Abstract
The purpose of this paper was to describe SystemCHANGE™-HIV, a novel self-management intervention for people living with HIV (PLWH) and provide evidence of its initial efficacy to improve physical activity. The rationale and design of the SystemCHANGE™-HIV intervention were reviewed. Intervention detail, including its historical use, learning exercises and content, were provided. Forty PLWH participated in this pilot study, using a randomized clinical trial design. Intervention participants increased their physical activity by 300 Metabolic Equivalent minutes per week, compared to the control condition. Additionally, 70% of intervention participants increased their physical activity, whereas 65% of control subjects either had no change or experienced a decrease in physical activity. SystemCHANGE™-HIV is an innovative intervention for PLWH, representing a new paradigm in health behavior change. Findings support its potential to increase physical activity in PLWH. Future work should refine and rigorously test the effect of this intervention.
Keywords: Self-Management, HIV, Intervention
Introduction
People living with HIV (PLWH) are having longer life expectancies and experiencing increased morbidity and mortality associated with chronic co-morbidities such as cardiovascular disease, cancer, pulmonary and musculoskeletal disorders [1–7]. This longevity is due to the development and widespread use of highly effective HIV antiretroviral therapy (ART). Recent epidemiological studies of PLWH treated with ART found that 19% of all deaths in PLWH were caused by non-AIDS malignancies, 16% from AIDS, 9% from non-AIDS infections and 6.5% from myocardial infarctions [8]. Research supports the relationship between improved cardiovascular disease, diabetes, infections and even cancer outcomes with self-management behaviors, or the daily decisions and subsequent behaviors people make to manage their illnesses and promote health [9–11], with most of the evidence targeting physical activity [12,13]. Interventions to improve physical activity in PLWH may have a significant, positive impact on both HIV disease progression and the development and progression of chronic co-morbidities.
While most self-management interventions for PLWH have focused on HIV medication adherence, recent evidence suggests that physical activity can also ameliorate the burden of chronic co-morbidities and are important targets of self-management interventions [3,12,14]. Studies on non-HIV infected adults and children have targeted physical activity as a means to improve self-management [15]. Reported outcomes included improved health status and decreased depression among persons with heart failure [16,17], decrease in body weight, BMI, and abdominal circumference in morbidly obese adults [18], and decreased pain and disability among adults with knee osteoarthritis [19]. While interventions targeting an increase in physical activity in PLWH have been found to be efficacious, almost all of these interventions have relied on exercise programs supervised by a trainer or exercise physiologist in controlled settings, not at home or in the community where the PLWH lives [6,20]. An important feature of self-management interventions is that they are able to be conducted by the individual daily, in their home environment [10], leaving an important gap in the literature describing how to improve and sustain physical activity in PLWH in a manner consistent with a self-management approach.
Purpose
Recognizing this need, we assessed the feasibility of a new, systems-based self-management intervention for PLWH called SystemCHANGE™-HIV. The purpose of our paper is twofold: 1) To provide a description of the rationale and design of the SystemCHANGE™-HIV intervention including its theoretical framework, an overview of SystemCHANGE™ paradigm, the history and content of the SystemCHANGE™-HIV intervention, and give an example of how the SystemCHANGE™ paradigm was used to develop an intervention to increase physical activity for PLWH; 2) To provide evidence of its initial efficacy to improve physical activity in 40 PLWH.
Rationale and design of the SystemCHANGE™-HIV intervention
Theoretical framework
Previous physical activity interventions were developed in accordance with behavior change theories (theory of reasoned action, health belief model, theory of planned behavior, social cognitive theory) that emphasized the roles of individual motivation, self-efficacy, and individual-level barriers in changing behavior. After decades of research, “these theories have changed little and have typically been found to explain only a small percentage of variability in physical activity behavior.”[21] Instead, we used the Socioecological Model to develop the SystemCHANGE™-HIV intervention. Socioecological factors at the individual, interpersonal, and environmental levels of one’s ecosystem are critical when modifying self-management behaviors [22–24]. These factors include individual factors such as clinical signs and symptoms, interpersonal factors such as peer and family relationships and household exercise and dietary routines, and environmental factors such as the physical setting of one’s home, workplace, shopping centers and recreational spaces (walk ability and layout, food displays, and safety). The influence these multi-level factors exert on one’s daily routine make the Socioecological Model an excellent theoretical framework when designing a self-management intervention. The Socioecological Model is based on findings that a single cause of health behaviors is unlikely, that processes leading to these behaviors involve combinations of factors at multiple environmental levels. This multi-level approach (individual, interpersonal, and environmental) has been effective in combating smoking, preventing HIV, and encouraging physical activity [25–28]. Recently, scholars have called for greater emphasis on these socioecological factors in programs for PLWH, [29–32] a population often characterized by a chaotic personal environment [33].
There has been considerable work exploring the best ways to facilitate health behavior change in PLWH. Most of these self-management programs in adult PLWH have been based on cognitive-behavioral skill-building [34–37]. These interventions have had moderate success in decreasing symptom severity and increasing self-efficacy, relaxation exercises, and ART adherence [34,35,37]. Factors contributing to their success include self-monitoring, goal-setting, problem-solving, and frequent contact [35,37]. However, beneficial effects have been difficult to sustain [37]; programs were not used extensively in “real-life” settings [36]; and environmental factors were not been accounted for.
