Abstract
Toward addressing the difficult problems of knee meniscus regeneration, a self-assembling process has been used to re-create the native morphology and matrix properties. A significant problem in such attempts is the recapitulation of the distinct zones of the meniscus, the inner, more cartilaginous and the outer, more fibrocartilaginous zones. In this study, an anisotropic and zonally variant meniscus was produced by self-assembly of the inner meniscus (100% chondrocytes) followed by cell seeding the outer meniscus (coculture of chondrocytes and meniscus cells). After 4 weeks in culture, the engineered, inner meniscus exhibited a 42% increase in both instantaneous and relaxation moduli and a 62% increase in GAG/DW, as compared to the outer meniscus. In contrast, the circumferential tensile modulus and collagen/DW of the outer zone was 101% and 129% higher, respectively, than the values measured for the inner zone. Furthermore, there was no difference in the radial tensile modulus between the control and zonal engineered menisci, suggesting that the inner and outer zones of the engineered zonal menisci successfully integrated. These data demonstrate that not only can biomechanical and biochemical properties be engineered to differ by the zone, but they can also recapitulate the anisotropic behavior of the knee meniscus.
Introduction
The meniscus, a fibrocartilage between the femur and tibia, absorbs shock, transmits loads, and stabilizes and lubricates the joint.1–3 The outer, fibrocartilaginous and vascularized region (red zone) and the inner, more cartilaginous and avascular region (white zone),4–6 are separated by a red-white zone with attributes from both the red and white zones. The inner zone lacks the intrinsic ability for self-repair and, thus, damage to the meniscus can be permanent, leading to a loss of functionality. More than 600,000 meniscus-related surgeries occur every year.7 Meniscal tears range from vertical, longitudinal, oblique, radial, and horizontal.8 Currently, partial meniscectomy is a treatment option.9 While this treatment alleviates symptoms temporarily, partial meniscectomies can lead to degeneration in the articular surfaces and, eventually, to osteoarthritis.10,11 Tissue engineering presents an exciting alternative to repair or replace meniscus injuries, while retaining native biomechanical functions.
The meniscus is comprised of water, (70–75% by wet weight or WW), collagen (20–22% by WW), sulfated glycosaminoglycans (GAG) (0.6–0.8% by WW), and DNA and adhesion molecules (0.10–0.12% by WW).12,13 This composition contributes to functional properties; the collagen and GAG content correlate with tensile and compressive properties, respectively.14,15 The inner and outer zones of the meniscus have different biochemical and biomechanical properties. Specifically, the inner meniscus zone contains a higher GAG content and exhibits a greater compressive stiffness, whereas the outer zone exhibits a higher tensile stiffness and a lower GAG content.16,17
Tissue engineering is an emerging option for the treatment of meniscus-related injuries that often culture cells in vitro on scaffolds, such as hydrogels, poly-glycolic acid, poly-L-lactic acid, and Teflon net.18–20 However, there are several limiting factors regarding scaffold use such as toxicity of degradation products, stress shielding, and phenotypic alteration.21,22 The self-assembling process is a scaffold-free tissue engineering method22 that can generate articular cartilage and fibrocartilage tissues.22–24 This method employs nonadherent and shape-specific agarose wells to form 3D constructs. A 50:50 coculture of self-assembled articular chondrocytes (ACs) to meniscus cells (MCs) is capable of recapitulating native meniscus geometry,23 although without reproducing the zonal differences observed in native tissue. Thus, the objective of this study was to create an anisotropic meniscus with zonal variations mimicking native tissue. Toward this end, this study applied a novel, spatially and temporally varying seeding technique that allowed for different zones to integrate with each other, while maintaining their distinct identities.
The hypotheses are (1) by culturing an inner meniscus zone comprised 100% ACs, a higher compressive stiffness can be engineered in the inner one-third, relative to the outer two-thirds, of the construct, (2) the outer two-thirds, comprised a 50:50 coculture of ACs and MCs, will have a higher circumferential tensile stiffness relative to the inner one-third, and (3) the two zones will remain distinct, while exhibiting integration, as assessed histologically and using tensile testing.
Materials and Methods
Mold fabrication
The shape of the rabbit meniscus, approximated over many menisci, was used to generate positive dies that were formed using rapid prototyping (Huntsman SL7811 HR, Laser Reproductions), as previously described.24,25 An additional positive die representing the inner one-third of the rabbit meniscus was similarly created. The positive die was plunged into molten 2% agarose (Fisher Scientific), and the agarose was allowed to set. Removal of the positive die resulted in two different sets of agarose wells: a meniscus-shaped well and another well that corresponded to only the inner one-third of the meniscus. The agarose wells were then saturated with chondrogenic media (CHG) over 2 days. CHG formulation was as follows: the Dulbecco's modified Eagle's medium (DMEM) with GlutaMAX (Invitrogen), 1% nonessential amino acids (NEAA), 1% penicillin–streptomycin–fungizone (PSF), 1% ITS+premix (consisting of insulin, human transferrin, and selenous acid) (BD Biosciences), 100 nM dexamethasone, 50 mg/mL ascorbate-2-phosphate, 40 mg/mL L-proline (Sigma), and 100 mg/mL sodium pyruvate (Fisher Scientific).
Cell isolation
Menisci and articular cartilage were sterilely isolated from stifle joints of eight skeletally immature calves (Research 87) at one joint per animal. Cartilage from the femoral and tibial surfaces was minced to 1 mm and washed three times in phosphate-buffered saline (PBS). Menisci were also minced to 1 mm and placed into 0.25% pronase (Sigma-Aldrich) for 1h. Cartilage and menisci were then digested separately in 0.2% collagenase type II (Worthington) for 18 h. MCs and ACs were strained at 70 μm. A series of washes using CHG and centrifugation removed the collagenase. Cells from all animals were mixed, counted with trypan blue exclusion, and frozen at −80°C until seeding. Cell numbers were also verified using a Coulter counter. Freezing media consisted of 70% DMEM with GlutaMAX (Invitrogen), 20% fetal bovine serum (FBS), and 10% dimethyl sulfoxide (Sigma-Aldrich).
