Abstract
Macrophages are central regulators of disease progression in both atherosclerosis and myocardial infarction. In atherosclerosis, macrophages are the dominant leukocyte population that influences lesional development. In myocardial infarction, which is caused by atherosclerosis, macrophages accumulate readily and play important roles in inflammation and healing. Molecular imaging has grown considerably as a field and can reveal biological process at the molecular, cellular, and tissue levels. Here we explore how various imaging modalities, from intravital microscopy in mice to organ-level imaging in patients, are contributing to our understanding of macrophages and their progenitors in cardiovascular disease.
INTRODUCTION
Macrophages are large myeloid cells that inhabit nearly all tissues and play vital roles in homeostasis and immunity. Over the last several years, our understanding of macrophage biology has grown substantially, thanks in large part to two related lines of investigation. First, we have begun to understand macrophage ontogeny at both the cellular and molecular levels. For many years it was believed that circulating monocytes give rise to macrophages.1 Yet, it has also been appreciated that macrophages can self renew in tissue, apparently without relying on monocytes.2 Recent studies, using sophisticated tools, have provided additional evidence for a disconnet between macrophages and monocytes.3,4 We now understand that some macrophages do derive from a hematopoietic stem cell lineage that involves specific progenitor intermediates and monocytes5, whereas other macrophages derive from primitive macrophages that have colonized tissue prior to definitive hematopoiesis.6 The second major and related line of investigation concerns macrophage functional heterogeneity. Macrophages have long been understood as highly “plastic” cells, capable of adapting to their environment. The observations that macrophages can polarize to various functional states7,8 as well as the identification of monocyte subsets9 has fostered the idea that macrophages are fated for specific functions, suggesting that harmful subsets can be therapeutically targeted while those that are beneficial can be spared. The recent profiling of various macrophage populations has revealed striking differences between macrophages of different tissues10. Investigators will need to be cautious about generalizing observations obtained with one type of macrophage (for example, peritoneal) to other types of macrophages (for example, microglial).
Macrophages are arguably the most important cells in atherosclerosis, a chronic inflammatory disease of leukocyte and lipid accumulation in the vessel wall that can cause myocardial infarction and stroke. Atherosclerotic lesions grow when circulating monocytes adhere to the activated endothelium at sites of lesion predilection, transmigrate, ingest oxidized lipoproteins, and differentiate to macrophages.11 Advances in macrophage biology have contributed to our understanding of atherosclerosis. Under conditions of hypercholesterolemia, specific populations of monocyte subsets accumulate in lesions preferentially.12,13 The large pool of circulating monocytes capable of lesional accumulation depends on hematopoietic stem cell proliferation14,15, extramedullary hematopoiesis16, and enhanced mobilization from the bone marrow.17 In lesions, macrophages perform vital functions that influence the course of disease (Figure 1). Macrophages release inflammatory cytokines and proteases that disrupt the extracellular matrix and prolong inflammation, accumulate lipids and dying cells, participate in efferocytosis, undergo autophagy, and, either as living foam cells or as cellular remnants of a tissue factor-rich lipid core, contribute significantly to lesion size and stability.18
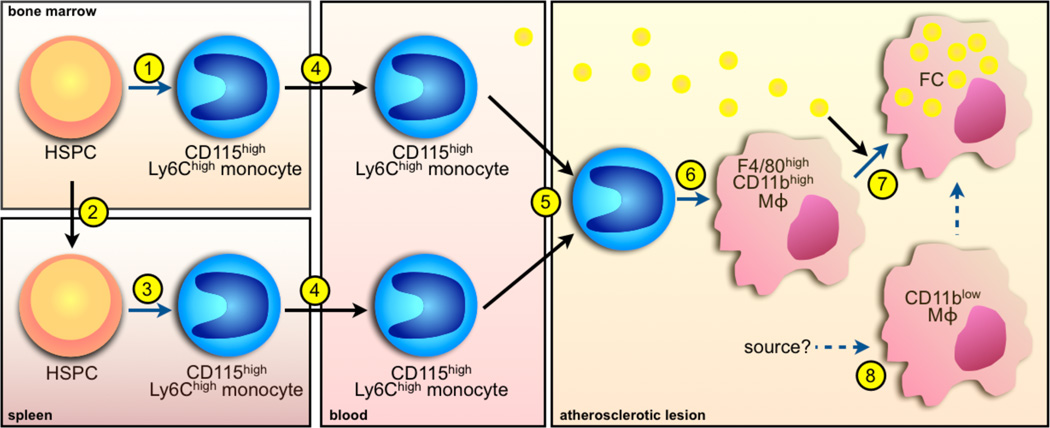
Figure 1. A simple model of the macrophage lineage in atherosclerosis.