SystemCHANGE™ paradigm
The SystemCHANGE™ (Change Habits by Applying New Goals and Experience) paradigm represents a new, innovative, and effective approach to changing health behaviors. This approach focuses on redesigning a person’s multi-level environment and their daily routines to encourage healthy behaviors by ingraining the new behavior in their regular activities [38–42]. The principles of the SystemCHANGE™ paradigm are listed in table 1. This approach is designed to assist individuals and their families/support networks/households to focus on changing the daily systems in their lives (routines, events, circumstances) that affect specificself-management behaviors. The System CHANGE™ intervention has been successful at decreasing sedentary behaviors [43] suggesting that the stimuli for behavioral change may be in the multi-level environment. The SystemCHANGE™ paradigm is currently being tested in additional populations and settings [43–45].
Table 1.
SystemCHANGE Principles.
| System Improvement specifies that change is best accomplished by: |
|
|
|
|
|
|
History and content of the SystemCHANGE™-HIV intervention
In early 2011 we conducted a qualitative study with the purpose of better understanding the process of self-management in adult PLWH [46]. We found a number of important self-management strategies including increasing physical activity, taking personal time, being an HIV advocate, regulating sleep schedules, adhering with medical appointments, and attending support groups. Participants reported that these were helpful self-management strategies but that these were often neglected by the traditional medical treatment plan, which tended to emphasize biomedical interventions. Participant’s desired interventions to improve these strategies that were tailored to their specific needs and context, including many socioecological barriers [46]. While there is variation in the economic status of PLWH in developed countries, it is largely a disease of poverty with people below the poverty line being 2.3 times as likely to be infected with HIV compared to people in the same community who lived above the poverty line [47]. Limited resources lead to socioecological barriers which can impact health [48]. These barriers include being a mobile population with inconsistent housing, having low employment rates, having few assets which can provide resources for social improvement, managing the burden of living with a stigmatized disease, and dealing with stress arising from complex family, legal, and financial troubles. Given these barriers, we determined that an intervention emphasizing the role of the multi-level environment, consistent with the Socioecological Model, including the development of the interpersonal and physical environment that would be supportive of behavior change, would be the most likely to improve and maintain self-management behavior in PLWH. After surveying existing interventions, the SystemCHANGE™ intervention paradigm was consistent with our conclusion.
Based on this work, we used the SystemCHANGE™ paradigm to develop an intervention that would assist both individual PLWH and their families/support network/households to change the daily systems (routines, events, circumstances) in their lives by focusing on the multi-level system factors, and not the cognitive-behavioral factors, that affect self-management. We developed the intervention to include challenges specifically faced by PLWH. For the majority of PLWH living with limited resources, these challenges are related to being immune compromised, lack of opportunities and safe venues in which to exercise, having chronically poor sleep hygiene, and high levels of fatigue. Other modifications were made which address the relationship between exercise, lipodystrophy, and body image. Additionally, the context of HIV was incorporated into the intervention by discussing the importance of exercise even when people are feeling ill, which can reduce individual’s motivation to be active.
The SystemCHANGE™ program was originally designed to facilitate sustained exercise behavior in patients undergoing cardiac rehabilitation. It used structured intervention sessions to make small changes at the individual, interpersonal, and environmental levels which could be self-monitored and incorporated into daily routines. The behavior change content and the strategies to facilitate sustained behavior change were the same employed in previous studies on SystemCHANGE™ [43]. Consistent with this previous work, our intervention taught a set of strategies emphasizing systems thinking, and that change is best accomplished by conducting a series of small, self-designed experiments to test ideas to improve health by modifying their immediate environment to change daily routines. The most successful ideas, based on the outcomes of these self-designed experiments, are then implemented, and the participant monitors the changes to assess sustainability of the health improvement strategy. Changes to the SystemCHANGE™ intervention were made to adapt it to the needs of PLWH.
These additional changes included discussions on how to maintain immune function and the impact of HIV stigma on behavior, which were not part of the original intervention program. Other logistical modifications were made including increasing the number of group sessions (from 5–10) and holding the sessions weekly instead of every other week following the assumption that regular meetings maintained for longer periods may improve outcomes. The five additional sessions added to the SystemCHANGE™-HIV intervention were based on previous work describing the context and strategies of HIV self-management, and its impact on the health of PLWH [46]. These sessions included content describing how to improve sleep hygiene, the relationship between mental wellness and HIV stigmatization, how to increase personal time, and how to use spiritual wellbeing to improve self-management. The five initial sessions of SystemCHANGE™-HIV closely followed the content of the original exercise- based SystemCHANGE™ studies. A detailed description of the intervention content is provided in table 2.
Table 2.
