Abstract
In articular cartilage repair, cells that will be responsible for the formation of repair tissue are often exposed to an osteochondral environment. To study cartilage repair mechanisms in vitro, we have recently developed a bovine osteochondral biopsy culture model in which cartilage defects can be simulated reproducibly. Using this model, we now aimed at studying the chondrogenic potential of human bone marrow-derived mesenchymal stem cells (hBMSCs) in an osteochondral environment. In contrast to standard in vitro chondrogenesis, it was found that supplementing transforming growth factor beta (TGFβ) to culture medium was not required to induce chondrogenesis of hBMSCs in an osteochondral environment. hBMSC culture in defects created in osteochondral biopsies or in bone-only biopsies resulted in comparable levels of cartilage-related gene expression, whereas culture in cartilage-only biopsies did not induce chondrogenesis. Subcutaneous implantation in nude mice of osteochondral biopsies containing hBMSCs in osteochondral defects resulted in the formation of more cartilaginous tissue than hBMSCs in chondral defects. The subchondral bone secreted TGFβ; however, the observed results could not be attributed to TGFβ, as either capturing TGFβ with an antibody or blocking the canonical TGFβ signaling pathway did not result in significant changes in cartilage-related gene expression of hBMSCs in the osteochondral culture model. Inhibition of BMP signaling did not prevent chondrogenesis. In conclusion, we demonstrate that chondrogenesis of hBMSCs is induced by factors secreted from the bone. We have strong indications that this is not solely mediated by members of the TGFβ family but other, yet unknown, factors originating from the subchondral bone appeared to play a key role.
Introduction
Human bone marrow-derived mesenchymal stem cells (hBMSCs) are widely used for tissue-engineering approaches because of their multi-lineage differentiation potential and expandability in vitro.1,2 For cartilage tissue engineering, hBMSCs can provide a more favorable cell source than articular chondrocytes, as the availability and in vitro expandability of chondrocytes are limited.3,4 In cartilage repair strategies based on either autologous chondrocytes or hBMSCs, the repair tissue formed is often of a fibro-cartilaginous nature, having inferior mechanical properties than native articular cartilage.5–7 Therefore, tissue-engineering strategies could provide a more successful solution for the regeneration of damaged cartilage.8 To eventually achieve this, more insight is required in the complex mechanisms involved in the chondrogenesis of hBMSCs.
Transforming growth factor beta (TGFβ) is generally recognized as a key regulator of in vitro chondrogenesis of hBMSCs: Without supplementing TGFβ to the specific differentiation media, hBMSCs will not differentiate toward cartilage.9–11 Environmental factors such as oxygen concentration, mechanical stimulation, or coculture with other cell types, such as chondrocytes, have been recognized to affect chondrogenesis of hBMSCs.12–14 In addition, differential activation of signaling pathways in hBMSCs affects the quality of the generated cartilaginous tissue in vitro.15 Thus, the micro-environment in which hBMSCs reside influences the chondrogenic potential of the cells.
Several in vivo studies involving orthotopic cartilage defects have demonstrated that implantation of hBMSCs without treatment of chondrogenesis-related growth factors before implantation results in the formation of cartilaginous tissue.16–19 Contrastingly, on ectopic implantation of hBMSCs, bone formation is reported, even when hBMSCs were stimulated to differentiate chondrogenically before implantation.20,21 These distinct responses of hBMSCs placed in different environments demonstrate that the micro-environment plays an important role in the induction and direction of differentiation of hBMSCs both in vitro and in vivo.
In an orthotopic cartilage defect, the surrounding cartilage, the subchondral bone, and the synovial fluid affect the local micro-environment. However, the specific effects that each joint tissue can have on the regeneration of cartilage are currently still unknown. We aimed at investigating the mechanisms involved in chondrogenesis of human hBMSCs in a simulated joint-like environment in vitro. Therefore, we used a bovine osteochondral biopsy model that was recently developed and validated.22 This enables us to study cartilage repair mechanisms in a well-characterized osteochondral environment in vitro.
In sharp contrast to the general hypothesis that TGFβ is essential for chondrogenesis of hBMSCs, we found that chondrogenesis of hBMSCs in this osteochondral environment was not dependent on the addition of TGFβ to the culture system. We identified the subchondral bone as the main source of secreted factors for chondrogenesis of hBMSCs. Subcutaneous implantation in nude mice of osteochondral biopsies with hBMSCs resulted in more newly formed cartilaginous tissue in osteochondral defects than in chondral defects, confirming the importance of the subchondral bone. Since TGFβ was our main candidate to induce chondrogenesis, we measured the presence of TGFβ in the culture media. Subsequently, we captured TGFβ secreted by subchondral bone using an antibody against TGFβ and blocked the canonical TGFβ signaling pathway by prevention of Smad2/3 phosphorylation. Neither of these strategies resulted in inhibition of chondrogenesis of hBMSCs. Altogether, our findings demonstrate that chondrogenesis of hBMSCs is stimulated by the bone and this is not solely mediated by TGFβ.
Materials and Methods
hBMSC isolation and expansion
Bone marrow aspirates from healthy donors and patients undergoing total hip replacement surgery after informed consent was obtained: For the healthy donors, all procedures for the collection of marrow have been approved by the Clinical Research Ethical Committee at the University College Hospital, Galway, Ireland (Ref: 2/08), and by the Institutional National University of Ireland Galway Research Ethics Committee (reference: 08/May/14); for the donors undergoing total hip replacement, all procedures have been approved by the local ethics committee of the Erasmus MC, University Medical Center Rotterdam (MEC 2004-142). Heparinized bone marrow aspirates were seeded at a density of 2–5×105 cells/cm2 in minimum essential medium—alpha (MEM-α; Gibco, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS; Lonza, Verviers, Belgium), 50 μg/mL gentamicine (Gibco) and 1.5 μg/mL fungizone (Gibco), 1 ng/mL fibroblast growth factor 2 (FGF2; AbD Serotec, Kidlington, United Kingdom), and 25 μg/mL ascorbic acid-2-phosphate (Sigma-Aldrich, St. Louis, MO). Nonadherent cells were washed off after 24 h, and adherent cells were further expanded. At subconfluence, hBMSCs were trypsinized and replated at a density of 2300 cells/cm2. Medium was refreshed twice per week. Passage 3 or 4 hBMSCs were used for experiments.