1. Hematopoietic stem and progenitor cells (HSPC) give rise to monocytes in the bone marrow, including the Ly-6Chighsubset. 2. During atherosclerosis, HSPC mobilize and seed secondary lymphoid organs such as the spleen. 3. In the spleen, HSPC give rise to monocytes in a process called extramedullary hematopoiesis. 4. Monocytes produced in the bone marrow and spleen enter the circulation. 5. Monocytes rely on various signals to enter the atherosclerotic lesion. 6. In the lesion, monocytes differentiate to macrophages. 7. Monocyte-derived macrophages ingest lipids and become lipidrich foam cells (FC). 8. There may also be monocyte-independent macrophages residing in the aorta that possibly contribute to FC formation. Black arrows depict cell or lipid movement whereas blue arrows depict differentiation.
Lesions that rupture in coronary arteries cause myocardial infarction. This ischemic event kills cardiomyocytes and triggers the influx of myeloid cells. Monocytes accumulating in the infarcted myocardium arrive from the bone marrow and spleen19 in two sequential phases: Ly-6Chigh monocytes arrive first in response to MCP-1, whereas Ly-6Clow monocytes arrive second in response to fractalkine.20 It is the coordinated action of both phases that leads to optimum healing. An imbalance in the phases that skews toward inflammation delays healing and causes heart failure.21 Myocardial infarction is an acute injury that mobilizes the bone marrow and the splenic reservoir for monocyte production, thus increasing atherosclerosis22, and fuelling an inflammatory cycle.
Molecular imaging encompasses many different modalities in which specific aspects of macrophage behavior at molecular, cellular, tissue and organ levels can be visualized.23 The main modalities currently employed in preclinical and clinical macrophage imaging include optical imaging, magnetic resonance imaging, and nuclear imaging (positron emission tomography and SPECT). Each of these modalities has distinct advantages and operates on a scale defined by its spatial resolution (Table 1). Imaging of macrophages has been employed to pursue two major goals: a) preclinical imaging to observe macrophage lineage biology and behavior in the undisturbed microenvironment, and b) clinical imaging to detect macrophage presence and function in inflamed tissue. The latter identifies patients at risk for complications and disease acceleration, and could therefore enable preventive measures. Ultimately, detecting disease early may spare patients myocardial infarction, heart failure and stroke.24 In this review, we will focus on imaging approaches that elucidate the development of macrophages, their dynamic paths to the site of inflammation, and their function and fate in the context of atherosclerosis and myocardial infarction.
Table 1.
Imaging modalities to study macrophage function in cardiovascular disease
| Modality | Imaging targets | Spatial resolution | Pros | Cons | Preclinical | clincal |
|---|---|---|---|---|---|---|
| Intravital microscopy | Single cell behavior in live animal, including motion, interaction, release, recruitment | µm | Cellular resolution, dynamic | Penetration depth <1mm, invasive | + | − |
| OCT | Cells in arterial vessel wall | µm | Highest resolution of clinical techniques | Limited specificity, not quantitative, invasive | + | + |
| FMT | Cell population dynamics, cellular function such as protease activity | 1mm | Quantitative, multiple targets, high thorughput | Limited to rodents | + | − |
| MRI | Cell population imaged with nanoparticles | 100µm to 0.5mm | Combined with excellent anatomic and functional data | Semiquatitative | + | + |
| PET SPECT | Cell population imaged with 18FDG or nanoparticles (preclinical), protease presence | 1mm to 1cm | Quantitative, highest sensitivity of macoscopic techniques | Expensive, radiation, low throughput | + | + |
Abbreviations: FMT, fluorescence molecular tomography; MRI, magnetic resonance imaging; OCT, optical coherence tomography; PET, positron emission tomography; SPECT, single-photon emission computed tomography; 18FDG, 18F-fluorodeoxyglucose.
IMAGING THE CRADLE
Many macrophage populations, especially in inflammatory plaque or in acute ischemic myocardial tissue, derive from monocytes. In the steady state, monocytes arise from hematopoietic stem and progenitor cells in the bone marrow, a protected site that harbors the hematopoietic stem cell niche. The purpose of this niche is to protect HSPC against the harsh extramedullary environment, allowing production of billions of blood cells in the steady state and inflammation.
