Products containing omega-3 fatty acids, such as krill oil and fish oils, have been effective in lowering triglyceride levels. Although no data have suggested that the low-density lipoprotein-cholesterol (LDL-C) increases associated with some omega-3 fatty acid formulations lead to adverse outcomes, these elevations in LDL-C levels may compromise the achievement of lipid targets. Thus, there is a need for agents that can lower triglyceride levels without increasing LDL-C levels.
Keywords: dyslipidemias, omega-3 fatty acids, eicosapentaenoic acid, docosahexaenoic acid, triglycerides
Abstract
The triglyceride (TG)-lowering benefits of the very-long-chain omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are well documented. Available as prescription formulations and dietary supplements, EPA and DHA are recommended by the American Heart Association for patients with coronary heart disease and hypertriglyceridemia. Dietary supplements are not subject to the same government regulatory standards for safety, efficacy, and purity as prescription drugs are; moreover, supplements may contain variable concentrations of EPA and DHA and possibly other contaminants. Reducing low-density lipoprotein-cholesterol (LDL-C) levels remains the primary treatment goal in the management of dyslipidemia. Dietary supplements and prescription formulations that contain both EPA and DHA may lower TG levels, but they may also increase LDL-C levels.
Two prescription formulations of long-chain omega-3 fatty acids are available in the U.S. Although prescription omega-3 acid ethyl esters (OM-3-A EEs, Lovaza) contain high-purity EPA and DHA, prescription icosapent ethyl (IPE, Vascepa) is a high-purity EPA agent. In clinical trials of statin-treated and non–statin-treated patients with hypertriglyceridemia, both OM-3-A EE and IPE lowered TG levels and other atherogenic markers; however, IPE did not increase LDL-C levels.
Results of recent outcomes trials of long-chain omega-3 fatty acids, fibrates, and niacin have been disappointing, failing to show additional reductions in adverse cardiovascular events when combined with statins. Therefore, the REDUCE–IT study is being conducted to evaluate the effect of the combination of IPE and statins on cardiovascular outcomes in high-risk patients. The results of this trial are eagerly anticipated.
INTRODUCTION
It is now established that omega-3 and omega-6 fatty acids play important roles in human health and disease. Both are considered essential fatty acids, because they are not endogenously synthesized and must be obtained from the diet. Long-chain omega-6 fatty acids include linoleic, gamma-linolenic, and arachidonic acids. Omega-3 fatty acids include the long-chain alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA).1
EPA and DHA are often called very-long-chain omega-3 fatty acids. The typical Western diet is rich in omega-6 fatty acids because of the abundance of linoleic acid present in corn, sunflower, and safflower oils.2 Conversely, omega-3 fatty acids account for only a small percentage of the daily dietary fat intake and are obtained from two main dietary sources—plants and fish.3,4 Plant oils from walnuts, flaxseed, and canola contain the omega-3 fatty acid ALA,5 which is a metabolic precursor of the very-long-chain omega-3 fatty acids EPA and DHA; however, the conversion from ALA to EPA and DHA in the body is inefficient.4 The most concentrated food source of EPA and DHA is fatty fish such as albacore tuna, salmon, mackerel, sardines, and herring.4,6
Following consumption, polyunsaturated fatty acids, such as the omega-3 and omega-6 fatty acids, are incorporated into cell membranes, where they modulate membrane protein function, cellular signaling, and gene expression. Dietary omega-3 fatty acids compete with omega-6 fatty acids for incorporation into cell membranes.4 When omega-6 fatty acids predominate in cell membranes, proinflammatory mediators such as thromboxanes, prostaglandins, and leukotrienes are produced via the cyclooxygenase and 5-lipoxygenase pathways. Conversely, the presence of omega-3 fatty acids promotes secretion of anti-inflammatory prostaglandins and less potent leukotrienes, resulting in a shift to a milieu of less inflammatory mediators.2 These proinflammatory and anti-inflammatory effects represent the primary pharmacological difference between omega-3 and omega-6 fatty acids.
In addition to their anti-inflammatory activity, very-long-chain omega-3 fatty acids have well-described effects on various risk factors for cardiovascular disease.7 Epidemiological and clinical studies support the cardiovascular benefits of EPA and DHA; however, there is less evidence to support the benefits of ALA.8,9 Potential mechanisms for the cardioprotective effects of omega-3 fatty acids include (Table 1):9
reduction of triglyceride (TG) levels.
attenuation of atherosclerotic plaques.
exertion of antidysrhythmic, antithrombotic, and anti-inflammatory effects.
lowering of systolic and diastolic blood pressures.
improvement in endothelial function.
Table 1.
Potential Cardiovascular Benefits of Omega-3 Fatty Acids
Antidysrhythmic effects
|
From Kwiterovitch PO Jr, ed. The Johns Hopkins Textbook of Dyslipidemia. Lippincott Williams & Wilkins; 2010:245–257. Adapted with permission.9
HDL-C = high-density lipoprotein-cholesterol; LDL-C = low-density lipo-protein-cholesterol; TG = triglyceride; VLDL-C = very-low-density lipoproteincholesterol.