Osteochondral culture model
Cartilage defects were created in bovine osteochondral biopsies as previously described.22 In short, osteochondral biopsies that were 8 mm in diameter and 5 mm in length were created using a hollow drill (Synthes, Oberdorf, Switzerland) from the four proximal sesamoïd bones of fresh metacarpal phalangeal joints of 3- to 8 month-old calves (Fig. 1A). Biopsies were incubated overnight in Dulbecco's-modified Eagle's medium with Glutamax (DMEM-HG; Gibco) supplemented with 10% FBS, 50 μg/mL gentamicine, and 1.5 μg/mL fungizone. Using a 6 mm-diameter dermal biopsy punch (Stiefel Laboratories, Durham, NC) and scalpel, cartilage defects were created of chondral, subchondral, and osteochondral nature as previously described.22 Biopsies were placed in 2% low-gelling agarose (gelling temperature 37°C–39°C; Eurogentec, Liege, Belgium) in such a way that the cartilage was above the agarose surface. By cutting the cartilage from the bone, we created cartilage-only explants and bone-only explants. To exclude cartilage remnants on the bone-only explants, about 1 mm of the subchondral bone was removed. In the bone-only and cartilage-only explants, 6 mm defects were also created.
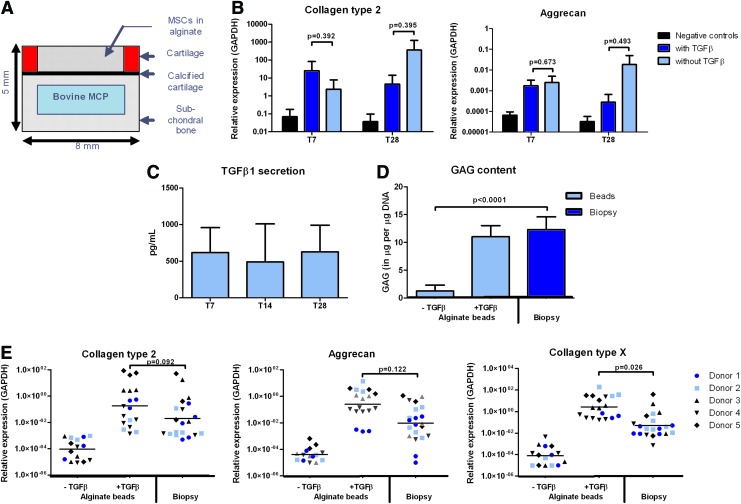
FIG. 1.
hBMSCs differentiate chondrogenically in an osteochondral environment in vitro without supplementing TGFβ to the culture media. (A) Schematic representation of the bovine osteochondral biopsy model with a simulated subchondral defect containing hBMSCs in alginate; (B) Collagen type 2 and aggrecan gene expression relative to GAPDH after 7 or 28 days of culture of hBMSCs from one donor in alginate in simulated subchondral defects with or without supplementing TGFβ (n=6, mean with standard deviation, Student's t-test); (C) TGFβ1 secretion measured in culture medium used for 72 h of osteochondral biopsies (n=3, mean with standard deviation) after 7, 14, or 21 days in culture to which no exogenous TGFβ was supplemented; (D) μg of GAGs normalized to μg of DNA measured either in hBMSCs from one donor cultured for 28 days in alginate beads with or without TGFβ or in alginate in simulated subchondral defects without TGFβ (n=6, mean with standard deviation, Student's t-test); (E) Collagen type 2, aggrecan and collagen type X gene expression relative to GAPDH of hBMSCs from five different donors (n=6 for MSC donor 1 and 2, n=3 for MSC donors 3, 4, and 5, generalized estimated equations model with correction for multiple testing). hBMSCs, human bone marrow-derived mesenchymal stem cells; TGFβ, transforming growth factor beta; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GAG, glycosaminoglycan. Color images available online at www.liebertpub.com/tea
hBMSCs in alginate in the osteochondral culture model
To culture hBMSCs in alginate inside the simulated subchondral cartilage defects in the osteochondral culture model, hBMSCs were resuspended in 1.2% low viscosity alginate (Keltone, San Diego, CA) in physiological saline at a density of 10×106 cells/mL. Simultaneously, 50 μL of alginate cell suspension and 50 μL 102 mM CaCl2 were added to the simulated cartilage defects, enabling in-situ gelation. Equal amounts of alginate cell suspension were used in all conditions. To study the roles of bone and cartilage, hBMSCs in alginate were cultured in defects in osteochondral biopsies, bone-only explants, or cartilage-only explants (Fig. 2A). To determine whether either living cells present in the osteochondral biopsies or factors released from the bone matrix affect the behavior of hBMSCs, biopsies were snap frozen in liquid nitrogen and stored at −80°C. Subsequently, six freeze-thaw cycles were performed, using a 60°C water bath and liquid nitrogen. hBMSCs in alginate were cultured in defects in these devitalized osteochondral biopsies. Unless stated otherwise, samples were cultured for 28 days at 37°C and 5% CO2 in 1.5 mL incomplete chondrogenic medium (ICM) per biopsy, consisting of DMEM-HG supplemented with insulin, transferrin, and selenium (ITS+1; B&D Bioscience, Bedford, MA), 40 μg/mL L-proline (Sigma-Aldrich), 1 mM sodium pyruvate (Gibco), 1.5 μg/mL fungizone, 50 μg/mL gentamicin, 25 μg/mL ascorbic acid-2-phosphate, and 10−7 M dexamethasone (Sigma-Aldrich). When 10 ng/mL TGFβ1 (R&D Systems, Minneapolis, MA) was added to this medium, it was referred to as complete chondrogenic medium (CCM). To evaluate whether induction of chondrogenesis in ICM was due to endogenously produced TGFβ, 1.5 μg/mL pan specific anti-TGFβ1,2,3 (anti-TGFβ, MAB1835; R&D Systems) was used to capture produced TGFβ. This dosage was determined based on previous measurements of TGFβ1 levels in used culture medium of osteochondral biopsies, combined with the manufacturer's instructions to use 0.25–1.25 μg/mL to neutralize 1 ng/mL TGFβ. To prevent phosphorylation of Smad2/3 (pSmad2/3) and activation of the canonical TGFβ signaling pathway, 10 ng/mL SB-505124 (Sigma-Aldrich) was added to ICM. About 10 ng/mL dorsomorphin (Biomol International, Exeter, United Kingdom) was added to ICM to prevent phosphorylation of Smad1/5/8 (pSmad1/5/8) and activation of the BMP-associated signaling pathway. The dosage of SB-505124 and dorsomorphin was based on previous research.15 Medium was refreshed thrice per week. Used medium was stored once per week at −80°C for later analysis of the concentration of TGFβ1 using an ELISA kit for human TGFβ1 (R&D Systems) according to the manufacturer's instructions. Samples were cultured for 28 days before harvesting the hBMSCs for mRNA isolation or biochemical assays. For western blot, samples were harvested 1.5 h after refreshing the medium after 4 days of culture.
