Abstract
Osteopontin (OPN) is a chemotactic factor which can be cleaved to the pro-inflammatory form by matrix metalloproteinases (MMPs). To test the hypothesis that OPN can modulate inflammatory liver injury during cholestasis, wild-type (WT) C57BL/6 and OPN knockout (OPN-KO) mice underwent bile duct ligation (BDL). OPN-KO mice showed significant reduction in liver injury (plasma ALT and necrosis) and neutrophil recruitment compared with WT animals at 24h but not 72h after BDL. In WT mice, a 4-fold increase in hepatic MMP-3 mRNA and elevated MMP activities and cleaved OPN levels were observed in bile. WT mice subjected to BDL in the presence of the MMP inhibitor BB-94 showed reduced liver injury, less neutrophil extravasation and diminished levels of cleaved OPN in bile. Thus, during obstructive cholestasis, OPN released from biliary epithelial cells could be cleaved by MMPs in bile. When the biliary system leaks, cleaved OPN enters the parenchyma and attracts neutrophils. In the absence of OPN, other chemoattractants, e.g. chemokines, mediate a delayed inflammatory response and injury. Taken together, our data suggest that OPN is the pro-inflammatory mediator that initiates the early neutrophil-mediated injury phase during obstructive cholestasis in mice.
Keywords: Inflammatory Liver Injury, Cholestasis, Bile duct ligation, Osteopontin, Neutrophils
1. INTRODUCTION
Cholestatic liver disease can be caused acutely by drug toxicities or obstruction of the bile duct by gallstones, and chronically by genetic defects or malignancies (Hirschfield et al., 2010). Acute obstructive cholestasis is characterized by hepatocellular injury, which is followed by bile duct proliferation, fibrosis and cirrhosis (Sellinger and Boyer, 1990). However, the molecular mechanisms of liver injury induced by obstructive cholestasis remain not well defined (Woolbright and Jaeschke, 2012). One hypothesis suggests that liver injury during BDL is caused by an inflammatory response involving neutrophil-mediated liver injury (Gujral et al., 2003, 2004b). However, for neutrophils to be a relevant factor in the pathophysiology, a prerequisite would be the formation of chemotactic agents that can activate and recruit neutrophils into the liver (Jaeschke, 2006). It was demonstrated that bile acids can induce the transcription factor early growth response factor-1 (egr-1), which regulates a number of pro-inflammatory genes including macrophage inflammatory protein-2 (MIP-2), keratinocyte chemoattractant (KC) and intercellular adhesion molecule-1 (ICAM-1) in hepatocytes (Allen et al., 2010, 2011; Kim et al., 2006). The fact that mice deficient in ICAM-1 or egr-1 showed reduced neutrophilic inflammation and less injury after BDL supported the importance of bile acid-induced inflammation in the pathophysiology (Gujral et al., 2004b; Kim et al., 2006). Most importantly, the pro-inflammatory effect was caused by bile acids at concentrations that are achieved in vivo after BDL (Zhang et al., 2012). However, an important, unanswered question remains which mediator(s) actually initiate this neutrophilic inflammatory response after BDL.
Osteopontin is a multifunctional glycophosphoprotein that can function as a neutrophil chemoattractant by binding to integrin receptors (Banerjee et al., 2008; Denhardt et al., 2001; Ramaiah and Rittling, 2008). In the liver, osteopontin is expressed in biliary epithelial cells and has been shown to be substantially induced in different models of biliary fibrosis including BDL (Banerjee et al., 2006b; De Minicis et al., 2007; Fickert et al., 2007, 2010). Osteopontin expression was also upregulated in biliary epithelium in human biliary atresia (Whitington et al., 2005). Because of the induction during BDL and its potent chemotactic properties, we tested the hypothesis that osteopontin may initiate the neutrophilic inflammatory response during BDL.
2. MATERIAL AND METHODS
2.1 Animals
Eight to twelve week old male WT (C57BL/6) and OPN knockout (OPN-KO) mice, which are on a C57BL/6 background, were purchased from Jackson Laboratories (Bar Harbor, ME). All animals were maintained in an environmentally controlled room with a 12 h light/dark cycle and allowed free access to food and water. The experimental protocol was approved by the Institutional Animal Care and Use Committee of the University of Kansas Medical Center and followed the criteria of the National Research Council for the care and use of laboratory animals in research.
2.2 Experimental design
WT and OPN-KO mice were subjected to BDL for 1 or 3 days as described previously (Gujral et al., 2003). Sham-operated animals served as controls (n=5 for each time point). For experiments with inhibition of MMPs, the MMP inhibitor Batimastat (BB-94) at a dose of 20 mg/kg was used (Wielockx et al., 2001). The compound was sonicated into suspension with PBS/0.01% Tween 20. WT mice subjected to BDL for 1-day were intraperitoneally injected with either BB-94 or vehicle, just after BDL and then 6 hours after BDL. The animals were sacrificed at various time points by cervical dislocation and exsanguination. Blood, bile and liver samples were collected at the time of sacrifice from the animals. Bile was obtained via rupture of the gall bladder into a plastic tube. Gall bladder was excluded from protein used in western blot assays. Bile was flash frozen in liquid nitrogen and stored at −80°C. Plasma was used to determine alanine aminotransferase (ALT) activities. Liver samples were either snap-frozen in liquid nitrogen or fixed in phosphate-buffered formalin. Formalin-fixed livers were embedded in paraffin, and 5μm sections were cut and used for histology.
2.3 Histology
Liver sections were stained with hematoxylin and eosin (H&E) for evaluation of liver injury. The tissue for histological analysis was always derived from similar mid-sections from the same liver lobes, i.e. the tissue sections used for H&E staining and immunohistochemical analysis were of similar size for all animals in all groups. For counting of the infarcts per section, the tissue sections were de-identified and evaluated blindly. To assess neutrophil accumulation in the liver, sections were stained for chloroacetate esterase, a marker for neutrophils (Jaeschke et al., 1990), using a Naphthol-ASD Chloroacetate Esterase Kit (Sigma, St. Louis, MO). Neutrophils present in the sinusoids and extravasated into the parenchyma were counted in 20 randomly selected high-power fields (HPF). The sum of sinusoidal and extravasated neutrophils were expressed as the total neutrophil sequestration in the liver (Gujral et al., 2003, 2004b).
