Abstract
The use of bioreactors for the in vitro culture of constructs for bone tissue engineering has become prevalent as these systems may improve the growth and differentiation of a cultured cell population. Here we utilize a tubular perfusion system (TPS) bioreactor for the in vitro culture of human mesenchymal stem cells (hMSCs) and implant the cultured constructs into rat femoral condyle defects. Using nanofibrous electrospun poly(lactic-co-glycolic acid)/poly(ɛ-caprolactone) scaffolds, hMSCs were cultured for 10 days in vitro in the TPS bioreactor with cellular and acellular scaffolds cultured statically for 10 days as a control. After 3 and 6 weeks of in vivo culture, explants were removed and subjected to histomorphometric analysis. Results indicated more rapid bone regeneration in defects implanted with bioreactor cultured scaffolds with a new bone area of 1.23±0.35 mm2 at 21 days compared to 0.99±0.43 mm2 and 0.50±0.29 mm2 in defects implanted with statically cultured scaffolds and acellular scaffolds, respectively. At the 21 day timepoint, statistical differences (p<0.05) were only observed between defects implanted with cell containing scaffolds and the acellular control. After 42 days, however, defects implanted with TPS cultured scaffolds had the greatest new bone area with 1.72±0.40 mm2. Defects implanted with statically cultured and acellular scaffolds had a new bone area of 1.26±0.43 mm2 and 1.19±0.33 mm2, respectively. The increase in bone growth observed in defects implanted with TPS cultured scaffolds was statistically significant (p<0.05) when compared to both the static and acellular groups at this timepoint. This study demonstrates the efficacy of the TPS bioreactor to improve bone tissue regeneration and highlights the benefits of utilizing perfusion bioreactor systems to culture MSCs for bone tissue engineering.
Introduction
Bioreactor systems are frequently used in the in vitro culture of stem cells for bone regeneration as these systems have been shown to be an important cell culture tool.1–6 In addition to increasing proliferation through increased perfusion of nutrients to cells,7 bioreactors expose cells to fluid shear stress which can induce upregulation of several key osteogenic signaling pathways.5,8 Despite these benefits in vitro, in vivo studies analyzing perfusion bioreactor systems are relatively few in number. In subcutaneous models bioreactor cultured human bone marrow stromal cells were shown to generate bone like tissue in mice9 and increase ectopic bone formation after being cultured on a hybrid hydrogel and collagen sponge scaffold.10 While in a bone defect model implanted with perfusion cultured bone marrow stromal cells, no significant differences in bone formation were observed between bioreactor and static cultured scaffolds.11 A more recent study was unable to demonstrate any benefit of perfusion bioreactor culture of human trabecular cells in repair of a rat mandible.12 However, in another study, perfusion bioreactor cultured constructs seeded with bone marrow stromal cells enhanced bone healing in a goat segmental tibia defect as compared to static controls.13 Perfusion bioreactor systems previously have been demonstrated to have beneficial effects on in vitro osteogenesis.14–18 To accurately assess bioreactor systems with demonstrated effectiveness in vitro, including increased proliferation and enhanced differentiation, these systems should be evaluated for the ability to enhance in vivo bone regeneration as well.
In this study, we utilize a tubular perfusion system (TPS) bioreactor in which individual scaffolds are tightly packed in a tubular growth chamber and media perfused through the growth chamber using a pump. This study aims to evaluate the effect that statically and TPS cultured human mesenchymal stem cells (hMSCs) have on bone regeneration in a rat femoral condyle defect. In this study, a synthetic electrospun poly(lactic-co-glycolic acid) (PLGA)/poly(ɛ-caprolactone) (PCL) scaffold is used. Electrospun scaffolds mimic the native ECM environment19 and the PLGA/PCL scaffolds used in this study are fabricated using a new wet-electrospinning method in which cylindrical scaffolds are formed through a loose accumulation of fibers allowing for cellular infiltration.20 While electrospun scaffolds fabricated via traditional means may often have fibers packed too tightly to allow for cellular infiltration,21 the loose accumulation of fibers produced by this new method allow for infiltration of cells throughout the scaffold both in vivo and in vitro.20 Culturing these scaffolds in the TPS bioreactor should provide for ample cellular infiltration in vitro and in vivo integration with the host tissue.
TPS culture has previously been shown to enhance the proliferation and differentiation of hMSCs;22 however, scaffolds cultured in this system have not been previously evaluated in vivo. Thus, the objective of the study is as follows: to elucidate the effect of dynamic culture on bone regeneration through implantation of nanofibrous scaffolds cultured statically or in the TPS bioreactor into rat femoral condyle defects.
