Abstract
Anterior cruciate ligament (ACL) injury and subsequent reconstructive surgery is increasing with an estimated 200,000 reconstructions performed yearly in the United States. Current treatment requires reconstruction with autograft or allograft tissue with inherent disadvantages. The development of tissue-engineered ligament replacements or scaffolds may provide an alternative treatment method minimizing these issues. The study of ligament fibroblast catabolic and anabolic responses to mechanical and biologic stimuli in three-dimensional (3D) cell culture systems is critical to the development of such therapies. A 3D cell culture system was used to measure the total content and active forms of matrix metalloproteinases (MMPs)-1, -3, and -13 to assess the potential role of the mechanical environment in regulation of matrix turnover by ligament fibroblasts. The production, retention, and secretion of MMPs by ACL fibroblasts in 3D culture were measured over a 14-day period. The total MMP content and MMP activity were determined. The level of all MMPs studied increased over 7–10 days and then reached a steady state or decreased slightly in both the collagen gels and the media. This system will now permit the study of externally applied cyclic and static strains, strain deprivation, and the potential combined role of the cytoskeleton and MMPs in matrix turnover in ligaments.
Introduction
Anterior cruciate ligament (ACL) reconstruction is the current gold standard for treatment of ACL rupture. Whereas the outcome of these surgical treatments has generally proven favorable, morbidity and rehabilitation of the graft harvest site (autografts) and immune rejection and disease transmission (allografts) have serious drawbacks.1 Synthetic ligament replacements made from woven fluorocarbons and polyesters were developed to address the weaknesses of grafts and became popular in the late 1980s to the early 1990s. Their popularity was due to ease of manufacture, excellent tensile strength, elimination of donor-site morbidity, and subsequent pain and rehabilitation. These products failed clinically due to material degradation over time, increased incidence of inflammation, and immune foreign body response.2 Thus, research investigating the potential for development of tissue-engineered ligament constructs or scaffolds, which could harness the potential of the ligament to heal, is warranted.
Tissue-engineered ligament replacements can potentially provide constructs which combine the benefits of autografts by using autologous cells or tissues, and the strength of synthetic ligaments through the development of strong biological scaffolds. The availability of three-dimensional (3D) gel culture systems permits the study of the behavior of ligament fibroblasts in a spatial orientation, which more closely resembles their position in situ,3 providing a valuable tool in the progression to engineered ligament substitutes. In the current study, we characterized the expression and activity of matrix metalloproteinases (MMPs) by ligament fibroblasts cultured in 3D collagen gels.
MMPs are the dominant enzymes involved in the regulation of matrix turnover, a key process in the maintenance of the structure and function of ligaments and repair after injury.4,5 Fibroblasts in intact ligaments synthesize mRNAs for specific MMPs, which are differentially regulated during repair following injury.6 Cultured ligament fibroblasts continue to synthesize MMPs as determined by gelatin zymography7 and mRNA quantitation.5,8–10 Characterization of the amount and activity of MMPs produced by the ligament fibroblasts is necessary to understand the role of the matrix, mechanical load, and inflammatory cytokines on the regulation and involvement of MMPs in matrix turnover. The use of 3D collagen gel cell culture provides a useful system for measuring the MMP activity and content in vitro. In this study, quantitation of the total content and active forms of MMP-1, -3, and -13 produced, retained, and secreted by ACL fibroblasts grown in 3D collagen gels was determined over a 14-day period. Previous studies in an in vitro rabbit model, conducted by our research group have demonstrated an increase in the expression of MMP-1 and -13 in response to ligament transection and subsequent stress deprivation.11 The MMPs selected for analysis in the current study were chosen for their documented role in matrix degradation and remodeling in ligaments.
Materials and Methods
Cell culture
Rabbit ACL fibroblasts were harvested from skeletally mature male New Zealand White rabbits sacrificed for other Institutional Animal Care and Use Committee (IACUC) approved studies at the Hospital for Special Surgery within 6 h of sacrifice. The ligaments were harvested under aseptic conditions and placed in a sterile medium (M199) (Gibco-Vitrogen Corporation, Carlsbad, CA) containing 10% of antibiotic–antimycotic (AbAm) solution (Gibco-Vitrogen Corporation). A portion of the ligament at both the tibial and femoral ends was trimmed and discarded. The outer synovial layer was removed by sharp dissection and the ligaments were cut into 0.5–1 cm pieces and placed into the aforementioned medium. The ligament segments were finely minced into 20–40 pieces and placed into T-75 flasks containing the M199 medium with 10% fetal bovine serum (FBS) (Gibco-Vitrogen Corporation) and 1% AbAm solution. The flasks were incubated at 37°C in a humidified atmosphere of 5% CO2–95% air in a cell culture incubator. The cells migrated out of the explants, attached to the flasks, and divided. The explant pieces were removed after 10–12 days. A confluent monolayer was routinely achieved in 2 weeks. Monolayers were then treated with 0.25% trypsin (Gibco-Vitrogen Corporation), then suspended in a cell freezing medium (90% FBS) and 10% dimethyl sulfoxide (Sigma-Aldrich, Inc. St. Louis, MO), and cryopreserved in liquid nitrogen until needed for experiments.
