Abstract
Objective
To compare the risk of cesarean wound disruption or infection after closure with surgical staples compared with subcuticular suture.
Methods
Women with viable pregnancies at 24 weeks of gestation or greater undergoing scheduled or unscheduled cesarean delivery were randomized to wound closure with surgical staples or absorbable suture. Staples were removed at postoperative days 3-4 for low transverse incisions and days 7-10 for vertical incisions. Standardized wound evaluations were performed at discharge (days 3-4) and 4-6 weeks post-operatively. The primary outcome was a composite of wound disruption or infection within 4-6 weeks. Secondary outcomes included operative time, highest pain score on analog scale, cosmesis score and patient scar satisfaction score. Analyses were by intent-to-treat.
Results
Of 398 patients, 198 were randomized to staples and 200 to suture (but four received staples). Baseline characteristics including body mass index, prior cesarean, labor, and type of skin incision were similar by group. The primary outcome incidence at hospital discharge was 7.1% for staples and 0.5% for suture; P <0.001 (RR 14.1; 95% CI 1.9-106). Of 350 (87.9%) with follow up at 4-6 weeks, the cumulative risk of the primary outcome at 4-6 weeks was 14.5% for staples and 5.9% for suture; P=0.008 (RR 2.5; 95% CI 1.2-5.0). Operative time, pain scores at 72-96 hours and at 6 weeks, cosmesis score, and patient satisfaction score did not differ by group.
Conclusion
Staples closure compared with suture is associated with significantly increased composite wound morbidity after cesarean delivery.
Introduction
Cesarean delivery is the most common major surgical procedure performed in the United States and elsewhere. Currently, approximately a third of pregnant women in the US and 15% worldwide deliver by cesarean, and this prevalence is on the rise.1 Given these trends, cesarean wound complications, such as disruption or infection, remain an important cause of post-cesarean morbidity at considerable costs to the patient and health system.2-5
The skin is typically closed with surgical staples or sutures after cesarean delivery. Until recently there has been little evidence regarding the best cesarean skin closure material.6 It has been postulated that sutures act as a foreign body and damage tissue leading to increased infections.7 Initial small studies regarding cesarean skin closure materials examined operative time, pain scores, cosmesis scores and/or patient satisfaction and yielded contradictory findings.8-9 One randomized controlled trial of wound disruption or infection (evaluated by phone interview supplemented with record review) at 2-4 weeks as the primary outcome and suggested increased rates with staple compared with suture closure.10 Given the paucity of trials that adequately examined wound morbidity outcomes of cesarean closure methods, the objective of our study was to compare the risk of cesarean wound disruption or infection after closure with surgical staples compared with absorbable subcuticular suture.
Materials and Methods
We conducted a single-center randomized controlled trial that included women with viable pregnancies (≥ 24 weeks) undergoing cesarean delivery at University Hospital, Birmingham, Alabama. All cesarean types were included - scheduled or unscheduled and primary or repeat cesareans. Women were excluded for the following reasons: inability to obtain informed consent, fetal demise, immune compromising disease (e.g. AIDS), chronic steroid use, contraindication to routine postpartum pain medications (ibuprofen, acetaminophen, narcotics) or planned postpartum visit at another facility. The study was approved by the Institutional Review Board of the University of Alabama at Birmingham and was registered at ClinicalTrials.gov (NCT01008449).
Eligible women were approached and consented at the time of admission for delivery. Those who required cesarean delivery underwent usual perioperative management (surgical skin preparation with povidone iodine solution and prophylactic antibiotics). Women were randomized to either surgical metallic staples (Ethicon Endosurgery Promixate PlusMD skin stapler) or 4-0 Monocryl (absorbable sutures) according to a predetermined computer generated block randomization scheme prepared by a study statistician. The block size was 4. Sequentially numbered and sealed opaque envelopes were prepared according to the randomization scheme and were delivered to a secure container in the operating room suite in order to maintain concealed treatment allocation. At the time of fascia closure, the next numbered envelope was pulled and opened by the circulating nurse to reveal the designated closure method. At this point the patient was considered randomized.