Materials and Methods
hMSC culture
hMSCs (Lonza) were expanded before implantation in control media consisting of DMEM (Gibco, Life Technologies) supplemented with 10% fetal bovine serum (Gibco), 1.0% v/v penicillin/streptomycin (Gibco), 0.1 mM nonessential amino acids (Gibco), and 4 mM L-glutamine (Gibco) using protocols set forth by the manufacturer and previously described.22,23 hMSCs were expanded in tissue culture polystyrene flasks with media changes every 3 days according to the manufacturer's specifications. Cells were cultured in an incubator at 37°C and 5% CO2 and passaged upon reaching 70–80% confluency using trypsin/EDTA (Gibco). hMSCs (P4) were then seeded on scaffolds and cultured in osteogenic media formulated as described in the literature by supplementing control media with 100 nM dexamethasone (Sigma-Aldrich), 10 mM β-glycerophosphate (Sigma-Aldrich), and 173 μM ascorbic acid (Sigma-Aldrich).22,23
PLGA/PCL scaffold fabrication
The polymers used for electrospinning, PLGA (Purasorb® PDLG 5010, inherent viscosity: 1.0 dL/g) and PCL (from LACTEL® Absorbable Polymers, inherent viscosity range: 1.0–1.3 dL/g) were purchased from Purac Biomaterials BV and Durect Corporation, respectively. The electrospinning solution was prepared by dissolving PLGA/PCL (3:1 weight ratio) in trifluoroethanol/hexafluoroisopropanol (9:1 volume ratio) at a concentration of 20% w/v. The experimental 3D scaffolds were fabricated using a modified electrospinning technique. Briefly, the electrospinning process was implemented by Esprayer ES-2000S (Fuence Co., Ltd.). The prepared solution was loaded into a syringe and fed into the nozzle at the tip of the syringe with a speed of 25 μL/min. A high voltage of 15 kV was applied at the nozzle to generate a stable polymer jet. A grounded bath filled with 100% ethanol was positioned 14 cm under the nozzle for fiber deposition. As ethanol is a wetting agent for both PLGA and PCL, the resulted PLGA/PCL fibers formed a loosen cotton ball shape in the ethanol bath. Every 60 s the fibers were collected and inserted into a Teflon mold 3 mm in diameter and 3 mm in depth. Subsequently, they were washed thoroughly in MilliQ water and freeze-dried for 3 days. Before the cell culture and animal experiment, all scaffolds were sterilized by γ-irradiation (Isotron). Scanning electron microscope images of the sample were then taken (Jeol SEM6340F) after the sample was sputter coated with gold–platinum.
hMSC seeding on PLGA/PCL scaffolds
hMSCs were removed from tissue culture flasks and resuspended in media at a concentration of 1.25×106 cells/mL. Concentrated cell solution was added to the scaffold resulting in the addition of 250,000 cells/scaffold. Scaffolds were briefly exposed to vacuum to draw cell solution into the scaffolds and rotated for 3 h in a cell culture incubator. Scaffolds were then moved to a 24-well plate. Unseeded cells were captured via centrifugation and reseeded on scaffolds. After additional 2 h incubation, control media was added to scaffolds. Cell containing scaffolds were incubated overnight and moved to 24-well plates or TPS bioreactor growth chambers. All groups were cultured in osteogenic media which was changed every 3 days and cultured in vitro for a total of 10 days before implantation. Scaffolds for no cell groups were treated following a similar manner and placed in 24-well plates in osteogenic media without the seeding of a cell population. This yielded three groups: PLGA/PCL scaffolds with hMSCs cultured in the bioreactor, PLGA/PCL scaffolds with hMSCs cultured statically, and PLGA/PCL scaffolds without cells cultured statically.
Bioreactor design
Dynamic culture was completed in the TPS bioreactor as described previously in the literature.22,24–26 Briefly a tubing circuit comprised primarily of platinum-cured silicone tubing (Cole Parmer) and PharMed BPT tubing (Cole Parmer) for the section that passes through the pump connected a growth chamber to a media reservoir (Fig. 1). The entire tubing circuit was sterilized via autoclave. The growth chamber was composed of platinum-cured silicone tubing (ID of 1/4′′) and contained the electrospun scaffolds. Media was pumped through the recirculating system using a peristaltic pump (Cole Parmer) at 1.0 mL/min. The entire system was placed in an incubator at 37°C for the duration of the study. Forty milliliters of osteogenic media was loaded into separate 125 mL Erlenmeyer flasks reservoirs for each growth chamber topped with rubber stoppers. Media was withdrawn and replaced from the reservoir through two tubes that penetrate the stopper and changed every 3 days.
FIG. 1.
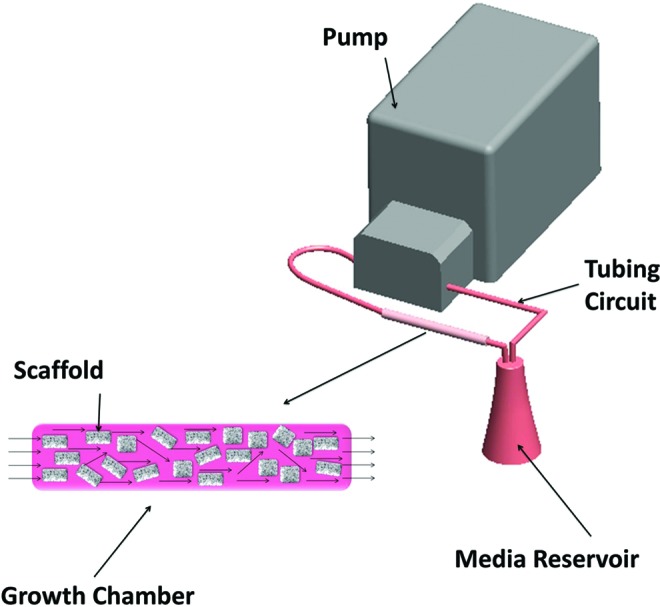
Schematic describing the TPS bioreactor. Media is delivered from the media reservoir by the peristaltic pump through the growth chamber. Note enlargement of growth chamber with PCL/PLGA scaffolds and media flow throughout the chamber illustrated. TPS, tubular perfusion system; PCL/PLGA, poly(ɛ-caprolactone)/poly(lactic-co-glycolic acid) Color images available online at www.liebertpub.com/tea
Surgical procedure for femoral condyle defect
Sixteen 8-week-old nude rats (Charles River Labs) were used in the study. The animal experiment was approved by the animal ethics committee of The Radboud University Nijmegen Medical Center. All surgeries were performed under general inhalation anesthesia (Isoflurane®) and sterile conditions. Preoperative, Rimadyl® (5.0 mg/kg) and Temgesic® (1.0 mg/kg) were administered to reduce postoperative pain. Subsequently, each animal was immobilized supine with the knee joint in a maximally flexed position and the hind limbs were shaved, washed, and disinfected with 10% povidone-iodine.