Preparation of 3D collagen gels
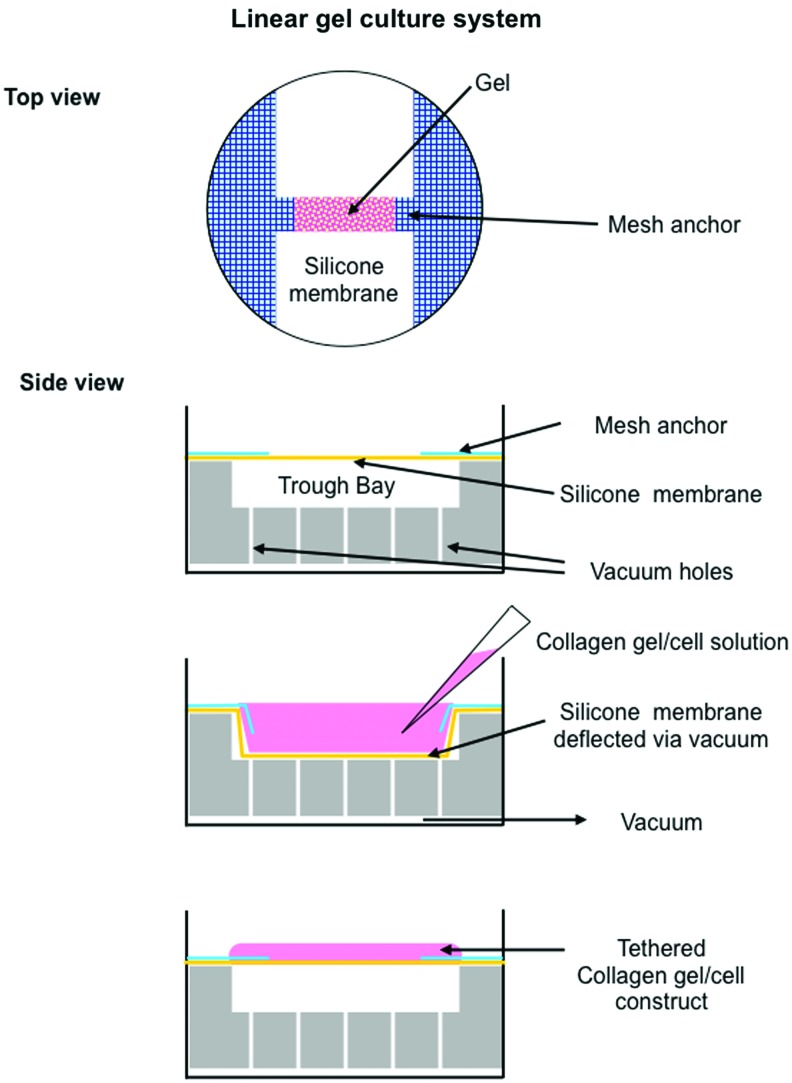
A liquid gel solution was formed by combining 8 mL PureCol collagen solution (2.9 mg/mL) (Advanced BioMatrix, Inc., San Diego, CA) with 1 mL 10× M199, 1 mL FBS, 100 mL AbAm, and pH was neutralized with 100 μL of 1N sodium hydroxide (NaOH) solution (Sigma-Aldrich, Inc.). Cells were suspended in the liquid at a final concentration of 1×105 cells/mL. Linear 3D cell-seeded gels were created using 200 μL of cell suspension in Flexcell Tissue-Train Culture plates with collagen type I-coated tabs using a trough-loading system (Flexcell International Corporation, Hillsborough, NC) resulting in linear gels that were tethered between the tabs (Fig. 1). Controls consisted of gels created in the same manner using 200 μL of gel solution without cells. Gels were set for 48 h and fed every 48 h with medium M199 containing 10% FBS, 1% AbAm, and 0.5% ascorbic acid (Sigma-Aldrich, Inc.).
FIG. 1.
Schematic of forming gels using the Flexcell Tissue Train system. Color images available online at www.liebertpub.com/tea
Sample preparation
The media and gels were collected at day 4, 5, 6, 7, 10, and 14. Gels were washed 3× with serum-free and phenol red-free 1× M199 (Gibco-Vitrogen Corporation), then incubated in 3 mL of the phenol red/serum-free medium for 24 h. Serum-free media were used to minimize the effect of serum proteins on MMP measurement and phenol red was eliminated to prevent interference with the spectrophotometric measurements. Supernatant media were collected and frozen at −80°C until activity assays and enzyme-linked immunosorbent assays (ELISAs) were performed. Gels were harvested and homogenized on ice in 300 μL of serum/phenol red-free medium and frozen at −80°C until activity assays and ELISAs were performed.
Enzyme-linked immunosorbent assay
The MMP-1, -3, and -13 content was determined using an ELISA in which all forms of MMPs present (latent, active and tissue inhibitor of metalloproteinase [TIMP]-bound) were measured, using high-binding microplates (Santa Cruz Biotechnology, Inc. Santa Cruz, CA). Specific antibodies that exhibited no immuno-cross reactivity were used: MMP-1 primary, SC58377 (Santa Cruz Biotechnology, Inc.); MMP-3 primary, AB38907 (AbCam, Cambridge, MA); MMP-13, MAB13424 (Santa Cruz Biotechnology, Inc.); and the secondary antibody SC2005 (Santa Cruz Biotechnology, Inc.). The antigen (MMP) was allowed to bind to the ELISA plates at room temperature for 30 min, washed 3× with phosphate-buffered saline (PBS), and blocked with 5% bovine serum albumin in PBS. The wells were then incubated with a primary antibody for 30 min, washed 5× with PBS containing 0.1% Tween 20, incubated with the horseradish peroxidase-linked secondary antibody for 30 min, washed 5× with PBS containing 0.1% Tween 20, and color developed with the tetramethylbenzidine peroxidase substrate (Sigma-Aldrich, Inc.). Optical densities were read at 450 nm with a TECAN Spectrafluor (Tecan Group Ltd., San Jose, CA). Protein levels were represented as ng/gel for gel lysates and ng/total media (3 mL) for media samples.