SystemCHANGE™ -HIV Pilot Study Interventiona
| TOPIC | CONTENT |
|---|---|
| Session 1: Systems Thinking, Self-Monitoring & Goal Setting | |
| Introduction to SystemCHANGE: HIV | Introduce participants; Background to intervention Schedule/Content Ground Rules |
| Systems Thinking | Introducing Systems as a way to think about habits |
| Determining process owners | Involving people in your process; Who impacts your system? Distinguishing between buddies and process owners; |
| Goal Setting: Describing what you want to accomplish | |
| Self-Monitoring | Introduction on self-monitoring: What and Why |
| Session 2: Lifestyle Routines, Systems Improvement Strategies and Social Support | |
| Introduce Team Member | |
| Review Systems Thinking and Discuss first team meeting | Key points for system thinking; Team meeting reports-surprises? |
| Lifestyle Routines | Cyclical nature of routines |
| Environmental changes | List of possible environmental changes |
| Social Support | Where, why and unusual places to find |
| Session 3: Physical Activity and Self Experiments | |
| Physical Activity Recommendations | Physical activity recommendations Weather related activity Community Resources |
| Demonstrate use of exercise log | Physical Activity log: exertion levels, sensations, emotions, safe exercise |
| PDSA Cycles | Plan-Do-Study-Act (PDSA) format for testing small changes |
| Session 4: Patterns of Exercise, Types of Fitness and Testing Small Experiments | |
| Patterns of Exercise | Graph exercise from own data in exercise diary |
| Types of Fitness | Health benefits of fitness Types of fitness HIV-related concerns |
| Storyboards | Benefits of publicizing change “journey” in home Structure and steps in making a storyboard |
| Session 5: Sleep | |
| Sleep Benefits | List reasons why sleep is important |
| Environmental Sleep Barriers | Discuss actigraph patterns List barriers that make sleep is hard |
| Recommendations, Sleep Hygiene | Standard recommendations Sleep hygiene strategies Sleep rhythms |
| Session 6: Mental Wellness Behaviors | |
| Mental Wellness Behaviors | Components of Mental Wellness Mental Wellness and HIV |
| Consequences of Poor Mental Wellness | |
| Session 7: Personal Time | |
| Personal Time Introduction | Overview of importance; Findings from pilot study |
| Strategies | Finding and effectively using personal time |
| Balancing Personal Time with Isolation | What is the difference between effectively using personal time and isolating? |
| Session 8: Spirituality and Altruism as Tools to Change the Environment | |
| Review Personal Time | Review main points on persona time |
| Spirituality | Share focus group findings How can spirituality be used to change environment? How does can it support changes and hinder changes |
| Altruism | Perspectives on benefits of altruism Discuss ways to “give back” |
| Session 9: Relapse Prevention and Continual Improvement | |
| Homework Review | Share storyboard |
| Reinforcement of Systems Thinking | Discuss how they are using Systems Improvement Strategies |
| Leaning form information about patterns of exercise- recognizing a lapse in behavior | Review graphs of Physical Activity, sleep, personal time |
| Life processes and all health behaviors | Healthy living is lifelong commitment |
| Session 10: Overview and Celebration | |
| Celebration | |
Weekly homework review and session evaluation and feedback were removed for brevity
Using the SystemCHANGE™-HIV intervention to increase physical activity
We include an example of how someone can use the SystemCHANGE™ principles and intervention to achieve a specific behavior goal relating to improving physical activity. The SystemCHANGE™ strategies first encourage participants to identify a specific goal relating to a behavior they wished to change. The second step is to systematically obtain a detailed understanding of one’s own current routines in order to have a clear idea of what environmental barriers exist to meeting the chosen goal. For an example, while keeping detailed diaries of their daily activities a number of participants noticed they were exercising less than they expected due to work and family responsibilities (interpersonal factors), lack of safe opportunities to exercise in their community, and poor weather (environmental factors). These participants concluded that specific exercise times and venues needed to be worked into their daily routines to overcome these barriers.
The third step is to design and implement short trials of possible steps for improvement. In this example, participants incorporated stretching and walking into their morning routines. The timing of the stretching intentionally coincided with another part of the participant’s daily routine-taking their medication to help trigger them to complete this activity. Another participant began taking brisk walks in the morning, often to visit a nearby relative, resulting in increased activity and increased socialization. The fourth step is to self-monitor outcomes of these trials. In the SystemCHANGE™-HIV intervention this was done by documenting exercise in personal logs provided by the researchers. The goal of the personal logs was to make participants aware of their exercise behaviors and empower individuals to make changes by being actively engaged in the self-monitoring process. The fifth step was to evaluate the success of the changes by graphically displaying and visually analyzing the data, which was accomplished through creating and displaying posters documenting the SystemCHANGE™-HIV process. The last step in the SystemCHANGE™ paradigm is to make provisions for maintaining improvements.
In our exercise example, if stretching and walking did not become part of daily routine, the participants may have wanted to use an individual-level cue such as a timer or alarm to remind them it was time to exercise. At the interpersonal level, participants could use the members of their household to maintain improvements by exercising with them (as several in our study did), to exercise at set points in the household routine (such as stretching or walking up and down stairs after dinner) or by parking further away or walking to a farther bus stop when members of the household run errands. At the environmental level, participants could use strategies such as campaigning for sidewalks in their neighborhood to encourage more walking.
Consistent with the socioecological framework, participants should employ strategies at multiple levels of the framework in order to improve and maintain their physical activity, which participants did. One example of using strategies from multiple levels of the socioecological framework was a participant who set an alarm three mornings a week (individual), to get up and drive to meet a friend (interpersonal) at a nearby park (environmental) in order to walk for about 45 minutes each morning, several days a week.
By using the above strategies and disease-specific content, participants were taught how to modify their immediate environment so that they successfully increased their physical activity, despite wavering motivation. Because the individual participants, and not the research team, determined and evaluated their own behavior change, this intervention allowed for individual tailoring, cultural adaptation, and validation. Participants were taught how to identify these behavioral events, track data about these events, and evaluate potential changes. Strategies to facilitate improvements in physical activity included flow charts of daily routines, worksheets identifying a range of the ecological causes and effects on the desired behavioral change, self-defined experiments to test change, diaries to track self-reported data about cause/effect on behavior, and individual storyboards to present one’s journey through change (Table 2). In summary, the SystemCHANGE™-HIV intervention is theoretically-grounded in the socioecological model, consistent with the SystemCHANGE™ paradigm and historical use of the paradigm, while also being tailored to the unique needs of PLWH. We will next discuss the results of a small pilot study testing the initial efficacy of the SystemCHANGE™ -HIV intervention on increasing physical activity in PLWH.
Initial efficacy of the SystemCHANGE™ -HIV intervention
To assess the initial efficacy of the SystemCHANGE™-HIV intervention, a pilot study utilizing a randomized clinical trial design was conducted in February-April, 2011.