A mid-line longitudinal para-patellar incision was made in the left and right hind limb. The knee joint capsule was incised longitudinally, and by lifting the patellar ligament gently and moving it laterally, the knee joint became fully exposed. This maneuver was facilitated by a slight extension of the knee. At the femoral inter-condylar notch, a cylindrical hole defect (2.5 mm in diameter and 3 mm in depth) was prepared parallel to the long axis of the femur, using a dental bur (Elcomed 100;W&H Dentalwerk Burmoos) with low rotational drill speeds (1200 rpm) and continuous external cooling with saline. Scaffolds were placed bilaterally into the predrilled bony defects, resulting in two implants per rat (Table 1). Scaffolds were rinsed in saline and one scaffold was press fit into each defect. After insertion of the implants, the soft tissue layers were closed with resorbable sutures (Vicryl® 4.0; Ethicon Products) and the skin with Vicryl 3.0 (Ethicon Products). To reduce postoperative pain, Temgesic (0.02 mg/kg) was administered subcutaneously for 2 days postoperatively. After 3 and 6 weeks, rats were sacrificed using carbon dioxide and condyles were retrieved and fixed in 10% neutral buffered formalin solution.
Table 1.
Surgical Implant Descriptions
| Subject number | Implanted scaffold |
|---|---|
| 1, 2, 3a, 4, 5, 6a |
PLGA/PCL bioreactor |
| 7, 8, 9, 10, 11b, 12b |
PLGA/PCL static |
| 11c, 12c, 13, 14, 15, 16 | PLGA/PCL no cell |
Note, that unless otherwise stated, each animal is implanted with the same scaffold group in each condyle. For all groups, five implants were analyzed on days 21 and 42.
No scaffold implanted in left condyle
Left condyle implanted with PLGA/PCL no cell
Right condyle implanted with PLGA/PCL static
PLGA, poly(lactic-co-glycolic acid); PCL, poly(ɛ-caprolactone).
Histomorphometric analysis
Samples were decalcified in an EDTA solution, dehydrated using increasing ethanol steps, and embedded in paraffin. Five-micrometer sections were sectioned transversely using a microtome (RM 2165; Leica). Sections were mounted on glass slides and stained with hematoxylin and eosin (H&E) and elastic van Gieson (EVG) stains using standard protocols. Images of stained sections were acquired by optical light microscopy (Zeiss Axio vert or Axio Imager Z1; Zeiss). H&E sections were utilized in the histomorphometric analysis. Images of the entire defect region were analyzed quantitatively by defining a region of interest (ROI) to the size (2.5 mm diameter) of the original defect using Adobe Photoshop (Fig. 2). The ROI was identified based on visual analysis of the image. ROI images were then evaluated using an image analysis system (Leica Qwin; Leica). Bone area was identified via stain color, selected and quantified using Qwin.
FIG. 2.
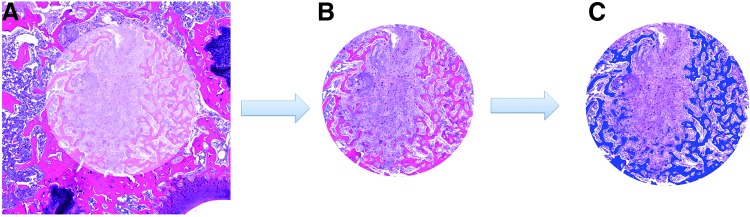
Schematic illustrating method to measure bone area. A region of interest was defined to the size of the original 2.5 mm diameter defect (A). New bone within the original defect was then identified using color (B). Identified new bone was selected using Qwin and the pixel area calculated as a percent of the total defect area (C).
Statistical analysis
Five replicate defects were created for each group at both timepoints. Three separate sections were stained and imaged from each of the defects. Images were taken of each of these sections and analyzed, yielding 15 samples for each group per timepoint. Data were analyzed using single factor ANOVA followed by Tukey's multiple comparison test assuming normal data distribution with a confidence of 95% (p<0.05). Mean values and standard deviation error bars are reported in each figure, as well as relevant statistical relationships.