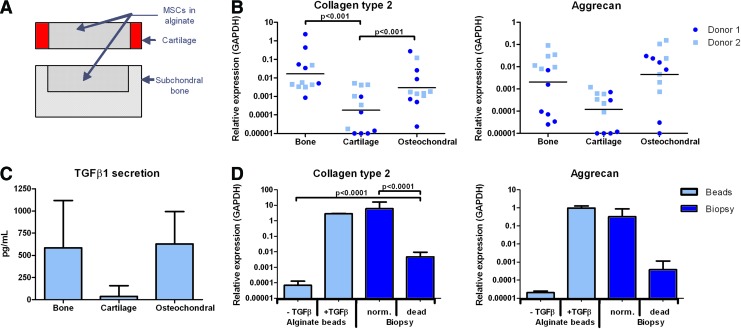
FIG. 2.
Chondrogenic differentiation of hBMSCs in an osteochondral environment in vitro is mediated by the subchondral bone. (A) Schematic representation of bovine cartilage-only and bone-only biopsies with a simulated defect containing hBMSCs in alginate; (B) Collagen type 2 and aggrecan gene expression relative to GAPDH of hBMSCs from two different donors in alginate cultured in bone-only biopsies, cartilage-only biopsies, or simulated subchondral defects in osteochondral biopsies (n=6 per hBMSC donor, generalized estimated equations model with correction for multiple testing); (C) TGFβ1 secretion of bone-only, cartilage-only, or osteochondral biopsies measured in culture medium used for 72 h after 28 days of culture in total (n=3, mean with standard deviation); (D) Collagen type 2 and aggrecan gene expression relative to GAPDH of hBMSCs from one donor cultured for 28 days in alginate beads with or without supplementing TGFβ or in alginate in simulated subchondral defects in osteochondral biopsies (norm.) or in devitalized osteochondral biopsies (dead) (n=6, mean with standard deviation, Student's t-test). Color images available online at www.liebertpub.com/tea
hBMSCs in alginate beads as controls
As controls for hBMSCs cultured in the osteochondral biopsy model, hBMSCs were cultured in alginate beads. hBMSCs were resuspended in alginate at a density of 10×106 cells/mL. The alginate-cell suspension was pressed through a 22-gauge needle in 102 mM CaCl2. Beads were washed twice in physiological saline and once in ICM. 100 μL medium was used per alginate bead, and 10 to 12 beads were cultured per well in 24-well plates. hBMSCs in alginate beads were cultured in CCM or ICM. Anti-TGFβ, SB-505124, or dorsomorphin was added to CCM as controls for the hBMSCs cultured in alginate in the osteochondral biopsy system. Medium was refreshed thrice per week. Samples were cultured for 28 days before harvesting for mRNA isolation or biochemical assays.
mRNA isolation and qRT-PCR
After 28 days of culture, alginate beads were dissolved using 55 mM sodium citrate (150 μL/bead; Sigma-Aldrich) in 20 mM ethylene diamintetraacetate (EDTA; Sigma-Aldrich). hBMSCs in alginate cultured in the osteochondral model, bone-only and cartilage-only explants were removed using a spatula and dissolved in 450 μL sodium citrate in EDTA. All samples were incubated at 4°C while rotating and subsequently centrifuging for 8 min at 1200 rpm. The supernatant was removed, and the samples were resuspended in 150 μL/bead or 500 μL/sample RNABee (TEL-TEST, Friendswood, TX). Chloroform (Sigma-Aldrich) was added at a quantity of 200 μL/mL RNABee. Further RNA isolation was performed using the RNeasy Microkit (Qiagen, Hilden, Germany) according to the manufacturer's instructions, including on-column DNAse treatment. RNA concentration and quality was measured using a NanoDrop ND1000 UV-VIS spectrophotometer (Isogen Life Science, de Meern, the Netherlands). cDNA was prepared using RevertAid First-Strand cDNA Synthesis Kit (MBI Fermentas, St. Leon-Rot, Germany) according to the manufacturer's instructions. qRT-PCR was performed in 20 μL reactions on an ABI Prism 7000 system (Applied Biosystems, Foster City, CA) using either Taqman Universal PCR mastermix (Applied Biosystems) or SybrGreen (Eurogentec). The expression of the cartilage-related genes collagen type 223 and aggrecan24 and the hypertrophy-related gene collagen type X23 was determined. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH23) was selected as a reference gene after comparison with two other housekeeping genes (data not shown). Relative gene expression was calculated using the 2−ΔCT method25.
Glycosaminoglycan content
After 28 days of culture, alginate was digested overnight at 56°C in 200 μg/mL papain in 50 mM EDTA supplemented with 5 mM L-cystein (Sigma-Aldrich). The amount of glycosaminoglycans (GAGs) was determined using dimethylmethylene blue (DMB) assay; the protocol was modified for measurements in alginate as previously reported: The pH of the DMB reagent was lowered to 1.75 using formic acid.26,27 A spectrophotometer (VersaMax; Molecular Devices, Sunnyvale, CA) was used to measure the metachromatic reaction of GAGs with DMB at 540 and 595 nm. Chondroitin sulfate C (Sigma-Aldrich) was used as a standard. The DNA content in papain-digested samples was determined after RNAse (Sigma) treatment using ethidium bromide (Gibco). Using a spectrofluorometer (Wallac 1420 Victor 2; Perkin-Elmer, Wellesley, MA), the extinction and emission were measured at 340 and 590 nm, respectively. Calf thymus DNA (Sigma-Aldrich) was used as a standard.
Western blot
After 4 days of preculture to enable stabilization of culture conditions, hBMSCs that were cultured in alginate beads were stimulated with TGFβ1 alone, TGFβ1, and anti-TGFβ or TGFβ1 and SB-505124. Alternatively, hBMSCs cultured in alginate in the osteochondral biopsy model were stimulated with TGFβ1, anti-TGFβ, and SB-505124 or left untreated as control. After 1.5 h of stimulation, alginate was dissolved and removed using cold sodium citrate and centrifugation as described in the mRNA isolation section. M-PER Protein extraction reagent (Thermo Scientific, Rockford, IL) with 1% protease inhibitor (Roche Diagnostics, Basel, Switzerland) was added. Total protein content was determined using a bicinchoninic acid assay kit (BCA assay; Pierce, Rockford, IL). Per sample, 10 μg of total protein lysate was subjected to gel electrophoresis using a 10% sodium dodecyl sulphate polyacryl amide gel and transferred to polyvinylidene fluoride membranes. Membranes were treated for 2.5 h with a blocking buffer consisting of 0.1% Tris/Tween (TBS-T) supplemented with 5% dried milk powder, washed in TBS-T, and incubated overnight at 4°C with the primary antibody anti-α-tubulin (1:1000; Cell Signaling Technology, Danvers, MA) or the primary antibody anti-pSmad2 (1:1000; Cell Signaling Technology). Subsequently, membranes were incubated for 1.5 h at room temperature with anti-horseradish peroxidase-conjugated secondary antibody (1:1000; Cell Signaling Technology). Blots were visualized using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, Durham, NC) according to the manufacturer's instructions. For anti-α-tubulin, exposure time was 1–5 s and for anti-pSmad2, exposure time was 2–4 min.
