was 3.9.
Interleukin-8 is a chemokine produced by macrophages and epithelial cells. It is also synthesized by endothelial cells, which store IL-8 in their storage vesicles, the Weibel-Palade bodies. IL-8, also known as neutrophil chemotactic factor, induces chemotaxis in target cells, primarily neutrophils but also other granulocytes, causing them to migrate toward the site of infection. IL-8 is also known to be a potent promoter of angiogenesis.15 In our studies, the normal L/S ratio was 10.
Interleukin 12 is produced by dendritic cells and macrophages in response to antigenic stimulation. IL-12 is involved in the differentiation of naive T cells into Th1 cells. It stimulates the production of interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α) from T and natural killer (NK) cells. IL-12 mediates enhancement of the cytotoxic activity of NK cells and CD8+ cytotoxic T lymphocytes. IL-12 also has anti-angiogenic activity.16 The lymph IL12 L/S ratio was 0.29.
Interleukin 15 (IL-15) is secreted by mononuclear phagocytes, induces cell proliferation of natural killer cells, and is constitutively expressed by monocytes, macrophages, dendritic cells (DC), keratinocytes, fibroblasts, and nerve cells.17 In our studies, the L/S ratio was 5.0.
Tumor necrosis factor can cause cytolysis of certain tumor cell lines. It is involved in the induction of cachexia, and is a potent pyrogen, causing fever by direct action or by stimulation of interleukin-1 secretion. It can stimulate cell proliferation and induce cell differentiation under certain conditions.18 In our studies, the L/S ratio was 1.9.
IL-1Ra is secreted by immune cells, epithelial cells, and adipocytes. This protein inhibits the activities of interleukin 1 alpha and beta and modulates a variety of interleukin 1-related immune and inflammatory responses.19 In our studies, the L/S ratio was 1.1
IL-10 is a cytokine with pleiotropic effects in immunoregulation and inflammation. It downregulates the expression of Th1cytokines, MHC class II antigens, and co-stimulatory molecules on macrophages. It also enhances B cell survival, proliferation, and antibody production. IL-10 can block NF-κB activity and is capable of inhibiting synthesis of pro-inflammatory cytokines such as IFN-γ, IL-2, IL-3, TNFα, and GM-CSF made by cells such as macrophages and regulatory T-cells. It also displays a potent ability to suppress the antigen-presentation capacity of antigen presenting cells.20 In our studies, the normal L/S ratio was 0.4.
TGF-beta acts as an antiproliferative factor in normal epithelial cells and at early stages of oncogenesis. It is secreted by many cell types, including macrophages. TGF-β appears to block the activation of lymphocytes and monocyte derived phagocytes.21 The TGFβ L/S ratio was 0.004.
Leptin is involved in the regulation of the inflammatory response. It has been further theorized that leptin's role as an inflammatory marker is to respond specifically to adipose-derived inflammatory cytokines. In terms of both structure and function, leptin resembles IL-6.22 The leptin L/S ratio in our studies was 2.6.
Adiponectin is a protein exclusively secreted from adipose tissue, mainly subcutaneous, into the bloodstream and is very abundant in plasma relative to many hormones. Adiponectin is secreted into the bloodstream where it accounts for approximately 0.01% of all plasma protein.23 In our studies, the normal L/S ratio was 0.34.
CCL21 is a small cytokine belonging to the CC chemokine family present in lymphatic endothelial cells of afferent vessels and lymph node sinuses. CCL21 elicits its effects by binding to a lymphocyte or dendritic cell surface chemokine receptor known as CCR7. It mediates Langerhans cell exit from the epidermis.24 In our studies, the normal L/S ratio was 3.0.
CCL27 is a small cytokine also known as cutaneous T-cell-attracting chemokine (CTACK). It is associated with homing of memory T lymphocytes to the skin, and plays a role in T cell-mediated inflammation of the skin. It elicits its chemotactic effects by binding to the chemokine receptor CCR10. CCL27 is expressed in the epidermis and endothelium of both infected and uninfected skin.25 In our studies, the normal L/S ratio was 0.28.
Macrophage inflammatory proteins (MIP) are major factors produced by macrophages after they are stimulated with bacterial endotoxins, dendritic cells, and lymphocytes They activate human granulocytes (neutrophils, eosinophils, and basophils). They also induce the synthesis and release of other pro-inflammatory cytokines such as interleukin 1, 6, and TNF-α from fibroblasts and macrophages.26 In our studies, the normal L/S ratio was 3.4.