Activity assay
The MMP-1, MMP-3, and MMP-13 activity was detected using SensoLyte 520 MMP Assay Kits (AnaSpec, San Jose, CA) following the manufacturer's instructions using a 5-FAM/QXL 520 FRET peptide as the substrate. Forty-five microliters of medium and gel extract from each sample was activated using 1 mM 4-aminophenylmercuric acetate (APMA) incubated at 37°C for 1 h. Standards were made using 1, 5, 10, 15, 20, and 25 ng of the MMP-1, MMP-3, and MMP-13 recombinant protein (Chemicon International, Temecula, CA) and were activated in the same manner. Controls using nonseeded gels were tested for all MMPs with and without exogenous activated MMP proteins. Fluorescence upon cleavage was monitored at excitation/emission wavelengths of 490/520 nm, measured at 5-min intervals for 25 cycles, and the results were expressed as ng MMP per sample. The fluorescence signal was measured by a Tecan Spectrafluor Plus microplate reader (Tecan, Crailshaim, Germany).
Statistical analysis
Student's paired t-tests for two sample means (Sigma Plot 11.2, San Jose, CA) were used to assess statistical significance. Paired t-tests for two sample means were used to determine significance, all results with p-values less than 0.05 are indicated with an asterisk. MMP values at different days were compared to day 4 as reference control. A minimum of three independent experiments were performed in duplicate.
Results
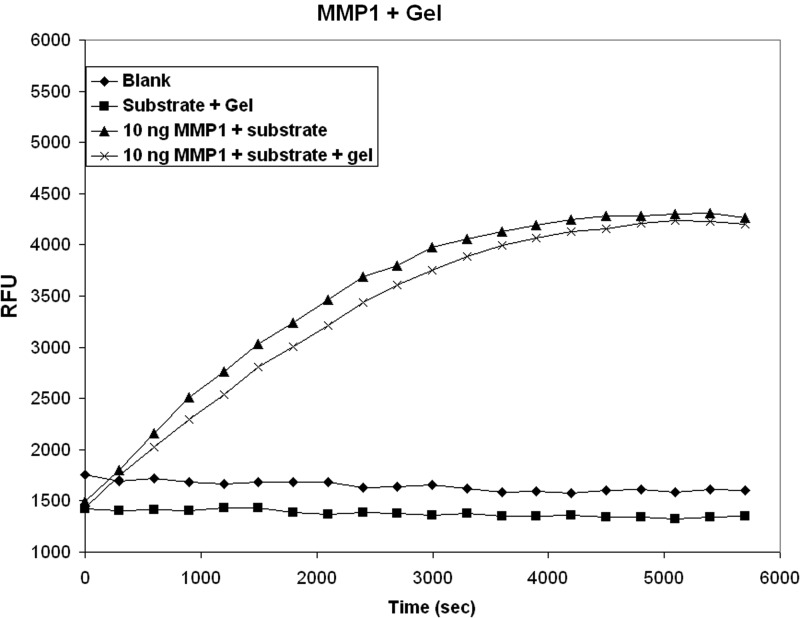
Collagen gels contracted throughout the 2-week period in culture and cells maintained a fibroblastic morphology and aligned linearly in the direction of the tethered gels. We have documented this observation in a previous study.3 MMP -1, -3, and -13 total content was first measured 4 days after cell attachment and stabilization of the gels. Day 4 values were used as reference for MMP content and activity. All measurements at subsequent time points are expressed as a percent increase or decrease compared to day 4 measurements. The control (noncell seeded) gels and media demonstrated no measurable MMP protein or activity. Control samples also had no effect when added to positive controls containing activated recombinant MMPs. A representative graph of MMP-1 activity derived from control and cell-seeded gels is shown in Figure 2.
FIG. 2.
The effect of control gel lysate on the activity of recombinant matrix metalloproteinase-1 (MMP-1). There was no activity detected from control gel lysate. There was also no significant effect of control gel lysate on activated recombinant MMP-1. RFU, relative fluorescence unit.