Intravital microscopy has unraveled some of the basic biological processes in hematopoietic tissues, including stem cell behavior, organization, and movement in the steady state and after interventions. The relatively thin cortex of the murine skull allows for serial confocal microscopy and two-photon video imaging of individual hematopoietic progenitor cells in live mice.25 Often, these cells are harvested from donor mice using flow sorting, and are labeled with fluorescent dyes prior to intravenous injection into recipients. Cells may also be harvested from mice that ubiquitously express a fluorescent protein. Several days after transfer, transplanted stem cells that have settled in bone marrow niches can be observed with cellular resolution (Figure 2A), and in relation to other components of the hematopoietic niche such as bone, blood vessels, or cells that instruct the niche's microenvironment. Motorized microscopy stages locate precise coordinates in the skull bone marrow, allowing to revisit the same location in serial experiments.25 With these techniques, dynamic processes such as cell division or departure can be visualized. The experiments have provided unprecedented insight into the life of macrophages’ most upstream progenitors, including hematopoietic stem cells. For instance, the location of progenitors, i.e. their proximity to bone or sinusoids, may depend on their stage of activity and maturity. Intravital microscopy also revealed that immunosuppressive T regulatory cells protect stem cells from immune attack after allotransplantation.26 Combining serial intravital imaging of medullary niches in the skull with laser photoconversion have revealed that certain catastrophic events such as myocardial infarction trigger the departure and relocation of fluorescently labeled HSPCs out of the bone marrow and into the spleen.22 The technology can quantify therapeutic targeting of macrophage progenitors in the bone marrow, for instance by measuring HSPC release in serial imaging sessions. In one such experiment, treatment with a beta 3 adrenergic receptor blocker, which acts on bone marrow niche cells supplying retention factors27, inhibited departure of HSPC after MI.22
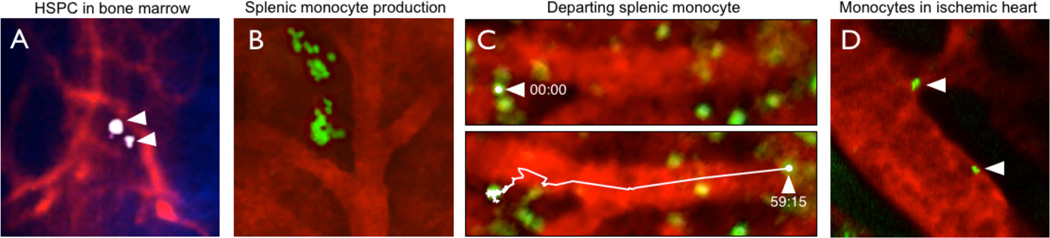
Figure 2. Intravital microscopy of the macrophage linage.
(A) HSPC in the bone marrow niche after adoptive transfer of FACS isolated, near infrared membrane labeled progenitors. (B) GFP+ progenitor cells in the spleen form a cluster in the red pulp. (C) Monocyte that departs the splenic reservoir after induction of myocardial infarction. Two movie frames (59 seconds apart) of video microscopy are shown. (D) Intravital microscopy of monocytes in the mouse heart.
Clinical reports have shown altered bone marrow activity after myocardial infarction. An18FFDG PET study in patients on day 7 after MI measured increased uptake of the tracer in the bone marrow, reflecting accentuated glucose uptake.28 Given the spatial resolution of PET, the signal changes could not be attributed to specific cell types. One could speculate that the higher PET signal reflected increased cellular proliferation after MI, as higher proliferation accelerates energy demand of bone marrow cells. There are more specific PET agents that could report directly on cell proliferation in the bone marrow, for instance18F-FLT (3'-deoxy-3'- fluorothymidine).29 Currently used in oncologic PET imaging of proliferating cancer cells, this agent should also enable tracking proliferative activity of hematopoietic tissue during atherosclerosis and after myocardial infarction, when the periphery's increased macrophage demand signals to hematopoietic tissue to accelerate macrophage progenitor production. These signals, which are currently being explored, could potentially serve as therapeutic targets; imaging hematopoietic tissue would be a suitable tool to test their therapeutic efficiency.
During atherosclerosis and myocardial infarction the bone marrow outsources the production of monocytes to extramedullary sites such as the spleen. Intravital imaging of the spleen is possible because motion of the organ can be easily controlled. A typical experiment involves the exteriorization of the spleen and imaging of the splenic red pulp up to 200 µm below the capsule (Figure 2B). It is important to maintain organ perfusion, which can be visualized with a blood pool agent. The use of CX3CR1GFP/+ mice allows to image monocytes and their descendants, whereas GFP expressed ubiquitously can be a tool for imaging endogenous or adoptively transferred leukocytes. In the steady state, z-stack projections of a CX3CR1GFP/+ spleen reveals clusters of green, relatively immotile cells in the tissue parenchyma.19 In response to myocardial infarction, this population departs quickly (Figure 2C), but is replaced in a few days with the help of extramedullary hematopoiesis.30 During chronic inflammation, the spleen becomes a hematopoietic organ that supports the production of monocytes in the red pulp.16,31 Fate-mapping studies employing adoptive transfer of GFP+ monocyte progenitors has revealed clusters of green cells in the red pulp (Figure 2B). These clusters bear a striking resemblance to monocyte clusters occupying the spleen in the steady state. It is possible that, even in the steady state, low level production of monocytes is occuring in the spleen. Traditional tools such as flow cytometry or immunofluorescence would have been inadequate at deciphering the three-dimensional organization of these cells in an intact organ; it was intravital microscopy that made identification of monocyte clusters and demonstration of local hematopoiesis possible.