Materials and Methods
Sample and participant selection
The randomized clinical trial was approved by the Institutional Review Board at University Hospitals, Case Medical Center. We recruited participants through Infectious Disease clinics, AIDS Service Organizations, and an HIV Research Registry, all based in Northeast Ohio. Participants met the following inclusion criteria: being ≥18 years of age, speaking fluent English, and having a confirmed HIV diagnosis. We had no exclusion criteria. As a pilot study intending only to explore initial efficacy of this newly-adapted intervention, we planned to enroll approximately 40 subjects, estimating 10% attrition. Forty-three subjects were screened using a standardized telephone screening to ensure they met the inclusion criteria, enrolled in the study and attended the first appointment.
Procedures
At the first scheduled appointment, 43 participants completed the baseline assessment, were randomized to either an intervention (SystemCHANGE™-HIV intervention) or control group (where they received a copy of and were oriented to the HIV Symptom Management Strategies: A Manual for People Living with HIV/AID). Those randomized to the intervention group immediately commenced with the first of ten weekly group SystemCHANGE™-HIV intervention sessions. Each of the weekly sessions was led by a trained interventionalist and followed the topics and content described in table 2. All participants were instructed to return for a follow-up assessment immediately following the conclusion of the intervention (10 weeks), were sent a reminder letter, and received a phone call from a research assistant reminding them of the date and time of this next assessment. Forty of the 43 enrolled subjected returned for the follow-up assessments (93% retention). Participants were paid $50 for completion of each of their assessments. A more detailed discussion of the procedures used in testing the initial efficacy of the SystemCHANGE™-HIV intervention is reported elsewhere [45].
Measurement
Our physical activity outcome was assessed using the 7-item International Physical Activity Questionnaire-Short Form (IPAQ-SF) - a widely used and extensively validated measure of self-reported physical activity [49]. In a large, international study (n=1,974, 12 countries), the IPAQ-SF was found to have good test-retest reliability (pooled ρ=0.70), concurrent validity (pooled ρ=0.67), and criterion validity against accelerometer (pooled ρ=0.30) [50]. The IPAQ-SF measures duration, frequency and intensity of self-reported physical activity over the past 7 days. Data were aggregated and analyzed using the recommended truncated methodology to account for over-reporting and scored as a continuous outcome reported in Metabolic Equivalent (MET) minutes per week.
Metabolic Equivalent (MET) minutes are a standardized measure of energy expenditure due to physical activity [51]. MET is the ratio of energy expenditure during physical activity to the rate of energy expended at rest. One MET is the rate of energy expenditure while a person is at rest; whereas a 3 MET activity expends 3 times the energy used by the one at rest. For example, if a person did a 3 MET activity for 30 minutes, he or she has done 3 x 30 (or 90) MET minutes of physical activity. These MET minutes are then summed over a 7-day period to obtain the value of MET minutes per week. The U.S. Department of Health and Human Services recommends that people get 500 to 1,000 MET-minutes of activity per week for a substantial mortality benefit but this response is dose-dependent, meaning the more MET minutes achieved, the greater the benefit [51]. Given that MET-minutes are the recommended standard reporting unit of energy expenditure, and that the IPAQ-SF has a validated scoring method transforming the results into MET-minutes per week, we reported our change in physical activity outcome in MET-minutes per week.
Demographic and clinical covariates were assessed using a brief 26-item demographic survey. It included questions on age, gender, race, ethnicity, education, employment status, income level, and health insurance. Participants also consented to allow the research team to abstract the data from their medical chart to obtain information on comorbid health conditions and medication history.
Descriptive statistics were used to summarize participant’s demographic and clinical characteristics. All analyses maintained subjects in the original treatment groups (intent to treat). The treatment effect on physical activity was estimated using ANCOVA models looking at the difference between baseline and end-of-study responses. Our analyses were adjusted for age, sex, and years since HIV diagnosis. These covariates were chosen a priori as they have been shown to affect physical activity in PLWH [6].
Results
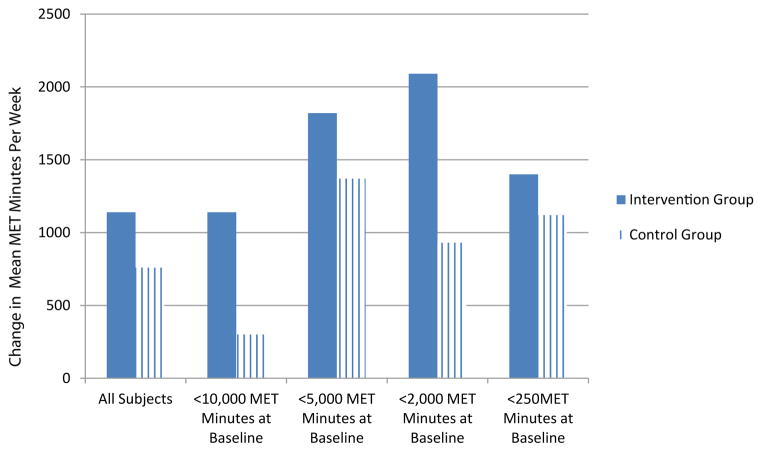
Forty adults living with HIV completed both the baseline and follow-up physical activity assessment using the IPAQ-SF. The pilot study was not powered for statistical significance; however, lessons learned from this study will inform larger studies which can statistically validate the findings. The demographic characteristics of our sample are reported in table 3. In determining the initial efficacy of the SystemCHANGE™-HIV intervention on physical activity, we found that those in the intervention group increased their physical activity by 300 Metabolic Equivalent (MET) minutes per week, compared to those in the control group (p=0.743). After adjusting for baseline physical activity level, age, sex, and years since HIV diagnosis, those in the intervention had an average of 380 MET minutes per week increase in physical activity, compared to those in the control group (p=0.687). This is equivalent to approximately a 2 hour increase in walking per week. Also, in reviewing individual difference scores, we found that 70% (n=14) of participants in the intervention group increased their weekly physical activity, whereas 65% (n=13) of participants in the control group either had no change or experienced a decrease in physical activity. We were also interested in estimating the differential effects of physical activity by baseline physical activity level in order to help determine which population of PLWH might benefit the most from the SystemCHANGE™-HIV intervention and found that those with a baseline physical activity of less than 2,000 MET minutes per week had the greatest benefit (an increase of 1180 MET Minutes per week; p=0.260). Complete results, including all subgroup analyses, are in table 4, Supplemental table 1, and figure 1.