Matrix metalloproteinase-1
Content
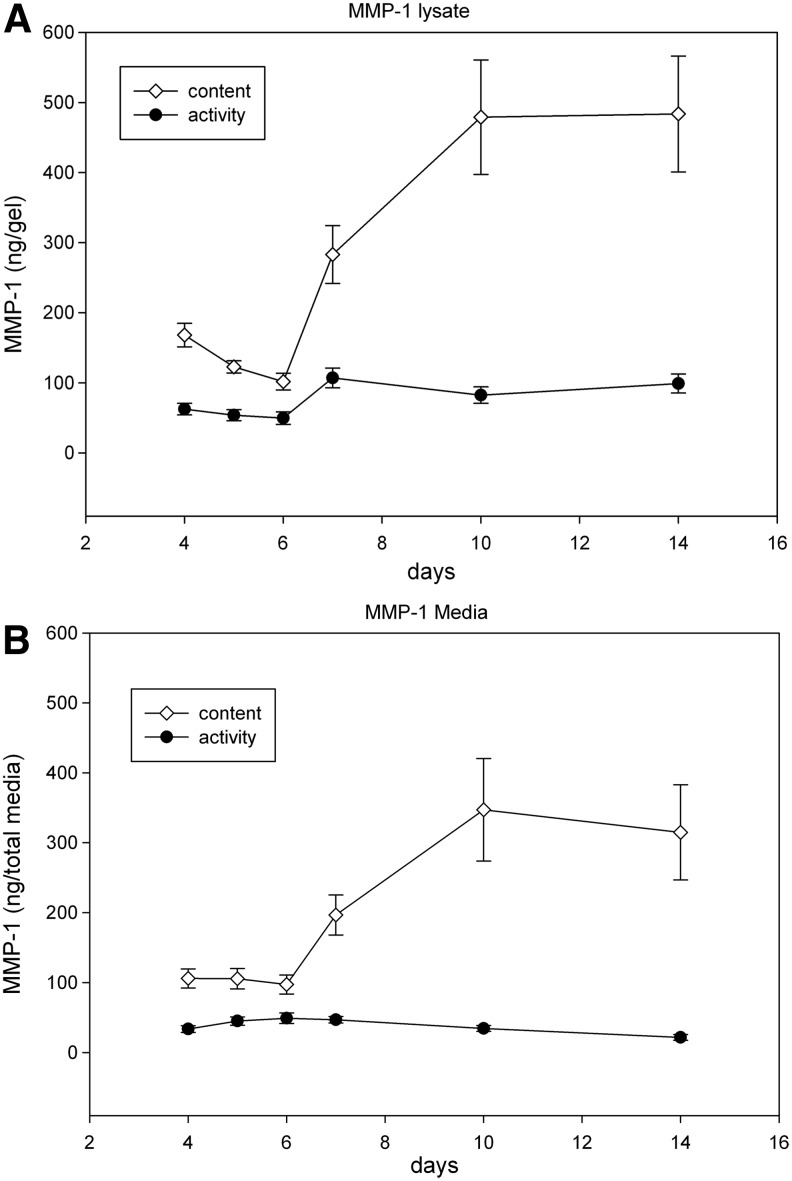
The total content of MMP-1 in lysates initially decreased from day 4 to 6, then gradually increased to 150% on day 10, reaching a plateau at day 14 (Fig. 3A). In media, the total content of MMP-1 remained constant from day 4 to 6, and gradually increased up to 250%, reaching a plateau by day 14 (Fig. 3B) (p<0.005).
FIG. 3.
The total content and active forms of MMP-1 produced by anterior cruciate ligament (ACL) fibroblasts cultured in three-dimensional (3D) collagen gels. (A) MMPs in the gel construct, (B) MMPs secreted in media. A minimum of three independent experiments in duplicate were performed. Data are expressed±SEM. p<0.005.
Activity
The active form of MMP-1 was initially stable from day 4 to 6, and then increased ∼50% at day 7 reaching a plateau at day 14. The active form of MMP-1 comprised 35–60% of the total from days 4 to 7 and 10–20% from days 10 to 14 (Fig. 3A) p<0.005. The active form of MMP-1 in media did not change significantly from day 4 to 14.
Matrix metalloproteinase-3
Content
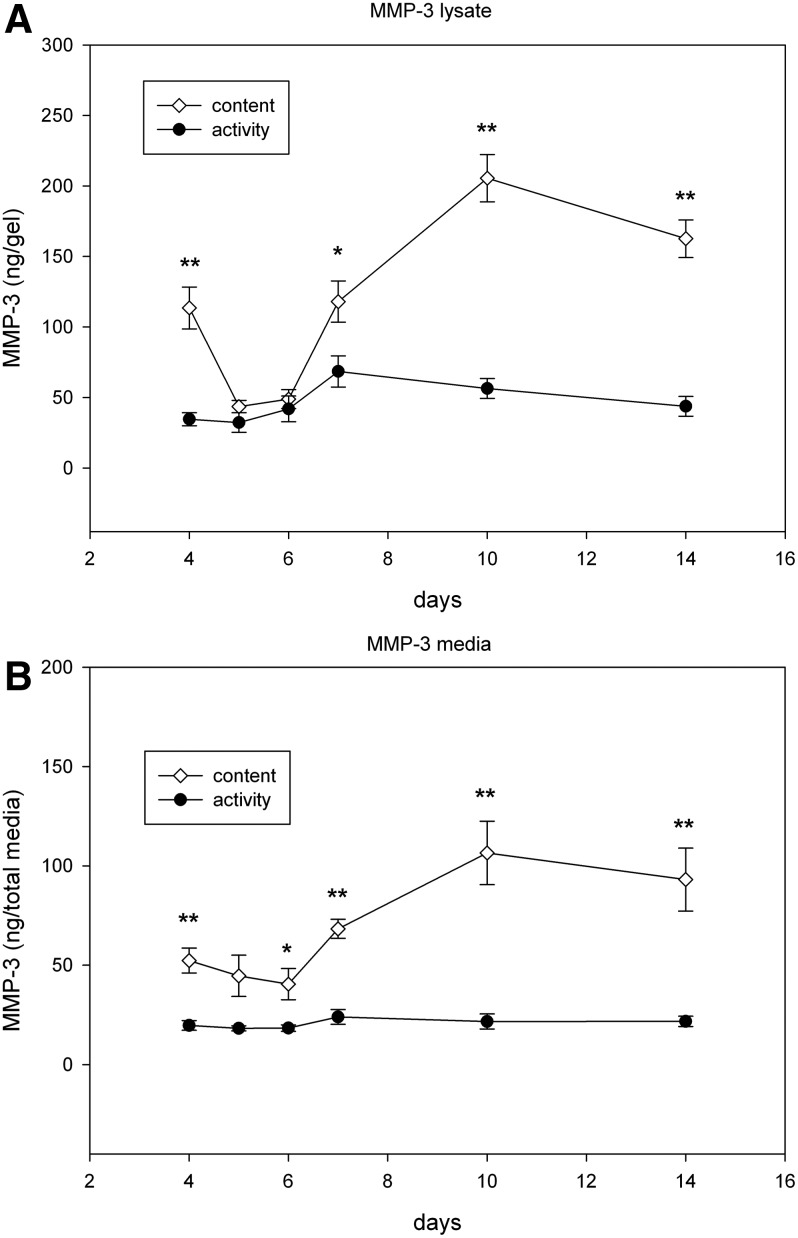
In the gels, the total content of MMP-3 decreased from day 4 to 6, then steadily increased to day 7, peaking at 80–90% by day 10, and remained high on day 14 (Fig. 4A) (*p<0.05, **p<0.001). In media, the total content of MMP-3 remained constant from day 4 to 7, increased by 100% on day 10, and then did not change significantly on day 14 (Fig. 4B) (*p<0.05, **p<0.001).