IMAGING MOVEMENT
Monocytes generated in the bone marrow exit by relying on the CCR2 chemokine receptor which responds to MCP1 produced by mesenchymal stem cells (MSCs) and their progeny.32–34 Monocytes generated in the spleen do not require CCR2 but utilize angiotensin-II to escape the red pulp (Figure 2C).19 Upon entering the circulation, monocytes can rapidly migrate and accumulate in atherosclerotic lesions or the infarcted myocardium (Figure 2D). Real-time tracking of the entire migration path is not yet possible because of the cells’ velocity in the blood stream. However, some monocytes patrol the vessel wall through weak adhesions. Tracking their migration has revealed irregular patterns of movement, sometimes against the flow of the blood.35 The patrolling behavior presumably allows monocytes to sense danger and alert other leukocytes. Neutrophils, as recently shown, utilize ‘slings’ to roll at high shear stress.36 Events in which leukocytes interact with the endothelium, such as adhesion, intravasation, and extravasation, can be particularly conducive to intravital microscopy, especially when visualizing small vessels whose motion can be readily stabilized (Figure 2C, D). In the microcirculation, leukocytes traverse the endothelial and pericyte layers back and forth several times before commiting for a final destination.37–39 This remarkable dynamism broadens our understanding of leukocyte accumulation and suggests that leukocytes may influence a particular tissue without necessarily differentiating or acquiring residence. It is also tempting to speculate that such leukocytes sample their environment and “report” at distant sites.
Although real-time tracking of monocyte migration in the blood has not been possible, pulsechase fate-mapping studies can reveal redistribution from one location to another. Tagging sorted monocytes with the radioactive cell-labeling agent 111In-oxine and re-injection into the blood showed continuous accumulation in the artery wall during atherosclerosis40,41, and a biphasic time-dependent accumulation of Ly-6Chigh and Ly-6Clow monocytes in the infarcted myocardium,20 The use of the CD45.1/CD45.2 allelic exclusion method12 or a combination of clodronate liposome and bead labeling13 has shown that Ly-6Chigh monocytes accumulate in atherosclerotic lesions preferentially. These imaging approaches have forged direct links between circulating cells and tissue macrophages in ways that would have not been possible earlier.
Intravital microscopy has proven useful in imaging cell movement in organs. Monocytes exiting the spleen can be visualized as they intravasate from the splenic parenchyma to the vessel (Figure 2C).19 Macrophages are predominantly found in the intima of atherosclerotic blood vessels, but there is also a population of macrophages readily found in the adventitia. In later stages of atherosclerosis, at least in the mouse, tertiary lymphoid organs develop next to the adventitia.42 A long-standing question was therefore whether monocytes can utilize the vasa vasora to shuttle to the adventitia and, subsequently, the intima, or whether monocyte entry is exclusively dependent on traversing the endothelium into the intima.43 Recent intravital microscopy studies have shown that microvessels are indeed major leukocyte entry points in advanced lesions.44 Because monocytes/macrophages are rarely detected in the media, it still remains to be determined whether relocation from the adventitia to the intima is possible. Perhaps a short transit time, rather than the absence of movement, can explain the paucity of these cells in the media.
IMAGING MACROPHAGES AT THE EFFECTOR SITE
For decades, observing the dynamic action of macrophages in their native environment was impossible. Vascular biology and heart failure research had to rely on snapshots that provided a macrophage still image in the tissue at the time of death. Molecular and cellular imaging techniques have recently overcome several decisive hurdles. We can now observe in vivo macrophage activity at the site of inflammation in the heart. One major obstacle of in vivo microscopy, which has been used to image the macrophage lineage with high resolution in other inflammatory settings and in lymph nodes, is the motion of the heart during the cardiac cycle. ECG-gated image acquisition, together with advanced tissue stabilisation, can now overcome these hurdles and for the first time visualize macrophages in the beating heart (Figure 2D).45,46 In conjunction with monocyte/macrophage labeling with exogenous dyes or fluorescent protein reporter gene techniques, these tools will likely provide key insights into recruitment, function and interaction of macrophages with other cells.47
Motion is likewise a major problem in imaging macrophage behavior in the atherosclerotic lesion by intravital microscopy. In order to observe how a cell behaves in 3 dimensional space, it is imperative that the tissue is immobilized. For these reasons, it is easier to image the spleen, liver or lymph nodes19,48,49, but much more difficult to image the aorta which is continuously moving as a result of the pulsatile blood flow. One way to bypass the problem is to perform microscopic imaging of an explanted aorta.47 While this approach technically constitutes ex vivo imaging, careful tissue handling keeps leukocytes in the vessel wall alive and motile for many hours. Such studies have shown that dendritic cells residing in the aorta are highly motile and readily interact with T cells, forming lasting and decisive partnerships.