Dietary Recommendations
Dietary guidelines recommended by The American Heart Association (AHA) for healthy individuals include consumption of fatty fish at least two times a week, along with plant-derived omega-3 fatty acids, including ALA from soybean products, walnuts, flaxseed oil, and canola oil.6 A combined EPA and DHA intake of 1 g/day, in the form of oily fish or EPA plus DHA supplements, is recommended for patients with documented coronary heart disease (CHD), although the decision to supplement should be made in consultation with a physician.6 The FDA recommends that the intake for consumers not exceed 3 g/day of EPA plus DHA with no more than 2 g/day from dietary supplementation.10
Glossary
Clinical Trials
ACCORD = Action to Control Cardiovascular Risk in Diabetes
AIM–HIGH = Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides—Impact on Global Health Outcomes
ALPHA OMEGA = Study of Omega-3 Fatty Acids and Coronary Mortality
ANCHOR = Effect of AMR101 (Icosapent Ethyl) on Triglyceride Levels in Patients on Statins With High Triglyceride Levels (≥200 and <500 mg/dL)
COMBOS = Combination of Prescription Omega-3 With Simvastatin
ECLIPSE = Epanova Compared to Lovaza In a Pharmacokinetic Single-dose Evaluation
ESPRIT = Epanova Combined with a Statin in Patients with Hypertriglyceridemia to Reduce Non-HDL Cholesterol
EVOLVE = Epanova for Lowering Very High Triglycerides
GISSI–HF = Gruppo Italiano per lo Studio della Sopravvivenza Nell’Insufficienza Cardiaca
GISSI–Prevenzione = Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico
HPS2–THRIVE = Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events
JELIS = Japan EPA Lipid Intervention Study
MARINE = Multicenter, Placebo-controlled, Randomized, Double-blind, 12-week Study With an Open-label Extension
OMEGA = Effect of Omega 3-Fatty Acids on the Reduction of Sudden Cardiac Death After Myocardial Infarction
ORD = Omega-3 fatty acids Randomized Double-blind study
ORIGIN = Outcome Reduction with Initial Glargine Intervention
REDUCE–IT = Reduction of Cardiovascular Events with EPA—Intervention Trial
Su.Fol.Om3 = Supplémentation en Folates et Omega-3
TRIFECTA = TrIal For Efficacy of CaPre on hypertriglyceridemia
Omega-3 Fatty Acids
ALA = alpha linolenic acid
DHA = docosahexaenoic acid
EE = ethyl esters
EPA = eicosapentaenoic acid
IPE = icosapent ethyl (e.g., Vascepa)
OM-3-A EE = omega-3 acid ethyl esters (e.g., Lovaza)
OM-3 FFA = omega-3 free fatty acids (e.g., Epanova)
The AHA recommends that EPA plus DHA supplements may be useful in patients with hypertriglyceridemia, but patients should not consume more than 3 g/day from supplements without a physician’s supervision.6 This article discusses very-long-chain omega-3 fatty acids.
Clinical Evidence
In 1999 and 2007, two major outcomes trials of cardiovascular disease documented the efficacy of omega-3 fatty acids in the primary and secondary prevention of CHD. GISSI–Prevenzione was a secondary outcomes study in which 11,324 postmyocar-dial infarction (MI) patients were randomly assigned to receive a fish oil supplement (0.85 g of EPA plus DHA), 0.3 g of vitamin E, both, or none.11 Patients taking omega-3 fatty acids had a 15% reduction (P = 0.02) in the primary endpoint (death, nonfatal MI, and stroke), including 20% and 30% decreases in total and cardiovascular mortality, respectively.11
In JELIS, 18,645 patients with hypercholesterolemia (80% in primary and 20% in secondary prevention) received statins alone or in combination with highly purified EPA (1.8 g/day).12 Although this study was limited to Japanese patients, most of whom were postmenopausal women, after 5 years patients receiving the combined treatment experienced a 19% relative reduction in major coronary events (P = 0.01). Beneficial effects were noted in both primary and secondary prevention subgroups, but these were significant only in the secondary prevention subgroup (P < 0.05).12
In contrast to GISSI and JELIS, more recent studies of omega-3 fatty acid supplementation, such as OMEGA (EPA 0.46 g/day plus DHA 0.38 g/day) and ALPHA OMEGA (EPA 0.4 g/day plus DHA), which investigated survivors of MI, yielded disappointing results.13,14 However, in a secondary analysis of 1,014 patients from the ALPHA OMEGA trial with previous MI and diabetes, EPA plus DHA decreased ventricular arrhythmia and the combined endpoint of ventricular arrhythmia and fatal MI.15 Because this was a post hoc subgroup analysis, the findings are limited.
The ORIGIN trial, which evaluated the effects of 0.9 g/day of omega-3 fatty acids ethyl esters (OM-3-A EEs) on cardiovascular outcomes in patients with diabetes, or at risk for diabetes, found no reductions in deaths from cardiovascular causes or other outcomes in patients with impaired fasting glucose, impaired glucose tolerance, or diabetes and additional cardiovascular risk factors.16 Notably, the investigational doses of omega-3 fatty acids were low (less than 1 g/day of EPA plus DHA), the median baseline dietary intake of EPA or DHA was 210 mg/day, and most patients also received concomitant evidence-based antihypertensive, antithrombotic, and lipid-modifying therapies.