FIG. 4.
The total content and active forms of MMP-3 produced by ACL fibroblasts cultured in 3D collagen gels. (A) MMPs in the gel construct, (B) MMPs secreted in media. A minimum of three independent experiments in duplicate were performed. Data are expressed±SEM. *p<0.05, **p<0.005.
Activity
The active form of MMP-3 in the gels did not change significantly from day 4 to 14 (Fig. 4A). The active form of MMP-3 in media did not change significantly from day 4 to 14 (Fig. 4B). The highest levels of active MMP compared with the total content were detected between 4 and 6 days after plating. In the media, peak activity was detected on day 5 (22%) and decreased to 7% by day 10. Levels in the gels peaked on day 6 (47%) and dropped to 17% by day 14.
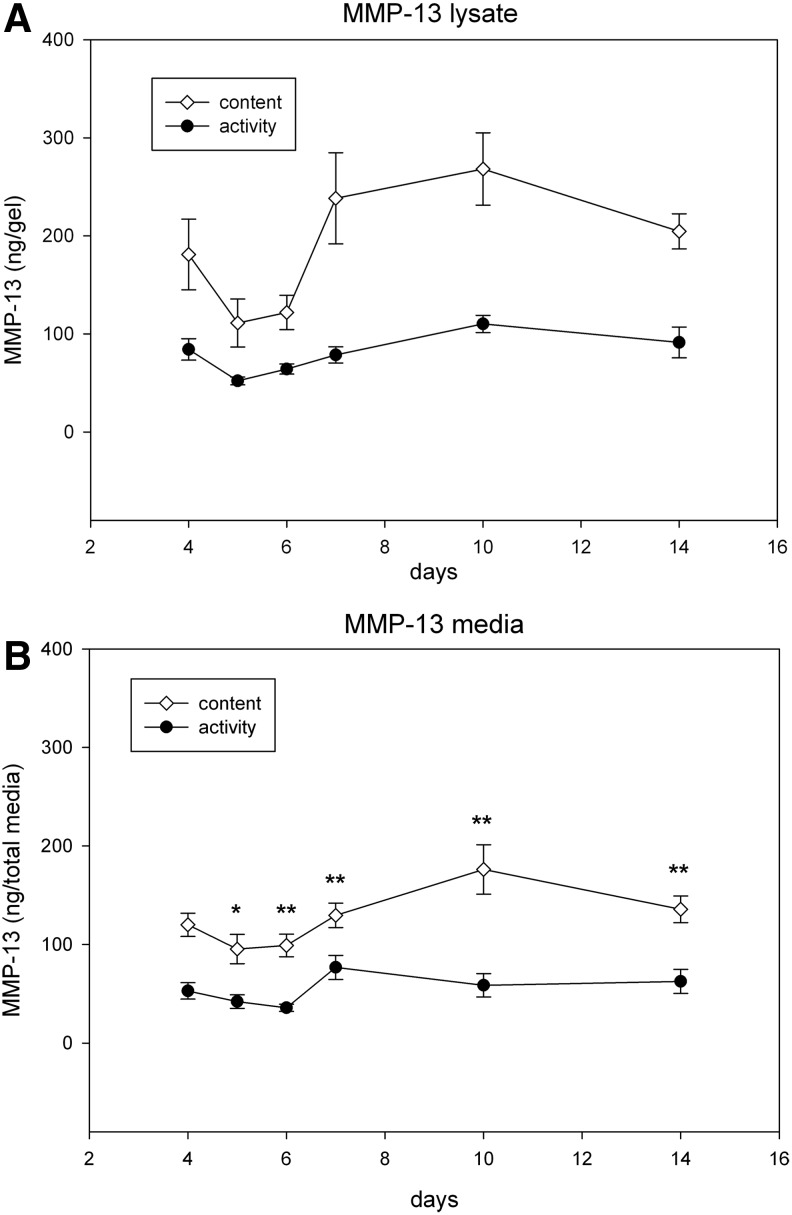
Matrix metalloproteinase-13
Content
In gels, the total content of MMP-13 decreased by 40% from day 4 to 5, gradually increasing and reaching a plateau from day 7 to 14 (Fig. 5A). p<0.005. In the media, the total content did not change significantly from days 4 to 14 (Fig. 5B).
FIG. 5.
The total content and active forms of MMP-13 produced by ACL fibroblasts cultured in 3D collagen gels. (A) MMP-13 in gels (B) MMP-13 secreted in media. A minimum of three independent experiments in duplicate were performed. Data are expressed±SEM. The activity fluctuated from day 4 to 14. In the media, activity ranged from 13% to 21% (p<0.003 in all samples), while in the lysate, activity ranged from 22% to 29%. *p<0.05, **p<0.001.