Cell seeding
Seeding for both control and regionally variant constructs employed the same batches of ACs and MCs. Viability after thawing was greater than 85%. All constructs were seeded using a self-assembling method, based on introducing a high-density cell suspension into nonadherent agarose molds. Cells sediment to the bottom of the molds after seeding and remain unattached to the agarose.26 Ring-shaped constructs were formed due to the meniscus-like anisotropy generated in these shapes, as previously described;23 in practice, each ring can generate two menisci.
Control 50:50 meniscus constructs
ACs and MCs were rapidly thawed and combined in CHG. Aliquots of 20 million cells in 200 μL were drawn from a cell suspension with 50% ACs and 50% MCs. Previous work has shown that this ratio of cells yields constructs with both collagen types I and II, consistent with the fibrocartilage matrix content.25,27,28 These cells were seeded into the meniscus-shaped agarose wells. After 7d, the intact control meniscus constructs were removed from the wells and placed into plastic six-well plates (BD Biosciences) coated with 2% agarose to prevent construct adhesion, cell migration, and construct deformation through outgrowth of their meniscus-shaped wells.
Regionally variant, zonal meniscus constructs
ACs were rapidly thawed and combined with CHG. Aliquots of 5 million ACs in 50 μL were seeded into the inner one-third agarose wells (t=0d). These inner one-third engineered constructs were cultured for 48 h before being transferred to meniscus-shaped agarose wells (t=2 day). At this time, additional ACs and MCs were rapidly thawed and combined in CHG. To form the zonal meniscus, these inner one-third engineered constructs were seeded with additional cells. Aliquots of 15 million cells, at 50% ACs and 50% MCs in 180 μL, were seeded into the meniscus-shaped agarose wells containing the inner one-third constructs. Thus, each zonal meniscus construct was seeded with a total of 20 million cells, 5 million ACs for the inner zone and 15 million ACs and MCs for the outer zone. At t=9 day, the intact zonal meniscus constructs were unconfined from their wells and transferred to agarose-coated, plastic six-well plates.
To summarize, after seeding was completed, each control or regionally variant construct contained 20 million cells in total. CHG was changed every 48 h throughout the culture period for both control and zonal engineered meniscus constructs.
Live-cell fluorescence staining
Fluorescence staining was used to assess cell migration and distribution. For the control meniscus constructs, ACs were labeled with CellTrace Far-Red DDAO-SE (Invitrogen), and MCs were labeled with CellTracker Orange CMTMR (Invitrogen). For the zonal meniscus constructs, the inner ACs were labeled with CellTracker Green CMFDA (Invitrogen); the two subpopulations of the outer cells were labeled identically to the control meniscus constructs. Briefly, each cell population was incubated at 37°C with 10 μM of cell dye for 45 min, washed and incubated at 37°C with CHG for 30 min, and washed once more with CHG. Constructs were then seeded as described above.
Construct processing
At t=4 weeks, constructs were weighed, photographed, and divided for further testing. For quantitative biochemistry and compression testing, 2 mm punches were used for the control meniscus constructs and 1.5 mm punches were used for both the inner and outer zones of the zonal meniscus construct. For circumferential tensile testing, samples were taken from the long edges of the control constructs as well as the inner and outer zones of the zonal meniscus constructs. For radial tensile testing, samples were designed to include the interface between the inner and outer zones; the same location and shape were used for controls. The remaining construct tissue was used for histology and biochemistry.
Quantitative biochemistry
Wet and dry weights of the samples designated for biochemical analysis were obtained. For the zonal constructs, samples were taken from the inner and outer zones and evaluated separately. Samples were digested for 18 h at 65°C using a 100 mM phosphate buffer, 5 mM EDTA, 5 mM N-acetyl-L-cysteine, and 125 μg/mL papain (Sigma). Total collagen was quantified by hydroxyproline.29 Sulfated GAG was quantified using the Blyscan kit (Biocolor, Newtownabbey, Northern Ireland). Total DNA was quantified using PicoGreen (Invitrogen).
Compression testing
Before testing, samples were photographed to determine the diameter and then placed in PBS. After height detection (set at 0.02 N) and preconditioning at 5% of the sample thickness for 15 cycles at a rate of 1% of the sample height per second, samples underwent unconfined stress relaxation at 10% strain using an Instron Model 5565 and were allowed to relax for 500 s. A customized Matlab curve fitting program was used to determine the relaxation modulus (Eo) and instantaneous modulus (Ei).30
Tensile testing
Tensile samples were evaluated in the circumferential and radial directions using the Instron Model 5565. For zonal constructs, circumferential testing was performed on both the inner and outer zones. Dog-bone-shaped samples were photographed from the top and side views to determine geometrical properties, and then secured to paper tabs with consistent gauge lengths. The ends of the paper tabs were gripped, and samples were pulled at a constant rate of 1% of the gauge length per second until failure. Stress–strain curves were generated from the load–displacement curves and sample cross-sectional areas. Using Matlab, Young's modulus (Ey) values for both the circumferential and radial directions were determined using linear regions of the stress–strain curves.