Table 3.
Demographic and Clinical Characteristics of Participants
| Control Group (n=22) Frequency (%)a |
Intervention Group (n=21) Frequency (%)a |
|
|---|---|---|
| Mean Age (+/−SD), years | 47.8 (6.4) | 49.1 (7.4) |
| Female | 9 (40.9) | 9 (42.9) |
| African American | 18 (90.0)b | 18(85.7) |
| White/Angelo | 2 (10.0)b | 2 (9.5) |
| Other | 0 | 1 (4.8) |
| Marital Status | ||
| Single | 17 (77.3) | 17 (81.0) |
| Divorced | 4 (18.2) | 2 (9.5) |
| Other | 1 (4.5) | 2 (9.5) |
| Education Level | ||
| 11th grade or less | 7 (31.8) | 6 (28.6) |
| High School or higher | 15 (68.2) | 15 (71.4) |
| Annual Income | ||
| No monthly income | 5 (22.7) | 3 (14.3) |
| $1–$599 | 6 (27.3) | 4 (19.1) |
| $600–$999 | 9 (40.9) | 13 (61.9) |
| $1000 or more | 2 (9.1) | 1 (4.8) |
| Currently Works for Pay | 3 (13.6)c | 1 (4.8) |
| Has Permanent Housing | 17 (77.3)d | 21 (100) |
| Has Health Insurance | 17 (77.3) | 20 (95.2) |
| Type of Health Insurance | ||
| Medicaid | 9 (40.9) | 11 (52.4) |
| Medicare | 2 (9.1) | 3 (14.3) |
| Private, not by work | 3 (13.6) | 2 (9.5) |
| Other | 1 (4.5) | 1 (4.8) |
| Mean Years Since HIV Diagnosis (+/− SD) | 13 (7.2) | 14 (5.8) |
| Currently Taking HIV Anti-Retroviral Medication | 21 (95.5) | 20 (95.3) |
| Have Comorbid Health Conditions | 18 (81.8) | 20 (95.2) |
Table 4.
Mean change in outcome variables by treatment assignment (n=40)
| Control Group | Intervention Group | Adjusted for baseline score and group assignment a | Further adjusted for demographic variablesb | |||||
|---|---|---|---|---|---|---|---|---|
| Endpoint | n | Mean Δ (+/− SD)a | n | Mean Δ (+/− SD) | Intervention – Control | p-value | Intervention – Control | p-value |
| Physical Activity (MET Minutes/Week) | 20 | 760 (2820) | 20 | 1140 (3470) | 300 (−1560, 2160) | 0.743 | 380 (−1520, 2280) | 0.687 |
| Subgroup analyses by baseline physical activity level | ||||||||
| Physical Activity (baseline <10,000 MET Minutes/Week) | 19 | 300 (2890) | 20 | 1140 (3470) | 510 (−1370, 2380) | 0.587 | ||
| Physical Activity (baseline <5,000 MET Minutes/Week) | 17 | 1370 (2350) | 17 | 1820 (3040) | 450 (−1490, 2380) | 0.640 | ||
| Physical Activity (baseline <2,000 MET Minutes/Week) | 13 | 930 (1790) | 14 | 2090 (3140) | 1180 (−930, 3280) | 0.260 | ||
| Physical Activity (baseline <250 MET Minutes/Week | 9 | 1120 (1880) | 8 | 1400 (1710) | 230 (−1690, 2160) | 0.799 | ||
Mean change from baseline to 10-week follow up;
Adjusted for age, sex, and yrs since HIV diagnosis
Discussion
SystemCHANGE™ is a novel evidence-based intervention paradigm which teaches participants to self-identify, self- monitor, and meet their own health goals. These goals are then met by making small changes to one’s environment so that ultimately, this goal is integrated into the daily routines of the individual. The unique focus of this paradigm is on the multi-level environment. An intervention approach, focusing on the impact that environment has on the spectrum of health decisions and behaviors, is a new approach to behavior change in PLWH, where most interventions have been theoretically grounded in the social cognitive theory. Recently scholars and policy makers have called for this exact shift in emphasis from social cognitive theory to socioecological theory in developing intervention strategies in PLWH [6,29–32,52]. We are among the first research groups to both heed this call and extend this theoretical model to often overlooked self-management behaviors in PLWH. Our results can be used to inform further refinement and expansion of this intervention to other important self-management behaviors for PLWH, including engagement in health care and HIV medication adherence. The SystemCHANGE™ intervention paradigm may also help us to better understand why existing interventions fail to sustain their positive benefits.