2.4 Real-Time PCR
mRNA expression of the selected genes (OPN, MMP-2, -3, -7, and -13) was quantified using real-time RT-PCR analysis as previously described (Bajt et al., 2008). Briefly, RNA was isolated using TRI reagent (Sigma-Aldrich, St. Louis, MO), and was reverse-transcribed into cDNA. After normalizing cDNA concentration, the SYBR green DNA PCR kit (Applied Biosystems) was used for real-time PCR analysis. Primer pairs used included as follows: 5′-GTATGACTCCACTCACGGCAAA-3′ and 3′-GGTCTCGCTCCTGGAAGATG-5′ for β-actin; 5′-ACACTTTCACTCCAATCGTCC-3′ and 3′-TGCCCTTTCCGTTGTTGTCC-5′ for OPN; 5′-CACCTGGTTTCACCCTTTCTG-3′ and 3′-AACGAGCGAAGGGCATACAA-5′ for MMP-2; 5′-CCCACCAAGTCTAACTCTCTGGAA-3′ and 3′-GGGTGCTGACTGCATCAAAGA-5′ for MMP-3; 5′-GGTCACCTACAGGATCGTATCATAT-3′ and 3′-CATCACTGCATTAGGATCAGAGGAA-5′ for MMP-7; and 5′-GACCTTGTGTTTGCAGAGCACTAC-3′ and 3′-TTCTCGGAGCCTGTCAACTGT-5′ for MMP-13. The relative differences in expression between groups were expressed using cycle time (Ct) values generated by the ABI 7900 instrument (Applied Biosystems). All genes evaluated were first normalized to β-actin, a housekeeping gene, as an internal control, and then expressed as a fold increase relative to control arbitrarily set as 1.0. Calculations are made by the 2^ (−ddCt) formula.
2.5 Immunohistochemistry
Sequential liver sections were deparaffinized in xylene and rehydrated in serial ethanol: water dilutions. Endogenous hydrogen peroxide was quenched and the tissue was probed for OPN using an anti-OPN antibody (anti-mouse OPN. Cat. # AF808, R&D systems, Minneapolis, MN). Stain was visualized using a donkey anti-goat HRP secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and DAB peroxidase (Vector Labs, Burlingame, CA) and counterstained with hematoxylin.
2.6 Western Blotting
OPN in bile and liver tissue was evaluated by Western Blot analysis, as described (Bajt et al., 2000). The following antibodies were used: a rabbit anti-OPN antibody (Abcam, Cambridge, MA), which recognizes both the full-length OPN (66 kD) and MMP-cleaved OPN (32 kD); a donkey anti-rabbit IgG (Santa Cruz) was used as secondary antibody. Each lane was loaded with 30 μg protein for bile or liver samples. Proteins were visualized by enhanced chemiluminescence (Amersham Pharmacia Biotech. Inc., Piscataway, NJ).
2.7 Gelatin zymography
Zymography of protease activity was performed as described (Ramachandran and Balasubramanian, 2000). Briefly, bile samples (100 μg protein) were separated on 12% polyacrylamide gels containing 0.2% gelatin. After electrophoresis, the gels were soaked in 2% Triton X-100, followed by incubation in reaction buffer (0.1M Tris-HCl, pH 7.5 with 10mM CaCl2) overnight at 37°C and then stained with Coomassie brilliant blue.
2.8 Statistical Analysis
Data are given as means ± SE. Comparison between two groups were performed with Student t test or 1-wayANOVA followed by Bonferroni t test for multiple groups. If the data were not normally distributed, the Mann-Whitney test was applied for comparison of 2 groups. P< 0.05 was considered significant.
3. RESULTS
3.1 Liver injury and neutrophil accumulation were reduced in OPN-KO mice 1-day after BDL
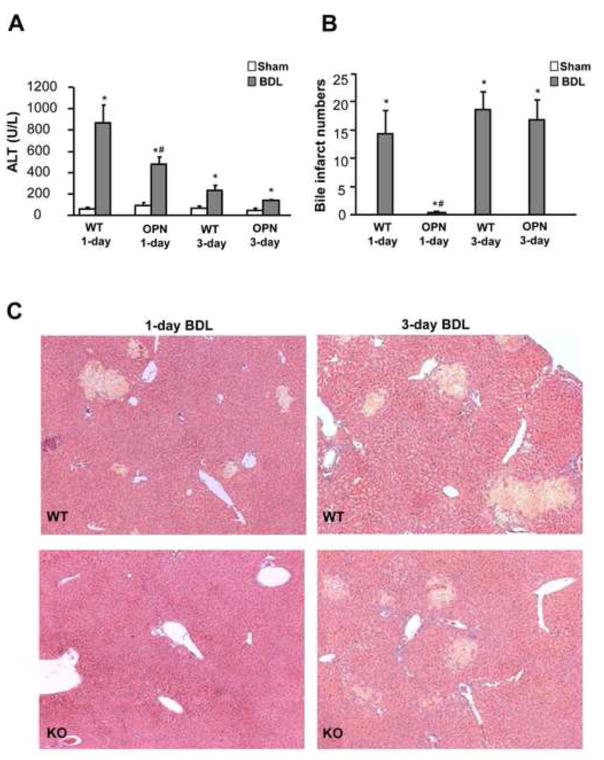
To investigate the role of OPN in the development of liver injury during obstructive cholestasis, BDL was carried out in WT and OPN-KO mice. Liver injury, as indicated by the increase in plasma ALT activities (Figure 1A), and the development of areas of necrosis (bile infarcts) (Figure 1B, C), was observed 1 day after BDL with no relevant further aggravation at 3 days. OPN-deficient mice were significantly protected against BDL-induced liver injury as indicated by the 45% lower ALT activities and the complete absence of bile infarcts at day 1 (Figure 1). However, plasma ALT activities (Figure 1A), the number of bile infarcts (Figure 1B,C) and their size distribution (not shown) were not significantly different 3 days after BDL suggesting that the development of liver injury was substantially delayed in OPN-deficient mice but not prevented.
Figure 1.
Liver injury after bile duct ligation (BDL) in mice. Wild-type (WT) animals or OPN knockout (KO) mice were sham-operated or subjected to BDL. The animals were sacrificed after 1 or 3 days. Plasma ALT activities were measured (A) and formalin-fixed liver sections were stained with H&E (C). The total number of bile infarcts was counted in each H&E-stained section (B). Data are expressed as mean ± SE of n=5 animals per group. *P<0.05 compared with sham; #P<0.05 compared with WT. Magnification, x50.