In the Su.Fol.Om3 trial, which enrolled 2,501 patients with previous acute coronary or cerebral ischemic events, no significant effects were found with 0.6 g/day of EPA plus DHA after a median of 4.7 years.17 Among the 1,863 patients with CHD in this trial, omega-3 fatty acid supplementation did not reduce the rates of “hard” coronary events (i.e., acute coronary syndrome, MI, or sudden coronary death) or coronary revascularization.18 However, the potential benefits of omega-3 fatty acids in patients with heart failure were shown in the randomized GISSI–HF trial, in which 6,975 patients with class II–IV heart failure received approximately 0.85 g/day of EPA plus DHA or placebo.19 After a median follow-up of 3.9 years, long-term treatment with omega-3 fatty acids reduced rates of all-cause mortality and cardiovascular-related hospital admissions.19 Although the benefit was smaller than expected, it was observed when the therapy was added to a recommended heart failure regimen.
Results of two meta-analyses, published in 2012, further complicate the potential role of omega-3 fatty acids in cardiovascular outcomes. In one meta-analysis,7 these products reduced cardiovascular events and death; in the other,20 they were not significantly associated with major cardiovascular events. So far, the evidence concerning the role of omega-3 fatty acids in cardiovascular outcomes is conflicting and might be related to factors such as the dose taken, the patient populations enrolled, and background therapy.
To help further understand the role of omega-3 fatty acids in cardiovascular outcomes, particularly the potential additive effects in patients currently receiving evidence-based anti-hypertensive, antithrombotic, and lipid-modifying therapy, new, prospective, randomized, controlled trials of omega-3 fatty acids (particularly at doses higher than 2 g/day) in patients receiving current evidence-based care are needed. The potential role of omega-3 fatty acid therapies in the management of dyslipidemia is the focal point of this review.
MANAGEMENT OF DYSLIPIDEMIA
Current standards of care in the management of hypertension, dyslipidemia, and hyperglycemia have led to substantial gains in the prevention of cardiovascular disease. Despite a multifactorial approach, including effective lowering of low-density lipoprotein-cholesterol (LDL-C) levels, significant cardiovascular risks persist for many patients.21 This risk is associated with atherogenic dyslipidemia, a condition characterized by elevated TG, apolipoprotein B, and non–high-density lipoprotein-cholesterol (non–HDL-C) levels, as well as low HDL-C levels. This profile is typically seen in patients with type-2 diabetes and metabolic syndrome.21 High TG levels are an independent risk factor for CHD and a marker of heightened cardiovascular risk beyond that predicted by LDL-C levels.22
The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III, or ATP III) defines normal TG levels as below 150 mg/dL; borderline–high levels as 150–199 mg/dL; high levels as 200–499 mg/dL; and very high levels as 500 mg/dL or greater.22 Data from the National Health and Nutrition Examination Survey (NHANES) reveal that one in five of adults in the U.S. have TG concentrations of 200 mg/dL or higher, and 1 in 50 adults have TG levels of 500 mg/dL or higher.23
The prevalence of all categories of TG elevation was higher among men than women and highest among Mexican-Americans when the groups were evaluated according to ethnicity.23 Over the past 20 years, a small increase in median TG levels of 3 mg/dL in men and 5 mg/dL in women has been reported, reflecting the increasing incidence of obesity, insulin resistance, and type-2 diabetes mellitus.24
The NCEP ATP III guidelines for cholesterol management were published in 2002.22 Recommendations for modification were published in 2004 following the publication of data from additional clinical trials with high-dose statins.25 The intensity of LDL-C–lowering treatment is directly related to the reduction in risk of cardiovascular events.22
ATP III recommends a two-step approach. The primary target is lowering elevated LDL-C levels; the emphasis then shifts to managing other lipoprotein risk factors.22,25 ATP III emphasizes therapeutic lifestyle changes as an essential first step to reduce risk through both lowering LDL-C and managing metabolic syndrome.22 If lifestyle changes are inadequate for achieving LDL-C goals, drug therapy is considered.22 The LDL-C goal for high-risk patients is below 100 mg/dL; a more stringent goal (below 70 mg/dL) is an option for patients at very high risk.25 Statin therapy remains the cornerstone of dyslipidemia management.22
Despite aggressive statin treatment to achieve target LDL-C levels, a residual risk for cardiovascular events of 65% to 75% is reported in statin studies.26 Factors contributing to residual risk other than LDL-C levels include components of non–HDL, such as very-low-density lipoprotein (VLDL), chylomicrons, VLDL remnants, and lipoprotein (a).26 To take into account the atherogenic potential of these lipids in patients with hypertriglyceridemia, NCEP ATP III introduced non–HDL-C as a secondary target of therapy in patients with elevated TG levels (200 mg/dL or higher). The non–HDL-C goal is 30 mg/dL higher than the LDL-C goal.22,25 Treatment of atherogenic dyslipidemia (TG levels of 150 mg/dL or higher and HDL-C levels below 40 mg/dL) focuses on lowering TG levels.22
Add-on therapy with niacin or fibrates is recommended in patients with atherogenic dyslipidemia who do not achieve non–HDL-C goals with optimized statin therapy.27 Omega-3 fatty acids may offer a potential alternative to niacin and fibrate agents, as discussed next.