Additionally, we found initial evidence demonstrating that the SystemCHANGE™-HIV intervention increases physical activity, as measured by the IPAQ-SF, up to recommended levels, but these results are not statistically significant. The results indicated that those in the intervention group increased their self-reported physical activity overall, and for each of the subgroups analyzed. The effect sizes reveal that two of the four subgroups improved physical activity up to recommended levels. Additionally, the finding that 70% of participants in the intervention group increased physical activity compared to 65% of participants in the control group reporting either no change or a decrease in physical activity is intriguing. The wide variability in the effect and effect sizes in the control group may be related to the limitations of a self-reported outcome and social desirability. The modest and homogenous effects in the intervention group may more accurately reflect changes attributable to participation in the SystemCHANGE™-HIV intervention. However, additional evidence is warranted before drawing that conclusion.
As a pilot study of a newly adapted intervention, we did not expect the results to yield statistical significance. As expected, we saw a trend towards improvement in physical activity that with further refinement could provide statistically significant evidence of the intervention’s efficacy. One important finding was that participants who reported a baseline physical activity level of less than 2,000 MET minutes per week improved their physical activity up to 1180 MET minutes per week. This suggests that future physical activity intervention studies should carefully consider baseline physical activity as either an inclusion or stratifying criterion. Additionally, the intervention participants who engaged in less than 250 MET Minutes per week at baseline reported the smallest amount of change, compared to those in the control group. The varied results by subgroup indicate that tailoring of the intervention to the individual PLWH’s unique situation. For example, for those PLWH who are the least physically active at baseline may require a larger dose of the intervention, perhaps in the form of increased group sessions or additional consultation with a behavior change specialist. However, this trend towards an increase in physical activity in the intervention group, compared to the control group, does suggest initial efficacy of the SystemCHANGE™-HIV intervention to improve physical activity.
This pilot study has limitations which must be considered. First, out outcomes were assessed via self-report of physical activity. To address this limitation we used a common, well-validated measure of physical activity, the IPAQ-SF. Additionally, we adopted the recommended truncated scoring procedure to address potential over-reporting of physical activity. However, our results could be more precisely assessed by using another, more objective measure of physical activity, preferably one that does not rely on subject recall. Potential assessment methods to be considered in future studies include accelerometry, heart rate monitoring, and ecological momentary assessments. Additionally, participants were, on average, middle-aged, often experiencing comorbid health conditions, and were experienced living with their HIV disease. While these demographics reflect a growing population of those living with HIV in the United States [3] and worldwide [8], it is important to recognize that these intervention effects may not be applicable to young people or those who are more recently diagnosed with HIV. Additional research specifically examining ways to increase physical activity in these populations is warranted. This research should consider the impact of social networks and support which exert great influence on the behavior of youth.
In conclusion, these results, with high variability in a small sample, indicate the need to further refine and rigorously test this intervention. If shown to be efficacious, this intervention could improve physical activity and other important self-management outcomes in an efficient and comprehensive manner in PLWH. Further research in the field of exercise and HIV could lead to a significant impact for the 1.2 million PLWH in the United States, and perhaps, the 32 million PLWH around the globe. With the widespread use of antiretroviral therapies HIV is quickly becoming a chronic disease in developed countries; understanding how to improve this chronic disease through exercise may help us understand ways to make crucial changes in lifestyle among other populations burdened with non-communicable disease. These changes, in turn, can make the difference between a healthy and productive life and one continually punctuated by episodes of illness. Globally, as PLWH begin to manage HIV over many decades and experience more chronic diseases, it is crucial to explore any paradigm which may help modify behavior to improve their overall health, not just slow HIV disease progression.
Figure 1.
Change in Physical Activity after Completing the SystemCHANGE™-HIV Intervention n=40
Acknowledgments
The project was supported by the National Institute for Allergy and Infectious Disease through Grant P30AI36219; National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grants 5KL2RR024990 and UL1RR024989; and the Association of Nurses in AIDS Care/Sigma Theta Tau. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The contents of this article are solely the views of the authors and do not represent the official views of the National Institutes of Health.
Footnotes
The authors have no financial interests or potential conflicts of interest to disclose.