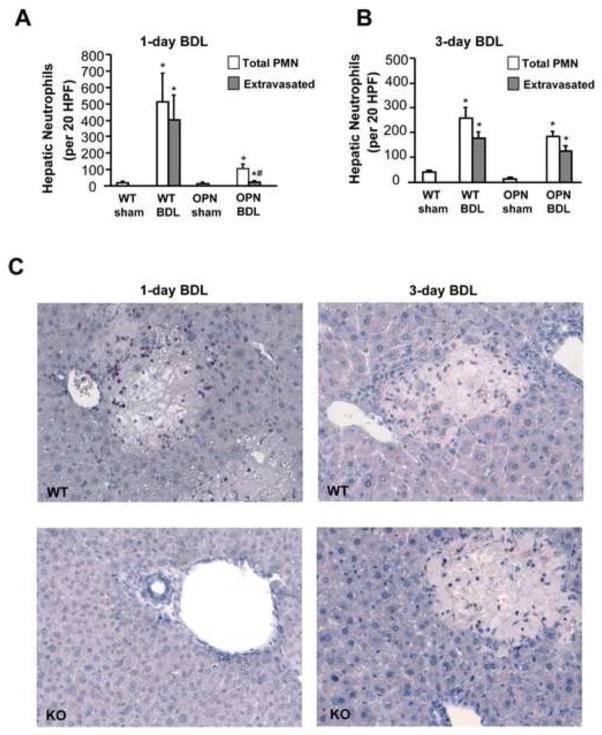
As BDL-mediated liver injury in mice is neutrophil-dependent (Gujral et al., 2003, 2004b), we evaluated if OPN is needed for neutrophil recruitment into the liver and extravasation into the parenchyma. Neutrophils were stained in tissue sections with the chloroacetate esterase method and counted in sinusoids and in the area of necrosis (extravasated). Compared to sham-operated animals, which had only a few neutrophils in sinusoids, WT mice had a substantial accumulation of neutrophils in the liver at 1 and at 3 days, most of which were located in and around the areas of necrosis (Figure 2). In contrast, only a limited number of neutrophils accumulated in livers of OPN-deficient mice and only very few extravasated after 1 day. Whereas the number of neutrophils declined in WT animals at 3 days compared to 1 day after BDL, the number increased in OPN-deficient mice (Figure 2B, C). Together with the injury data, these observations suggest that the inflammatory injury in WT animals occurs mainly during the first 24 h after BDL and in OPN-deficient mice between 1 and 3 days after BDL.
Figure 2.
Hepatic neutrophil (PMN) accumulation after BDL in mice. WT animals or OPN KO mice were sham-operated or subjected to BDL for 1 or 3 days. Neutrophils present in sinusoids and extravasated in the parenchymal tissue were counted in 20 high-power fields (HPF) (A: 1-day BDL; B: 3-day BDL). Formalin-fixed liver sections were stained for chloroacetate esterase activities to assess hepatic neutrophil accumulation (C). Data are expressed as the total number of neutrophils in liver sections and the number of extravasated neutrophils (present in areas of necrosis). Data represent means ± SE of 5 animals per group. *P<0.05 compared with sham; #P<0.05 compared with WT. Magnification, x200.
3.2 Induction of OPN and MMP-3 gene expression
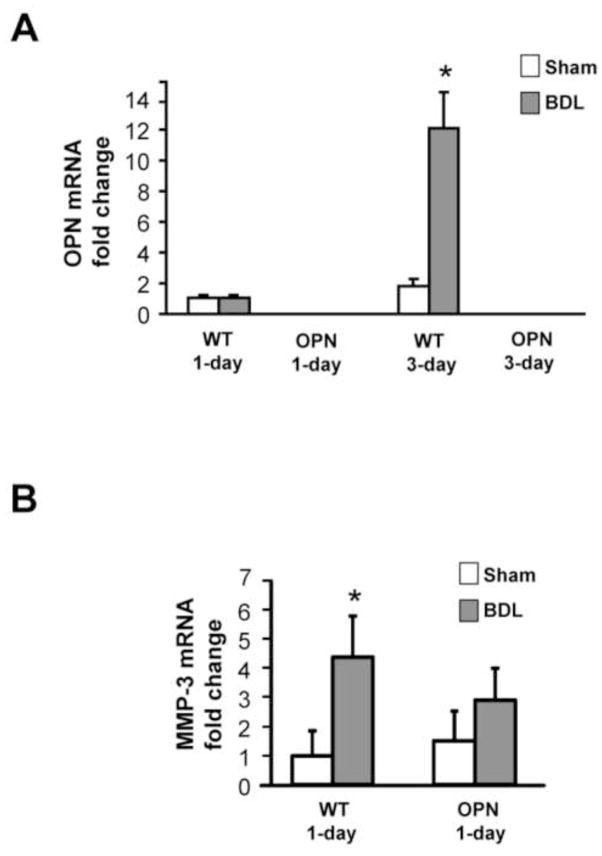
To further investigate the mechanism of protection in OPN-KO mice compared to WT mice, real-time RT-PCR analysis was performed for hepatic OPN mRNA expression 1 or 3 days after BDL. As expected, no OPN mRNA was detected in OPN-KO mice (Figure 3A). No difference in OPN mRNA was observed between WT sham and WT BDL animals 1 day after BDL (Figure 3A) or earlier (data not shown). However, livers from WT mice 3 days after BDL showed a more than 10-fold increase in OPN mRNA expression compared with WT sham mice (Figure 3A). To examine whether MMPs could be upstream mediators of OPN activation, mRNA levels of MMP-2, -3, -7, and -13 were measured in the liver samples. A 4-fold increase in MMP-3 mRNA was observed 1 day after BDL in WT but not in OPN-KO mice (Figure 3B), while MMP-2 and -13 mRNA did not change (data not shown) and MMP-7 mRNA was not detectable under these conditions.
Figure 3.
Real-time RT-PCR analysis of OPN and MMP-3. Real-time PCR was performed on 5 individual samples per group, and the results were normalized to β-actin. Data from sham-operated 1-day WT were set as 1 and the results of the BDL animals were expressed as fold increase over controls (A and B). Data represent means ± SE of 5 animals per group. *P<0.05 compared with sham. #P<0.05 compared with vehicle.
3.3 OPN is expressed exclusively in BDECs and its expression is increased post BDL
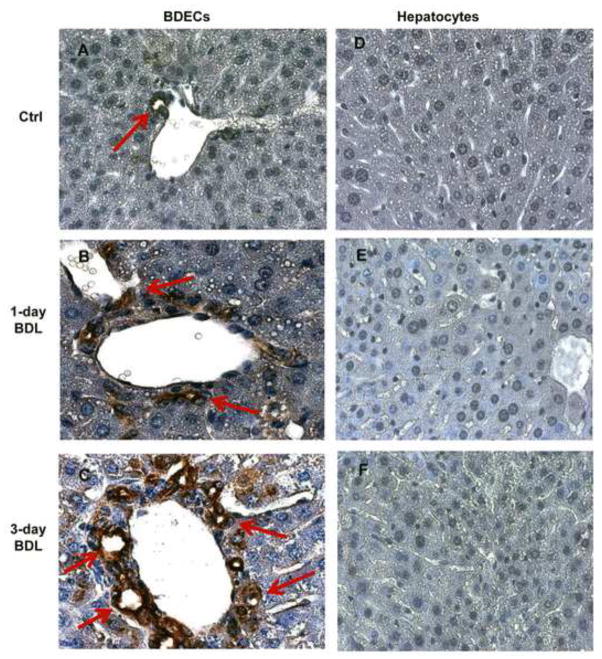
To define where OPN was expressed in the liver, immunohistochemistry using an anti-OPN antibody was used on liver tissue sections from control mice, and mice 1 day and 3 days post BDL (Figure 4). While there was no evidence for OPN expression in hepatocytes from any of the time points, there was extensive staining of bile duct epithelial cells (BDECs) in all samples. There was a dramatic increase in OPN expression in BDECs by 72h post BDL, consistent with mRNA data.