Results
Scaffold properties
PLGA/PCL scaffolds were fabricated using electrospinning to produce a cylindrical design that could be press fitted into the defect (Fig. 3a). Scaffolds were fabricated to be cylinders 3 mm in diameter and 3 mm in length. Scanning electron microscope images of PLGA/PCL scaffolds indicated scaffolds had a fiber diameter of approximately 2 μm (Fig. 3b). Scaffolds maintained size and shape after 10 days of culture and could be easily manipulated using forceps (Fig. 3c).
FIG. 3.

Image of cylindrical PCL/PLGA scaffold (a). SEM image showing fibers of PCL/PLGA scaffold (b). This scaffold can then be press fit in the defect (c). SEM, scanning electron microscope.
Bioreactor culture and surgery
PLGA/PCL scaffolds were cultured in the TPS bioreactor with no leaks or contamination and were easily loaded into the tubular growth chamber. A defect 2.5 mm wide and 3.0 mm deep was successfully created using a frontal approach as not to penetrate the medullary space. Gross wound inspection at day 21 showed no noticeable inflammation at the exterior of the defect site.
Histological images of bone regeneration
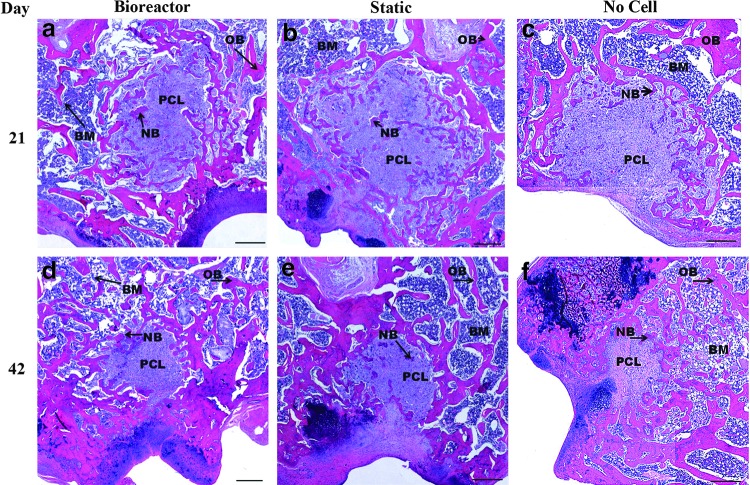
Light microscopy analysis using H&E histological images indicated some regeneration of the defect after 21 days of culture (Fig. 4). Increased bone regeneration was observed in cell containing scaffolds with bone regeneration occurring both at the edge and center of scaffolds (Fig. 4a, b). Defects with bioreactor cultured scaffolds appear to have a greater amount of bone infiltration and bone area within the defect site compared to other groups. Scaffolds containing no hMSC population exhibited minimal bone regeneration largely originating from the original bone on the edge of the defect with no bone islands observed in the center of the defect (Fig. 4c). By day 42 scaffold area was reduced through scaffold degradation and largely replaced with newly formed bone (Fig. 4d–f). The defect has not yet completely healed and some remaining scaffold is readily apparent in all groups. Bioreactor cultured scaffolds appeared to have less remaining scaffold area and greater new bone area than the two control groups.
FIG. 4.
10×objective images of H&E stained defect implanted with PLGA/PCL scaffolds after 3 weeks (a–c) and 6 weeks (d–f ) of in vivo implantation. Before implantation scaffolds were cultured in vitro in the TPS bioreactor with an hMSC population (a, d), in static culture with an hMSC population (b, e), or in static culture with no cell population (c, f ). Scale bar represents 500 μm. PCL, PLGA/PCL scaffold; NB, new bone; OB, original bone; BM, bone marrow; H&E, hematoxylin and eosin; hMSC, human mesenchymal stem cell.
Upon closer inspection of the defect, there appeared to be minimal tissue response to PLGA/PCL scaffolds as there was not a significant presence of inflammatory cells (Fig. 5). Mineralized bone can be observed within the defect site as labeled on the images and blood vessels can be observed throughout the scaffold. EVG staining was completed to verify H&E identification of bone and further evaluate blood vessel infiltration (Fig. 6). Results confirmed H&E based identification of bone formation and further permitted observation of blood vessel formation. By day 21 blood vessels can be readily observed penetrating the scaffold in both the bioreactor and static cell containing groups, while less blood vessel infiltration was observed in the day 21 no cell group. Bone islands were observed within the scaffolds of these groups with more bone islands appearing on day 42. Bone growth in the no cell group was restricted to the edge of the scaffolds at both timepoints, though increased blood vessel infiltration was evident by day 42 in this group.
FIG. 5.
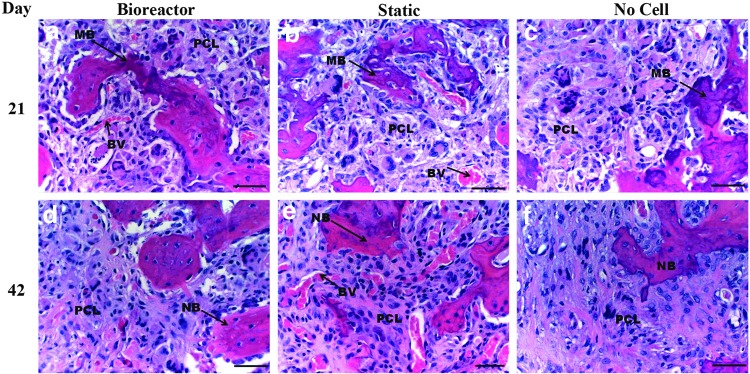
40×objective images of H&E stained defect implanted with PLGA/PCL scaffolds after 3 weeks (a–c) and 6 weeks (d–f ) of in vivo implantation. Before implantation scaffolds were cultured in vitro in the TPS bioreactor with an hMSC population (a, d), in static culture with an hMSC population (b, e), or in static culture with no cell population (c, f ). In images (a, b) note mineralization formation and blood vessel infiltration within PLGA/PCL scaffold. In image (c) note mineralized bone formation around the edge of the scaffold. Bone in growth continues to penetrate scaffold after 42 days (d–f ). Note minimal tissue response to material. Scale bar represents 50 μm. PCL, PLGA/PCL scaffold; NB, new bone; MB, mineralized bone; BV, blood vessel.