References
- 1.Chu C, Umanski G, Blank A, Meissner P, Grossberg R, et al. Comorbidity-related treatment outcomes among HIV-infected adults in the Bronx, NY. J Urban Health. 2011;88:507–516. doi: 10.1007/s11524-010-9540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engels EA, Biggar RJ, Hall HI, Cross H, Crutchfield A, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123:187–194. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 3.High KP, Brennan-Ing M, Clifford DB, Cohen MH, Currier J, et al. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr. 2012;60(Suppl 1):S1–18. doi: 10.1097/QAI.0b013e31825a3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kraft-Terry SD, Stothert AR, Buch S, Gendelman HE. HIV-1 neuroimmunity in the era of antiretroviral therapy. Neurobiol Dis. 2010;37:542–548. doi: 10.1016/j.nbd.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lescure FX, Omland LH, Engsig FN, Roed C, Gerstoft J, et al. Incidence and impact on mortality of severe neurocognitive disorders in persons with and without HIV infection: a Danish nationwide cohort study. Clin Infect Dis. 2011;52:235–243. doi: 10.1093/cid/ciq041. [DOI] [PubMed] [Google Scholar]
- 6.Yahiaoui A, McGough EL, Voss JG. Development of evidence-based exercise recommendations for older HIV-infected patients. J Assoc Nurses AIDS Care. 2012;23:204–219. doi: 10.1016/j.jana.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Moore RD, Keruly JC, Bartlett JG. Improvement in the health of HIV-infected persons in care: reducing disparities. Clin Infect Dis. 2012;55:1242–1251. doi: 10.1093/cid/cis654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hasse B, Ledergerber B, Furrer H, Battegay M, Hirschel B, et al. Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis. 2011;53:1130–1139. doi: 10.1093/cid/cir626. [DOI] [PubMed] [Google Scholar]
- 9.Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26:1–7. doi: 10.1207/S15324796ABM2601_01. [DOI] [PubMed] [Google Scholar]
- 10.Richard AA, Shea K. Delineation of self-care and associated concepts. J Nurs Scholarsh. 2011;43:255–264. doi: 10.1111/j.1547-5069.2011.01404.x. [DOI] [PubMed] [Google Scholar]
- 11.Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA. 2002;288:2469–2475. doi: 10.1001/jama.288.19.2469. [DOI] [PubMed] [Google Scholar]
- 12.Jones G, Hawkins K, Mullin R, Nepusz T, Naughton DP, et al. Understanding how adherence goals promote adherence behaviours: a repeated measure observational study with HIV seropositive patients. BMC Public Health. 2012;12:587. doi: 10.1186/1471-2458-12-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kokkinos P, Myers J. Exercise and physical activity: clinical outcomes and applications. Circulation. 2010;122:1637–1648. doi: 10.1161/CIRCULATIONAHA.110.948349. [DOI] [PubMed] [Google Scholar]
- 14.de Bruin M, Sheeran P, Kok G, Hiemstra A, Prins JM, et al. Self-regulatory processes mediate the intention-behavior relation for adherence and exercise behaviors. Health Psychol. 2012;31:695–703. doi: 10.1037/a0027425. [DOI] [PubMed] [Google Scholar]
- 15.Conn VS, Hafdahl AR, Brown SA, Brown LM. Meta-analysis of patient education interventions to increase physical activity among chronically ill adults. Patient Educ Couns. 2008;70:157–172. doi: 10.1016/j.pec.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flynn KE, Piña IL, Whellan DJ, Lin L, Blumenthal JA, et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1451–1459. doi: 10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riegel B, Moser DK, Anker SD, Appel LJ, Dunbar SB, et al. State of the science: promoting self-care in persons with heart failure: a scientific statement from the American Heart Association. Circulation. 2009;120:1141–1163. doi: 10.1161/CIRCULATIONAHA.109.192628. [DOI] [PubMed] [Google Scholar]
- 18.Tumiati R, Mazzoni G, Crisafulli E, Serri B, Beneventi C, et al. Home-centred physical fitness programme in morbidly obese individuals: a randomized controlled trial. Clin Rehabil. 2008;22:940–950. doi: 10.1177/0269215508092788. [DOI] [PubMed] [Google Scholar]
- 19.McKnight PE, Kasle S, Going S, Villanueva I, Cornett M, et al. A comparison of strength training, self-management, and the combination for early osteoarthritis of the knee. Arthritis Care Res (Hoboken) 2010;62:45–53. doi: 10.1002/acr.20013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Brien K, Nixon S, Tynan AM, Glazier R. Aerobic exercise interventions for adults living with HIV/AIDS. Cochrane Database Syst Rev. 2010:CD001796. doi: 10.1002/14651858.CD001796.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brassington G. Health-Enhancing Physical Activity. In: Baum A, Revenson T, Singer J, editors. Handbook of Health Psychology. Taylor & Francis Group, LLC; New York: 2012. p. 358. [Google Scholar]
- 22.Brownson RC, Hoehner CM, Day K, Forsyth A, Sallis JF. Measuring the built environment for physical activity: state of the science. Am J Prev Med. 2009;36:S99–123. doi: 10.1016/j.amepre.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaiser BL, Brown RL, Baumann LC. Perceived influences on physical activity and diet in low-income adults from two rural counties. Nurs Res. 2010;59:67–75. doi: 10.1097/NNR.0b013e3181c3bd55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King DK, Glasgow RE, Toobert DJ, Strycker LA, Estabrooks PA, et al. Self-efficacy, problem solving, and social-environmental support are associated with diabetes self-management behaviors. Diabetes Care. 2010;33:751–753. doi: 10.2337/dc09-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brownson RC, Haire-Joshu D, Luke DA. Shaping the context of health: a review of environmental and policy approaches in the prevention of chronic diseases. Annu Rev Public Health. 2006;27:341–370. doi: 10.1146/annurev.publhealth.27.021405.102137. [DOI] [PubMed] [Google Scholar]
- 26.DiClemente RJ, Salazar LF, Crosby RA. A review of STD/HIV preventive interventions for adolescents: sustaining effects using an ecological approach. J Pediatr Psychol. 2007;32:888–906. doi: 10.1093/jpepsy/jsm056. [DOI] [PubMed] [Google Scholar]