Activity
The active form of MMP-13 in the gels did not significantly change from day 4 to 14 (Fig. 5A). In media, activity of MMP-13 did not change significantly from day 4 to 14 (Fig. 5B). *p<0.05, **p<0.001.
Discussion
Regulation of matrix turnover is a key process, required for ligament development and repair following injury. This process of collagen synthesis and breakdown will prove critical in the successful development of functional tissue-engineered ligaments. Since MMPs play an important role in these processes, the characterization of MMPs present in normal and injured ligament tissue has been widely investigated using both in vivo and in vitro systems.
Several published studies have measured MMP mRNA expression in ACL tissue and monolayer culture. Foos et al. reported mRNA expression for MMP-1, -2, -3, -7, -9, -11, -14, -17, and -18 in normal ACL, whereas genes for 8, 10, 12, 13, 15, and 16 were not expressed.12 Roseti et al. reported expression of MMP-13 in normal human ACL fibroblasts, with higher expression found in fibroblasts isolated from tendon replacements following reconstruction.13 Tang et al. detected significant increases in mRNA levels of several MMPs, including MMP-1, -2, and -9 in ACL fibroblasts after an in vitro injury.14 In contrast Spindler et al. did not observe an increase in mRNAs for collagenase (MMP-1) and 72 kDa gelatinase (MMP-2) in specimens of ruptured human ACL.15 Attia et al. reported a 28-fold increase in mRNA for MMP-13 and a 3-fold increase in mRNA for MMP-1after complete transection of the AM bundle of the ACL in a rabbit partial ACL model.11 Bramono et al. reported an elevation of total mRNA levels for MMP-1, 2, and 9 postinjury in ACL tissue from patients undergoing ACL reconstruction.6 Zhang et al. compared the expression of MMPs between ACL and medial collateral ligament (MCL) fibroblasts in monolayers. They observed that all MMPs were expressed except MMP-8, 10, 12, 13, 15, 18, 20, and 26. MMP-7 mRNA was present in MCL, but not in ACL fibroblasts and mRNA for MMP-1, 2, 14, 17, 23A and 23B were higher in MCL than ACL fibroblasts. MMP-3 mRNA was higher in ACL than MCL fibroblasts.5 Another report from the same laboratory reported a sixfold increase in MMP-2 in injured ACL fibroblasts compared to MCL fibroblasts.14 Several other investigators have determined the regulation of MMP gene expression at the mRNA level in ligament fibroblasts by mechanical load and cytokines.16 Whereas understanding regulation at the transcriptional level is necessary, the activity of MMPs must also be investigated. MMP regulation is dependent on a number of factors, including the activation of latent forms and deactivation by binding to TIMPS.17,18 Several commercially available monoclonal and polyclonal antibodies raised against human, bovine, and murine MMPs-1, -3, and -13 were tested in the ELISA system for quantitating the rabbit MMPs. In spite of considerable sequence homology, many of the polyclonal and monoclonal antibodies did not react with rabbit MMPs. The antibodies against MMP-1, -3, and -13 used in these studies reacted with high affinity to specific rabbit MMPs and did not exhibit immunological cross reactivity with other MMPs, permitting us to measure each MMP in gel lysate and media. The MMP assay kits used in our studies were based on the generation of fluorogenic products when the quenched substrate was cleaved by a specific MMP. The MMP activities were linear with time up to 2 h and proportional to enzyme concentrations. A 20–30% cross reactivity of the MMP-3 substrate with MMP-1 was observed and no correction has been made for this cross reaction in our present studies.
In the present study, MMP-1, -3, and -13 were actively produced by ligament fibroblasts in 3D collagen gels, which were tethered but not dynamically loaded. During the first 4 days of culture, the ligament fibroblasts aligned parallel to the long axis of the gel, followed by subsequent gel contracture. Whereas collection of cell proliferation data was not performed for this study, we have previously shown that ACL fibroblast proliferation is negligible in this 3D gel system.19 To minimize the effect of serum proteins on MMP activity readings and to prevent the interference of phenol-red during spectrophotometric analyses, samples were washed and incubated 24 h before harvest with the serum-free, phenol red-free medium. Even though the cells continued to produce measurable amounts of MMPs, an effect on this production due to serum depletion cannot be ruled out. The dominant MMP measured was MMP-1, followed by MMP-13 and MMP-3, respectively. Despite increases in the total MMP content, the measurement of active enzyme rapidly stabilized by day 6–8, reflecting dynamic control of these enzymes in vitro. It is possible that the dynamic changes in the mechanical environment of the gel contracture influenced the cellular production and activation of the MMPs. The differences observed in active forms (expressed as percent of total) of the MMPs may also reflect differences in cellular production and binding of TIMPs. The active form of MMPs measured in this study includes both active MMPs and MMPs activated by APMA. The molecular mechanisms involved in differential retention/secretion of the three MMPs from the gel matrix into the medium are not clear and cannot be explained by simple diffusion. The volume of the media was over 100 times greater than of the gel, whereas the concentration of all MMPs within the gels was more than 10-fold than that in the culture media. The dynamic remodeling and gel contraction complicates this analysis. It is plausible, that with gel contraction, diffusion of the MMP from gel to media is impeded. Alternatively, binding of the MMP to the collagen gel or to secreted proteins may also limit diffusion from gel to media. A study of this response in a gel or scaffold that is resistant to contraction may facilitate this analysis.
The contraction of cell-seeded collagen gels has been previously described.20–25 In this study, the MMP production and activity was measured in cells cultured in 3D collagen gels under static conditions, therefore no extrinsic load was applied to the gels. Gels remained tethered throughout the experiment. As the fibroblasts aligned and realigned the collagen of the gel, the wet weight of the gels decreased with time in culture. By determining the baseline MMP expression, the next step would be to study the effect of applied mechanical load to characterize the changes in MMP activity and production in response to mechanical stimulation.