FIG. 6.
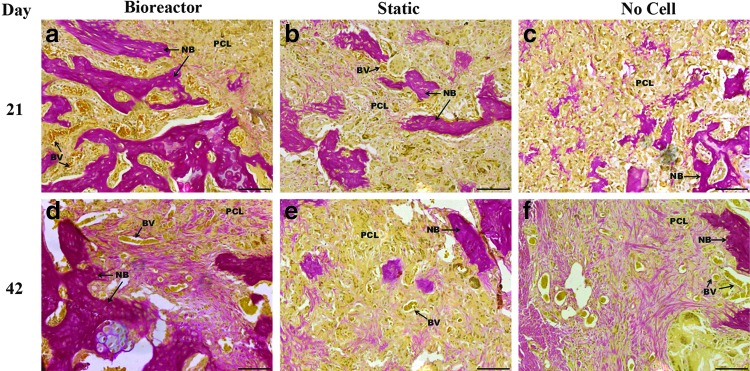
20×objective images of elastic van Gieson stained defect implanted with PLGA/PCL scaffolds after 3 weeks (a–c) and 6 weeks (d–f ) of in vivo implantation. Before implantation scaffolds were cultured in vitro in the TPS bioreactor with an hMSC population (a, d), in static culture with an hMSC population (b, e), or in static culture with no cell population (c, f ). Blood vessels can be observed frequently with scaffolds with the exception of the day 21 no cell group. Scale bar represents 100 μm. PCL, PLGA/PCL scaffold; NB, new bone; BV, blood vessel.
Histomorphometric analysis of defect site
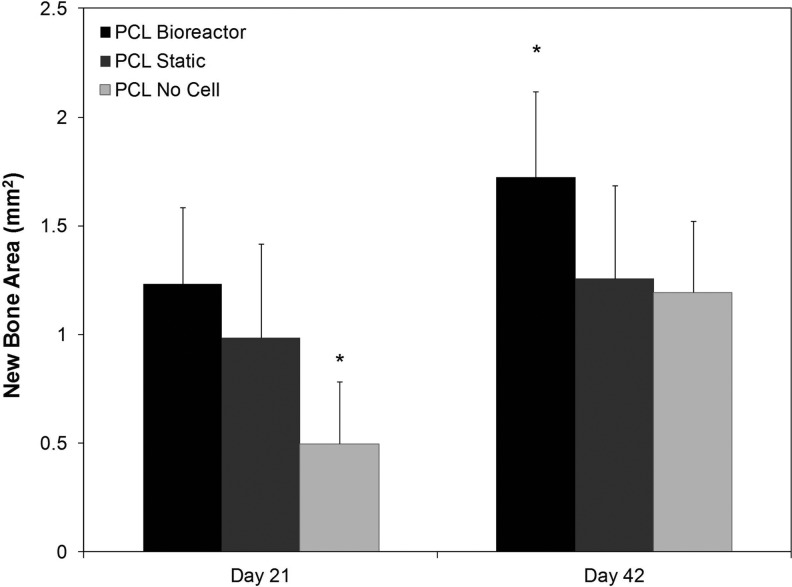
Histomorphometric analysis (detailed in Fig. 2) of new bone growth area within the PLGA/PCL implanted defects indicated that on day 21 new bone area was significantly (p<0.05) higher in defects implanted with hMSC containing scaffolds than those implanted with acellular scaffolds (Fig. 7). Defects implanted with TPS bioreactor cultured constructs had the highest overall new bone area with 1.23±0.35 mm2 at 21 days, but statistical testing revealed that the difference between these and statically cultured hMSC containing scaffolds (0.99±0.43 mm2 of bone area in the original defect site) was not significant (p>0.05). Both cell containing groups had a statistically significant (p<0.05) increase in new bone area as compared to defects implanted with a scaffold that did not contain cells. New bone area in this group was 0.50±0.28 mm2. Total defect area was 4.9 mm2, but the native bone in this region is trabecular and bone marrow was not quantified; thus, a fully healed defect would have a bone area less than 4.9 mm2. By day 42 all groups had an increased amount of bone area as compared to day 21 scaffolds. Defects implanted with bioreactor cultured PLGA/PCL scaffolds again had the highest amount of bone area in the original defect site with 1.72±0.40 mm2 of new bone area as compared to day 42 static and no cell groups. This was significantly higher than defects implanted with scaffolds that did not contain hMSCs, which had a new bone area of 1.19±0.33 mm2. Defects implanted with statically cultured hMSC containing scaffolds had a new bone area of 1.26±0.43 mm2, which was statistically lower than defects implanted with bioreactor cultured MSCs. By day 42, no statistical difference remained between defects implanted with acellular scaffold and those implanted with statically cultured cells.