Figure 4.
OPN expression in bile duct epithelial cells (BDECs) and hepatocytes. WT animals were subjected to BDL for 1 or 3 days and liver section were stained by immunohistochemistry for OPN using an anti-OPN antibody. BDECs show increased expression of OPN after BDL (B, C). While there was extensive staining of BDECs (red arrows), no OPN expression was observed in hepatocytes (D, E, F). Magnification, x100.
3.4 OPN is secreted by BDECs into bile and cleaved by MMPs in response to BDL
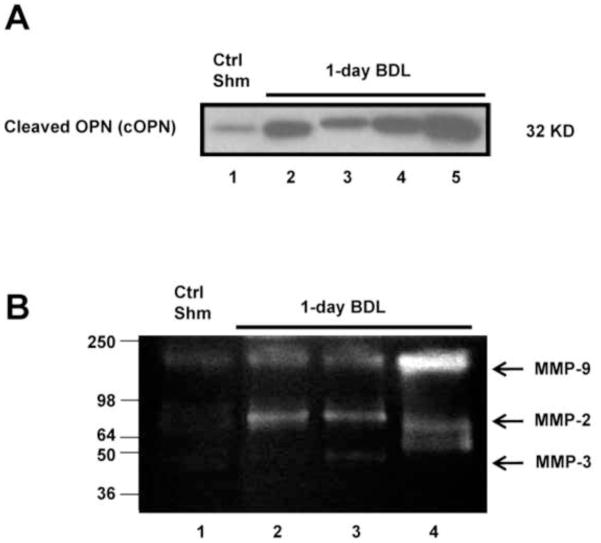
To examine regulation of remote liver injury by OPN during cholestasis, bile samples from WT mice 1 day after BDL were analyzed by western blot and gel zymography. Because bile in gallbladder is very limited in sham-operated mice, bile samples from the 4 control mice were pooled (Figure 5A, lane 1). Interestingly, no full-length OPN (fOPN) was found in bile either 1 day after BDL or in sham-operated mice (data not shown). Importantly, cleaved OPN (cOPN) was significantly increased in 1-day BDL WT mice (Figure 5A, lane 2–5) when compared with sham-operated animals (Figure 5A, lane 1). At the same time, elevated MMP-2, -3 and -9 enzymatic activities were found in the bile samples of 1-day BDL mice (Figure 5B), indicating that MMPs were present in bile that could cleave full-length OPN.
Figure 5.
OPN protein levels and MMP enzymatic activity in bile samples after BDL. Bile samples from 4 pooled sham-operated animals and from 4 individual mice subjected to 1day BDL were analyzed for cleaved OPN (cOPN) by western blotting (A) and for MMP activities by gel zymography (B).
3.5 The MMP inhibitor BB-94 protected against liver injury and PMN extravasation after BDL
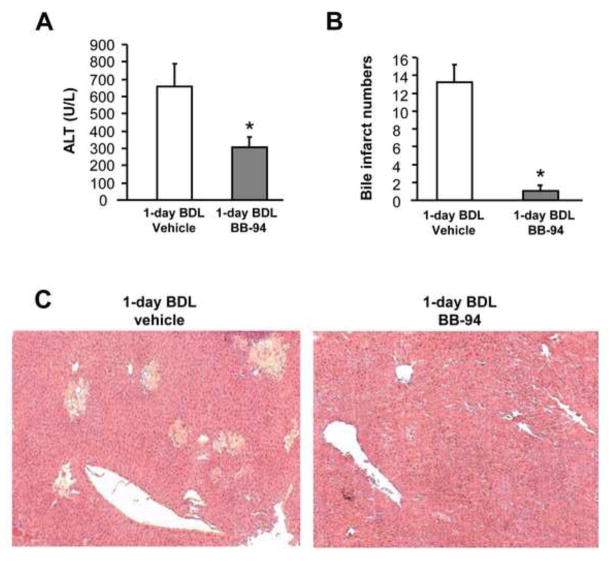
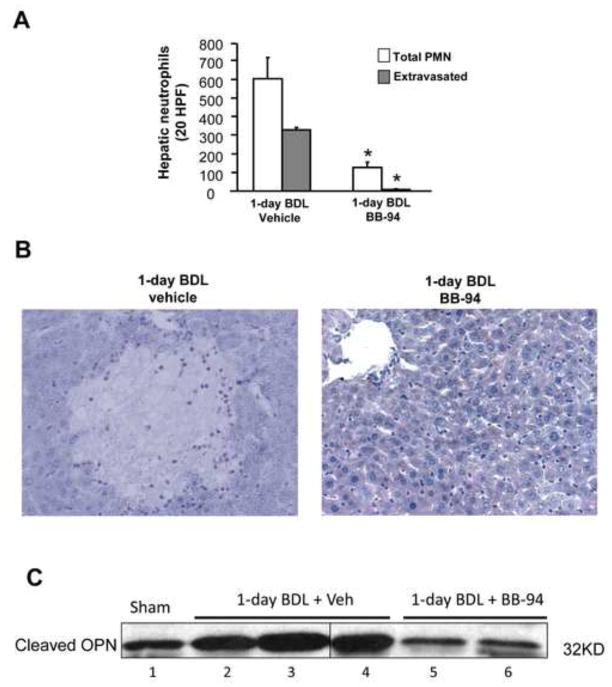
Since increased MMP-3 mRNA and cleaved OPN were observed in 1-day BDL mice, we used BB-94, a potent broad spectrum MMP inhibitor (Wielockx et al., 2001) to assess a potential contribution of MMPs in the pathophysiology. Compared to vehicle controls, the MMP inhibitor treated animals had 54% reduced plasma ALT levels (Figure 6A) and almost no areas of necrosis (bile infarcts) were detected (Figure 6B, C). Accordingly, very few neutrophils were seen in liver sections of BB-94 treated mice (Figure 7B), resulting in a dramatic decrease of total and extravasated neutrophil counts in liver sections (Figure 7A). Next, we determined whether the MMP inhibitor influences cleavage of full-length hepatic OPN to attenuate liver injury. In agreement with the previous experiment, an increase in cleaved OPN was observed in bile after BDL compared to sham-operated animals. This increase was attenuated by the MMP inhibitor BB-94 (Figure 7C). Again, no full length OPN was detectable in bile of any animal (not shown). These data suggest that MMPs are involved in the initiation of the inflammatory response after BDL most likely by generation of the pro-inflammatory cleaved form of OPN.
Figure 6.