EPA AND DHA: LIPID-LOWERING AND DIFFERENTIAL EFFECTS
The American Heart Association, addressing TGs and cardiovascular disease, recommends 2 to 4 g/day of EPA plus DHA for patients who need to lower TG levels.24 More recently, the Endocrine Society suggested that omega-3 fatty acids alone or in combination with statins could be considered a treatment option for patients with moderate-to-severe TG levels.28 Omega-3 fatty acids administered as fish oil supplements or as prescription OM-3-A EE lowers plasma TG levels by 25% to 34%.29,30 Studies of omega-3 fatty acids, including those involving 2 or more weeks of therapy, have generally found no significant differences in absorption after administration as ethyl esters or in natural forms such as TGs.31–33
The magnitude of this effect is dependent on both the fish oil dose and the baseline TG level.34 As reported in several studies, however, decreased TG levels, achieved by omega-3 fatty acids, have been accompanied by elevated LDL-C levels.29,35–37
In a meta-analysis of 21 studies, fish oil supplementation was associated with an average 6-mg/dL increase in LDL-C levels.34 Subsequent examination of the distinct effects of EPA and DHA, administered as monotherapy, revealed differential effects of these omega-3 fatty acids on lipid levels. DHA-containing supplements or therapies, compared with EPA-containing supplements, were associated with more significant increases in LDL-C. In a review of studies of head-to-head comparisons (n = 6), DHA treatment was associated with net increases in LDL-C (3.3%) and HDL-C levels (5.9%) compared with EPA.38 However, as noted by the authors, only four of the studies included statistical analyses of LDL-C. Three studies indicated no difference,39–41 and one study showed a significant increase in LDL-C with DHA (8%; P = 0.02) but not with EPA.42
A meta-analysis of studies investigating the differential effects of EPA or DHA monotherapy showed that compared with placebo, DHA significantly raised mean LDL-C levels by 7.23 mg/dL, for a 95% confidence interval (CI) of 3.98 to 10.5, whereas EPA nonsignificantly reduced mean LDL-C levels.43 When studies that directly compared EPA and DHA were analyzed (n = 6), DHA significantly increased mean LDL-C levels by 4.63 mg/dL (95% CI, 2.15–7.10) more than EPA did, and mean HDL-C levels were increased by 3.74 mg/dL (95% CI, 2.42–5.05) with DHA but not with EPA.43
A head-to-head study (ORD), published in 2013, was conducted to evaluate the lipid-lowering effects of 2 g/day or 4 g/day of 1-g capsules containing 0.465 g of EPA ethyl ester plus 0.375 g of DHA ethyl ester versus 1.8 g/day of EPA ethyl ester alone in 611 patients with hypertriglyceridemia (i.e., TG at or above 150 mg/dL and less than 750 mg/dL).44 TG levels were reduced by 11.2% and 10.8%, respectively, for the comparable doses of EPA ethyl ester 1.8 g/day and EPA ethyl ester plus DHA ethyl ester 2 g/day, with a least-squares mean difference of 0.37 (95% CI, −4.25 to 4.98).
Treatment-related adverse events occurring in 1% of patients or more in any group included diarrhea, flatulence, and elevated LDL-C levels. The degree of the increase in LDL-C and stratification, according to treatment group with or without statins, was not specified.
All three treatments produced small increases in HDL-C levels and small decreases in LDL-C levels, compared with baseline, but EPA ethyl ester produced more reductions in LDL-C than either dose of EPA ethyl ester plus DHA ethyl ester. Point estimates for 2 g/day and 4 g/day versus EPA ethyl ester 1.8 g/day were 2.14 [95% CI, −0.6 to −4.9] and 3.17 [95% CI, 0.2−6.1]). However, as the authors acknowledged, the study was limited to Japanese patients undergoing lifestyle modifications and LDL-C increases have been observed in other studies of prescription omega-3 fatty acids.
In summary, increased LDL-C levels with preparations that include both EPA and DHA may complicate the ability to achieve LDL-C goals in patients with dyslipidemia. Omega-3 fatty acids that do not raise LDL-C levels might be a more appropriate option for these patients. In several studies, EPA, independently of DHA, lowered TG levels, atherogenic lipo-proteins, and markers of vascular function without raising LDL-C levels.45–50
EPA AND DHA PRODUCTS ON THE MARKET
The daily omega-3 fatty acid requirement for patients with documented CHD (1 g/day) or for those requiring lower TG levels (2–4 g/day) is difficult to achieve by fish consumption alone; therefore, a fish oil supplement should be considered.30 Omega-3 fatty acids are available as dietary supplements and as prescription formulations containing either a mixture of both EPA and DHA ethyl esters or as purified EPA ethyl ester.