FIG. 7.
New bone area occurring in original defect area after implantation of PLGA/PCL scaffold as calculated by histomorphometric analysis. Note increased amounts of bone formation in cell containing groups on day 21 and the highest amount of bone formation in TPS cultured group on day 42. The symbol (*) indicates statistical significance from all other groups within a timepoint (p<0.05).
Discussion
Perfusion bioreactor systems have frequently been used to improve in vitro culture of MSCs for bone tissue engineering purposes.1,2,4,6,27,28 Constructs cultured in perfusion bioreactors have frequently been demonstrated to produce bone subcutaneously9,10,29 and evidence of perfusion cultured constructs enhancing bone repair exists;13 however, continued research is needed to fully demonstrate this point. Here we evaluate a perfusion system that has been previously shown to increase proliferation and enhance differentiation of hMSCs in vitro as compared to static cultured controls.22,25 The femoral condyle defect utilized in this study was chosen as a nonload bearing small animal model defect for cylindrical scaffolds. As the focus of the study was to compare dynamic and static cultured scaffolds, empty defects were not analyzed; however, a similar defect has been shown to not heal naturally after 6 weeks.30,31
The nanofibrous scaffold used in the study appeared to foster tissue integration and vascular invasion from the host as demonstrated by the presence of blood vessels within the implanted scaffold. In cell containing scaffolds greater amounts of bone formation after 3 weeks in vivo were observed as compared to scaffolds without a cell population. This bone formation was highest in bioreactor cultured PLGA/PCL scaffolds and statistically different bone area was observed between defects implanted with scaffolds without a cell population and those with, but not between dynamic and statically cultured cells. In cell containing groups, new bone could be observed forming in the interior of PLGA/PCL scaffolds, while in acellular scaffolds bone formation was primarily restricted to the exterior portions of the scaffold. This indicates either increased bone formation directly from the implanted hMSC population, or an ability of this cell population to recruit native cells to the defect area. Mineralization can be observed in these scaffolds possibly indicating formation of bone through an intramembranous ossification process.
Bone healing was accelerated in condyles implanted with bioreactor cultured scaffolds as day 21 bone area was higher in the bioreactor group compared to the day 42 no cell group and only slightly lower than the day 42 static cultured group. By day 42 bioreactor cultured PLGA/PCL scaffolds maintained the highest levels of new bone formation with a significant increase over the other two groups. Bioreactor cultured scaffolds may have contained a larger and more evenly distributed cell population; thus, leading to the increased amounts of bone regeneration in that group. The group implanted with statically cultured cells had statistically similar new bone area as acellular scaffolds, likely resulting as endogenous bone healing begins to more significantly repair the acellular group. The implanted scaffolds are visually reduced in area indicating significant scaffold degradation after 42 days. Prior studies on degradation indicate the PLGA component to be nearly completely degraded by 56 days and the PCL component to retain only 20% of its original molecular weight.32 The PLGA component does produce acidic byproducts during the degradation process,32 but a significant inflammatory response as indicated by the presence of macrophages and multinuclear cells was not observed at either timepoint.
Another aspect that should be considered in this study is the duration of in vitro culture time. Shorter in vitro culture times would require less time and money to culture cells and have been demonstrated to improve MSC in vivo bone forming potential.33 The influence of in vitro culture time on in vivo bone formation is frequently investigated as MSCs cultured for 4 weeks in osteogenic media were unable to form bone in vivo. Conversely, these same cells were able to form bone via the endochondral ossification pathway after 4 weeks of chondrogenic differentiation.34 Therefore, it may be beneficial to determine if varying bioreactor in vitro culture time could result in an improvement of bone healing via increased release of osteogenic signaling molecules from the implanted cell population. Here 10 days is chosen as a timepoint where the cell population has committed to an osteoblastic lineage but not fully differentiated into osteoblasts.22
Bone regeneration using in vitro cultured MSCs has been a topic of recent research as demonstrated by a study in which regeneration of critical sized sheep long bone defects was achieved after implantation of a scaffold containing MSCs.35 While this study demonstrated a successful use of MSCs to aid in the healing of bone defects, no bioreactor was used. However, using PLGA scaffolds containing MSCs cultured in a bioreactor, improved bone healing was observed in rat calvarial defects.36 This study did not directly compare bioreactor cultured scaffolds to statically cultured scaffolds as the current study is one of only a few studies completed to directly compare bioreactor and statically cultured scaffolds. However, in one such study comparing scaffolds cultured in a bioreactor system and those cultured statically on the ability to repair a segmental goat tibia defect, perfusion cultured constructs outperformed static constructs as demonstrated by micro-CT to quantify bone repair.13 The results of this work are supported by the findings in that study and demonstrate perfusion culture of MSC containing scaffolds to enhance in vivo osteogenesis. As a possible mechanism for this enhanced osteogenesis, a recent study demonstrated scaffold induced BMP/Smad signaling upregulation promoted bone regeneration by MSCs in a bone defect model.37 In previous studies involving both bulk22,25 and macroporous24 scaffolds, TPS culture has been shown to upregulate BMP expression in hMSCs. This upregulation could then lead to enhanced osteogenesis of the implanted cell population and release of secreted factors to enhance bone formation by the host.