Histology
Portions of constructs were frozen at −20°C in HistoPrep (Fisher Scientific) with orientation marked. For zonal constructs, inner and outer regions were also noted. Samples were sectioned at 14 μm onto glass slides, warmed at 37°C overnight, and fixed in formalin. Safranin-O/fast green for GAG distribution and picrosirius red for collagen were applied.31
Statistical analysis
Each group consisted of n=8 for biochemical and biomechanical assessment. Data were analyzed using single-factor ANOVA (p<0.05) with control, inner meniscus zone, and outer meniscus zone levels, and the Tukey's HSD post hoc test was then used to determine differences among groups when warranted. The Student's t-test (p<0.05) was performed for geometric properties, wet weight, and other data as noted in specific figures.
Results
Gross morphology and histology
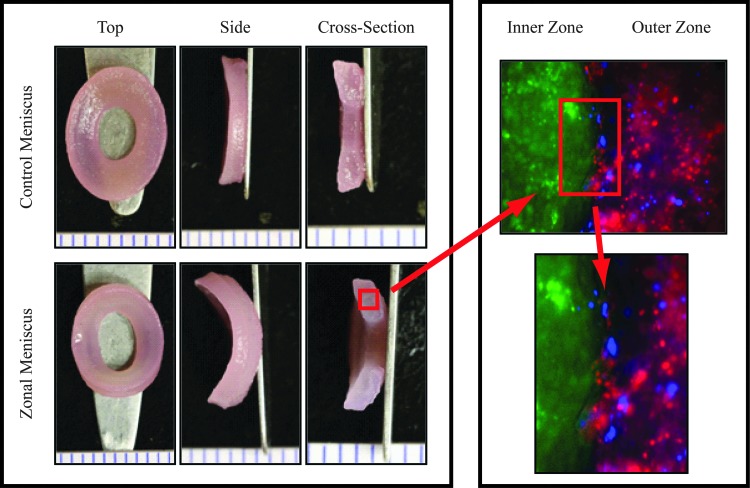
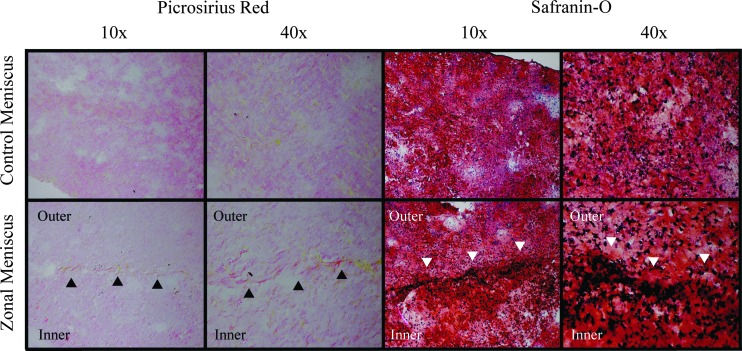
Figure 1 shows representative images of all groups. Although slight, significant size differences between the control and zonal constructs were observed (Table 1 and Fig. 1), all constructs exhibited a wedge-shaped cross-sectional profile resembling native tissue. The zonal constructs are comprised of two zones of cells, as denoted by fluorescence cell imaging; the inner zone cells (green fluorescence) remained separated from the outer zone cells even after 4weeks in culture. At the interface of the two zones, there are cells that may have migrated from their respective zones into the other (Fig. 1). Since the agarose casting method created a flat edge for integration, the absence of a sharp edge between the inner and outer zones implies either cell migration or matrix interdigitation. Histologically, there were no differences between the outer zone of the zonal meniscus constructs and the control meniscus constructs for both Safranin-O and picrosirius red staining (Fig. 2). However, the inner zone of the zonal constructs displayed apparent differences for picrosirius red when compared to the control and outer meniscus zone. This trend was also observed in the biochemical and biomechanical data.
FIG. 1.
Gross morphological images of control and zonal engineered meniscus constructs. Images display a top, side, and cross-sectional view for each group. A corresponding fluorescent cell image denoting articular chondrocytes (ACs) and meniscus cells (MCs) for the zonal engineered menisci is shown on the right. The inner zone ACs are labeled in green, while the outer zone ACs and MCs are labeled blue and red, respectively. Fluorescently labeled cell images indicate matrix interdigitation of the inner and outer zones.
Table 1.
Geometric Properties of Control and Zonal Meniscus Constructs
| Min. inner (mm) | Min. outer (mm) | Maj. inner (mm) | Maj. outer (mm) | Thickness (mm) | Wet weights (mg) | |
|---|---|---|---|---|---|---|
| Control meniscus |
3.33±0.15 |
4.5±0.30 |
8.61±0.19 |
11.63±0.33* |
1.94±0.24 |
101.6±6.37 |
| Zonal meniscus | 3.53±0.16* | 5.57±0.09* | 8.45±0.18 | 11.1±0.41 | 2.4±0.16* | 106.62±5.32 |
Construct wet weights are also provided. Values are shown as mean±standard deviation. Groups were evaluated using the Student's t-test (p<0.05). Asterisks (*) indicate significant differences between groups.
FIG. 2.
Picrosirius red staining for control and zonal engineered constructs imaged at 10× and 40× are shown on the left. Safranin-O staining for control and zonal engineered constructs imaged at 10× and 40× are shown on the right. Arrowheads indicate the interface between inner and outer zones. Color images available online at www.liebertpub.com/tea
Quantitative biochemistry
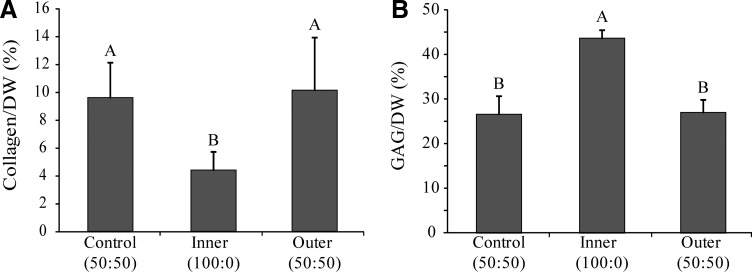
GAG/DW exhibited significant differences among groups, p<0.0001 (Fig. 3). The inner zone (43.7%±1.8%) contained significantly higher GAG, compared to the control group (26.5%±4.3%) and the outer zone (27.0%±3.0%), respectively.