Dietary Supplements
Numerous dietary fish oil supplements are available, but these preparations are not subject to the same regulatory standards or manufacturing oversight used by the FDA to establish the efficacy and safety of prescription products.51,52 The U.S. Dietary Supplementation Health and Education Act of 1994 stipulates that supplement labels may not claim to diagnose, mitigate, treat, cure, or prevent diseases.53 In contrast to prescription formulations that contain known and consistent amounts of active compounds, the amount of EPA and DHA per recommended serving can vary widely among supplement products.54 Reports regarding the accuracy of the stated amount of EPA and DHA in supplement labels have been inconsistent. In one report of 15 products, the amount of omega-3 fatty acids was stated on all labels;55 another report on 24 products, however, pointed out three supplements containing a lower amount of omega-3 fatty acids than the company claimed.56 Patients may thus find it difficult to navigate these issues of accuracy in supplement labels and may be further challenged in interpreting the importance of EPA and DHA doses.
The concentrations of EPA and DHA in omega-3 fatty acid supplements range from a modest level of less than 20% to more than 80%.56 Yet patients may require a median intake of 11.2 servings per day (one to three soft gels, capsules, or packets per serving) to achieve the higher recommended doses,52,54 and this regimen may interfere with patient adherence. The larger daily doses can also result in an additional intake of vitamins, saturated fat, and cholesterol, depending on the source of the oil.54
For example, cod liver oil contains fat-soluble vitamins and cholesterol. Although other sources, such as salmon oil, might also be high in cholesterol, oils derived from algae and fish may have low or no levels of cholesterol, depending on purity.54 By comparison, prescription drugs containing omega-3 fatty acids contain no cholesterol. Questions as to the presence of environmental contaminants (e.g., heavy metals, polychlorinated biphenyls, and pesticides) may arise with regard to fatty fish consumption; however, an evidence-based review concluded that omega-3 fatty acid supplements and prescription drugs do not contain sufficient amounts of contaminants to pose a potential health risk.57
Consumers, pharmacists, and clinicians should be aware of these differences between omega-3 fatty acid supplements and prescription products when selecting an appropriate agent. It is important for pharmacists to inform patients about the differences in product purity, potency, dosage, and the respective roles of EPA and DHA. Patients with a lipid disorder should not be encouraged to treat themselves with dietary supplements. The use of prescription drugs should always be supervised by a physician.
FDA-Approved Agents
Omega-3-Acid Ethyl Esters (Lovaza)
A prescription product containing high-purity omega-3-acid ethyl esters (OM-3-A EE), currently marketed as Lovaza (GlaxoSmithKline) following a name change from Omacor in 2007,58 is approved by the FDA as an adjunct to diet to reduce very high TG levels (500 mg/dL or higher) in adults (Table 2).59 Each 1-g capsule of OM-3-A EE contains ethyl esters of EPA (0.465 g) and DHA (0.375 g).59 Patients take a once-daily dose of 4 g or two 2-g doses (two capsules twice daily).59
Table 2.
Prescription Omega-3 Fatty Acid Products
| Trade name | Lovaza | Vascepa |
|---|---|---|
| Generic name | Omega-3-acid ethyl esters | Icosapent ethyl |
| Indication | Adjunct to diet to reduce TG levels in adult patients with severe hypertriglyceridemia (≥500 mg/dL) | Adjunct to diet to reduce TG levels in adult patients with severe hypertriglyceridemia (≥500 mg/dL) |
| Description | Each 1-g capsule contains at least 0.9 g of the ethyl esters of omega-3 fatty acids (EPA, about 0.465 g, and DHA, about 0.375 g) | Each capsule contains 1 g of icosapent ethyl, an ethyl ester of the omega-3 fatty acid EPA |
| Chemical structure | EPA ethyl ester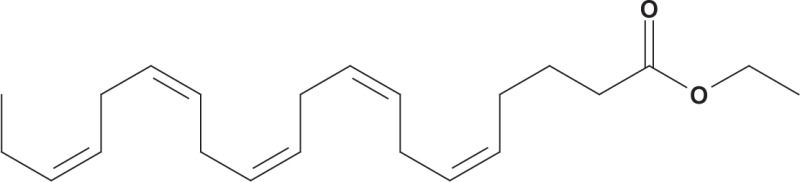 DHA ethyl ester DHA ethyl ester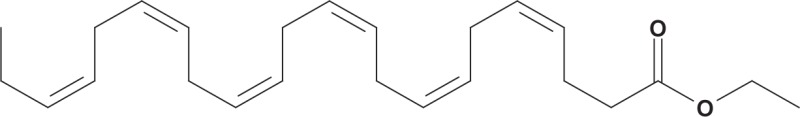
|
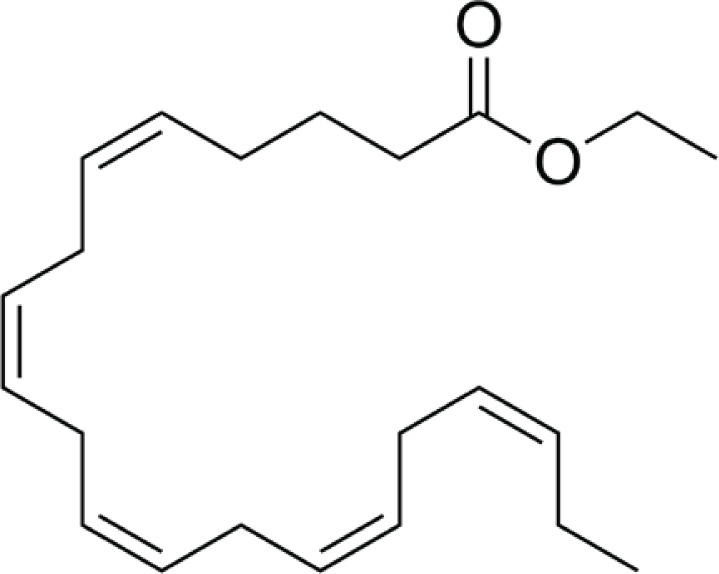
|
| Dosage | Daily oral dose of 4 g taken as a single 4-g dose (four capsules once daily) or as two 2-g doses (two capsules twice daily); in clinical studies, Lovaza was administered with meals | Daily oral dose of 4 g taken as two capsules twice daily with food |
In two randomized, double-blind, placebo-controlled trials in patients with severe hypertriglyceridemia (TG range, 500–2,000 mg/dL), OM-3-A EE (4 g/day) reduced TG levels by 45%, compared with a 16% increase with placebo (P < 0.0001) after 16 weeks of treatment60 and by 38.9%, compared with a 7.8% reduction with placebo (P = 0.001) after 6 weeks of treatment.35 After 6 and 16 weeks of treatment, OM-3-A EE was associated with LDL-C increases of 16.7% (P = 0.01) and 32% (P = 0.001); HDL-C increases of 5.9% (P = 0.02) and 13% (P = 0.004); and VLDL-C decreases of 29.2% (P = 0.002) and 32% (P = 0.001), respectively.35,60 (P values represent comparisons with placebo.)