Effect of MMP inhibitor BB-94 on BDL-induced liver injury. WT mice were sham-operated or subjected to BDL for 1 day. Some animals were treated intraperitoneally with 2 doses of 20 mg/kg of the MMP inhibitor Batimastat (BB-94); the first dose was administered just after BDL and the second one 6 hours after BDL. Vehicle-treated mice served as controls. Liver injury was assessed by plasma ALT activities (A) and histologically in H&E stained sections (C). The number of bile infarcts was counted in each tissue section (B). Data are expressed as mean ± SE of n=5 animals per group. *P<0.05 compared with vehicle-treated mice. Magnification, x50.
Figure 7.
Effect of MMP inhibitor BB-94 on hepatic neutrophil (PMN) accumulation after BDL.WT mice were sham-operated or subjected to BDL for 1day. Some animals were treated intraperitoneally with 2 doses of 20 mg/kg of the MMP inhibitor Batimastat (BB-94); the first dose was administered just after BDL and the second one 6 hours after BDL. Vehicle-treated mice served as controls. Total and extravasated PMNs were counted in 20 high power fields (A) of naphthol-ASD chloroacetate esterase-stained tissue sections (B). Data represent means ± SE of 5 animals per group. *P<0.05 compared with vehicle-treated mice. Magnification, x200. (C) OPN protein levels in bile samples after sham-operation or BDL. Bile samples from pooled sham-operated animals and from individual mice subjected to 1day BDL treated with either vehicle or BB-94 were analyzed for cleaved OPN by western blotting.
4. DISCUSSION
4.1 Osteopontin as an inflammatory mediator in BDL
The objective of this investigation was to evaluate if osteopontin could be involved in the inflammatory response after BDL in mice. Previous studies demonstrated that osteopontin, especially the cleaved form, can be an effective chemotactic mediator for neutrophils (Banerjee et al., 2008; Ramaiah and Rittling, 2008). In fact, it has been shown that cleaved osteopontin is responsible for neutrophil recruitment in a rat model of alcoholic hepatitis (Apte et al., 2005; Banerjee et al., 2006a,b). As the initial injury during BDL was strongly attenuated in OPN-deficient mice, we can conclude that OPN is a critical mediator of the early injury phase after BDL. Because liver injury after BDL in mice is mainly caused by neutrophils (Gujral et al., 2003, 2004b) and hepatic neutrophil accumulation was absent in OPN-KO mice at 24 h, OPN was likely the major mediator involved in neutrophil activation and recruitment during the early injury phase. The fact that neutrophil cytotoxicity is not impaired in OPN-KO neutrophils (Koh et al., 2007) supports the conclusion that OPN acted primarily as a chemotactic mediator after BDL. However, at 3 days after BDL, no significant difference in necrosis or neutrophil accumulation was observed suggesting that other inflammatory mediators may have taken over in OPN-KO mice at later time points. Consistent with these observations, elimination of OPN had no significant effect on liver injury or fibrosis after 8 weeks of BDL (Fickert et al., 2010). Identification of inflammatory mediators playing roles at later time points requires further studies. However, CXC chemokines are likely candidates as these mediators are induced by bile acids in hepatocytes (Allen et al., 2011; Zhang et al., 2012).
An interesting observation was the fact that virtually no bile infarcts (areas of necrosis) were observed in OPN-KO mice at 24 h after BDL but there was a mild increase of ALT. After BDL, bile leaks back into the parenchyma in areas where the increased pressure leads to ruptures in the biliary system (Fickert et al., 2002). Thus, the low ALT increase indicates that there was some stress on hepatocytes from this bile leakage but not enough to cause necrosis. This is the perfect scenario for a neutrophil-mediated injury. Neutrophils recruited into the liver require a chemotactic signal from the parenchyma to extravasate and to attack and kill the stressed cells (Chosay et al., 1997; Gujral et al., 2004a). However, a neutrophil can only affect the overall injury in a situation where the stressed cells would not have died but recovered in the absence of neutrophils (Jaeschke, 2006). If the cells die from a primary insult such as after acetaminophen exposure, neutrophil recruitment has no impact on the injury (Cover et al., 2006; Jaeschke et al., 2012). The fact that there was no neutrophil recruitment and cell necrosis in OPN-deficient mice early after BDL indicates that OPN-mediated neutrophil activation and recruitment was a critical event in mediating cell death of the stressed hepatocytes.
4.2 Release and processing of OPN after BDL
As OPN is a critical mediator of inflammation after BDL, the question is where is OPN formed and how does it recruit neutrophils into the parenchyma to cause these characteristic areas of necrosis (bile infarcts). In normal livers, OPN is mainly located in biliary epithelial cells (Apte et al., 2005; Fickert et al., 2007). However, OPN can also be expressed in hepatocytes during prolonged cholestasis (Fickert et al., 2007, 2010) or alcoholic hepatitis (Apte et al., 2005; Banerjee et al., 2006b). In our experiments, there was no relevant OPN expression in hepatocytes but increased OPN level in BDECs after BDL. The data suggest that the OPN is derived from biliary epithelial cells during acute obstructive cholestasis. These cells are under considerable stress due to the increased pressure in the biliary tract after BDL. The fact that OPN levels in bile substantially increased after BDL suggests that OPN had to be released by biliary epithelial cells into bile. Because most of the OPN in bile was present as the cleaved form, this indicates that OPN was either cleaved within biliary epithelial cells or that the intact form was released and the processing occurred in bile. OPN is known to be cleaved by thrombin (Senger et al., 1994) but also by matrix metalloproteinases, especially MMP-3 and MMP-7 (Agnihotri et al., 2001). Characteristic OPN fragments caused by MMP activities include peptides of 40 and 32kD (Agnihotri et al., 2001) similar to what was observed in our study. In general, MMP-cleaved OPN fragments appear to be more potent activators and chemoattractants of leukocytes (Agnihotri et al., 2001). Co-expression of OPN and induction of MMPs has been reported for wound healing and inflammation (Agnihotri et al., 2001; Dobaczewski et al., 2010; Giachelli et al., 1998; Mori et al., 2008). Our data demonstrate that MMP-3 was induced in the liver early after BDL. In addition, we could show that increased MMP-2, -3 and -9 activities were detectable in bile. Thus, it appears likely that MMPs including MMP-3 were released into bile and cleaved OPN to form a potent chemoattractant for neutrophils. The fact that the general MMP inhibitor Batimastat (BB-94) reduced OPN cleavage in bile and effectively reduced neutrophil recruitment and liver injury after BDL supports this conclusion.