Macrophages are polyfunctional in atherosclerotic lesions and in the infarcted myocardium. Among the most studied are phagocytosis and proteolysis, major "professional" activities of macrophages in inflammatory tissue. The phagocytic capacity of macrophages can be harnessed by using nanoparticles as imaging agents. A variety of materials may form suitable nanoparticles that are taken up by macrophages, most often lipid composites and dextran polymers. Usually sized between 5 and 100 nm in diameter, these nanomaterials can be derivatized with imaging beacons detectable with all major modalities. Magnetic nanoparticles frequently contain iron oxide cores or gadolinium chelates, both of which change the hydrogen behavior and consecutively contrast on MRI. While first explored in rodents with atherosclerosis50,51 or acute MI52,53 (Figure 3A), iron oxide nanoparticles have already been used in patients with atherosclerosis54 and myocardial infarction55 to detect macrophages in inflamed plaque and myocardium, respectively. Advantages of macrophage MRI include the excellent spatial resolution and soft tissue contrast of the modality. Conventional cardiovascular MRI is firmly established in clinical care due to these properties, and one can easily envision nanoparticle-facilitated macrophage imaging as a valuable add-on. While iron oxide nanoparticle MRI is usually more sensitive than gadolinium-based techniques, iron oxide nanoparticles also have limitations. These include the negative contrast, i.e. the nanoparticles lead to signal decay. Decreased MRI signal can also be caused by cardiac motion and flow; hence, specific solutions such as bright iron MRI and pre/post injection imaging have been developed.56 19Fluorine MRI is noteworthy for its supreme specificity, as19F does not naturally occur in the body and detected signal must derive from injected nanoparticles engulfed by macrophages and other phagocytes.57
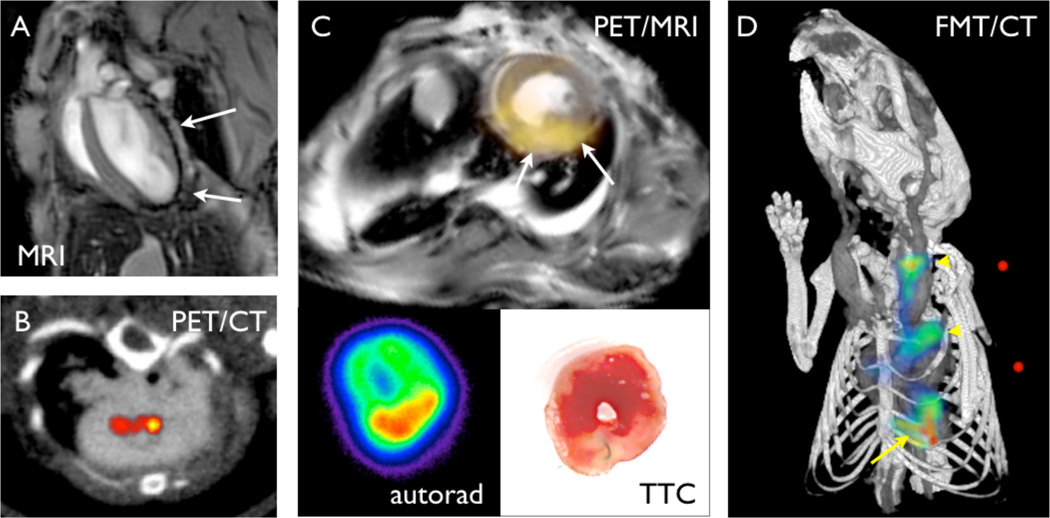
Figure 3. Non-invasive organ-level macrophage imaging.
(A) Macrophage imaging in the infarct with iron oxide nanoparticle-facilitated MRI in a mouse after coronary ligation. The arrows point at signal decrease in the infarct area. (B) PET/CT imaging in atherosclerotic lesions of an apoE−/− mouse using 64Cu labeled nanoparticles. (C) PET/MRI of infarct macrophages with 64Cu labeled nanoparticles. The lower panels show ex vivo autoradiography and the pale infarct on triphenyltetrazolium chloride (TTC) staining. (D) Fluorescence molecular tomography of protease activity in an apoE−/−mouse with atherosclerosis (arrow heads) and myocardial infarction (arrow).