The cesarean technique was left to the discretion of the provider but generally followed usual practice at our center including perioperative prophylactic antibiotics (Azythromycin and cefazolin) after cord clamp, closure of the fascia with #1 PDS a running stitch, saline irrigation of the subcutaneous layer and use of cautery to obtain hemostasis. In addition, the subcutaneous layer was closed with 3-0 vicryl for all women with a subcutaneous layer >2.0 cm. Women in the surgical staples group had the skin edges everted for staple placement. Those in the subcuticular suture group had absorbable sutures placed in one continuous closure with knots buried at the lateral edges of the wound. Skin closures were generally performed by resident physicians with attending supervision. First-year residents did not perform skin closures for study participants in the first 3 months of their training and required a minimum of 10 observed skin closures and approval by supervisory obstetric physician before performing skin closures for this study. The wound was dressed with an abdominal pad and Elastoplast tape (Steri-Strips [thin adhesive strips] were not placed at skin closure). The wound dressing was removed on postoperative day 1. Women showered by 24 hours post-op. Staples were removed and thin adhesive strips placed on postoperative days 3 or 4 prior to hospital discharge for low transverse abdominal incisions; patients with vertical incisions in the staple group returned on postoperative days 7-10 for removal.
A standardized physical examination of the wound was performed by trained obstetric providers (residents or attending) at hospital discharge (postoperative days 3-4) and at the postpartum examination (4-6 weeks postop) for patients in both groups. For patients who did not return for their postpartum visit at 4-6 weeks, a standardized phone assessment was implemented by trained study personnel; any report of a wound complication was validated by medical record review. The primary outcome was a composite of wound disruption or infection occurring within 4-6 postoperative weeks. Wound disruption was defined as subcutaneous skin dehiscence (from any cause including seroma or hematoma) or fascial dehiscence. Wound infection was defined as purulent drainage, cellullitis, abscess or wound requiring drainage, debridement and antibiotics associated with a clinical diagnosis of infection. Key pre-specified secondary outcomes included: operative time (from skin incision to end of skin closure), analog pain score at postoperative days 3-4 (the highest pain score as recorded by nursing staff at a minimum of every 8 hours between 72-96 hours postop) and postoperative weeks 4-6, cosmesis score (as defined by the Stony Brook Scar Evaluation Scale11) 4-6 weeks post-op, and patient satisfaction score (patients rated general appearance, location and comfort of scar on a 1 (worst) to 5 (best) scale) 4-6 weeks post-op.
Assuming a conservative baseline composite primary outcome of 8%, α=0.05 and power of 80%, we initially estimated that a sample size of 1204 (602 per group) was required for a 50% reduction in the primary composite outcome. During the course of the study, newly reported data with similar exposure groups indicated a higher rate of wound complication prompting us to re-evaluate our postulated baseline rate.10 Examination of our institutional data (external to the study) suggested a more realistic rate of 12-14%. However, after further considering i) the new reports and metaanalyses suggesting potential benefits of suture closure on wound morbidity and ii) the logistics required to continue this study, our research review group (comprising senior investigators, biostatisticians and our research center leadership) decided to stop enrollment at approximately 400 subjects. Therefore the study was stopped without any interim data analyses to compare arms.
Statistical analyses were conducted using SAS version 9.1 (SAS Institute, Inc., Cary, NC). The randomization code was kept confidential by a single statistician until the end of study after the database had been cleaned and locked for data analysis. At this time the code was imported into the database. The chi-square test of association and Fisher's exact test were used for analysis of categorical data. The Pearson's chi-square test was used where applicable. Where assumptions for this procedure were not met, Fisher's exact test was used. Quantitative measures were analyzed using the two-tailed unpaired Student t-test and the Wilcoxon rank sum test. Statistical significance was defined as P ≤ 0.05 without adjustments for multiple comparisons. The analysis, while occurring earlier than originally planned, was the final planned analysis. Hence, no adjustments were made to preserve alpha for subsequent analyses. Relative risks and 95% confidence intervals are presented for primary and secondary outcomes. All analyses were by intent-to-treat.