In vivo implantation of osteochondral biopsies with hBMSCs in alginate
Passage 3 hBMSCs from three healthy donors were resuspended in 1.2% alginate, which was solidified in simulated chondral or osteochondral defects in osteochondral biopsies (n=3 per donor per defect type) as described earlier for the in vitro experiments. Equal amounts of alginate cell suspension were used in all conditions: 50 μL of alginate per defect containing 10×106 hBMSCs/mL. Alginate without cells was solidified in both defect types (n=3 per defect type) as a control. Biopsies were cultured overnight to allow stabilization of the system. Simultaneously, osteochondral biopsies with hBMSCs from all three donors in both defect types in alginate were cultured in vitro as controls (n=3 per donor per defect type) as described earlier and harvested for mRNA isolation after 28 days. Four osteochondral biopsies were implanted subcutaneously per female NMRI nu/nu mouse (Charles River, Wilmington, MA) under isoflurane anesthesia. The osteochondral biopsies were covered using an 8 mm-diameter Neuro-Patch membrane (Braun, Melsungen, Germany) to prevent in-growth of host tissue. Before surgery and 6–10 h after surgery, mice received 0.05 mg/kg bodyweight of Temgesic (Reckitt Benckiser, Slough, United Kingdom). During surgery, mice received 9 mg/kg bodyweight of Ampi-dry (Dopharma, Raamsdonksveer, The Netherlands). After 12 weeks, mice were euthanized by cervical dislocation. Osteochondral biopsies were explanted and fixed in 4% formalin. After at least 1 week of fixation, biopsies were decalcified using 10% formic acid (Sigma-Aldrich) for 3 weeks. Subsequently, biopsies were embedded in paraffin, sectioned in 6 mm sections, and subjected to histology. Animal experiments were conducted with approval of the animal ethics committee (EMC2353, protocol number 116-11-06).
Histology and quantification
For safranin-O staining, paraffin sections were first stained with 0.1% light green for 8 min, subsequently washed in 1% acetic acid, and stained with 0.1% safranin-O (Fluka, St. Gallen, Switzerland) for 12 min. Newly formed tissue was discriminated visually from native tissue. The surface area of the simulated cartilage defect was measured, and the surface area of newly formed safranin-O-positive tissue was determined using ImageJ software (National Institutes of Health, Bethesda, MA).
Data analysis
Unpaired data were analyzed using Student's t-test. Normality of paired data was verified with Kolmogorov–Smirnov and Shapiro–Willk normality tests using SPSS 15.0. When necessary, logarithmic transformation was performed to obtain normal distribution of the data. For paired data that were normally distributed, a generalized estimated equations model was used. Correction for multiple testing was performed using the false discovery rate. If paired data were not normally distributed, a Kruskal–Wallis test was performed followed by the Mann–Whitney U test. For all statistical analyses, differences were considered statistically significant for p<0.05.
Results
hBMSCs differentiate in the osteochondral model without the addition of TGFβ
After 28 days of culture in CCM (with TGFβ) or ICM (without TGFβ), chondrogenesis of hBMSCs in alginate in simulated subchondral defects was assessed. Interestingly, chondrogenesis of hBMSCs in subchondral defects was observed after culture in ICM. Both after 7 and 28 days of culture, no significant differences were observed between hBMSCs in alginate in simulated subchondral defects cultured in CCM or ICM in terms of collagen type 2 and aggrecan gene expression as well as in GAG production (Fig. 1B, D). Throughout culture, TGFβ1 secretion by the osteochondral biopsies was measured in culture medium, and it was found that about 600 pg/mL TGFβ1 was secreted by the biopsies in 72 h (Fig. 1C). For hBMSCs from five different donors, no significant differences were observed in collagen type 2 and aggrecan gene expression between culture in alginate beads in CCM and culture in alginate in simulated subchondral defects in ICM (Fig. 1E). Collagen type X gene expression was significantly lower in hBMSCs cultured in alginate in simulated subchondral defects than in hBMSCs cultured in alginate beads (Fig. 1E). Based on these findings, for further experiments, ICM was used for hBMSCs cultured in alginate in osteochondral biopsies.
Subchondral bone and not cartilage stimulates hBMSC chondrogenesis
To assess the specific roles of bone and cartilage in the osteochondral biopsy model, bone-only and cartilage-only explants were prepared. hBMSCs were cultured for 28 days in alginate in simulated defects in osteochondral biopsies, bone-only, or cartilage-only explants (Fig. 2A). Collagen type 2 and aggrecan gene expression levels in hBMSCs cultured in bone-only explants or osteochondral biopsies were found to be comparable, where the levels in hBMSCs cultured in cartilage-only explants were significantly lower (p≤0.001, Fig. 2B). Significantly more TGFβ1 was produced by osteochondral biopsies and bone-only biopsies versus cartilage-only explants in 72 h after 28 days of culture (p=0.03, Fig. 2C).
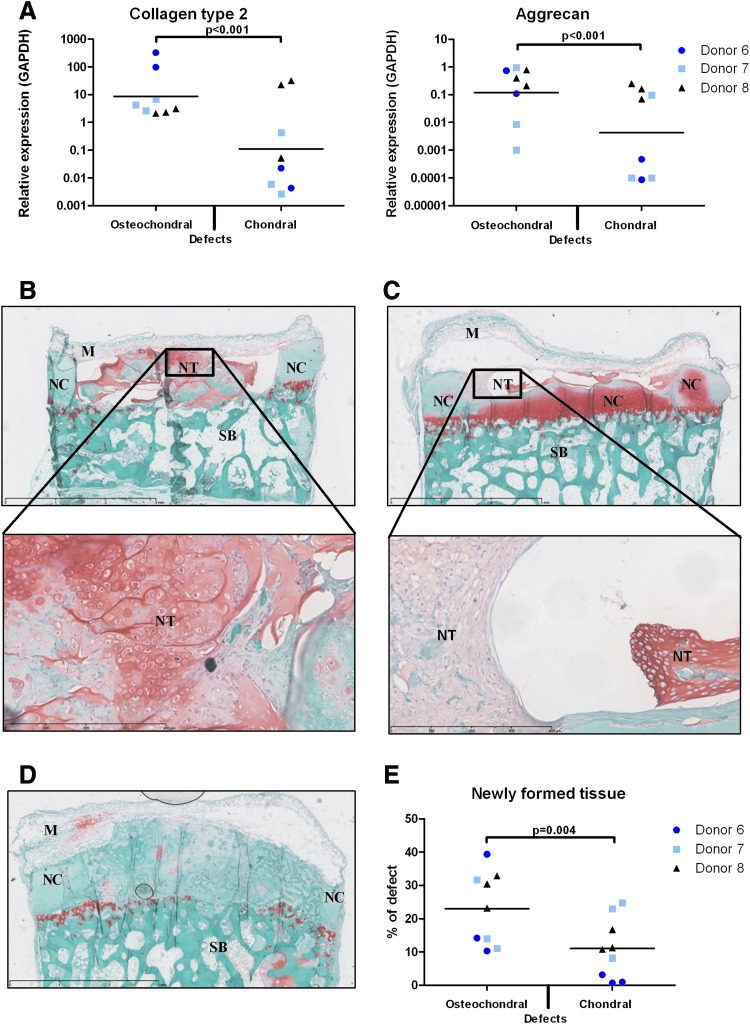
The stimulating role of subchondral bone in chondrogenesis of hBMSCs was confirmed by the results of the in vivo experiment: Subcutaneous implantation of osteochondral biopsies with hBMSCs from three different donors in alginate in chondral defects resulted in the formation of significantly less cartilaginous repair tissue than hBMSCs in osteochondral defects (p=0.004, Fig. 3B–E). The newly formed tissue by hBMSCs in osteochondral defects appeared more cartilage like in terms of intensity of safranin-O staining as well as in cell morphology than the newly formed tissue in chondral defects (Fig. 3B, C). This was also reflected in gene expression levels of accompanying in vitro controls: The expression of collagen type 2 (p<0.001) and aggrecan (p<0.001) was significantly higher in hBMSCs cultured in alginate in osteochondral defects than in hBMSCs cultured in alginate in chondral defects (Fig. 3A).