FIG. 3.
Construct biochemical properties. (A) Collagen per dry weight. (B) GAG per dry weight. Data are presented as mean±standard deviation. Significance among groups was determined using single-factor ANOVA and the Tukey's HSD post hoc test (p<0.05). Groups with different letters denote significance.
Collagen/DW exhibited statistically significant variations among groups, p<0.0014 (Fig. 3). The control group (9.6%±2.5%) and the outer zone (10.2%±3.8%) were significantly higher than the inner zone (4.4%±1.4%).
Construct hydration exhibited significant differences among groups. The control group (92.6%±1.7%) was significantly higher than the inner zone (89%±1.5%) and outer zone (89.6%±0.9%). Additionally, the control group exhibited the highest amount of DNA/DW (15.3±2.4 μg/mg), as compared to the outer and inner zones, which showed an average of 12.6±2.2 μg/mg and 8.6±0.8 μg/mg, respectively.
Biomechanical testing
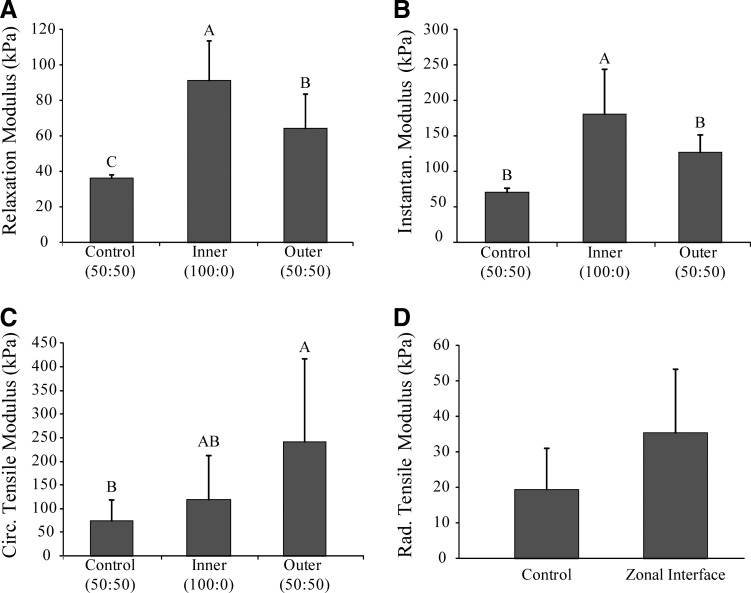
Results for the compressive and tensile testing for the control and zonal meniscus constructs are shown in Figures 4 and 5. The inner zone had the highest compression properties for both instantaneous and relaxation moduli (180.5±64.3 kPa and 91.6±22.2 kPa, respectively). Compressive properties of the control group and outer zone were significantly lower (p<0.0001) for the instantaneous modulus (71.1±6.0 and 126.9±25.1 kPa, respectively) and relaxation modulus (36.1±2.4 and 64.4±19.4 kPa, respectively). Conversely, for circumferential tensile testing, the outer meniscus zone exhibited a higher value (240.5±178.1 kPa) compared to the inner meniscus zone (119.4±93.4 kPa) and a significantly higher value (p<0.0467) than the control group (74.4±44.8 kPa). Additionally, the zonal meniscus constructs showed a radial tensile modulus of 35.5±18.0 kPa, which trended higher than the control group (19.4±11.9 kPa). The similar radial tensile modulus values between the zonal and control meniscus constructs suggest that the inner and outer zones of the engineered zonal meniscus constructs have successfully integrated. These data demonstrate that the two zones, which were seeded separately, fully integrated together since the interface between them was biomechanically indistinguishable from constructs that do not contain an interface.
FIG. 4.
Construct biomechanical properties. (A) Compressive, 10% relaxation modulus. (B) Compressive, 10% instantaneous modulus. (C) Circumferential Young's modulus. (D) Radial Young's modulus. Data are presented as mean±standard deviation. Significance among groups was determined using single-factor ANOVA and the Tukey's HSD post hoc test (p<0.05). Groups with different letters denote significance.
FIG. 5.
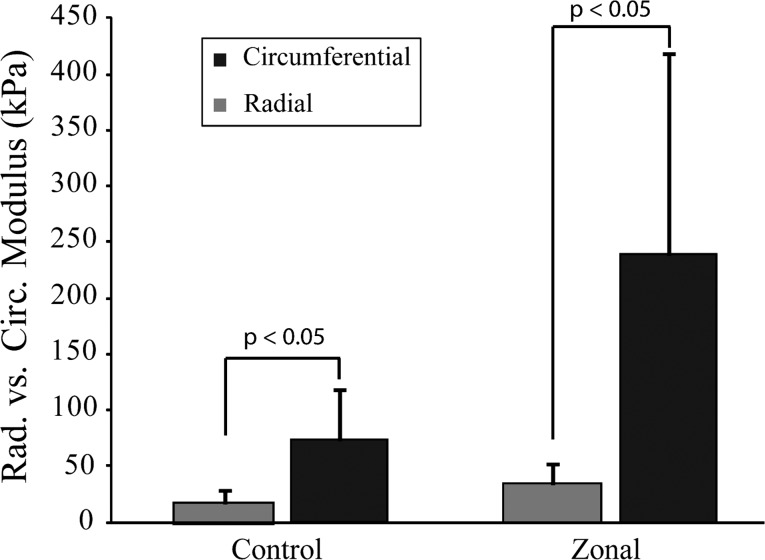
Comparison of radial and circumferential tensile modulus values for control and zonal engineered menisci to evaluate anisotropy. Data are presented as mean±standard deviation. Significance between groups was determined using the Student's t-test.