Results
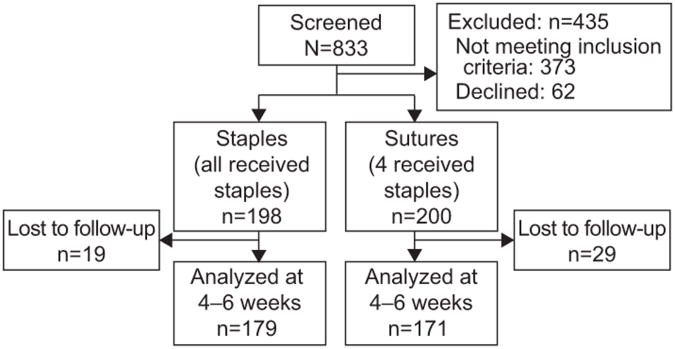
From August 2009 through November 2010 a total of 833 patients were screened and 62 declined participating. Among the 771 who consented, 373 were excluded from randomization because they did not meet inclusion criteria primarily because of a vaginal delivery. The remaining 398 were randomized: 198 to staples and 200 to suture (Figure 1). Four women randomized to the suture group actually received staples but they were analyzed in the suture group according to the intent to treat principle. The baseline characteristics of the randomized cohort including BMI (≈36 kg/m2), race/ethnicity (predominantly African American) and prior cesarean (47-49%) were similar between study groups. Of the 350 (88%) who had post-operative follow-up at 4-6 weeks, 179 were in the staple and 171 in the suture group. Baseline characteristics remained similar by group as shown in Table 1. Ninety-nine percent of women received peri-operative antibiotic prophylaxis and 30% received intrapartum antibiotics (mainly for GBS positive status and/or chorioamnionitis).
Figure 1. Study Flow Diagram.
Table 1. Baseline Characteristics of Patients With 4-6 Week Follow-up.
| Staples (n=179) | Suture (n=171) | P | |
|---|---|---|---|
|
| |||
| Age | 26.7 ± 6.1 | 26.9 ± 5.9 | 0.622* |
|
| |||
| Body mass index | 36.8 ± 8.1 | 35.9 ± 8.5 | 0.255* |
|
| |||
| Race | |||
| African American | 66.5 | 56.7 | 0.106† |
| White | 15.1 | 22.2 | |
| Hispanic | 17.9 | 18.7 | |
| Other | 0.6 | 2.3 | |
|
| |||
| Smoking | 10.6 | 12.3 | 0.624 |
|
| |||
| Primiparous | 30.7 | 33.9 | 0.523 |
|
| |||
| Prior cesarean | 46.9 | 51.5 | 0.396 |
|
| |||
| Chronic hypertension | 13.4 | 11.7 | 0.629 |
|
| |||
| Diabetes | 19.6 | 18.7 | 0.841 |
|
| |||
| Human immunodeficiency virus | 1.1 | 0 | 0.499† |
|
| |||
| Chorioamnionitis | 10.1 | 11.7 | 0.622 |
|
| |||
| Labor or induction | 49.7 | 53.2 | 0.513 |
|
| |||
| Intrapartum antibiotics | 30.7 | 28.1 | 0.586 |
|
| |||
| Vertical midline incision | 8.4 | 8.8 | 0.896 |
|
| |||
| Intraoperative antibiotics | 98.3 | 98.8 | >0.999† |
|
| |||
| Steroids | 8.4 | 7.6 | 0.789 |
|
| |||
| Intraoperative bilateral tubal ligation | 33.5 | 27.5 | 0.221 |
Data are mean ± standard deviation or % unless otherwise specified.
Wilcoxon rank sum test.
Fisher Exact test
The cumulative incidence of the primary composite outcome and its components at the time of hospital discharge and at 4-6 weeks are presented in Table 2. The primary outcome at hospital discharge was 7.1% (n=14) for staples and 0.5% (n=1) for suture; P<0.001 (RR 14.1; 95% CI 1.9-106); by 4-6 weeks it was 14.5% for staples and 5.9% for suture; P=.008 (RR 2.5; 95% CI 1.2-5.0). Not presented in the tables, examination of the size of the disruptions revealed that disruptions longer than 1 cm were more frequent in women with staples (6.2% vs. 0%, P=0.009) as were disruptions of depth deeper than 0.5 cm (4.5% vs. 0.6%, P=0.037). Of note, among the 36 patients with the primary outcome (within 4-6 weeks), the outcome was ascertained through an in-person evaluation in 23 (63.9%) while 13 (36.1%) were based on phone follow-up complemented by medical record review.