MMPs are known to degrade all components of the extracellular matrix but they are also involved in the modulation of cytokine and chemokine activity (Van Lint and Libert, 2007; Manicone and McGuire, 2008). MMPs can cleave cytokines and chemokines and generate chemotactic gradients or inactivate these pro-inflammatory mediators (Van Lint and Libert, 2007). Although our data strongly suggest that neutrophil recruitment and injury during the first 24 h is dominated by MMP-cleaved OPN, it cannot be excluded that additional substrates of MMPs are involved at later stages. In fact, the formation of chemokines such as MIP-2 and KC in hepatocytes after exposure to bile acids (Allen et al., 2011) may provide opportunity for MMPs to activate these chemokines and form a chemotactic gradient that promotes the initial inflammatory response. The fact that in the absence of OPN, there is a delayed neutrophil recruitment and injury after BDL supports the involvement of additional chemoattractants such as MIP-2 and KC in the pathophysiology.
A recent paper also demonstrated a protective effect of a MMP inhibitor 14 days after BDL (Kahraman et al., 2009). However, in contrast to our study, these authors focused on long-term effects of the pan-MMP inhibitor CTS-1027 showing reduced liver injury (apoptosis and bile infarcts) and less fibrosis (Kahraman et al., 2009). The authors suggested that MMPs induced hepatocellular apoptosis (Kahraman et al., 2009). However, a role of apoptosis in BDL-induced liver injury has been disputed (Gujral et al., 2004c; Fickert et al., 2005; Nalapareddy et al., 2009; Woolbright et al., 2013). Thus, it is unlikely that the MMP inhibitor worked through this mechanism. On the other hand, the anti-fibrotic effect of the MMP inhibitor may be related to reduced extracellular matrix degradation by MMPs secreted by activated stellate cells. In addition, the long-term effect on tissue injury could also be related to inhibition of pro-inflammatory cytokine and chemokine formation (Van Lint and Libert, 2007). Thus, the mechanism of protection by the MMP inhibitor in the study by Kahraman et al. (2009) is different compared to the very early effect on OPN cleavage described in our manuscript. In addition, in both studies pan-MMP inhibitors were used, which raises the possibility that different MMPs might be involved in the early versus late phase of the injury after BDL.
4.3 Direct cytotoxicity of bile acids after BDL
In addition to inflammation, direct cytotoxicity of bile acids has been proposed as mechanism of cholestatic liver injury (Higuchi and Gores, 2003; Perez and Briz, 2009). However, the fact that impairment of neutrophil cytotoxicity drastically reduces bile infarcts without affecting cholestasis suggests that in this mouse model bile acids may not cause direct cytotoxicity (Gujral et al., 2003, 2004b). Consistent with these findings, even mM concentrations of the most abundant bile acids found in mice after BDL (taurocholic acid, tauromuricholic acid and muricholic acid) do not cause hepatocyte cell death (Allen et al., 2011; Zhang et al., 2012). In contrast, these bile acids trigger formation of neutrophil chemoattractants (CXC chemokines) in mouse hepatocytes (Allen et al., 2010, 2011). These data support the hypothesis that bile acids leaking back into the parenchyma after BDL-induced rupture of cholangioles trigger formation of neutrophil chemotactic factors, which may amplify the inflammatory response initiated by cOPN.
4.4 Initiation of the inflammatory response after BDL - Conclusions
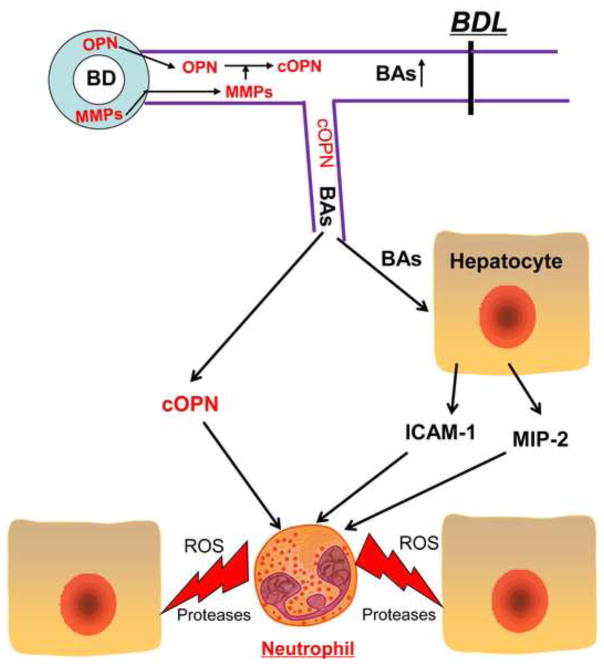
Based on published findings and the new data presented in this manuscript the following picture emerges for the early inflammatory response after BDL (Figure 8). After obstruction of the bile duct, the pressure in the biliary systems rapidly increases leading to stress on the biliary epithelial cells. OPN and MMPs are released into bile and MMPs generate fragments of OPN, which are chemotactic mediators for leukocytes. Due to the excessive pressure in the biliary system, ruptures in the biliary system occur at the level of the cholangioles, which results in the leakage of bile into the parenchyma (Fickert et al., 2002). Cleaved OPN acts directly as a chemoattractant for neutrophils, leading to the recruitment of neutrophils into the liver and extravasation in the area of bile leakage. Hepatocytes exposed to MMPs and the high levels of bile acids such as TCA trigger transcriptional activation of inflammatory genes including ICAM-1 and CXC chemokines and potentially some very mild injury that does not directly result in cell death. However, the extravasating neutrophils attack and kill these stressed cells thereby generating the characteristic bile infarcts (areas of necrosis). In OPN-deficient mice, the initial chemotactic signal is missing causing the delay of the inflammatory response and injury. However, high levels of BAs trigger transcriptional activation of CXC chemokines in hepatocytes, which requires some time to fully develop (Allen et al., 2011; Zhang et al., 2012). These chemokines, although not the most potent activators of neutrophils (Bajt et al., 2001), may eventually provide a sufficiently strong chemotactic signal to trigger neutrophil extravasation and induce a delayed inflammatory injury. Thus, the MMP-mediated cleavage of OPN represents the early pro-inflammatory signal, which induces a neutrophil-mediated injury phase after BDL in mice.
Figure 8.
Proposed mechanism of inflammatory liver injury in the early phase of obstructive cholestasis. After bile duct ligation (BDL), osteopontin (OPN) released from biliary epithelial cells could be cleaved by matrix metalloproteinases (MMPs) in bile. When the growing pressure in the biliary system results in rupture of cholangioles, cleaved OPN enters the parenchyma, and then attracts and activates neutrophils. The process is facilitated by intercellular adhesion molecule-1 (ICAM-1) expression on hepatocytes, which is induced by high concentrations of bile acids (BAs) leaking into the parenchyma. If OPN is absent, CXC chemokines such as MIP-2, which are also induced by BAs in hepatocytes, can promote neutrophil activation and recruitment resulting in a similar but delayed inflammatory injury.
Highlights.