- 27.Sallis JF, Cervero RB, Ascher W, Henderson KA, Kraft MK, et al. An ecological approach to creating active living communities. Annu Rev Public Health. 2006;27:297–322. doi: 10.1146/annurev.publhealth.27.021405.102100. [DOI] [PubMed] [Google Scholar]
- 28.Warner KE. The need for, and value of, a multi-level approach to disease prevention: the case of tobacco control. In: Smedley BD, Syme SL, editors. Promoting Health: Intervention Strategies From Social And Behavioral Research. Natl Acad; Washington, DC: 2000. pp. 417–449. [Google Scholar]
- 29.Auerbach J. Transforming social structures and environments to help in HIV prevention. Health Aff (Millwood) 2009;28:1655–1665. doi: 10.1377/hlthaff.28.6.1655. [DOI] [PubMed] [Google Scholar]
- 30.Feaster DJ, Brincks AM, Mitrani VB, Prado G, Schwartz SJ, et al. The efficacy of Structural Ecosystems Therapy for HIV medication adherence with African American women. J Fam Psychol. 2010;24:51–59. doi: 10.1037/a0017954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hankins CA, de Zalduondo BO. Combination prevention: a deeper understanding of effective HIV prevention. AIDS. 2010;4:S70–80. doi: 10.1097/01.aids.0000390709.04255.fd. [DOI] [PubMed] [Google Scholar]
- 32.Szapocznik J, Feaster DJ, Mitrani VB, Prado G, Smith L, et al. Structural ecosystems therapy for HIV-seropositive African American women: effects on psychological distress, family hassles, and family support. J Consult Clin Psychol. 2004;72:288–303. doi: 10.1037/0022-006X.72.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong MD, Sarkisian CA, Davis C, Kinsler J, Cunningham WE. The association between life chaos, health care use, and health status among HIV-infected persons. J Gen Intern Med. 2007;22:1286–1291. doi: 10.1007/s11606-007-0265-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gifford AL, Laurent DD, Gonzales VM, Chesney MA, Lorig KR. Pilot randomized trial of education to improve self-management skills of men with symptomatic HIV/AIDS. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18:136–144. doi: 10.1097/00042560-199806010-00005. [DOI] [PubMed] [Google Scholar]
- 35.Parsons JT, Golub SA, Rosof E, Holder C. Motivational Interviewing and Cognitive-Behavioral Intervention to Improve HIV Medication Adherence among Hazardous Drinkers: A Randomized Controlled Trial. J Acquir Immune Defic Syndr. 2007;46:443–450. doi: 10.1097/qai.0b013e318158a461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swendeman D, Ingram BL, Rotheram-Borus MJ. Common elements in self-management of HIV and other chronic illnesses: an integrative framework. AIDS Care. 2009;21:1321–1334. doi: 10.1080/09540120902803158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Webel AR. Testing a peer-based symptom management intervention for women living with HIV/AIDS. AIDS Care. 2010;22:1029–1040. doi: 10.1080/09540120903214389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alemi F, Moore S, Baghi H. Self-experiments and analytical relapse prevention. Qual Manag Health Care. 2008;17:53–65. doi: 10.1097/01.QMH.0000308638.04850.48. [DOI] [PubMed] [Google Scholar]
- 39.Alemi F, Neuhauser D, Ardito S, Headrick L, Moore S, et al. Continuous self-improvement: systems thinking in a personal context. Jt Comm J Qual Improv. 2000;26:74–86. doi: 10.1016/s1070-3241(00)26006-9. [DOI] [PubMed] [Google Scholar]
- 40.Humpel N, Owen N, Leslie E. Environmental factors associated with adults’ participation in physical activity: a review. Am J Prev Med. 2002;22:188–199. doi: 10.1016/s0749-3797(01)00426-3. [DOI] [PubMed] [Google Scholar]
- 41.Moore SM, Charvat JM, Gordon NH, Pashkow F, Ribisl P, et al. Effects of a CHANGE intervention to increase exercise maintenance following cardiac events. Ann Behav Med. 2006;31:53–62. doi: 10.1207/s15324796abm3101_9. [DOI] [PubMed] [Google Scholar]
- 42.Moore SM, Charvat JM. Using the CHANGE intervention to enhance long-term exercise. Nurs Clin North Am. 2002;37:273–283. doi: 10.1016/s0029-6465(01)00008-1. [DOI] [PubMed] [Google Scholar]
- 43.Moore SM, Charvat JM, Alemi F, Gordon N, Ribisl P, et al. Improving Lifestyle Exercise in Cardiac Patients: Results of the SystemCHANGE Trial. American Heart Association Scientific Sessions; Orlando, FL: 2011. [Google Scholar]
- 44.Cuttler L, Borawski E, Moore S. Targeting Obesity and Blood Pressure in Urban Youth. National Heart Lung and Blood Institute; Cleveland, Ohio: 2012. [Google Scholar]
- 45.Webel AR, Moore SM, Hanson JE, Patel SR, Schmotzer B, et al. Improving Sleep Hygiene Behavior in Adults Living with HIV/AIDS: A Randomized Control Pilot Study of the SystemCHANGE-HIV Intervention. Appl Nurs Res. 2013 doi: 10.1016/j.apnr.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Webel AR, Dolansky MA, Henry AG, Salata RA. A qualitative description of women’s HIV self-management techniques: context, strategies, and considerations. J Assoc Nurses AIDS Care. 2012;23:281–293. doi: 10.1016/j.jana.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.CDC. Today’s HIV/AIDS Epidemic. HIV Fact Sheet 2012 [Google Scholar]
- 48.Beltran VM, Harrison KM, Hall HI, Dean HD. Collection of social determinant of health measures in U.S. national surveillance systems for HIV, viral hepatitis, STDs, and TB. Public Health Rep. 2011;3:41–53. doi: 10.1177/00333549111260S309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ainsworth BE, Macera CA, Jones DA, Reis JP, Addy CL, et al. Comparison of the 2001 BRFSS and the IPAQ Physical Activity Questionnaires. Med Sci Sports Exerc. 2006;38:1584–1592. doi: 10.1249/01.mss.0000229457.73333.9a. [DOI] [PubMed] [Google Scholar]
- 50.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 51.Physical Activity Guidelines Steering Committee. Physical Activity Guidelines for Americans. Department of Health and Human Services; Washington, DC: 2008. [Google Scholar]
- 52.Lee KA, Gay C, Portillo CJ, Coggins T, Davis H, et al. Types of sleep problems in adults living with HIV/AIDS. J Clin Sleep Med. 2012;8:67–75. doi: 10.5664/jcsm.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]