Table 2. Cumulative Incidence of the Primary Composite Outcome and Its Components.
| Staples n (%) | Suture n (%) | Relative Risk (95% CI) | |
|---|---|---|---|
| At hospital discharge | n=198 | n=200 | |
|
|
|||
| Composite outcome | 14 (7.1) | 1 (0.5) | 14.1 (1.9-106) |
| Infection* | 0 (0) | 1 (0.5) | n/a |
| Disruption* | 14 (7.1) | 1 (0.5) | 14.1 (1.9-106) |
| At 4-6 weeks | n=179 | n=171 | |
|
|
|||
| Composite outcome | 26 (14.5) | 10 (5.9) | 2.5 (1.2-5.0) |
| Infection* | 4 (2.2) | 6 (3.5) | 0.6 (0.2-2.2) |
| Disruption* | 24 (13.4) | 6 (3.5) | 3.8 (1.6-9.1) |
CI, confidence interval; N/A, not applicable (due to small numbers).
Each subcategory includes women who had both a wound infection and wound disruption.
Stratified analyses of the cumulative primary outcome at 4-6 weeks by selected baseline variables (Table 3) revealed that the primary composite outcome was generally more frequent with staple closure regardless of sub-group including women with BMI <30 (15.8% vs. 0%, P=0.007) and BMI ≥ 30 (14.2% vs. 8.1%, P=0.117) as well as presence or absence of prior cesarean, labor or attempted induction, and chorioamnionitis. Also, only 30 patients (8.6%) had a vertical incision (15 in each group); the primary outcome occurred in 1 patient per group (p-value >0.999). Results of secondary study outcomes are presented in Table 4. Total operative time, analog pain scores at 72-96 hours and 4-6 weeks, cosmesis score, and scar satisfaction scores did not differ by skin closure method.
Table 3. Primary Outcome Incidence Stratified by Selected Characteristics.
| Staples n* (%) | Suture n* (%) | P | |
|---|---|---|---|
| BMI 30 or higher | 141 (14.2) | 124 (8.1) | 0.117 |
| BMI lower than 30 | 38 (15.8) | 46 (0) | 0.007 |
| Prior cesarean | 84 (13.1) | 88 (5.7) | 0.094 |
| No prior cesarean | 95 (15.8) | 83 (6.0) | 0.040 |
| Labor or attempted induction | 89 (16.0) | 91 (5.5) | 0.015 |
| No labor or attempted induction | 90 (12.2) | 80 (6.3) | 0.183 |
| Vertical incision | 15 (6.7) | 15 (6.7) | >0.999 |
| 6.7 | 6.7 | ||
| Pfannensteil incision | 164 (15.2) | 156 (5.8) | 0.006 |
| Chorioamnionitis | 18 (16.7) | 20 (10) | 0.652 |
| No chorioamnionitis | 161 (14.3) | 151 (5.3) | 0.008 |
n represents denominator (percent of denominator with primary outcome)
BMI, body mass index
Table 4. Prespecified Secondary Outcomes.
| Staples (n=179) | Suture (n=171) | P | |
|---|---|---|---|
| Procedure time in minutes | 37 (29-53) 8-133 | 40 (28-55) 9-121 | 0.345 |
| Analog pain score at 72-96 hours | 5 (3-7) 0-10 | 5 (4-7) 0-10 | 0.285 |
| Analog pain score at 4-6 weeks* | 0 (0-1) 0-10 | 0 (0-2) 0-7 | 0.066 |
| Composite Cosmesis Score*† | 3 (3-4) 0-5 | 4 (3-5) 2-5 | 0.750 |
| Satisfaction with appearance of scar | 4 (4-5) 2-5 | 4 (4-5) 1-5 | 0.842 |
| Satisfaction with comfort of scar | 4 (4-5) 1-5 | 4 (4-5) 1-5 | 0.894 |
| Satisfaction with location of scar | 4 (4-5) 1-5 | 4 (4-5) 1-5 | 0.539 |
Data are median (interquartile range) and range unless otherwise specified.