Nuclear imaging with PET is highly sensitive and quantitative.23 The limitations of PET include the exposure to radiation and the lower spatial resolution. However, by combining nuclear imaging with CT and MRI, spatial information can be gained in a hybrid fashion (Figure 3B, C). Copper-64 and flourine-18 labeled nanoparticles were used to image macrophages in mice with atherosclerotic disease (Figure 3B) and aortic aneurysms.58,59
Macrophages are major sources of proteases. In atherosclerotic plaques, proteases are of central interest because they can destroy the stabilizing extracellular matrix and render a plaque more vulnerable.60 In acute myocardial infarcts, protease activity is necessary to clear away necrotic cardiac tissue. However, the tissue-destabilizing function of proteases may also lead to infarct expansion, rupture, and dilation of the left ventricle.61 Hence, protease presence and activity have been explored to detect disease-promoting macrophage activity in vivo. One prominent approach used radiolabeled small molecule inhibitors of proteases to image the presence of these enzymes in atherosclerotic plaques62,63 and in acute myocardial infarcts.64 As these imaging agents consist of small molecules, the hurdles for clinical translation should be comparatively reasonable. Indeed, metalloproteinases were recently successfully imaged in a large animal model of myocardial infarction.65 An alternative approach that reports on protease activity rather than presence employs cleavable optical prodrugs that are activated when they become a substrate of proteases. Here, fluorochromes that are detectable with near infrared imaging are closely approximated to each other by linkers that are protease substrates. The specificity to certain proteases such as cathepsins or metalloproteinases can be tailored by using enzyme specific peptide sequences. While whole animal imaging is feasible in the mouse and has detected protease activity in murine infarcts21 and plaques66 (Figure 3D), the limited penetration of optical imaging will curtail potential clinical applications. However, catheter-based detection of protease activity in human coronary plaque may be feasible, as demonstrated in experiments in rabbits.67
CLINICAL DEVELOPMENTS
Will macrophage imaging ever become a clinical tool? Likely it will, as it has already been used in patients with cardiovascular disease and gained a foothold in the clinical trial arena.68 18F-FDG imaging, while not specific to macrophages, is one option that is clinically available. PET imaging studies in animal models of atherosclerosis and myocardial infarction reported correlation of macrophage density with 18F-FDG activity in the plaque69,70 and acute infarcts71, likely due to the high glucose uptake into these active cells. A similar correlation was reported for 18F-FDG activity in carotid plaque and subsequent histological enumeration of macrophages in patients that were imaged before endarterectomy.72 In addition to the limited specificity of 18F-FDG to macrophages, the high uptake of the tracer into myocytes in the heart poses a problem. Because the quantitative and sensitive character of PET is particularly helpful in clinical trials, other, more specific macrophage agents (for instance nanoparticle based) may need to be pursued. Iron oxide core nanoparticle MRI is another example for clinical macrophage imaging in patients with atherosclerosis or myocardial infarction. The typical decay of signal in T2-weighted MR imaging was observed in carotid arteries of patients with atherosclerosis54 and in patients on day 7 after acute myocardial infarction.55 Microscopic detection of macrophages in coronary arteries of patients is currently pursued with optical coherence tomography, a technique that relies on catheter-based optical imaging.73 The latest technical advances of this intracoronary imaging modality have pushed the spatial resolution to 1 µm, and should therefore allow to distinguish single cells in the coronary plaque.74 These examples highlight that macrophage imaging has arrived in patients. However, it is still a long road to implementation into routine clinical decision making. The obvious advantages which may motivate this development include the early detection of inflammatory macrophage activity at a disease stage when therapeutic measures can prevent complications such as MI and heart failure. In clinical studies, surrogate macrophage imaging markers may reduce the size of current therapeutic trials to more reasonable and cost effective dimensions. Before we get to this point, individual imaging approaches will have to undergo rigorous clinical validation.
PERSPECTIVES AND CONCLUSIONS
In the 1966 science fiction film “Fantastic Voyage” a team of experts travels in a submarine shrunken to 1 µm in length inside the body of a scientist who is comatose because an attempted assasination left him with a clot in the brain. Their mission is to destroy the clot and exit the patient before the submarine begins to revert to its original size. The characters face many obstacles as they travel throught the heart, ear, lungs, and brain, before completing their task and escaping through a tear duct. The movie reflects the ultimate aspiration of biomedical imaging: to visualize, in vivo, the workings of our cells and organs. Nearly half a century later, we are realizing at least parts of that dream. Increasingly sophisticated imaging technologies combined with genetic approaches that target molecules and processes of interest are providing us with glimpses of the dynamic microcosm that animates us. We will continue to be challenged as the underlying biological questions push the limits of our technologies. Properties of light and other radiation, detection capabilities, and many physical and biological constraints will need to be accomodated, manipulated, developed, or overcome so that we can continue to make meaningful discoveries about the cellular and sub-cellular world
Acknowledgments
This work was supported in part by NHLBI grants R01HL095612, R01HL095629, R01HL096576.