Three-dimensional gel systems offer certain advantages to monolayer and whole tissue models in the investigation of the regulation of matrix turnover by MMPs. By manipulating gel components, the composition, structure, and density of specific matrix proteins, the mechanical and structural properties of the gel or scaffold, and the effect of these changes on MMPs can be determined. The measurement of the total amount and active forms of MMPs contained within the gel can be compared to proteins secreted into the media as has been done in the present study. Cells cultured in 3D gels maintain a spatial orientation similar to cells in situ, in contrast to cells cultured in monolayer that exhibit a random alignment. The effect of mechanical load, cytokines, and the exploration of signaling pathways involved in the regulation of matrix turnover and MMPs can also be accomplished in this potentially more physiologically relevant model.26 It has been shown that ACL fibroblasts cultured in 3D gels upregulate specific integrins in response to mechanical strain, as seen in intact ligaments.3 In addition, fibroblasts cultured in 3D gels will facilitate characterization of MMPs in response to mechanical load in vitro. This will result in an improved understanding of the regulation of matrix turnover in response to mechanical stimulation, which will aid in the development of novel strategies for engineering ligament replacements.27
A potentially significant issue encountered in studies performed with cells grown in 3D gels is contraction of the gel. In the present study, the width of the gel was significantly reduced by day 5 and continued to contract throughout the duration of the experiment. Several studies have suggested that general MMP inhibitors can prevent fibroblast-induced gel contraction.20,24,28,29 Although specific MMPs involved have not been identified, MMP-2 and -9 have been implicated.30,31 Other studies have suggested that fibroblasts exhibit contractile force independent of MMPs32 through alteration in the cytoskeleton and cell–cell interactions.33 It has also been suggested that collagen reorganization as opposed to degradation is the major cause of gel contraction.34 Additional studies are needed to determine the mechanisms involved in gel contraction observed in our system. Studies are ongoing utilizing inhibitors of the cell cytoskeleton, which may shed light on this issue. Further investigation utilizing this model with gels constructed from different materials, application of mechanical strain, and the addition of growth factors may lead to advances in the development of biological equivalents for ligament and tendon replacement. In addition, a detailed understanding of cellular responses to the mechanical and biologic environment may help surgeons to predict the healing potential of partial ligament injury, where an appropriate balance between an anabolic healing response and the matrix turnover necessary for successful remodeling is required.
In summary, MMP-1, -3, and -13 are actively produced by ligament fibroblasts in 3D culture. The production, secretion, and activation of the MMPs is a highly complex dynamic system, which is responsive to the biologic and mechanical environment. The design of a biologic substrate or scaffold for ACL reconstruction will require a detailed understanding of the response of ACL fibroblasts or stem cells to the surrounding environment.
Acknowledgments
This work was supported by NIH RO1AR049339 awarded to Jo A. Hannafin. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number CO6-RR12538-01 from the NCRR, NIH.
Disclosure Statement
No competing financial interests exist.
References
- 1.Vangsness C.T., Jr., Wagner P.P., Moore T.M., and Roberts M.R.Overview of safety issues concerning the preparation and processing of soft-tissue allografts. Arthroscopy 22,1351, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Ge Z., Yang F., Goh J.C., Ramakrishna S., and Lee E.H.Biomaterials and scaffolds for ligament tissue engineering. J Biomed Mater Res Part A 77,639, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Henshaw D.R., Attia E., Bhargava M., and Hannafin J.A.Canine ACL fibroblast integrin expression and cell alignment in response to cyclic tensile strain in three-dimensional collagen gels. J Orthop Res 24,481, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Woessner J.F., Jr., Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J 5,2145, 1991 [PubMed] [Google Scholar]
- 5.Zhang J., Yang L., Tang Z., Xue R., Wang Y., Luo Z., Huang W., and Sung K.L.Expression of MMPs and TIMPs family in human ACL and MCL fibroblasts. Connect Tissue Res 50,7, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Bramono D.S., Richmond J.C., Weitzel P.P., Chernoff H., Martin I., Volloch V., Jakuba C.M., Diaz F., Gandhi J.S., Kaplan D.L., and Altman G.H.Characterization of transcript levels for matrix molecules and proteases in ruptured human anterior cruciate ligaments. Connect Tissue Res 46,53, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Zhou D., Lee H.S., Villarreal F., Teng A., Lu E., Reynolds S., Qin C., Smith J., and Sung K.L.Differential MMP-2 activity of ligament cells under mechanical stretch injury: an in vitro study on human ACL and MCL fibroblasts. J Orthop Res 23,949, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Lavagnino M., Arnoczky S.P., Tian T., and Vaupel Z.Effect of amplitude and frequency of cyclic tensile strain on the inhibition of MMP-1 mRNA expression in tendon cells: an in vitro study. Connect Tissue Res 44,181, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Kydd A.S., Achari Y., Lu T., Sciore P., Rattner J.B., and Hart D.A.The healing rabbit medial collateral ligament of the knee responds to systemically administered glucocorticoids differently than the uninjured tissues of the same joint or the uninjured MCL: a paradoxical shift in impact on specific mRNA levels and MMP-13 protein expression in injured tissues. Biochim Biophys Acta 1741,289, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Maeda E., Shelton J.C., Bader D.L., and Lee D.A.Differential regulation of gene expression in isolated