The combined results of these two studies, as reported in the Lovaza prescribing information,59 are shown in Table 3. The increase in LDL-C is a potential disadvantage, because this product may prevent patients from achieving target LDL-C levels. It is therefore recommended that LDL-C levels be monitored periodically during treatment with OM-3-A EE.61
Table 3.
Pivotal Studies of Lovaza and Vascepa for the Treatment of Very High Triglyceride Levels
| Lovaza (Omega-3-Acid Ethyl Esters)59 | Vascepa (Icosapent Ethyl)63 | |||||||
|---|---|---|---|---|---|---|---|---|
| Study | Two studies35,60 | MARINE49 | ||||||
| Lipid inclusion criteria | TG ≥ 500 and ≤2,000 mg/dL | TG ≥ 500 and ≤2,000 mg/dL | ||||||
| Duration | 6 and 16 weeks | 12 weeks | ||||||
| 4 g/day (n = 42) | Placebo (n = 42) | 4 g/day (n = 76) | Placebo (n = 75) | |||||
| BL | % Chga | BL | % Chga | BL | % Chga | BL | % Chga | |
| TG (mg/dL) | 816 | −44.9 | 788 | +6.7 | 680 | −26.6 | 703 | +9.7 |
| Differenceb | −51.6 | −33.1c (−47, −22) | ||||||
| LDL-C (mg/dL) | 89 | +44.5 | 108 | −4.8 | 91 | −4.5 | 86 | −3.0 |
| Difference | +49.3 | −2.3 (−13, +8) | ||||||
| Non–HDL-C (mg/dL) | 271 | −13.8 | 292 | −3.6 | 225 | −7.7 | 229 | +7.8 |
| Difference | −10.2 | −17.7 (−25, −11) | ||||||
| TC (mg/dL) | 296 | −9.7 | 314 | −1.7 | 254 | −7.3 | 256 | +7.7 |
| Difference | −8.0 | −16.3 (−22, −11) | ||||||
| HDL-C (mg/dL) | 22 | +9.1 | 24 | 0.0 | 27 | −3.5 | 27 | 0 |
| Difference | +9.1 | −3.6 (−9, +2) | ||||||
| VLDL-C (mg/dL) | 175 | −41.7 | 175 | −0.9 | 123 | −19.5 | 124 | +13.7 |
| Difference | −40.8 | −28.6d (−43, −14) | ||||||
| Apo B (mg/dL) | — | — | — | — | 121 | −3.8 | 118 | +4.3 |
| Difference | — | −8.5d (−14, −3) | ||||||
% Chg = median percentage change from baseline.
Difference for omega-3 acid ethyl esters: omega-3 acid ethyl esters median % change – placebo median % change. Difference for icosapent ethyl: median of (icosapent ethyl % change – placebo % change) (Hodges–Lehmann estimate, 95% confidence intervals)
P < 0.001 (Wilcoxon rank-sum test; primary efficacy endpoint).
P < 0.05 (Wilcoxon rank-sum test; key secondary endpoints determined to be statistically significant according to prespecified multiple comparison procedure).
Apo B = apolipoprotein B; BL = baseline; HDL-C = high-density lipoprotein-cholesterol; LDL-C = low-density lipoprotein-cholesterol; MARINE = Multicenter, Placebo-controlled, Randomized, Double-blind, 12-week Study With an Open-label Extension; non–HDL-C = non-high-density lipoprotein-cholesterol; TC = total cholesterol; TG = triglyceride; VLDL-C = very-low-density lipoprotein-cholesterol.