FIG. 3.
Chondrogenic differentiation of hBMSCs in a simulated osteochondral environment in vivo is also mediated by subchondral bone. (A) Collagen type 2 and aggrecan gene expression relative to GAPDH of hBMSCs cultured in vitro in alginate beads in complete chondrogenic medium or in alginate in osteochondral biopsies with simulated chondral or osteochondral defects without supplementing TGFβ for 28 days (hBMSCs from 3 donors, n=3 per donor, generalized estimated equations model with correction for multiple testing); (B–D) Osteochondral biopsies with hBMSCs in alginate in simulated osteochondral (B) or chondral (C) defects or controls with osteochondral defects filled with alginate without cells (D) were implanted subcutaneously in nude mice for 12 weeks. Representative safranin-O stained sections, scale bars in upper pictures represent 3 mm, scale bars in magnified pictures represent 400 μm. M, NeuroPatch membrane; NC, native bovine cartilage; NT, newly formed tissue; SB, subchondral bone. GAGs have been lost from the NC during the experiment, most likely due to lack of mechanical stimulation; (E) Quantification of newly formed safranin-O-positive tissue relative to the defect area (hBMSCs from 3 donors, n=3 per donor, generalized estimated equations model with correction for multiple testing); Color images available online at www.liebertpub.com/tea
Chondrogenesis is partly caused by actively produced factors
When hBMSCs were cultured in alginate in osteochondral biopsies that were devitalized by subjecting them to six repeated freeze-thaw cycles, chondrogenesis was partly inhibited (Fig. 2D): Collagen type 2 gene expression was significantly higher than in hBMSCs cultured in alginate beads without supplementing TGFβ and significantly lower than in hBMSCs cultured in alginate in normal osteochondral biopsies. This implies that a part of the factors that were responsible for the chondrogenesis of hBMSCs in the osteochondral biopsies were released from the subchondral bone matrix and that a part of it was actively produced or activated by cells in the osteochondral biopsies.
Inhibition of TGFβ signaling does not block chondrogenesis
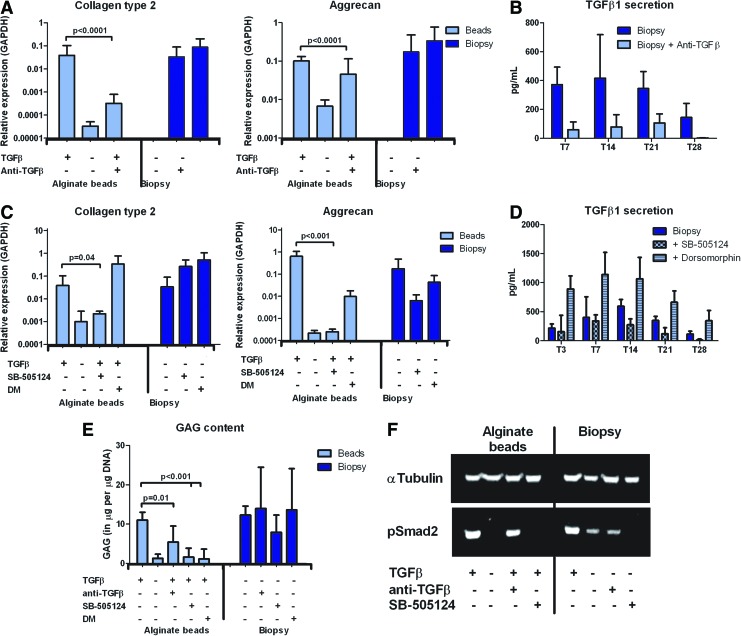
Anti-TGFβ was used to capture TGFβ secreted by the osteochondral tissue. Strikingly, even though the anti-TGFβ captured about 80% of the produced TGFβ (Fig. 4B), no significant differences were observed in collagen type 2 and aggrecan gene expression as well as GAG production of hBMSCs cultured in alginate in the osteochondral biopsy model (Fig. 4A, E). Controls in which hBMSCs in alginate beads were cultured in the presence of both anti-TGFβ and TGFβ demonstrated the effectiveness of the antibody: Collagen type 2 (p<0.0001) and aggrecan (p<0.0001) gene expression and GAG production (p=0.01) were significantly lower when the antibody was used (Fig. 4A, E). Western blot for pSmad2 was in line with these results: A decrease in pSmad2 was observed when hBMSCs in alginate beads were stimulated with both anti-TGFβ and TGFβ (Fig. 4F). When the canonical TGFβ signaling pathway was blocked by prevention of Smad2/3 phosphorylation using SB-505124, chondrogenesis of hBMSCs cultured in alginate in the osteochondral biopsies remained unchanged (Fig. 4C, E). This was confirmed by western blot for pSmad2: When hBMSCs in alginate in the osteochondral biopsies were treated with SB-505124, no pSmad2 was detected, indicating that the canonical TGFβ pathway was successfully blocked (Fig. 4F). Controls in alginate beads cultured in CCM reflected the effectiveness of SB-505124 by significantly decreasing collagen type 2 (p=0.04) and aggrecan (p<0.001) gene expression as well as GAG production (p<0.001).
FIG. 4.