When developing a successful cell based bone tissue engineering strategy, it is vital to study the extent to which an implanted cell population contributes to bone regeneration after culture in different in vitro conditions. In this study, the increased bone regeneration in cell containing scaffolds, especially those cultured in a bioreactor compared to acellular scaffolds clearly demonstrates the implanted cell population enhances bone regeneration; however, the mechanisms by which this enhancement occurs are not fully elucidated. Though dynamic culture has been shown to improve differentiation and proliferation in vitro, the degree to which in vitro results translate to in vivo bone regeneration is dependent on many factors.29 Scaffold material,29,38 culture conditions,10,29 and culture time11 have all been demonstrated to have an effect on in vivo bone formation after bioreactor culture; thus, these studies should be completed with greater frequency to aid in understanding the optimal culture conditions of these complex systems. In this study we observed elevated levels of new bone formation after 21 days of implantation of TPS bioreactor cultured scaffolds leading to significantly higher bone regeneration in this group at 42 days compared to controls. This demonstrates the efficacy of using a TPS bioreactor for the culture of hMSCs to aid in vivo bone regeneration and repair. Continued research in this area could lead to a greater understanding of how the in vitro culture environment translates to bone regeneration in a defect model.
Acknowledgments
The authors would like to thank Ms. Natasja van Dijk, Mr. René van Rheden, and Ms. Coby van Run-van Breda for their assistance with the histological procedures. This research was supported by the National Institutes of Health (R01 AR061460).
Disclosure Statement
No competing financial interests exist.
References
- 1.McCoy R.J., and O'Brien F.J.Influence of shear stress in perfusion bioreactor cultures for the development of three-dimensional bone tissue constructs: a review. Tissue Eng Part B Rev 16,587, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Yeatts A.B., and Fisher J.P.Bone tissue engineering bioreactors: dynamic culture and the influence of shear stress. Bone 48,171, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Rodrigues C.A., Fernandes T.G., Diogo M.M., da Silva C.L., and Cabral J.M.Stem cell cultivation in bioreactors. Biotechnol Adv 29,815, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Salter E., Goh B., Hung B., Hutton D., Ghone N., and Grayson W.L.Bone tissue engineering bioreactors: a role in the clinic? Tissue Eng Part B Rev 18,62, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Yeatts A.B., Choquette D.T., and Fisher J.P.Bioreactors to influence stem cell fate: augmentation of mesenchymal stem cell signaling pathways via dynamic culture systems. Biochim Biophys Acta 1830,2470, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rauh J., Milan F., Gunther K.P., and Stiehler M.Bioreactor systems for bone tissue engineering. Tissue Eng Part B Rev 17,263, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Volkmer E., Drosse I., Otto S., Stangelmayer A., Stengele M., Kallukalam B.C., et al. Hypoxia in static and dynamic 3D culture systems for tissue engineering of bone. Tissue Eng Part A 14,1331, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Kim J., and Ma T.Perfusion regulation of hMSC microenvironment and osteogenic differentiation in 3D scaffold. Biotechnol Bioeng 109,252, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Janssen F.W., van Dijkhuizen-Radersma R., Van Oorschot A., Oostra J., de Bruijn J.D., and Van Blitterswijk C.A.Human tissue-engineered bone produced in clinically relevant amounts using a semi-automated perfusion bioreactor system: a preliminary study. J Tissue Eng Regen Med 4,12, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Hosseinkhani H., Hosseinkhani M., Tian F., Kobayashi H., and Tabata Y.Ectopic bone formation in collagen sponge self-assembled peptide-amphiphile nanofibers hybrid scaffold in a perfusion culture bioreactor. Biomaterials 27,5089, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Sikavitsas V.I., van den Dolder J., Bancroft G.N., Jansen J.A., and Mikos A.G.Influence of the in vitro culture period on the in vivo performance of cell/titanium bone tissue-engineered constructs using a rat cranial critical size defect model. J Biomed Mater Res A 67A,944, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Schliephake H., Zghoul N., Jager V., van Griensven M., Zeichen J., Gelinsky M., et al. Bone formation in trabecular bone cell seeded scaffolds used for reconstruction of the rat mandible. Int J Oral Maxillofac Surg 38,166, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Wang C., Wang Z., Li A., Bai F., Lu J., Xu S., et al. Repair of segmental bone-defect of goat's tibia using a dynamic perfusion culture tissue engineering bone. J Biomed Mater Res A 92,1145, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Grayson W.L., Frohlich M., Yeager K., Bhumiratana S., Chan M.E., Cannizzaro C., et al. Engineering anatomically shaped human bone grafts. Proc Natl Acad Sci U S A 107,3299, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holtorf H.L., Jansen J.A., and Mikos A.G.Flow perfusion culture induces the osteoblastic differentiation of marrow stromal cell-scaffold constructs in the absence of dexamethasone. J Biomed Mater Res A 72A,326, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Janssen F.W., Oostra J., van Oorschot A., and van Blitterswijk