The COMBOS study evaluated the effects of adding OM-3-A EE to stable simvastatin (e.g., Zocor) therapy (40 mg/day) in patients with persistent hypertriglyceridemia (at or above 200 mg/dL and less than 500 mg/dL) (Table 4; see page 687).37 After an 8-week lead-in phase with simvastatin, patients received OM-3-A EE or placebo for an additional 8 weeks.
Table 4.
Clinical Studies of Lovaza and Vascepa in Statin-Treated Patients with High Triglyceride Levels
| Lovaza (Omega-3-Acid Ethyl Esters)59 | Vascepa (Icosapent Ethyl)50 | |||||||
|---|---|---|---|---|---|---|---|---|
| Study | COMBOS37 (N = 256) | ANCHOR50 (N = 702) | ||||||
| Lipid and statin inclusion criteria | TG ≥ 200 and <500 mg/dL; mean LDL-C ≤ 10% above NCEP ATP III goal | TG ≥ 200 and <500 mg/dL; LDL-C ≥40 and <115 mg/dL with optimized statin therapy | ||||||
| Duration | 8 weeks | 12 weeks | ||||||
| Treatment | 4 g/day plus simvastatin 40 mg/day (n = 122) | Placebo plus simvastatin 40 mg/day (n = 132) | 4 g/day plus statina (n = 226) | Placebo plus statina (n = 227) | ||||
| BL | % Chgb | BL | % Chgb | BL | % Chgb | BL | % Chgb | |
| TG (mg/dL) | 268 | −29.5 | 271 | −6.3 | 265 | −17.5 | 259 | 5.9 |
| Difference, Pc | −23.2, P < 0.0001 | −21.5, P < 0.0001 | ||||||
| LDL-C (mg/dL) | 91 | 0.7 | 88 | −2.8 | 82 | 1.5 | 84 | 8.8 |
| Difference, P | 3.5, P = 0.05 | −6.2, P = 0.007 | ||||||
| Non–HDL-C (mg/dL) | 137 | −9.0 | 141 | −2.2 | 128 | −5.0 | 128 | 9.8 |
| Difference, P | −6.8, P < 0.0001 | −13.6, P < 0.0001 | ||||||
| TC (mg/dL) | 184 | −4.8 | 184 | −1.7 | 167 | −3.2 | 168 | 9.1 |
| Difference, P | −3.1, P < 0.05 | −12.0, P < 0.0001 | ||||||
| HDL-C (mg/dL) | 46 | +3.4 | 43 | −1.2 | 37 | −1.0 | 39 | 4.8 |
| Difference, P | +4.6, P < 0.05 | −4.5, P = 0.0013 | ||||||
| VLDL-C (mg/dL) | 52 | −27.5 | 52 | −7.2 | 44 | −12.1 | 42 | 15 |
| Difference, P | −20.3, P < 0.05 | −24.4, P < 0.0001 | ||||||
| Apo B (mg/dL) | 86 | −4.2 | 87 | −1.9 | 93 | −2.2 | 91 | 7.1 |
| Difference, P | −2.3, P < 0.05 | −9.3, P < 0.0001 | ||||||
Various regimens (and patient numbers) of simvastatin, atorvastatin, or rosuvastatin.
% Chg = median percentage change from baseline.
Difference for omega-3 acid ethyl esters: omega-3 acid ethyl esters median % change minus placebo median % change. Difference for icosapent ethyl: median of (icosapent ethyl % change – placebo % change) (Hodges-Lehmann estimate; two-tailed 95% confidence interval).
ANCHOR = Effect of AMR101 (Ethyl Icosapentate) on Triglyceride Levels in Patients on Statins With High Triglyceride Levels (≥ 200 and < 500 mg/dL); Apo B = apolipoprotein B; COMBOS = Combination of Prescription Omega-3 With Simvastatin; HDL-C = high-density lipoprotein-cholesterol; LDL-C = low-density lipoprotein-cholesterol; NCEP–ATP III = National Cholesterol Education Program–Adult Treatment Panel III; non–HDL-C = non–high-density lipoprotein-cholesterol; TC = total cholesterol; TG = triglyceride; VLDL-C = very-low-density lipoprotein-cholesterol.
The reduction from baseline in non–HDL-C (the primary outcome variable) was significantly greater with OM-3-A EE plus simvastatin than with simvastatin alone (−9.0% vs. −2.2%, respectively; P < 0.0001).59 Treatment with OM-3-A EE also significantly reduced TG levels (−29.5% vs. −6.3%) and VLDL-C levels (−27.5% vs. −7.2%) and significantly increased HDL-C levels compared with placebo (3.4% vs. −1.2%) (all comparisons, P < 0.05).59
An increase of 0.7% in LDL-C levels was noted for OM-3-A EE compared with a decrease of 2.8% for placebo;37 this was reported as a net increase of 3.5% (P = 0.05).59 OM-3-A EE was also associated with reduced levels of apolipoprotein B, remnant-like particle cholesterol, apolipoprotein CIII, and lipoprotein-associated phospholipase A2 levels,37,62 as well as changes in the size and composition of lipoproteins.