Chondrogenic differentiation of hBMSCs in an osteochondral environment in vitro is not solely mediated by TGFβ. (A) Collagen type 2 and aggrecan gene expression relative to GAPDH of hBMSCs from two different donors cultured for 28 days in alginate beads with or without TGFβ and anti-TGFβ or in simulated subchondral defects with or without anti-TGFβ (n=6 per MSC donor, mean with standard deviation, Kruskal–Wallis test followed by the Mann–Whitney U test); (B) TGFβ secretion of osteochondral biopsies cultured with or without anti-TGFb measured in medium used for 72 h (n=3, mean with standard deviation); (C) Collagen type 2 and aggrecan gene expression relative to GAPDH of hBMSCs from two different donors cultured for 28 days in alginate beads with or without TGFβ or in simulated subchondral defects without TGFβ. Samples were cultured with the pSmad2/3 inhibitor SB-505124 or the pSmad1/5/8 inhibitor DM (n=6 per MSC donor, mean with standard deviation, Kruskal–Wallis test followed by the Mann–Whitney U test); (D) TGFβ secretion of osteochondral biopsies measured in medium used for 72 h with or without the pSmad2/3 inhibitor SB-505124 or the pSmad1/5/8 inhibitor DM (n=3, mean with standard deviation); (E) GAG production relative to DNA concentration of hBMSCs from one donor cultured in alginate beads with or without TGFβ or in alginate in simulated subchondral defects without TGFβ. Samples were cultured with anti- TGFβ, SB-505124, or DM (n=6, mean with standard deviation, Student's t-test); (F) western blot for α-tubulin and phosphorylated Smad 2 (pSmad2) on hBMSCs cultured for 4 days in alginate beads or in alginate in simulated subchondral defects harvested after 1.5 h of stimulations with or without TGFβ, anti-TGFβ, and/or SB-505124. DM, dorsomorphin. Color images available online at www.liebertpub.com/tea
Apart from TGFβ, the bone is known to contain high levels of other members of the TGFβ family, BMPs. To verify whether signaling of BMPs played a role in chondrogenesis of hBMSCs in an osteochondral environment, the BMP-associated signaling pathway was blocked via prevention of pSmad1/5/8 using dorsomorphin. This did not affect chondrogenesis of hBMSCs in alginate in osteochondral biopsies (Fig. 4C, E). The secretion of TGFβ increased when dorsomorphin was added to the ICM of the osteochondral biopsies (Fig. 4D).
Discussion
Cartilage defects heal poorly due to the low intrinsic repair capacity of articular cartilage. Therefore, solutions that stimulate repair and help prevent the development of osteoarthritis (OA) are a major research topic in orthopedics. As a part of this process, hBMSCs are exhaustively investigated as a potential cell source for cartilage tissue-engineering purposes based on their multipotency, expandability in vitro, and the possibility to use autologous cells. When hBMSCs are cultured in a 3D in vitro setting, for example in pellets or in alginate beads, TGFβ is essential to induce chondrogenesis.9–11,15 In the present study, we have found that when hBMSCs were cultured in alginate in an osteochondral environment, chondrogenesis is induced independent of TGFβ.
We initiated this study by culturing hBMSCs in alginate in simulated subchondral defects in a bovine osteochondral biopsy model with and without the addition of TGFβ to the culture media, hypothesizing that TGFβ would be required to induce chondrogenesis. Strikingly, in the osteochondral culture system, chondrogenesis of hBMSCs was present even when no TGFβ was supplemented. The osteochondral culture model appeared to provide a favorable micro-environment for chondrogenesis of hBMSCs. This finding corresponds with clinical outcomes of the microfracture procedure: A bone marrow clot fills a cartilage defect and hBMSCs, either present or recruited, are held responsible for the spontaneous generation of cartilaginous repair tissue, without the supplementation of any exogenous factors.28,29 This led to our renewed hypothesis: TGFβ secreted by the osteochondral biopsies themselves was responsible for the induction of chondrogenesis in the hBMSCs. This was supported by measurements of significant TGFβ1 levels in the culture media throughout culture.
To study whether the induction of chondrogenesis could be addressed to a specific part of the osteochondral culture system, the separate roles of bone and cartilage were studied by culturing hBMSCs in alginate in simulated defects in bone-only or cartilage-only biopsies. The findings that the subchondral bone played the most important role and also produced the majority of the TGFβ1 fitted the hypothesis. Again, this corresponds with the clinical outcomes of the microfracture procedure, as it is known that careful removal of the calcified cartilage layer before puncturing the subchondral plate is required.30 This implies that both in our culture system as well as in a clinical setting, the subchondral bone plays an important role in the generation of repair tissue.
To validate our findings, osteochondral biopsies containing hBMSCs in alginate in either osteochondral or chondral simulated defects were implanted subcutaneously in nude mice. More newly formed cartilage-like tissue was observed in osteochondral defects than in chondral defects. This validates our culture model, and it also confirms the in vitro finding that subchondral bone plays a critical role in the stimulation of chondrogenesis of hBMSCs in an osteochondral environment. These findings correspond with recent clinical and animal studies in which autologous hBMSCs were injected in the knee joints of patients or animals with OA. These studies showed a decrease in the size of cartilage lesions and enhanced regeneration of the cartilage using hBMSCs with exposure to growth factors before or after implantation.19,31,32 This is in contrast with other studies, suggesting that treatment with growth factors before or after implantation is required to achieve regeneration of osteochondral defects by hBMSCs.33,34 Despite the fact that hBMSCs were shown to undergo chondrogenic differentiation, we cannot exclude the fact that other cells might have contributed to the formation of repair tissue. It is possible that cells from the subchondral bone of the osteochondral biopsy or from the murine host contributed to the formed repair tissue, as it is known that hBMSCs can secrete trophic factors which can recruit other cells.41 Either way, the presence of hBMSCs was crucial for the formation of cartilaginous repair tissue, as in control defects with alginate without hBMSCs, no formation of safranin-O positive repair tissue was observed.
To study whether the factor(s) that induce chondrogenesis in the osteochondral culture system are actively produced by cells or secreted from the matrix of the subchondral bone during culture, hBMSCs in alginate were cultured in devitalized osteochondral biopsies. The results from this experiment suggest that not only a part of the factor or factors involved were secreted by the bone matrix, but also they were at least partly, actively produced by the cells present in the subchondral bone of the osteochondral biopsies.
After these observations, we aimed at confirming whether TGFβ was actually the key player behind the observed effect, as among the possible factors that can be released by the bone, TGFβ is the most likely candidate to induce chondrogenesis, whereas BMPs and vascular endothelial growth factor (VEGF), for example, are more associated with bone formation.35 To achieve this, an antibody against TGFβ was used, which resulted in the majority of the secreted TGFβ being captured. Surprisingly, chondrogenesis of hBMSCs in alginate in simulated subchondral defects remained unchanged, which was in sharp contrast to our hypothesis. Even though levels were low, the possibility remained that the residual TGFβ was responsible for the observed chondrogenesis. To rule out this option, we aimed at inhibiting TGFβ signaling.