C.A.A perfusion bioreactor system capable of producing clinically relevant volumes of tissue-engineered bone: in vivo bone formation showing proof of concept. Biomaterials 27,315, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Sikavitsas V.I., Bancroft G.N., Lemoine J.J., Liebschner M.A.K., Dauner M., and Mikos A.G.Flow perfusion enhances the calcified matrix deposition of marrow stromal cells in biodegradable nonwoven fiber mesh scaffolds. Ann Biomed Eng 33,63, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Zhao F., Chella R., and Ma T.Effects of shear stress on 3-D human mesenchymal stem cell construct development in a perfusion bioreactor system: experiments and hydrodynamic modeling. Biotechnol Bioeng 96,584, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Szpalski C., Wetterau M., Barr J., and Warren S.M.Bone tissue engineering: current strategies and techniques—part I: Scaffolds. Tissue Eng Part B Rev 18,246, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Yang W., Yang F., Wang Y., Both S.K., and Jansen J.A.In vivo bone generation via the endochondral pathway on three-dimensional electrospun fibers. Acta Biomater 9,4505, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Sill T.J., and von Recum H.A.Electrospinning: applications in drug delivery and tissue engineering. Biomaterials 29,1989, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Yeatts A.B., and Fisher J.P.Tubular perfusion system for the long-term dynamic culture of human mesenchymal stem cells. Tissue Eng Part C Methods 17,337, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Guo L., Kawazoe N., Hoshiba T., Tateishi T., Chen G., and Zhang X.Osteogenic differentiation of human mesenchymal stem cells on chargeable polymer-modified surfaces. J Biomed Mater Res A 87,903, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Pisanti P., Yeatts A.B., Cardea S., Fisher J.P., and Reverchon E.Tubular perfusion system culture of human mesenchymal stem cells on poly-L-lactic acid scaffolds produced using a supercritical carbon dioxide-assisted process. J Biomed Mater Res A 100A,2563, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeatts A.B., Geibel E.M., Fears F.F., and Fisher J.P.Human mesenchymal stem cell position within scaffolds influences cell fate during dynamic culture. Biotechnol Bioeng 109,2381, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeatts A.B., Gordon C.N., and Fisher J.P.Formation of an aggregated alginate construct in a tubular perfusion system. Tissue Eng Part C Methods 17,1171, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Carpentier B., Layrolle P., and Legallais C.Bioreactors for bone tissue engineering. Int J Artif Organs 34,259, 2010 [DOI] [PubMed] [Google Scholar]
- 28.El Haj A.J., and Cartmell S.H.Bioreactors for bone tissue engineering. Proc Inst Mech Eng H 224,1523, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Schliephake H., Zghoul N., Jager V., van Griensven M., Zeichen J., Gelinsky M., et al. Effect of seeding technique and scaffold material on bone formation in tissue-engineered constructs. J Biomed Mater Res A 90,429, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Gan J.H.Y.Characterisation of bone defect models in immunodeficient animals [Ph.D. Thesis]. Department of Biomedical Engineering, The University of New South Wales, Sydney, 2005 [Google Scholar]
- 31.Torricelli P., Fini M., Giavaresi G., Rimondini L., and Giardino R.Characterization of bone defect repair in young and aged rat femur induced by xenogenic demineralized bone matrix. J Periodontol 73,1003, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Ji W., Yang F., Seyednejad H., Chen Z., Hennink W.E., Anderson J.M., et al. Biocompatibility and degradation characteristics of PLGA-based electrospun nanofibrous scaffolds with nanoapatite incorporation. Biomaterials 33,6604, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Castano-Izquierdo H., Alvarez-Barreto J., van den Dolder J., Jansen J.A., Mikos A.G., and Sikavitsas V.I.Pre-culture period of mesenchymal stem cells in osteogenic media influences their in vivo bone forming potential. J Biomed Mater Res A 82A,129, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Farrell E., Both S.K., Odorfer K.I., Koevoet W., Kops N., O'Brien F.J., et al. In-vivo generation of bone via endochondral ossification by in-vitro chondrogenic priming of adult human and rat mesenchymal stem cells. BMC Musculoskelet Disord 12,31, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manassero M., Viateau V., Deschepper M., Oudina K., Logeart-Avramoglou D., Petite H., et al. Bone regeneration in sheep using acropora coral, a natural resorbable scaffold, and autologous mesenchymal stem cells. Tissue Eng Part A 19,2013 [DOI] [PubMed] [Google Scholar]
- 36.Zong C., Xue D., Yuan W., Wang W., Shen D., Tong X., et al. Reconstruction of rat calvarial defects with human mesenchymal stem cells and osteoblast-like cells in poly-lactic-co-glycolic acid scaffolds. Eur Cells Mater 20,2010 [DOI] [PubMed] [Google Scholar]
- 37.Liu H., Peng H., Wu Y., Zhang C., Cai Y., Xu G., et al. The promotion of bone regeneration by nanofibrous hydroxyapatite/chitosan scaffolds by effects on integrin-BMP/Smad signaling pathway in BMSCs. Biomaterials 34,2013 [DOI] [PubMed] [Google Scholar]
- 38.Rai B., Lin J.L., Lim Z.X.H., Guldberg R.E., Hutmacher D.W., and Cool S.M.Differences between in vitro viability and differentiation and in vivo bone-forming efficacy of human mesenchymal stem cells cultured on PCL-TCP scaffolds. Biomaterials 31,7960, 2010 [DOI] [PubMed] [Google Scholar]