Discussion
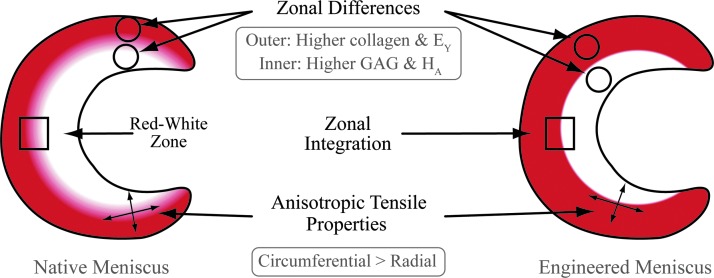
In this study, a self-assembling process was employed to produce neo-fibrocartilage tissue that mimicked the different zones of the meniscus both biomechanically and biochemically (Fig. 6). The global objective was to create an anisotropic meniscus with zonal variations akin to native tissues by applying a novel, spatially and temporally varying seeding technique. The first hypothesis, that culturing an inner meniscus zone with 100% ACs would result in higher compressive properties for this zone relative to the outer zone, was confirmed. A second hypothesis, that the outer meniscus zone, which comprised 50% ACs and 50% MCs, would display higher tensile properties relative to the inner meniscus zone, was also confirmed. Last, the third hypothesis, that the inner and outer meniscus zones will show integration, was validated through histology and tensile testing. Previous work in the field has successfully produced tissue-engineered meniscus constructs,32,33 but to our knowledge, this is the first study to engineer menisci with both inner and outer zones by integrating the two together.
FIG. 6.
Schematic of native and engineered menisci demonstrating recapitulative biomechanical and biochemical differences in the inner and outer zones. Color images available online at www.liebertpub.com/tea
Engineering a zonally variant meniscus is important if the neotissue is to function in the joint, where nonhomogeneous loading is encountered. Specifically, the native inner meniscus is stiffer and stronger in compression as compared to its outer counterpart.8 Previous work that engineered menisci using varying cell ratios, from 100% ACs to 100% MCs showed that tissues formed using 100% ACs had significantly higher compressive properties relative to those formed using AC and MC cocultures.23 Utilizing this knowledge, the meniscus constructs formed in this experiment were engineered using an inner zone that contained 100% ACs. The result was an inner zone with 42% higher instantaneous and relaxation compressive modulus values than the outer zone. In addition to recapitulating zonal differences, the zonal meniscus constructs also compare well with the compressive properties of native tissue. For instance, the anterior meniscus has an aggregate modulus of 160±40 kPa and the central and posterior portions have an aggregate modulus of 100±30kPa.34 Consequently, it is exciting to note that the control group, outer zone, and inner zone exhibited instantaneous moduli of 71.1±6.0, 126.9±25.1 kPa, and 180.5±64.3 kPa, respectively, which match native tissue values.
Since it is well-established that cartilage compressive properties correlate with the GAG content, the differences in compressive properties seen in this experiment can likely be attributed to the differences in GAG content caused by seeding the inner zone with a higher percentage of chondrocytes. In previous studies, chondrocytes have been shown to process aggrecan differently from fibrochondrocytes during matrix production.35 In this study, the GAG content for the inner meniscus zone was 61% and 64% higher compared to the outer meniscus zone and the control group, respectively. Consequently, the higher GAG content for the inner meniscus zone supports the higher compressive moduli relative to the outer meniscus zone. Indeed, the magnitude of GAG difference (1.6-fold) is reflected in the difference in compressive properties (1.4-fold). In the native tissue, a higher proteoglycan content and compressive properties are seen in the inner one-third of the meniscus to reflect its function under higher compressive loads.17,36,37 The zonal differences seen here are thus recapitulative of those of the native meniscus.
In contrast to the compressive loading experienced by the inner one-third of the native meniscus, the outer two-thirds are subjected to higher tensile forces. Due to the wedge-shaped cross section of the meniscus and the anterior and posterior horns, menisci are displaced radially and subjected to circumferential tensile loads.1,38 Structurally, this is reflected in circumferential collagen fibers.13 Replicating this feature is thus important for engineered menisci. In this experiment, the engineered zonal constructs displayed a 101% higher circumferential tensile in its outer zone, compared to the inner zone. A corresponding 129% increase in the total collagen content in the outer zone was also observed relative to the inner meniscus zone. While the tissue-engineered zonal meniscus constructs demonstrate regional variations, the tensile stiffness still falls short compared to native tissue.39 Currently, this is a common problem not only for engineered fibrocartilage such as the meniscus, but also for other orthopedic soft tissues, such as tendons and ligaments. Recently, tensile properties for engineered cartilage have been enhanced by increasing collagen crosslinks. Specifically, copper, a cofactor for lysyl oxidase (LOX), and hypoxia, which regulates LOX, have been manipulated to enhance crosslinks and therefore tensile properties.40,41 Additionally, collagen crosslinks have been used to enhance cartilage integration.42 Conceivably, the same system can be exploited to enhance the tensile properties of the zonal interface of the menisci engineered here.