Cholestatic liver injury occurs partially by neutrophil mediated hepatic necrosis
Osteopontin-deficient mice are protected against early cholestatic liver injury
Metalloproteinases cleave osteopontin to a pro-inflammatory form in bile
MMP inhibitor prevents neutrophil recruitment and early cholestatic liver injury
Osteopontin mediates neutrophil recruitment leading to cholestatic liver injury
Acknowledgments
This investigation was supported in part by National Institutes of Health Grants AA12916 and DK070195(H.J.), DK073566 (B.L.C.), and by a Center of Biomedical Research Excellence (COBRE) grant P20 RR021940 from the National Center for Research Resources (NCRR), and the National Institute of General Medical Sciences (8 P20 GM103549-07) of the National Institutes of Health. Additional support came from an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20 GM12345 and, from the “Training Program in Environmental Toxicology” T32 ES007079-26A2 from the National Institute of Environmental Health Sciences.
Abbreviations
- ALT
alanine aminotransferase
- BDECs
bile duct epithelial cells
- BDL
bile duct ligation
- H&E
hematoxylin and eosin
- HPF
high-power fields
- ICAM-1
intercellular adhesion molecule-1
- KO mice
gene knock-out mice
- MMP
matrix metalloproteinase
- OPN
osteopontin
- WT
wild type
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agnihotri R, Crawford HC, Haro H, Matrisian LM, Havrda MC, Liaw L. Osteopontin, a novel substrate for matrix metalloproteinase-3 (stromelysin-1) and matrix metalloproteinase-7 (matrilysin) J Biol Chem. 2001;276:28261–28267. doi: 10.1074/jbc.M103608200. [DOI] [PubMed] [Google Scholar]
- Allen K, Jaeschke H, Copple BL. Bile acids induce inflammatory genes in hepatocytes: a novel mechanism of inflammation during obstructive cholestasis. Am J Pathol. 2011;178:175–186. doi: 10.1016/j.ajpath.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen K, Kim ND, Moon JO, Copple BL. Upregulation of early growth response factor-1 by bile acids requires mitogen-activated protein kinase signaling. Toxicol Appl Pharmacol. 2010;243:63–67. doi: 10.1016/j.taap.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte UM, Banerjee A, McRee R, Wellberg E, Ramaiah SK. Role of osteopontin in hepatic neutrophil infiltration during alcoholic steatohepatitis. Toxicol Appl Pharmacol. 2005;207:25–38. doi: 10.1016/j.taap.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Farhood A, Jaeschke H. Effects of CXC chemokines on neutrophil activation and sequestration in hepatic vasculature. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1188–G1195. doi: 10.1152/ajpgi.2001.281.5.G1188. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Lawson JA, Vonderfecht SL, Gujral JS, Jaeschke H. Protection against Fas receptor-mediated apoptosis in hepatocytes and nonparenchymal cells by a caspase-8 inhibitor in vivo: evidence for a postmitochondrial processing of caspase-8. Toxicol Sci. 2000;58:109–117. doi: 10.1093/toxsci/58.1.109. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Yan HM, Farhood A, Jaeschke H. Plasminogen activator inhibitor-1 limits liver injury and facilitates regeneration after acetaminophen overdose. Toxicol Sci. 2008;104:419–427. doi: 10.1093/toxsci/kfn091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Apte UM, Smith R, Ramaiah SK. Higher neutrophil infiltration mediated by osteopontin is a likely contributing factor to the increased susceptibility of females to alcoholic liver disease. J Pathol. 2006a;208:473–485. doi: 10.1002/path.1917. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Burghardt RC, Johnson GA, White FJ, Ramaiah SK. The temporal expression of osteopontin (SPP-1) in the rodent model of alcoholic steatohepatitis: a potential biomarker. Toxicol Pathol. 2006b;34:373–384. doi: 10.1080/01926230600806543. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Lee JH, Ramaiah SK. Interaction of osteopontin with neutrophil alpha(4)beta(1) and alpha(9)beta(1) integrins in a rodent model of alcoholic liver disease. Toxicol Appl Pharmacol. 2008;233:238–246. doi: 10.1016/j.taap.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Chosay JG, Essani NA, Dunn CJ, Jaeschke H. Neutrophil margination and extravasation in sinusoids and venules of liver during endotoxin-induced injury. Am J Physiol. 1997;272:G1195–G1200. doi: 10.1152/ajpgi.1997.272.5.G1195. [DOI] [PubMed] [Google Scholar]
- Cover C, Liu J, Farhood A, Malle E, Waalkes MP, Bajt ML, Jaeschke H. Pathophysiological role of the acute inflammatory response during acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2006;216:98–107. doi: 10.1016/j.taap.2006.04.010. [DOI] [PubMed] [Google Scholar]
- De Minicis S, Seki E, Uchinami H, Kluwe J, Zhang Y, Brenner DA, Schwabe RF. Gene expression profiles during hepatic stellate cell activation in culture and in vivo. Gastroenterology. 2007;132:1937–1946. doi: 10.1053/j.gastro.2007.02.033. [DOI] [PubMed] [Google Scholar]
- Denhardt D, Giachelli CM, Rittling SR. Role of osteopontin in cellular signaling and toxicant injury. Annu Rev Pharmacol Toxicol. 2001;41:723–749. doi: 10.1146/annurev.pharmtox.41.1.723. [DOI] [PubMed] [Google Scholar]
- Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol. 2010;48:504–511. doi: 10.1016/j.yjmcc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fickert P, Stöger U, Fuchsbichler A, Moustafa T, Marschall HU, Weiglein AH, Tsybrovskyy O, Jaeschke H, Zatloukal K, Denk H, Trauner M. A new xenobiotic-induced mouse model of sclerosing cholangitis and biliary fibrosis. Am J Pathol. 2007;171:525–536. doi: 10.2353/ajpath.2007.061133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fickert P, Thueringer A, Moustafa T, Silbert D, Gumhold J, Tsybrovskyy O, Lebofsky M, Jaeschke H, Denk H, Trauner M. The role of osteopontin and tumor necrosis factor alpha receptor-1 in xenobiotic-induced cholangitis and biliary fibrosis in mice. Lab Invest. 2010;90:844–852. doi: 10.1038/labinvest.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fickert P, Trauner M, Fuchsbichler A, Zollner G, Wagner M, Marschall HU, Zatloukal K, Denk H. Oncosis represents the main type of cell death in mouse models of cholestasis. J Hepatol. 2005;42:378–383. doi: 10.1016/j.jhep.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Fickert P, Zollner G, Fuchsbichler A, Stumptner C, Weiglein AH, Lammert F, Marschall HU, Tsybrovskyy O, Zatloukal K, Denk H, Trauner M. Ursodeoxycholic acid aggravates bile infarcts in bile duct-ligated and Mdr2 knockout mice via disruption of cholangioles. Gastroenterology. 2002;123:1238–1251. doi: 10.1053/gast.2002.35948. [DOI] [PubMed] [Google Scholar]