Monocyte chemotactic protein-1 (MCP-1) is a small inducible cytokine called CCL2 recruiting monocytes, T lymphocytes, dendritic cells to the sites of inflammation produced by either tissue injury or infection. It is produced by the lymphatic endothelial cells. However, it does not attract neutrophils or eosinophils.27 In our studies, the normal L/S ratio was 1.0.
Vascular endothelial growth factor (VEGF) is a signal protein produced by cells that stimulate vasculogenesis and angiogenesis. It is an important signaling protein involved in both vasculogenesis (the de novo formation of the embryonic circulatory system) and angiogenesis (the growth of blood vessels from pre-existing vasculature). The members of the VEGF family are VEGF-A, B, C, and D. VEGF C is regulating lymphangiogenesis.28 In our studies, the normal the L/S ratio of VEGF was 5.3 and of VEGF C 1.3.
Keratinocyte growth factor (KGF), also known as FGF7, is a growth factor present in the epithelialization phase of wound healing. In this phase, keratinocytes are covering the wound, forming the epithelium. KGF binds to fibroblast growth factor receptor 2b (FGFR2b).29 In our studies, the normal L/S ratio was 6.0.
Macrophage colony-stimulating factor (M-CSF) is a secreted cytokine that influences hematopoietic stem cells to differentiate into macrophages or other related cell types.30 In our studies, the normal L/S ratio of M-CSF was 1.6 and that of G-CSF 1.6.
IFN-γ, or type II interferon, is a cytokine that is critical for innate and adaptive immunity against viral and intracellular bacterial infections and for tumor control. IFN-γ is produced predominantly by natural killer (NK) and natural killer T (NKT) cells as part of the innate immune response, and by CD4 Th1 and CD8 cytotoxic T lymphocyte (CTL) effector T cells once antigen-specific immunity develops.31 In our studies, the normal L/S ratio was 0.15.
Matrix metallopeptidase 9 (MMP-9) is an enzyme involved in the breakdown of extracellular matrix in normal physiological processes, such as tissue remodeling, and metastasis. Most MMPs are secreted as inactive proproteins that are activated when cleaved by extracellular proteinases. They degrade type IV and V collagens.32 In our studies, the normal L/S ratio was 0.03.
TIMP metallopeptidase inhibitor 1, also known as TIMP1, a tissue inhibitor of metalloproteinases is a glycoprotein that is expressed from the several tissues. In addition to its inhibitory role against most of the known MMPs, the encoded protein is able to promote cell proliferation in a wide range of cell types, and may also have an anti-apoptotic function.
TIMP metallopeptidase inhibitor 2 is thought to be a metastasis suppressor. In addition to an inhibitory role against metalloproteinases, it has a unique role among TIMP family members in its ability to suppress the proliferation of endothelial cells directly. It suppresses the proliferation of quiescent tissues in response to angiogenic factors, and by inhibiting protease activity in tissues undergoing remodeling of the extracellular matrix.33,34 In our studies, the normal L/S ratio was 2.5 and that of TIMP2 3.5.
Stem Cell Factor (also known as SCF, kit-ligand, KL, or steel factor) is a cytokine that binds to the c-Kit receptor. Soluble and transmembrane SCF is produced by fibroblasts and endothelial cells.35 In our studies, the normal L/S ratio was 2.25.
BLC (CXCL13), also known as a B lymphocyte chemoattractant, elicits its effects by interacting with chemokine receptor CXCR5. CXCL13 and its receptor CXCR5 control the organization of B cells within follicles of lymphoid tissues and is expressed highly in the liver, spleen, lymph nodes, and gut of humans.36 In our studies, the normal L/S ratio was 1.0.
The generally higher concentrations of humoral factors in normal lymph compared with serum raise the question of which tissue cells contribute to the level of lymph cytokines, chemokines, and growth factors, and how these humoral factors may regulate metabolism and proliferation of tissues keratinocytes, fibroblasts, lymphatic and blood endothelial, recirculating immune and lymph node cells.37,38
Conclusions
Taken together, lymph drained from normal human leg tissues contains an array of regulatory proteins originating from plasma but also produced locally by parenchymal, endothelial, and migrating immune cells. The concentration of most of these proteins is higher than in serum. Only few are represented at levels below 50% of those of plasma. Generally, high concentrations of cytokines, chemokines, growth factors, and enzymes point to the autonomous regulation of growth and metabolic processes, with only partial contribution by factors supplied from plasma. Knowledge of how the regulatory proteins work at the tissue level is still limited. Culturing tissue cells in lymph, its natural environment, would give more insight into the tissue processes.