Akin to the native meniscus, further evidence of anisotropy was seen when comparing the circumferential to radial tensile properties. For the control group, the circumferential tensile modulus was approximately four times the radial tensile modulus. In contrast, the circumferential tensile modulus for the engineered outer meniscus zone was approximately seven times the tensile modulus tested in the radial direction (Fig. 5). For reference, the tensile modulus in the circumferential direction of the human meniscus is approximately ten times the radial tensile modulus.43,44 Few other studies have generated fibrocartilage with anisotropic properties, and these are often formed using scaffolds.45,46 Previously, the zonal differences of the meniscus were engineered with compressive values of approximately 150 kPa (inner zone) and 50 kPa (outer zone) by using a rotary Cell Culture System (RCCS).47 In comparison, this study produced similar instantaneous moduli for the inner and outer zones (180.5±64.3 kPa and 126.9±25.1 kPa, respectively). A difference between the two studies is in scaffold use, a hyaluronan-based mesh was used to produce zonal meniscus tissue in RCCS culture, whereas the current study recapitulated the native meniscus geometry in a scaffold-free manner. Significantly, the scaffold-free method not only generates a zonal meniscus, but also results in anisotropic behavior similar to the native meniscus.
Interestingly, the biomechanical data suggest that the presence of an inner meniscus zone consisting solely of ACs may affect the resulting properties of the outer meniscus zone. Specifically, while the outer meniscus contained the same mixture of cells as the controls, different biomechanical properties were observed between these two groups. With respect to compressive stiffness, the outer meniscus zone exhibited a relaxation modulus intermediate to the inner meniscus zone and the control group. This may be explained by cell signaling factors, excreted by the AC-only inner meniscus, acting on the outer meniscus zone.48 The outer meniscus zone also exhibited higher circumferential tensile and radial moduli compared to control. Since the inner zone contained ACs that are more metabolically active than fibrochondrocytes in vitro, it is possible that the tissue produced in the inner zone expanded and pushed against the outer zone. It is known that engineered cartilaginous constructs respond to mechanical forces49; the inner zone pushing against the outer zone would thus accentuate the deviatoric stresses previously determined to lead to circumferential alignment and tensile properties.23,44 To summarize, the presence of two distinct zones in the engineered menisci facilitated the development of biomechanical properties. Whether this is due to paracrine signaling or due to mechanotransduction would require additional investigations.
One of the main hypotheses examined in this study is that the two separately seeded zones would integrate. Previous studies have produced articular cartilage constructs with different zones in an attempt to recapitulate native tissue,50–52 but functional integration between the zones of engineered menisci has not been demonstrated. In this study, the problem was overcome in vitro as inner and outer menisci were integrated as shown by histology, fluorescent cell labeling, and radial tensile testing (Figs. 1, 2, and 4). Compared to the control, robust integration was seen despite the additional manipulations that caused cell loss during the formation of the zonal meniscus.
The inner and outer zones successfully integrated yet remained distinct. As shown in Figure 2, the Safranin-O staining displays interdigitation of the extracellular matrix of the inner and outer zone. Furthermore, more nuclei are seen at the interface between the zones, suggesting that the higher cell density at this location facilitated matrix integration (Fig. 2, arrows). Integration may have also been assisted by cell migration (from the inner to the outer zone and vice versa) and subsequent matrix production to result in an interdigitated interface (Fig. 1). Finally, through biomechanical assessment, the two integrated zones behaved as if the interface between them did not exist, that is, no significant difference for the radial tensile modulus was observed between the integrated construct and the control (35.5±18.0 kPa and 19.4±11.9 kPa, respectively).
Cartilaginous tissues are notoriously difficult to integrate due to (1) rich GAG content that repels the tissues, (2) dense collagen, and (3) a dearth of metabolically active cells at the interface.8,53,54 It is likely that the integration seen here was facilitated by the following reasons. First, since the outer meniscus contained comparatively lower GAG content than the inner meniscus, a lower barrier for integration of this zone may exist. Second, a dense collagen network may not exist at the time the two zones were integrated. It has been shown previously in self-assembled constructs that, up to 10d, the collagen type and content are still in flux.55 Finally, at these early time periods, the cells are also more metabolically active.55
Engineering a zonal meniscus construct has potential clinical implications to both total and partial meniscectomies. For example, in the ovine model, a hyaluronic-acid-polycaprolactone scaffold with autologous chondrocytes was used to replace the entire meniscus to result in a higher morphological score as compared to total meniscectomy, with signs of integration and vessel ingrowth.56 The zonal meniscus constructs in this study can similarly be envisioned as full meniscus replacements. It is also possible to trim the zonal meniscus construct into an appropriate implant, as alternatives to partial meniscectomy, to address meniscal tears in either the vascular or avascular zones.
Conclusion
In conclusion, zonal variations of meniscus constructs can be achieved using a spatially and temporally varying seeding technique. Results indicate that the constructs were able to show significant differences in biomechanical and biochemical properties with respect to zone. To our knowledge, this is the first time a meniscus construct has been engineered by fully integrating two distinct zones within a meniscus construct, while simultaneously mimicking zonal variations in biomechanical and biochemical properties and anisotropy. Future studies will explore varying stimulation treatments aimed to maximize the efficacy of these meniscus constructs. Overall, this study presents a novel approach in tissue engineering for designing an anisotropic, zonal meniscus construct.
Acknowledgments
The authors wish to acknowledge funding from R01AR047839 and R01DE019666.
Disclosure Statement
No competing financial interests exist.