- Giachelli CM, Lombardi D, Johnson RJ, Murry CE, Almeida M. Evidence for a role of osteopontin in macrophage infiltration in response to pathological stimuli in vivo. Am J Pathol. 1998;152:353–358. [PMC free article] [PubMed] [Google Scholar]
- Gujral JS, Farhood A, Bajt ML, Jaeschke H. Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct-ligated mice. Hepatology. 2003;38:355–363. doi: 10.1053/jhep.2003.50341. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Hinson JA, Farhood A, Jaeschke H. NADPH oxidase-derived oxidant stress is critical for neutrophil cytotoxicity during endotoxemia. Am J Physiol Gastrointest Liver Physiol. 2004a;287:G243–G252. doi: 10.1152/ajpgi.00287.2003. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Liu J, Farhood A, Hinson JA, Jaeschke H. Functional importance of ICAM-1 in the mechanism of neutrophil-induced liver injury in bile duct-ligated mice. Am J Physiol Gastrointest Liver Physiol. 2004b;286:G499–G507. doi: 10.1152/ajpgi.00318.2003. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Liu J, Farhood A, Jaeschke H. Reduced oncotic necrosis in Fas receptor-deficient C57BL/6J-lpr mice after bile duct ligation. Hepatology. 2004c;40:998–1007. doi: 10.1002/hep.20380. [DOI] [PubMed] [Google Scholar]
- Higuchi H, Gores GJ. Bile acid regulation of hepatic physiology: IV. Bile acids and death receptors. Am J Physiol Gastrointest Liver Physiol. 2003;284:G734–G738. doi: 10.1152/ajpgi.00491.2002. [DOI] [PubMed] [Google Scholar]
- Hirschfield GM, Heathcote EJ, Gershwin ME. Pathogenesis of cholestatic liver disease and therapeutic approaches. Gastroenterology. 2010;139:1481–1496. doi: 10.1053/j.gastro.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Jaeschke H. Mechanisms of Liver Injury. II Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1083–G1088. doi: 10.1152/ajpgi.00568.2005. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Farhood A, Smith CW. Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. FASEB J. 1990;4:3355–3359. [PubMed] [Google Scholar]
- Jaeschke H, Williams CD, Ramachandran A, Bajt ML. Acetaminophen hepatotoxicity and repair: the role of sterile inflammation and innate immunity. Liver Int. 2012;32:8–20. doi: 10.1111/j.1478-3231.2011.02501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahraman A, Bronk SF, Cazanave S, Werneburg NW, Mott JL, Contreras PC, Gores GJ. Matrix metalloproteinase inhibitor, CTS-1027, attenuates liver injury and fibrosis in the bile duct-ligated mouse. Hepatol Res. 2009;39:805–813. doi: 10.1111/j.1872-034X.2009.00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ND, Moon JO, Slitt AL, Copple BL. Early growth response factor-1 is critical for cholestatic liver injury. Toxicol Sci. 2006;90:586–595. doi: 10.1093/toxsci/kfj111. [DOI] [PubMed] [Google Scholar]
- Koh A, da Silva AP, Bansal AK, Bansal M, Sun C, Lee H, Glogauer M, Sodek J, Zohar R. Role of osteopontin in neutrophil function. Immunology. 2007;122:466–475. doi: 10.1111/j.1365-2567.2007.02682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manicone AM, McGuire JK. Matrix metalloproteinases as modulators of inflammation. Semin Cell Dev Biol. 2008;19:34–41. doi: 10.1016/j.semcdb.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori R, Shaw TJ, Martin P. Molecular mechanisms linking wound inflammation and fibrosis: knockdown of osteopontin leads to rapid repair and reduced scarring. J Exp Med. 2008;205:43–51. doi: 10.1084/jem.20071412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalapareddy P, Schüngel S, Hong JY, Manns MP, Jaeschke H, Vogel A. The BH3-only protein bid does not mediate death-receptor-induced liver injury in obstructive cholestasis. Am J Pathol. 2009;175:1077–1085. doi: 10.2353/ajpath.2009.090304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MJ, Briz O. Bile-acid-induced cell injury and protection. World J Gastroenterol. 2009;15:1677–1689. doi: 10.3748/wjg.15.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Balasubramanian KA. Protease activation during surgical stress in the rat small intestine. J Surg Res. 2000;92:283–290. doi: 10.1006/jsre.2000.5841. [DOI] [PubMed] [Google Scholar]
- Ramaiah SK, Rittling S. Pathophysiological role of osteopontin in hepatic inflammation, toxicity, and cancer. Toxicol Sci. 2008;103:4–13. doi: 10.1093/toxsci/kfm246. [DOI] [PubMed] [Google Scholar]
- Sellinger M, Boyer JL. Physiology of bile secretion and cholestasis. Prog Liver Dis. 1990;9:237–259. [PubMed] [Google Scholar]
- Senger DR, Perruzzi CA, Papadopoulos-Sergiou A, Van de Water L. Adhesive properties of osteopontin: regulation by a naturally occurring thrombin-cleavage in close proximity to the GRGDS cell-binding domain. Mol Biol Cell. 1994;5:565–574. doi: 10.1091/mbc.5.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lint P, Libert C. Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. J Leukoc Biol. 2007;82:1375–1381. doi: 10.1189/jlb.0607338. [DOI] [PubMed] [Google Scholar]
- Whitington PF, Malladi P, Melin-Aldana H, Azzam R, Mack CL, Sahai A. Expression of osteopontin correlates with portal biliary proliferation and fibrosis in biliary atresia. Pediatr Res. 2005;57:837–844. doi: 10.1203/01.PDR.0000161414.99181.61. [DOI] [PubMed] [Google Scholar]
- Wielockx B, Lannoy K, Shapiro SD, Itoh T, Itohara S, Vandekerckhove J, Libert C. Inhibition of matrix metalloproteinases blocks lethal hepatitis and apoptosis induced by tumor necrosis factor and allows safe antitumor therapy. Nat Med. 2001;7:1202–1208. doi: 10.1038/nm1101-1202. [DOI] [PubMed] [Google Scholar]
- Woolbright BL, Antoine DJ, Jenkins RE, Bajt ML, Park BK, Jaeschke H. Plasma biomarkers of liver injury and inflammation demonstrate a lack of apoptosis during obstructive cholestasis in mice. Toxicol Appl Pharmacol. 2013 Oct 3; doi: 10.1016/j.taap.2013.09.023. pii: S0041-008X(13)00423-7. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolbright BL, Jaeschke H. Novel insight into mechanisms of cholestatic liver injury. World J Gastroenterol. 2012;18:4985–4993. doi: 10.3748/wjg.v18.i36.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hong JY, Rockwell CE, Copple BL, Jaeschke H, Klaassen CD. Effect of bile duct ligation on bile acid composition in mouse serum and liver. Liver Int. 2012;32:58–69. doi: 10.1111/j.1478-3231.2011.02662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]