Author Disclosure Statement
No competing financial interests exist.
This work was supported by grant No. NN404 113139 from the National Center of Science, Poland.
References
- 1.Olszewski WL, Engeset A, Łukasiewicz H. Immunoglobulins, complement and lysozyme in leg lymph of normal men. Scand J Clin Lab Invest 1977;37:669–674 [DOI] [PubMed] [Google Scholar]
- 2.Olszewski WL, Engeset A. Immune proteins, enzymes and electrolytes in human peripheral lymph. Lymphology 1978;11:156–164 [PubMed] [Google Scholar]
- 3.Olszewski WL. Immune proteins, cell interactions and immunoregulatory hormones: Lymph versus blood. Lymphology 1987;20:239–243 [PubMed] [Google Scholar]
- 4.Olszewski WL, Loe K, Engeset A. Immune proteins and other biochemical constituents of peripheral lymph in patients with malignancy and postirradiation lymphedema. Lymphology 1992;25:166–171 [PubMed] [Google Scholar]
- 5.Olszewski WL, Jamal S, Łukomska B, Manokaran G, Grzelak I. Immune proteins in peripheral tissue fluid-lymph in patients with filarial lymphedema of the lower limbs. Lymphology 1992;25:166–171 [PubMed] [Google Scholar]
- 6.Olszewski WL. Collection and physiological measurements of peripheral lymph and interstitial fluid in man. Lymphology 1977;10:137–145 [PubMed] [Google Scholar]
- 7.Olszewski WL, Grzelak I, Ziółkowska A, Engeset A. Epidermal cell thymocyte activity factor/interleukin 1 (ETAF/IL)-like activity in lymph drained from normal human skin. Lymphology 1988;21:118–123 [PubMed] [Google Scholar]
- 8.Olszewski WL, Grzelak I, Ziolkowska A, Engeset A. Effect of local hyperthermia on lymph immune cells and lymphokines of normal human skin. J Surg Oncol 1989;11:109–116 [DOI] [PubMed] [Google Scholar]
- 9.Olszewski WL, Pazdur J, Kubasiewicz E, Zaleska M, Cooke CJ, Miller NE. Lymph draining from foot joints in rheumatoid arthritis provides insight into local cytokine and chemokine production and transport to lymph nodes. Arthritis Rheum 2001;44:541–549 [DOI] [PubMed] [Google Scholar]
- 10.Olszewski WL, Engeset A, Sokołowski J. Lymph flow and protein in the normal male leg during lying, getting up, and walking. Lymphology 1977;10:178–183 [PubMed] [Google Scholar]
- 11.Nanjee MN, Cooke CJ, Wong JS, Hamilton RL, Olszewski WL, Miller NE. Composition and ultrastructure of size subclasses of normal human peripheral lymph lipoproteins: quantification of cholesterol uptake by HDL in tissue fluids. J Lipid Res 2001;4:639–648 [PubMed] [Google Scholar]
- 12.Nanjee MN, Cooke CJ, Olszewski WL, Miller NE. Lipid and apolipoprotein concentrations in prenodal leg lymph of fasted humans. Associations with plasma concentrations in normal subjects, lipoprotein lipase deficiency, and LCAT deficiency. J Lipid Res 2000;41:1317–1327 [PubMed] [Google Scholar]
- 13.Blanton RA, Kupper TS, McDougall JK, Dower S. Regulation of interleukin 1 and its receptor in human keratinocytes. Proc Natl Acad Sci 1989;86:1273–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F. Principles of interleukin-6-type cytokine signalling and its regulation. Biochem J 2003;374:1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Utgaard JO, Jahnsen FL, Bakka A, Brandtzaeg P, Haraldsen G. Rapid secretion of prestored interleukin 8 from Weibel-Palade bodies of microvascular endothelial cells. J Exp Med 1998;188:1751–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaliński P, Hilkens CM, Snijders A, Snijdewint FG, Kapsenberg ML. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J Immunol 1997;159:28–35 [PubMed] [Google Scholar]
- 17.Steel JC, Waldmann TA, Morris JC. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol Sci 2012;33:35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bashir MM, Sharma MR, Werth VP. TNF-alpha production in the skin. Arch Dermatol Res 2009; 30: 87–91 [DOI] [PubMed] [Google Scholar]
- 19.Dinarello CA. The interleukin-1 family: 10 years of discovery. FASEB J 1994;8:1314–1325 [PubMed] [Google Scholar]