References
- 1.Walker P.S., and Erkman M.J.The role of the menisci in force transmission across the knee. Clin Orthop Relat Res 109, 184, 1975 [DOI] [PubMed] [Google Scholar]
- 2.Hoshino A., and Wallace W.A.Impact-absorbing properties of the human knee. J Bone Joint Surg Br 69, 807, 1987 [DOI] [PubMed] [Google Scholar]
- 3.Radin E.L., de Lamotte F., and Maquet P.Role of the menisci in the distribution of stress in the knee. Clin Orthop Relat Res 185, 290, 1984 [PubMed] [Google Scholar]
- 4.Vangsness C.T., Jr., Akl Y., Marshall G.J., Subin W., and Smith C.F.The effects of the neodymium laser on meniscal repair in the avascular zone of the meniscus. Arthroscopy 10, 201, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Arnoczky S.P., and Warren R.F.The microvasculature of the meniscus and its response to injury. An experimental study in the dog. Am J Sports Med 11, 131, 1983 [DOI] [PubMed] [Google Scholar]
- 6.Rubman M.H., Noyes F.R., and Barber-Westin S.D.Arthroscopic repair of meniscal tears that extend into the avascular zone. A review of 198 single and complex tears. Am J Sports Med 26, 87, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Rodkey W.G., Stone K.R., and Steadman J.R.Prosthetic meniscal replacement. In: Finerman G.A.M., and Noyes F.R., eds. Biology and Biomechanics of the Traumatized Synovial Joint: The knee as a model. Rosemont: American Academy of orthopaedic Surgeons, 1992 [Google Scholar]
- 8.Athanasiou K.A., and Sanchez-Adams J.Engineering the Knee Meniscus. San Rafael, CA: Morgan & Claypool, 2009 [Google Scholar]
- 9.Stone K.R.Current and future directions for meniscus repair and replacement. Clin Orthop Relat Res 367Suppl, S273, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Cox J.S., Nye C.E., Schaefer W.W., and Woodstein I.J.The degenerative effects of partial and total resection of the medial meniscus in dogs' knees. Clin Orthop Relat Res 109, 178, 1975 [DOI] [PubMed] [Google Scholar]
- 11.McDermott I.D., and Amis A.A.The consequences of meniscectomy. J Bone Joint Surg Br 88, 1549, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Herwig J., Egner E., and Buddecke E.Chemical changes of human knee joint menisci in various stages of degeneration. Ann Rheum Dis 43, 635, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sweigart M.A., and Athanasiou K.A.Toward tissue engineering of the knee meniscus. Tissue Eng 7, 111, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Kempson G.E., Muir H., Pollard C., and Tuke M.The tensile properties of the cartilage of human femoral condyles related to the content of collagen and glycosaminoglycans. Biochim Biophys Acta 297, 456, 1973 [DOI] [PubMed] [Google Scholar]
- 15.Responte D.J., Natoli R.M., and Athanasiou K.A.Collagens of articular cartilage: Structure, function, and importance in tissue engineering. Crit Rev Biomed Eng 35, 363, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Tanaka T., Fujii K., and Kumagae Y.Comparison of biochemical characteristics of cultured fibrochondrocytes isolated from the inner and outer regions of human meniscus. Knee Surg Sports Traumatol Arthrosc 7, 75, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Nakano T., Dodd C.M., and Scott P.G.Glycosaminoglycans and proteoglycans from different zones of the porcine knee meniscus. J Orthop Res 15, 213, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Aufderheide A.C., and Athanasiou K.A.Comparison of scaffolds and culture conditions for tissue engineering of the knee meniscus. Tissue Eng 11, 1095, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Leenslag J.W., Pennings A.J., Veth R.P.H., Nielsen H.K.L., and Jansen H.W.B.A porous composite, based on a biodegradable poly(l-lactide)-polyurethane matrix and reinforced with carbon-fibers, for reconstruction of meniscus lesions. Makromol Chem Rapid Commun 5, 815, 1984 [Google Scholar]
- 20.Toyonaga T., Uezaki N., and Chikama H.Substitute meniscus of teflon-net for the knee joint of dogs. Clin Orthop Relat Res 179, 291, 1983 [PubMed] [Google Scholar]
- 21.Grande D.A., Halberstadt C., Naughton G., Schwartz R., and Manji R.Evaluation of matrix scaffolds for tissue engineering of articular cartilage grafts. J Biomed Mater Res 34, 211, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Zhao X.Q., Hu J.F., and Yu J.Comparative analysis of eubacterial DNA polymerase iii alpha subunits. Genomics Proteomics Bioinform 4, 203, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aufderheide A.C., and Athanasiou K.A.Assessment of a bovine co-culture, scaffold-free method for growing meniscus-shaped constructs. Tissue Eng 13, 2195, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Huey D.J., and Athanasiou K.A.Maturational growth of self-assembled, functional menisci as a result of tgf-beta1 and enzymatic chondroitinase-abc stimulation. Biomaterials 32, 2052, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunja N.J., Huey D.J., James R.A., and Athanasiou K.A.Effects of agarose mould compliance and surface roughness on self-assembled meniscus-shaped constructs. J Tissue Eng Regen Med 3, 521, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu J.C., and Athanasiou K.A.A self-assembling process in articular cartilage tissue engineering. Tissue Eng 12, 969, 2006 [DOI] [PubMed] [Google Scholar]
- 27.MacBarb R.F., Makris E.A., Hu J.C., and Athanasiou K.A.A chondroitinase-abc and tgf-beta1 treatment regimen for enhancing the mechanical properties of tissue-engineered fibrocartilage. Acta Biomater 9, 4626, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hadidi P., and Athanasiou K.A.Enhancing the mechanical properties of engineered tissue through matrix remodeling via the signaling phospholipid lysophosphatidic acid. Biochem Biophys Res Commun 433, 133, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woessner J.F.Determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys 93, 440, 1961 [DOI] [PubMed] [Google Scholar]