From 4-6 week follow-up only.
Stony Brook Scar evaluation score
Discussion
Overall, we observed that surgical staples were significantly associated with a higher incidence of cumulative composite wound morbidity than absorbable sutures at hospital discharge and up to 4-6 weeks after cesarean delivery. The difference was mainly due to more wound disruptions among those randomized to staples. This observation remains robust in several sub-groups or when the outcome is restricted to disruptions >1cm in length or >0.5cm in depth which may be considered more clinically important and typically led to additional scheduled clinic follow-up visits. There were no differences in total operative time, post-operative pain, cosmesis or patient satisfaction.
Postoperative wound complications for women undergoing cesarean delivery constitute a major cause of morbidity and they are costly to both the patient and health system.3-4 Prior to the initiation of our study, few studies had objectively evaluated the potential impact of cesarean skin closure technique and materials on wound disruption or infection but focused primarily on pain or cosmesis.8-9 Recently, a number of reports including clinical trials and meta-analyses have addressed wound morbidity.7,10,12 Our study is an important contribution to this developing literature. It is one of the 2 largest studies of this issue, several key outcomes are examined including an objective and clinically important primary outcome, and a considerable proportion of women were evaluated in-person at 4-6 weeks. Specifically, our findings are consistent with the recent trial in which staple closure was associated with significantly higher self-reported wound morbidity compared with suture, a finding observed in both meta-analyses.7, 10, 12 Although not statistically significant in our study, the 4-minute lower median procedure time we observed seems consistent with recent reports of a 3-9 minute shorter operative time with staple closure.7, 9-10, 12 Our results indicating no differences in pain score, cosmesis score, and patient satisfaction with scar is also consistent with the findings from meta-analyses.7, 12 In addition, prior studies were limited to planned cesareans whereas our study included women undergoing post-labor cesarean, enhancing the generalizability of our findings.9-10 Furthermore, our study has a higher prevalence of obese participants for whom the risk of wound morbidity is highest.
We acknowledge a number of limitations. Up to 12% of women in our study lacked follow-up outcome information at 4-6 weeks. However, there were not material differences in characteristics among those who followed up and those who did not. It may be argued that wound complications involving complete wound disruption, readmission or debridement may be more appropriate outcomes to evaluate. However, such outcomes are rare and a large proportion may occur independently of closure method. Still our results indicate that larger wound complications that would typically lead to additional clinic visits were more frequent with staples. Also, a very small proportion of women had vertical skin incisions limiting the generalizability of our findings to this sub-group. Finally, the decision to stop the trial is a legitimate concern. Based on the new studies and observations in our study population overall, we concluded that we had significantly underestimated the baseline incidence of wound morbidity. Further consideration of the ethical implications of the new studies suggesting that suture is beneficial and the logistics of continuing enrollment let to the decision to stop the study. Thus, although a formal DSMB was not in place for this trial, our research review committee provided oversight and monitored recruitment and evolving data. Of note, the decision was made without an interim review comparing outcomes by group (information linking the sequential randomization numbers to closure method was imported into the database at the end of the study).
The magnitude of the observed difference in cumulative incidence of wound morbidity between staples and suture closure methods was higher at the time of hospital discharge compared to 4-6 weeks post-op. This suggests that a higher proportion of wound complications following staples occurred by the time of hospital discharge while most complications following suture occur afterwards. Some may postulate that removal of staples later than post-operative days 3-4 may reduce the observed discrepancy in wound morbidity vis-à-vis suture closure. However, product information (Ethicon EndoSurgery) recommends removal of staples at 3-4 days. Among prior reports, timing of removal was on day 3 in one study,9 whereas in another it was left to the discretion of the provider (staples were associated with increased wound morbidity).10 Our anecdotal experience suggests that many providers remove staples prior to hospital discharge for most patients. This avoids significant provider and patient time and costs involved in a clinic (or home) visit for staple removal. Therefore, while it may be academically attractive to evaluate whether staple removal after hospital discharge is associated with a similar incidence of wound morbidity compared with sutures, pragmatically suture closure would remain advantageous in terms of costs to the patient and health system. Furthermore, we estimate that the price of a stapler (not even including a staple removal kit) is at least 2.5 times the price of the absorbable suture. Finally, a potential modest benefit in operative time with staple closure is likely to be grossly offset by the time required for subsequent removal. In sum, our results support the use of suture over staples among women undergoing cesarean delivery, particularly after a horizontal skin incision.