References
- 1.van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968;128:415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Furth R, Diesselhoff-den Dulk MM. Dual origin of mouse spleen macrophages. J Exp Med. 1984;160:1273–1283. doi: 10.1084/jem.160.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jenkins SJ, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ginhoux F, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fogg DK, et al. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 6.Schulz C, et al. A Lineage of Myeloid Cells Independent of Myb and Hematopoietic Stem Cells. Science. 2012 doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 7.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kadl A, et al. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res. 2010;107:737–746. doi: 10.1161/CIRCRESAHA.109.215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 10.Gautier EL, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012 doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerrity RG. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981;103:181–190. [PMC free article] [PubMed] [Google Scholar]
- 12.Swirski FK, et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tacke F, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy AJ, et al. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J Clin Invest. 2011;121:4138–4149. doi: 10.1172/JCI57559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yvan-Charvet L, et al. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robbins CS, et al. Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation. 2012;125:364–374. doi: 10.1161/CIRCULATIONAHA.111.061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Westerterp M, et al. Regulation of hematopoietic stem and progenitor cell mobilization by cholesterol efflux pathways. Cell Stem Cell. 2012;11:195–206. doi: 10.1016/j.stem.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swirski FK, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nahrendorf M, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panizzi P, et al. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J Am Coll Cardiol. 2010;55:1629–1638. doi: 10.1016/j.jacc.2009.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dutta P, et al. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–329. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nahrendorf M, et al. Multimodality cardiovascular molecular imaging, Part II. Circ Cardiovasc Imaging. 2009;2:56–70. doi: 10.1161/CIRCIMAGING.108.839092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leuschner F, Nahrendorf M. Molecular imaging of coronary atherosclerosis and myocardial infarction: considerations for the bench and perspectives for the clinic. Circ Res. 2011;108:593–606. doi: 10.1161/CIRCRESAHA.110.232678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lo Celso C, et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92–96. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujisaki J, et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 2011;474:216–219. doi: 10.1038/nature10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 28.Assmus B, et al. Acute myocardial infarction activates progenitor cells and increases Wnt signalling in the bone marrow. Eur Heart J. 2012;33:1911–1919. doi: 10.1093/eurheartj/ehr388. [DOI] [PubMed] [Google Scholar]
- 29.Shields AF, et al. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med. 1998;4:1334–1336. doi: 10.1038/3337. [DOI] [PubMed] [Google Scholar]
- 30.Leuschner F, et al. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med. 2012;209:123–137. doi: 10.1084/jem.20111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cortez-Retamozo V, et al. Origins of tumor-associated macrophages and neutrophils. Proc Natl Acad Sci U S A. 2012;109:2491–2496. doi: 10.1073/pnas.1113744109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 33.Shi C, et al. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity. 2011;34:590–601. doi: 10.1016/j.immuni.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsou CL, et al. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Auffray C, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 36.Sundd P, et al. ‘Slings’ enable neutrophil rolling at high shear. Nature. 2012;488:399–403. doi: 10.1038/nature11248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 38.Proebstl D, et al. Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J Exp Med. 2012;209:1219–1234. doi: 10.1084/jem.20111622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voisin MB, Woodfin A, Nourshargh S. Monocytes and neutrophils exhibit both distinct and common mechanisms in penetrating the vascular basement membrane in vivo. Arterioscler Thromb Vasc Biol. 2009;29:1193–1199. doi: 10.1161/ATVBAHA.109.187450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kircher MF, et al. Noninvasive in vivo imaging of monocyte trafficking to atherosclerotic lesions. Circulation. 2008;117:388–395. doi: 10.1161/CIRCULATIONAHA.107.719765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swirski FK, et al. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc Natl Acad Sci U S A. 2006;103:10340–10345. doi: 10.1073/pnas.0604260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grabner R, et al. Lymphotoxin beta receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE−/− mice. J Exp Med. 2009;206:233–248. doi: 10.1084/jem.20080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mulligan-Kehoe MJ. The vasa vasorum in diseased and nondiseased arteries. Am J Physiol Heart Circ Physiol. 2010;298:H295–H305. doi: 10.1152/ajpheart.00884.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eriksson EE. Intravital microscopy on atherosclerosis in apolipoprotein e-deficient mice establishes microvessels as major entry pathways for leukocytes to advanced lesions. Circulation. 2011;124:2129–2138. doi: 10.1161/CIRCULATIONAHA.111.030627. [DOI] [PubMed] [Google Scholar]