- 30.Allen K.D., and Athanasiou K.A.Viscoelastic characterization of the porcine temporomandibular joint disc under unconfined compression. J Biomech 39, 312, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg L.Chemical basis for the histological use of safranin o in the study of articular cartilage. J Bone Joint Surg Am 53, 69, 1971 [PubMed] [Google Scholar]
- 32.Ionescu L.C., Lee G.C., Huang K.L., and Mauck R.L.Growth factor supplementation improves native and engineered meniscus repair in vitro. Acta Biomater 8, 3687, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puetzer J.L., Ballyns J.J., and Bonassar L.J.The effect of the duration of mechanical stimulation and post-stimulation culture on the structure and properties of dynamically compressed tissue-engineered menisci. Tissue Eng Part A 18, 1365, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Sweigart M.A., Zhu C.F., Burt D.M., DeHoll P.D., Agrawal C.M., Clanton T.O., et al. Intraspecies and interspecies comparison of the compressive properties of the medial meniscus. Ann Biomed Eng 32, 1569, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Wilson C.G., Nishimuta J.F., and Levenston M.E.Chondrocytes and meniscal fibrochondrocytes differentially process aggrecan during de novo extracellular matrix assembly. Tissue Eng Part A 15, 1513, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vanderploeg E.J., Wilson C.G., Imler S.M., Ling C.H., and Levenston M.E.Regional variations in the distribution and colocalization of extracellular matrix proteins in the juvenile bovine meniscus. J Anat 221, 174, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scott P.G., Nakano T., and Dodd C.M.Isolation and characterization of small proteoglycans from different zones of the porcine knee meniscus. Biochim Biophys Acta 1336, 254, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Bylski-Austrow D.I., Ciarelli M.J., Kayner D.C., Matthews L.S., and Goldstein S.A.Displacements of the menisci under joint load: An in vitro study in human knees. J Biomech 27, 421, 1994 [DOI] [PubMed] [Google Scholar]
- 39.Fithian D.C., Kelly M.A., and Mow V.C.Material properties and structure-function relationships in the menisci. Clin Orthop Relat Res 252, 19, 1990 [PubMed] [Google Scholar]
- 40.Makris E.A., Macbarb R.F., Responte D.J., Hu J.C., and Athanasiou K.A.A copper sulfate and hydroxylysine treatment regimen for enhancing collagen cross-linking and biomechanical properties in engineered neocartilage. FASEB J 27, 2421, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Makris E.A., Hu J.C., and Athanasiou K.A.Hypoxia-induced collagen crosslinking as a mechanism for enhancing mechanical properties of engineered articular cartilage. Osteoarthritis Cartilage 21, 634, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Athens A.A., Makris E.A., and Hu J.C.Induced collagen cross-links enhance cartilage integration. Plos One 8, e60719, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tissakht M., and Ahmed A.M.Tensile stress-strain characteristics of the human meniscal material. J Biomech 28, 411, 1995 [DOI] [PubMed] [Google Scholar]
- 44.AufderHeide A.C., and Athanasiou K.A.Mechanical stimulation toward tissue engineering of the knee meniscus. Ann Biomed Eng 32, 1161, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Balint E., Gatt C.J., Jr., and Dunn M.G.Design and mechanical evaluation of a novel fiber-reinforced scaffold for meniscus replacement. J Biomed Mater Res A 100, 195, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mandal B.B., Park S.H., Gil E.S., and Kaplan D.L.Multilayered silk scaffolds for meniscus tissue engineering. Biomaterials 32, 639, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marsano A., Wendt D., Raiteri R., Gottardi R., Stolz M., Wirz D., et al. Use of hydrodynamic forces to engineer cartilaginous tissues resembling the non-uniform structure and function of meniscus. Biomaterials 27, 5927, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Hoben G.M., Koay E.J., and Athanasiou K.A.Fibrochondrogenesis in two embryonic stem cell lines: effects of differentiation timelines. Stem Cells 26, 422, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Nagel T., and Kelly D.J.Mechanically induced structural changes during dynamic compression of engineered cartilaginous constructs can potentially explain increases in bulk mechanical properties. J R Soc Interface 9, 777, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu H., Grynpas M., and Kandel R.A.Composition of cartilagenous tissue with mineralized and non-mineralized zones formed in vitro. Biomaterials 18, 1425, 1997 [DOI] [PubMed] [Google Scholar]
- 51.Klein T.J., Schumacher B.L., Schmidt T.A., Li K.W., Voegtline M.S., Masuda K., et al. Tissue engineering of stratified articular cartilage from chondrocyte subpopulations. Osteoarthritis Cartilage 11, 595, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Ng K.W., Wang C.C., Mauck R.L., Kelly T.A., Chahine N.O., Costa K.D., et al. A layered agarose approach to fabricate depth-dependent inhomogeneity in chondrocyte-seeded constructs. J Orthop Res 23, 134, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Khan I.M., Gilbert S.J., Singhrao S.K., Duance V.C., and Archer C.W.Cartilage integration: Evaluation of the reasons for failure of integration during cartilage repair. A review. Eur Cell Mater 16, 26, 2008 [DOI] [PubMed] [Google Scholar]
- 54.DiMicco M.A., and Sah R.L.Integrative cartilage repair: Adhesive strength is correlated with collagen deposition. J Orthop Res 19, 1105, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Ofek G., Revell C.M., Hu J.C., Allison D.D., Grande-Allen K.J., and Athanasiou K.A.Matrix development in self-assembly of articular cartilage. Plos One 3, e2795, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kon E., Filardo G., Tschon M., Fini M., Giavaresi G., Marchesini Reggiani L., et al. Tissue engineering for total meniscal substitution: animal study in sheep model—results at 12 months. Tissue Eng Part A 18, 1573, 2012 [DOI] [PubMed] [Google Scholar]