Acknowledgments
The authors thank Jan Grant, RN for contributing to data collection, and the UAB residents in obstetrics and gynecology for assistance with patient enrollment.
At the time of the study, Dr. Tita was funded by an NIH Women's Reproductive Health Research (WRHR) Grant at UAB (5 K12 HD01258-09).
Footnotes
This study was presented in part at the 32nd Annual Meeting of the Society for Maternal-Fetal Medicine in Dallas, TX February 9-11, 2012.
Financial Disclosure: The authors did not report any potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martin JA, Hamilton BE, Ventura SJ, Osterman MJK, Kirmeyer S, Matthews TJ, et al. Births: Final Data for 2009. National Vital Statistics Reports. 2011;60:1. [PubMed] [Google Scholar]
- 2.Owen J, Andrews WW. Wound complications after cesarean sections. Clin Obstet Gynecol. 1994;37:842–55. doi: 10.1097/00003081-199412000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Smyth ET, Emmerson AM. Surgical site infection surveillance. J Hosp Infect. 2000;45:173–84. doi: 10.1053/jhin.2000.0736. [DOI] [PubMed] [Google Scholar]
- 4.Reilly J, Twaddle S, McIntosh J, Kean L. An economic analysis of surgical wound infection. J Hosp Infect. 2001;49:245–249. doi: 10.1053/jhin.2001.1086. [DOI] [PubMed] [Google Scholar]
- 5.Ramsey PS, White AM, Guinn DA, Lu GC, Ramin SM, Davies JK, et al. Subcutaneous Tissue Reapproximation, Alone or in Combination With Drain, in Obese Women Undergoing Cesarean Delivery. Obstet Gynecol. 2005;105:967–73. doi: 10.1097/01.AOG.0000158866.68311.d1. [DOI] [PubMed] [Google Scholar]
- 6.Alderdice F, McKenna D, Dornan J. Techniques and materials for skin closure in caesarean section. Cochrane Database of Systematic Reviews. 2003;(2) doi: 10.1002/14651858.CD003577. Art. No.: CD003577. [DOI] [PubMed] [Google Scholar]
- 7.Tuuli MG, Rampersad RM, Carbone JF, Stamilio D, Macones GA, Odibo AO. Staples compared with subcuticular suture for skin closure after cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol. 2011;117(3):682–90. doi: 10.1097/AOG.0b013e31820ad61e. Review. Erratum in: Obstet Gynecol. 2011;1171440. [DOI] [PubMed] [Google Scholar]
- 8.Frishman GN, Schwartz T, Hogan JW. Closure of Pfannenstiel Skin Incisions. J Reprod Med. 1997;42:627–30. [PubMed] [Google Scholar]
- 9.Rousseau JA, Girard K, Turcot-Lemay L, Thomas N. A randomized study comparing skin closure in cesarean sections: staples vs subcuticular sutures. Am J Obstet Gynecol. 2009;200:265.e1–4. doi: 10.1016/j.ajog.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 10.Basha SL, Rochon ML, Quiñones JN, Coassolo KM, Rust OA, Smulian JC. Randomized controlled trial of wound complication rates of subcuticular suture vs staples for skin closure at cesarean delivery. Am J Obstet Gynecol. 2010;203:285.e1–8. doi: 10.1016/j.ajog.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Singer AJ, Arora B, Dagum A, Valentine S, Hollander JE. Development and validation of a novel scar and evaluation scale. Plast Reconstr Surg. 2007;120:1892–7. doi: 10.1097/01.prs.0000287275.15511.10. [DOI] [PubMed] [Google Scholar]
- 12.Clay FS, Walsh CA, Walsh SR. Staples vs. subcuticular sutures for skin closure at cesarean delivery: a metaanalysis of randomized controlled trials. Am J Obstet Gynecol. 2011;204:378–83. doi: 10.1016/j.ajog.2010.11.018. Epub 2010 Dec 31. [DOI] [PubMed] [Google Scholar]