- 45.Lee S, et al. Real-time in vivo imaging of the beating mouse heart at microscopic resolution. Nat Commun. 2012;3:1054. doi: 10.1038/ncomms2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li W, et al. Intravital 2-photon imaging of leukocyte trafficking in beating heart. J Clin Invest. 2012;122:2499–2508. doi: 10.1172/JCI62970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koltsova EK, et al. Dynamic T cell-APC interactions sustain chronic inflammation in atherosclerosis. J Clin Invest. 2012;122:3114–3126. doi: 10.1172/JCI61758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murooka TT, et al. HIV-infected T cells are migratory vehicles for viral dissemination. Nature. 2012;490:283–287. doi: 10.1038/nature11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McDonald B, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 50.Amirbekian V, et al. Detecting and assessing macrophages in vivo to evaluate atherosclerosis noninvasively using molecular MRI. Proc Natl Acad Sci U S A. 2007;104:961–966. doi: 10.1073/pnas.0606281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jaffer FA, et al. Cellular imaging of inflammation in atherosclerosis using magnetofluorescent nanomaterials. Mol Imaging. 2006;5:85–92. [PubMed] [Google Scholar]
- 52.Naresh NK, et al. Monocyte and/or macrophage infiltration of heart after myocardial infarction: MR imaging by using T1-shortening liposomes. Radiology. 2012;264:428–435. doi: 10.1148/radiol.12111863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sosnovik DE, et al. Fluorescence tomography and magnetic resonance imaging of myocardial macrophage infiltration in infarcted myocardium in vivo. Circulation. 2007;115:1384–1391. doi: 10.1161/CIRCULATIONAHA.106.663351. [DOI] [PubMed] [Google Scholar]
- 54.Trivedi RA, et al. In vivo detection of macrophages in human carotid atheroma: temporal dependence of ultrasmall superparamagnetic particles of iron oxide-enhanced MRI. Stroke. 2004;35:1631–1635. doi: 10.1161/01.STR.0000131268.50418.b7. [DOI] [PubMed] [Google Scholar]
- 55.Alam SR, et al. Ultrasmall superparamagnetic particles of iron oxide in patients with acute myocardial infarction: early clinical experience. Circ Cardiovasc Imaging. 2012;5:559–565. doi: 10.1161/CIRCIMAGING.112.974907. [DOI] [PubMed] [Google Scholar]
- 56.Sosnovik DE, Nahrendorf M, Weissleder R. Magnetic nanoparticles for MR imaging: agents, techniques and cardiovascular applications. Basic Res Cardiol. 2008;103:122–130. doi: 10.1007/s00395-008-0710-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flogel U, et al. In vivo monitoring of inflammation after cardiac and cerebral ischemia by fluorine magnetic resonance imaging. Circulation. 2008;118:140–148. doi: 10.1161/CIRCULATIONAHA.107.737890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nahrendorf M, et al. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117:379–387. doi: 10.1161/CIRCULATIONAHA.107.741181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nahrendorf M, et al. Detection of macrophages in aortic aneurysms by nanoparticle positron emission tomography-computed tomography. Arterioscler Thromb Vasc Biol. 2011;31:750–757. doi: 10.1161/ATVBAHA.110.221499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 61.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev. 2007;87:1285–1342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 62.Razavian M, et al. Atherosclerosis plaque heterogeneity and response to therapy detected by in vivo molecular imaging of matrix metalloproteinase activation. J Nucl Med. 2011;52:1795–1802. doi: 10.2967/jnumed.111.092379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schafers M, et al. Scintigraphic imaging of matrix metalloproteinase activity in the arterial wall in vivo. Circulation. 2004;109:2554–2559. doi: 10.1161/01.CIR.0000129088.49276.83. [DOI] [PubMed] [Google Scholar]
- 64.Su H, et al. Noninvasive targeted imaging of matrix metalloproteinase activation in a murine model of postinfarction remodeling. Circulation. 2005;112:3157–3167. doi: 10.1161/CIRCULATIONAHA.105.583021. [DOI] [PubMed] [Google Scholar]
- 65.Sahul ZH, et al. Targeted imaging of the spatial and temporal variation of matrix metalloproteinase activity in a porcine model of postinfarct remodeling: relationship to myocardial dysfunction. Circ Cardiovasc Imaging. 2011;4:381–391. doi: 10.1161/CIRCIMAGING.110.961854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nahrendorf M, et al. Hybrid in vivo FMT-CT imaging of protease activity in atherosclerosis with customized nanosensors. Arterioscler Thromb Vasc Biol. 2009;29:1444–1451. doi: 10.1161/ATVBAHA.109.193086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jaffer FA, et al. Two-dimensional intravascular near-infrared fluorescence molecular imaging of inflammation in atherosclerosis and stent-induced vascular injury. J Am Coll Cardiol. 2011;57:2516–2526. doi: 10.1016/j.jacc.2011.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fayad ZA, et al. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet. 2011;378:1547–1559. doi: 10.1016/S0140-6736(11)61383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tawakol A, et al. Noninvasive in vivo measurement of vascular inflammation with F-18 fluorodeoxyglucose positron emission tomography. J Nucl Cardiol. 2005;12:294–301. doi: 10.1016/j.nuclcard.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 70.Vucic E, et al. Regression of Inflammation in Atherosclerosis by the LXR Agonist R211945: A Noninvasive Assessment and Comparison With Atorvastatin. JACC Cardiovasc Imaging. 2012;5:819–828. doi: 10.1016/j.jcmg.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee WW, et al. PET/MRI of inflammation in myocardial infarction. J Am Coll Cardiol. 2012;59:153–163. doi: 10.1016/j.jacc.2011.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tawakol A, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006;48:1818–1824. doi: 10.1016/j.jacc.2006.05.076. [DOI] [PubMed] [Google Scholar]
- 73.Tearney GJ, et al. Three-dimensional coronary artery microscopy by intracoronary optical frequency domain imaging. JACC Cardiovasc Imaging. 2008;1:752–761. doi: 10.1016/j.jcmg.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu L, et al. Imaging the subcellular structure of human coronary atherosclerosis using micro-optical coherence tomography. Nat Med. 2011;17:1010–1014. doi: 10.1038/nm.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]