Earlier, we demonstrated that when canonical TGFβ signaling is blocked by prevention of Smad2/3 phosphorylation using the inhibitor SB-505124 in hBMSCs in pellet culture in the presence of TGFβ, chondrogenesis was inhibited.15 The use of an antibody against TGFβ to capture the majority of the produced TGFβ did not prevent chondrogenesis of bMSCs in osteochondral defects. However, the use of the antibody against TGFβ resulted in some remaining Smad2 phosphorylation, indicating that we might not have blocked TGFβ signaling pathways sufficiently. Therefore, we used SB-505124, which resulted in a complete prevention of Smad2 phosphorylation, where chondrogenesis remained uninhibited, thus indicating that chondrogenesis was mediated by factors other than TGFβ. When the canonical BMP signaling was inhibited via prevention of Smad1/5/8 phosphorylation by dorsomorphin in our culture system, the situation remained the same as observed earlier: Chondrogenesis of hBMSCs was uninhibited. These findings suggest that chondrogenesis of hBMSCs in our osteochondral culture system is not solely mediated by TGFβ and that the effect is also not addressable to BMPs.
It is known that apart from TGFβ and BMPs, other growth factors reside in the extracellular bone matrix, such as insulin-like growth factor (IGF), and that various growth factors, such as FGF, IGF, platelet-derived growth factor (PDGF), epidermal growth factor, and VEGF, can be produced by different cell types residing in the bone.36,37 During healing of bone fractures, it is known that a variety of growth factors are released by the extracellular bone matrix or produced by cells residing in the bone: For example, as an acute response to a fracture, interleukin (IL)-1, IL-6, TGFβ, and PDGF are released.38,39 In the homeostasis of bone, many of these and other factors are required to be present in a delicate balance, as bone is continuously remodeled. It is known, for example, that sclerostin, an inhibitor of Wnt signaling, is important in generating bone tissue, where its production by osteocytes can be inhibited by oncostatin M.40 Since all of these factors are likely to be present in our osteochondral culture system, the observed chondrogenesis might be attributed to one of these factors or a combination of multiple factors.
The bovine biopsies used in the osteochondral culture system originate from 3- to 8 month-old calves. It is obvious that this young and healthy material is likely to provide a different environment than old and/or diseased material, such as osteoarthritic biopsies. Apart from degeneration of the cartilage, in OA, the subchondral bone undergoes major changes. The effects of these changes on cartilage repair mediated by bMSCs are difficult to predict and will require further studies. Altogether, our study stresses that chondrogenesis of hBMSCs in an osteochondral environment is a complex process. Conventional culture systems such as pellet culture or alginate beads might not be sufficiently representative to truly reach an understanding of the complexity of the differentiation process. Using an osteochondral culture model, we not only have identified the subchondral bone as a key player in cartilage regeneration by hBMSCs, but also that the commonly recognized in vitro regulator of chondrogenesis TGFβ is not solely responsible for the observed effects. It is evident that more studies are required to truly identify the now unidentified key players in the induction of chondrogenesis of hBMSCs not only in a healthy, but also in a diseased osteochondral environment, for example, in OA.
Acknowledgments
The research leading to these results has received funding from the European Union's 7th Framework Program under grant agreement nos. NMP3-SL-2010-245993 and HEALTH-2007-B-223298, from the Dutch Arthritis Association (LLP11), the Dutch government to the Netherlands Institute for Regenerative Medicine (NIRM, grant no. FES0908), and the Science Foundation, Ireland (grant no. 09/SRC/B1794). The research in this article was performed within the framework of the Erasmus Postgraduate School Molecular Medicine. The authors would like to thank Stefan Sandker and René van der Bel for conducting pilot experiments for the current study during their internships as a part of the master technical medicine of the University of Twente, Enschede, the Netherlands.
Disclosure Statement
No competing financial interests exist.
References
- 1.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., and Marshak D.R.Multilineage potential of adult human mesenchymal stem cells. Science 284, 143, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Caplan A.I.Mesenchymal stem cells. J Orthop Res 9, 641, 1991 [DOI] [PubMed] [Google Scholar]
- 3.Pelttari K., Lorenz H., Boeuf S., Templin M.F., Bischel O., Goetzke K., Hsu H.Y., Steck E., and Richter W.Secretion of matrix metalloproteinase 3 by expanded articular chondrocytes as a predictor of ectopic cartilage formation capacity in-vivo. Arthritis Rheum 58, 467, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Tuan R.S.Stemming cartilage degeneration: adult mesenchymal stem cells as a cell source for articular cartilage tissue engineering. Arthritis Rheum 54, 3075, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Shapiro F., Koide S., and Glimcher M.J.Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am 75, 532, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Buckwalter J.A.Articular cartilage injuries. Clin Orthop Relat Res 21, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Roberts S., McCall I.W., Darby A.J., Menage J., Evans H., Harrison P.E., and Richardson J.B.Autologous chondrocyte implantation for cartilage repair: monitoring its success by magnetic resonance imaging and histology. Arthritis Res Ther 5, R60, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bos P.K., van Melle M.L., and van Osch G.J.V.M.Articular cartilage repair and the evolving role of regenerative medicine. Open Access Surgery 3, 109, 2010 [Google Scholar]
- 9.Johnstone B., Hering T.M., Caplan A.I., Goldberg V.M., and Yoo J.U.In-vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res 238, 265, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Mackay A.M., Beck S.C., Murphy J.M., Barry F.P., Chichester C.O., and Pittenger M.F.Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng Winter 4, 415, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Cals F.L., Hellingman C.A., Koevoet W., Baatenburg de Jong R.J., and van Osch G.J.Effects of transforming growth factor-beta subtypes on in-vitro cartilage production and mineralization of human bone marrow stromal-derived mesenchymal stem cells. J Tissue Eng Regen Med 6, 68, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Potier E., Noailly J., and Ito K.Directing bone marrow-derived stromal cell function with mechanics. J Biomech 43, 807, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Khan W.S., Adesida A.B., Tew S.R., Lowe E.T., and Hardingham T.E.Bone marrow-derived mesenchymal stem cells express the pericyte marker 3G5 in culture and show enhanced chondrogenesis in hypoxic conditions. J Orthop Res 28, 834, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Fischer J., Dickhut A., Rickert M., and Richter W.Human articular chondrocytes secrete parathyroid hormone-related protein and inhibit hypertrophy of mesenchymal stem cells in coculture during chondrogenesis. Arthritis Rheum 62, 2696, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Hellingman C.A., Davidson E.N., Koevoet W., Vitters E.L., van den Berg W.B., van Osch G.J., and van der Kraan P.M.Smad signaling determines chondrogenic differentiation of bone-marrow-derived mesenchymal stem cells: inhibition of Smad1/5/8P prevents terminal differentiation and calcification. Tissue Eng Part A 17, 1157, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Tay L.X., Ahmad R.E., Dashtdar H., Tay K.W., Masjuddin T., Ab-Rahim S., Chong P.P., Selvaratnam L., and Kamarul T.Treatment outcomes of alginate-embedded allogenic mesenchymal stem cells versus autologous chondrocytes for the repair of focal articular cartilage defects in a rabbit model. Am J Sports Med 40, 83, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Qi Y., Zhao T., Xu K., Dai T., and Yan W.The restoration of full-thickness cartilage defects with mesenchymal stem cells (HBMSCs) loaded and cross-linked bilayer collagen scaffolds on rabbit model. Mol Biol Rep 39, 1231, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Li Q., Tang J., Wang R., Bei C., Xin L., Zeng Y., and Tang X.Comparing the chondrogenic potential in-vivo of autogeneic mesenchymal stem cells derived from different tissues. Artif Cells Blood Substit Immobil Biotechnol 39, 31, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Steck E., Fischer J., Lorenz H., Gotterbarm T., Jung M., and Richter W.Mesenchymal stem cell differentiation in an experimental cartilage defect: restriction of hypertrophy to bone-close neocartilage. Stem Cells Dev 18, 969, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Pelttari K., Winter A., Steck E., Goetzke K., Hennig T., Ochs B.G., Aigner T., and Richter W.Premature induction of hypertrophy during in-vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum 54, 3254, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Farrell E., Both S.K., Odorfer K.I., Koevoet W., Kops N., O'Brien F.J., Baatenburg de Jong R.J., Verhaar J.A., Cuijpers V., Jansen J., Erben R.G., and van Osch G.J.In-vivo generation of bone via endochondral ossification by in-vitro chondrogenic priming of adult human and rat mesenchymal stem cells. BMC Musculoskelet Disord 12, 31, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Vries-van Melle M.L., Mandl E.W., Kops N., Koevoet W.J., Verhaar J.A., and van Osch G.J.An osteochondral culture model to study mechanisms involved in articular cartilage repair. Tissue Eng Part C Methods 18, 45, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farrell E., van der Jagt O.P., Koevoet W., Kops N., van Manen C.J., Hellingman C.A., Jahr H., O'Brien F.J., Verhaar J.A., Weinans H., and van Osch G.J.Chondrogenic priming of human bone marrow stromal cells: a better route to bone repair? Tissue Eng Part C Methods 15, 285, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Clockaerts S., Bastiaansen-Jenniskens Y.M., Feijt C., Verhaar J.A., Somville J., De Clerck L.S., and Van Osch G.J.Peroxisome proliferator activated receptor alpha activation decreases inflammatory and destructive responses in osteoarthritic cartilage. Osteoarthritis Cartilage 19, 895, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Schmittgen T.D., and Livak K.J.Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3, 1101, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Farndale R.W., Sayers C.A., and Barrett A.J.A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res 9, 247, 1982 [DOI] [PubMed] [Google Scholar]
- 27.Enobakhare B.O., Bader D.L., and Lee D.A.Quantification of sulfated glycosaminoglycans in chondrocyte/alginate cultures, by use of 1,9-dimethylmethylene blue. Anal Biochem 243, 189, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Steadman J.R., Rodkey W.G., Briggs K.K., and Rodrigo J.J.[The microfracture technic in the management of complete cartilage defects in the knee joint] Die Technik der Mikrofrakturierung zur Behandlung von kompletten Knorpeldefekten im Kniegelenk. Orthopade 28, 26, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Knutsen G., Engebretsen L., Ludvigsen T.C., Drogset J.O., Grontvedt T., Solheim E., Strand T., Roberts S., Isaksen V., and Johansen O.Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am 86-A, 455, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Frisbie D.D., Morisset S., Ho C.P., Rodkey W.G., Steadman J.R., and McIlwraith C.W.Effects of calcified cartilage on healing of chondral defects treated with microfracture in horses. Am J Sports Med 34, 1824, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Emadedin M., Aghdami N., Taghiyar L., Fazeli R., Moghadasali R., Jahangir S., Farjad R., and Baghaban Eslaminejad M.Intra-articular injection of autologous mesenchymal stem cells in six patients with knee osteoarthritis. Arch Iran Med 15, 422, 2012 [PubMed] [Google Scholar]
- 32.Mokbel A.N., El Tookhy O.S., Shamaa A.A., Rashed L.A., Sabry D., El and Sayed A.M.Homing and reparative effect of intra-articular injection of autologus mesenchymal stem cells in osteoarthritic animal model. BMC Musculoskelet Disord 12, 259, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J., Chen H., Li P., Diao H., Zhu S., Dong L., Wang R., Guo T., Zhao J., and Zhang J.Simultaneous regeneration of articular cartilage and subchondral bone in-vivo using HBMSCs induced by a spatially controlled gene delivery system in bilayered integrated scaffolds. Biomaterials 32, 4793, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Yoshioka T., Mishima H., Kaul Z., Ohyabu Y., Sakai S., Ochiai N., Kaul S.C., Wadhwa R., and Uemura T.Fate of bone marrow mesenchymal stem cells following the allogeneic transplantation of cartilaginous aggregates into osteochondral defects of rabbits. J Tissue Eng Regen Med 5, 437, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Cao X., and Chen D.The BMP signaling and in-vivo bone formation. Gene 357, 1, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urist M.R.Bone: formation by autoinduction. Science 150, 893, 1965 [DOI] [PubMed] [Google Scholar]
- 37.Van Blitterswijk C.A.Tissue Engineering. London: Academic Press Elsevier, 2008 [Google Scholar]
- 38.Bolander M.E.Regulation of fracture repair by growth factors. Proc Soc Exp Biol Med 200, 165, 1992 [DOI] [PubMed] [Google Scholar]
- 39.Joyce M.E., Jingushi S., Scully S.P., and Bolander M.E.Role of growth factors in fracture healing. Prog Clin Biol Res 365, 391, 1991 [PubMed] [Google Scholar]
- 40.Krause C., Korchynskyi O., de Rooij K., Weidauer S.E., de Gorter D.J., van Bezooijen R.L., Hatsell S., Economides A.N., Mueller T.D., Lowik C.W., and ten Dijke P.Distinct modes of inhibition by sclerostin on bone morphogenetic protein and Wnt signaling pathways. J Biol Chem 285, 41614, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caplan A.I., and Correa D.The MSC: an injury drugstore. Cell Stem Cell 9, 11, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]