- 20.Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol 2004;22:929–979 [DOI] [PubMed] [Google Scholar]
- 21.Jiang CK, Magnaldo T, Ohtsuki M, Freedberg IM, Bernerd F, Blumenberg M. Epidermal growth factor and transforming growth factor alpha specifically induce the activation- and hyperproliferation-associated keratins 6 and 16. Proc Natl Acad Sci USA 1993;90:6786–6790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brennan AM, Mantzoros CS. Drug Insight: The role of leptin in human physiology and pathophysiology—Emerging clinical applications. Nat Clin Pract Endocrinol Metab 2006;2:318–327 [DOI] [PubMed] [Google Scholar]
- 23.Díez JJ, Iglesias P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur J Endocrinol 2003;148:293–300 [DOI] [PubMed] [Google Scholar]
- 24.Randolph GJ. Dendritic cell migration to lymph nodes: Cytokines, chemokines, and lipid mediators. Semin Immunol 2001;13:267–274 [DOI] [PubMed] [Google Scholar]
- 25.Morales J, Homey B, Vicari AP, Hudak S, Oldham E, Hedrick J, Orozco R, Copeland NG, Jenkins NA, McEvoy LM, Zlotnik A. CTACK, a skin-associated chemokine that preferentially attracts skin-homing memory T cells. Proc Natl Acad Sci USA 1999;96:14470–14475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maurer M, von Stebut E. Macrophage inflammatory protein-1. Int J Biochem Cell Biol 2004;36:1882–1886 [DOI] [PubMed] [Google Scholar]
- 27.Mancardi S, Vecile E, Dusetti N, Calvo E, Stanta G, Burrone OR, Dobrina A. Evidence of CXC, CC and C chemokine production by lymphatic endothelial cells. Immunology 2003;108:523–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin HY, Smith ML, Toy KJ, et al. VEGF-C mediates cyclic pressure-induced endothelial cell proliferation. Physiol Genomics 2002;11:245–251 [DOI] [PubMed] [Google Scholar]
- 29.Rotolo S, Ceccarelli S, Romano F, Frati L, Marchese C, Angeloni A. Silencing of keratinocyte growth factor receptor restores 5-fluorouracil and tamoxifen efficacy on responsive cancer cells. PLoS ONE 2008;3:e2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mann A, Breuhahn K, Schimacher P, Blessing M. Keratinocyte-derived granulocyte-macrophage colony stimulating factor accelerates wound healing: Stimulation of keratinocyte proliferation, granulation tissue formation, and vascularization. J Invest Dermatol 2001;117:1382–1390 [DOI] [PubMed] [Google Scholar]
- 31.Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol 2007;96:41–101 [DOI] [PubMed] [Google Scholar]
- 32.Lindeman JH, Abdul-Hussien H, van Bockel JH, Wolterbeek R, Kleemann R. Clinical trial of doxycycline for matrix metalloproteinase-9 inhibition in patients with an abdominal aneurysm: Doxycycline selectively depletes aortic wall neutrophils and cytotoxic T cells. Circulation 2009;119:2209–2216 [DOI] [PubMed] [Google Scholar]
- 33.Reichenstein M, Reich R, LeHoux JG, Hanukoglu I. ACTH induces TIMP-1 expression and inhibits collagenase in adrenal cortex cells. Mol Cell Endocrinol 2004;215:109–114 [DOI] [PubMed] [Google Scholar]
- 34.Zucker S, Drews M, Conner C, Foda HD, DeClerck YA, Langley KE, Bahou WF, Docherty AJ, Cao J. Tissue inhibitor of metalloproteinase-2 (TIMP-2) binds to the catalytic domain of the cell surface receptor, membrane type 1-matrix metalloproteinase 1 (MT1-MMP). J Biol Chem 1998;273:1216–1222 [DOI] [PubMed] [Google Scholar]
- 35.Broudy VC. Stem cell factor and hematopoiesis. Blood 1997;90:1345–1364 [PubMed] [Google Scholar]
- 36.Ansel KM, Harris RB, Cyster JG. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity”. Immunity 2002;6:67–76 [DOI] [PubMed] [Google Scholar]
- 37.Chaitanya GV, Franks SE, Cromer W, et al. Differential cytokine responses in human and mouse lymphatic endothelial cells to cytokines in Vitro. Lymphat Res Biol 2010;8:155–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fang C, Miguel MA, Avis I, Martinez A, Zudaire E, Cuttitta F. Non-peptide small molecule regulators of lymphangiogenesis. Lymphat Res Biol 2009;7:189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]