tendon fascicles exposed to cyclic tensile strain in vitro. J Appl Physiol 106,506, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Attia E., Brown H., Henshaw R., George S., and Hannafin J.A.Patterns of gene expression in a rabbit partial anterior cruciate ligament transection model: the potential role of mechanical forces. Am J Sports Med 38,348, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Foos M.J., Hickox J.R., Mansour P.G., Slauterbeck J.R., and Hardy D.M.Expression of matrix metalloprotease and tissue inhibitor of metalloprotease genes in human anterior cruciate ligament. J Orthop Res 19,642, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Roseti L., Buda R., Cavallo C., Desando G., Facchini A., and Grigolo B.Ligament repair: a molecular and immunohistological characterization. J Biomed Mater Res Part A 84,117, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Tang Z., Yang L., Xue R., Zhang J., Wang Y., Chen P.C., and Sung K.L.Differential expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in anterior cruciate ligament and medial collateral ligament fibroblasts after a mechanical injury: involvement of the p65 subunit of NF-kappaB. Wound Repair Regen 17,709, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Spindler K.P., Clark S.W., Nanney L.B., and Davidson J.M.Expression of collagen and matrix metalloproteinases in ruptured human anterior cruciate ligament: an in situ hybridization study. J Orthop Res 14,857, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Majima T., Lo I.K., Randle J.A., Marchuk L.L., Shrive N.G., Frank C.B., and Hart D.A.ACL transection influences mRNA levels for collagen type I and TNF-alpha in MCL scar. J Orthop Res 20,520, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Yan C., and Boyd D.D.Regulation of matrix metalloproteinase gene expression. J Cell Physiol 211,19, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Chakraborti S., Mandal M., Das S., Mandal A., and Chakraborti T.Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem 253,269, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Wright V., Attia E., Bohnert K., Brown H., Bhargava M., and Hannafin J.A.Activation of MKK3/6, SAPK, and ATF-2/c-jun in ACL fibroblasts grown in 3 dimension collagen gels in response to application of cyclic strain. J Orthop Res 29,397, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Arnoczky S.P., Lavagnino M., Gardner K.L., Tian T., Vaupel Z.M., and Stick J.A.In vitro effects of oxytetracycline on matrix metalloproteinase-1 mRNA expression and on collagen gel contraction by cultured myofibroblasts obtained from the accessory ligament of foals. Am J Vet Res 65,491, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Weiser L., Bhargava M., Attia E., and Torzilli P.A.Effect of serum and platelet-derived growth factor on chondrocytes grown in collagen gels. Tissue Eng 5,533, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Garvin K.A., Hocking D.C., and Dalecki D.Controlling the spatial organization of cells and extracellular matrix proteins in engineered tissues using ultrasound standing wave fields. Ultrasound Med Biol 36,1919, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu S., Cui D., Yang X., Hu J., Wan W., and Zeng J.The crucial role of collagen-binding integrins in maintaining the mechanical properties of human scleral fibroblasts-seeded collagen matrix. Mol Vis 17,1334, 2011 [PMC free article] [PubMed] [Google Scholar]
- 24.Margulis A., Nocka K.H., Wood N.L., Wolf S.F., Goldman S.J., and Kasaian M.T.MMP dependence of fibroblast contraction and collagen production induced by human mast cell activation in a three-dimensional collagen lattice. Am J Physiol Lung Cell Mol Physiol 296,L236, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Raub C.B., Putnam A.J., Tromberg B.J., and George S.C.Predicting bulk mechanical properties of cellularized collagen gels using multiphoton microscopy. Acta Biomater 6,4657, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ravanti L., Heino J., Lopez-Otin C., and Kahari V.M.Induction of collagenase-3 (MMP-13) expression in human skin fibroblasts by three-dimensional collagen is mediated by p38 mitogen-activated protein kinase. J Biol Chem 274,2446, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Vunjak-Novakovic G., Altman G., Horan R., and Kaplan D.L.Tissue engineering of ligaments. Annu Rev Biomed Eng 6,131, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Bildt M.M., Bloemen M., Kuijpers-Jagtman A.M., and Von den Hoff J.W.Matrix metalloproteinase inhibitors reduce collagen gel contraction and alpha-smooth muscle actin expression by periodontal ligament cells. J Periodontal Res 44,266, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Myers S.A., and Wolowacz R.G.Tetracycline-based MMP inhibitors can prevent fibroblast-mediated collagen gel contraction in vitro. Adv Dent Res 12,86, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Davis G.E., Pintar Allen K.A., Salazar R., and Maxwell S.A.Matrix metalloproteinase-1 and -9 activation by plasmin regulates a novel endothelial cell-mediated mechanism of collagen gel contraction and capillary tube regression in three-dimensional collagen matrices. J Cell Sci 114,917, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Defawe O.D., Kenagy R.D., Choi C., Wan S.Y., Deroanne C., Nusgens B., Sakalihasan N., Colige A., and Clowes A.W.MMP-9 regulates both positively and negatively collagen gel contraction: a nonproteolytic function of MMP-9. Cardiovasc Res 66,402, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillips J.A., Vacanti C.A., and Bonassar L.J.Fibroblasts regulate contractile force independent of MMP activity in 3D-collagen. Biochem Biophys Res Commun 312,725, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Kasugai S., and Ogura H.The effects of cytoskeletal inhibitors on the collagen gel contraction by dog periodontal ligament fibroblasts in vitro. Arch Oral Biol 38,785, 1993 [DOI] [PubMed] [Google Scholar]
- 34.Guidry C., and Grinnell F.Studies on the mechanism of hydrated collagen gel reorganization by human skin fibroblasts. J Cell Sci 79,67, 1985 [DOI] [PubMed] [Google Scholar]