Treatment was generally well tolerated, with 19 of 20,35 22 of 22,60 and 116 of 12337 patients in the OM-3-A EE groups completing the two pivotal trials and the COMBOS trial, respectively. In the OM-3-A EE patients, adverse reactions occurring in 3% of patients or more, and at an incidence greater than in the placebo group, included eructation, dyspepsia, and taste distortion.59
Periodic liver function testing is recommended in patients with hepatic impairment during therapy with OM-3-A EE. LDL-C levels should also be monitored. A possible association between OM-3-A EE and more frequent recurrences of atrial fibrillation or flutter has been noted.59 In COMBOS, fasting blood glucose levels were significantly increased with OM-3-A EE.37
Icosapent Ethyl (Vascepa)
Icosapent ethyl (IPE; Vascepa, Amarin), formerly called AMR101, is a high-purity prescription form of EPA ethyl ester that was approved in 2012 by the FDA as an adjunct to diet to reduce TG levels in adults with severe hypertriglyceridemia (500 mg/dL or higher) (see Table 2). The daily dose is 4 g, taken as two capsules twice daily with food. Each capsule contains 1 g of IPE.63
In the MARINE study, 229 patients with severe hypertriglyceridemia (TG between 500 and 2,000 mg/dL) were randomly assigned to receive IPE 4 g/day or 2 g/day or placebo.49 Results from the 4-g/day and placebo arms are shown in Table 3.
Compared with placebo, changes in TG levels from baseline at week 12 with IPE 4 g/day and 2 g/day were 33.1% (P < 0.0001) and 19.7% (P = 0.005), respectively. Among statin-treated patients, placebo-adjusted TG level decreases were 65% (P = 0.0001) with IPE 4 g/day and 40.7% (P = 0.03) with IPE 2 g/day; the decline in TG levels was also greater in patients with higher baseline TG levels (above 750 mg/dL). Importantly, no significant increase in LDL-C levels was observed in either the overall intent-to-treat population or in the subgroup with TG levels above 750 mg/dL.
The efficacy of IPE in improving lipid parameters was further demonstrated in a second 12-week randomized, placebo-controlled trial (ANCHOR) that enrolled statin-treated patients at high cardiovascular risk with well-controlled LDL-C levels and persistently high TG levels (at or above 200 mg/dL and less than 500 mg/dL) (Table 4).50
Compared with placebo, IPE 4 g/day and 2 g/day reduced median TG levels from baseline by 21.5% (P < 0.0001) and 10.1% (P = 0.0005), respectively, without increasing LDL-C levels. The 4 g/day dose decreased LDL-C levels by 6.2% (P = 0.007) and significantly reduced other lipid parameters, compared with a light liquid paraffin placebo, including apolipoprotein B (9.3%; P < 0.0001), VLDL-C (24.4%; P < 0.0001), lipoprotein-associated phospholipase A2 (19.0%; P < 0.0001), and high-sensitivity C-reactive protein (hsCRP) (22.0%; P = 0.0005).50 Thus, IPE provided additional benefits beyond those achieved with optimized statin therapy.
A large proportion of patients who were treated with IPE in clinical practice have diabetes or are receiving statin treatment. Notably, IPE had no significant effect on glucose metabolism or hepatic or renal function in these studies.49,50 IPE was well tolerated, with a safety profile similar to that of placebo. More than 90% of patients completed the 12-week studies, and the incidence of eructations was less than that in the placebo group. Arthralgia was experienced in 2.3% of patients. Periodic liver function testing is recommended in patients with hepatic impairment, but LDL-C levels do not need to be monitored.63
Emerging Agents
Omega-3 Free Fatty Acids (Epanova)
OM-3 FFA (Epanova, Omthera/AstraZeneca), a soft-coated gelatin capsule, contains a mixture of the free fatty acid forms of EPA and DHA and is currently in phase 3 trials for the treatment of severe hypertriglyceridemia and mixed dyslipidemia.64 OM-3 FFA contains 50% to 60% EPA and 15% to 25% DHA free fatty acids.65 In the ECLIPSE study, which assessed the relative bioavailability of 4 g of OM-3 FFA or OM-3-A EE, the bioavailability of OM-3 FFA was found to be greater than that of OM-3-A EE under certain dietary conditions.66
A phase 2/3 study, EVOLVE, investigated the TG-lowering efficacy of OM-3 FFA (2, 3, or 4 g daily) compared with placebo in patients with severe hypertriglyceridemia (at or above 500 and less than 2,000 mg/dL). Preliminary results demonstrated significant reductions in TG levels in all dose groups; LDL-C levels were increased by 21%, 16%, and 26% with 2, 3, and 4 g/day, respectively.67
Another trial, ESPRIT, also investigated OM-3 FFA in statin-treated patients with persistent hypertriglyceridemia and at high risk for cardiovascular disease.68 There was a 7% reduction (P < 0.001) in non–HDL-C levels, a 21% reduction (P < 0.001) in TG levels, and a nonsignificant 1% increase in LDL-C levels in patients receiving 4 g/day. However, in the group receiving 2 g/day, LDL-C levels were significantly increased by 5% (P < 0.025).
Results are anticipated for a clinical trial (Effect of Multiple Doses of Epanova on the Multiple-Dose Pharmacokinetics of Simvastatin in Healthy Normal Subjects). Approximately 52 patients were enrolled, and pharmacokinetic parameters were the primary outcome measures.69
Krill Oil
Extracted from Antarctic krill, the oil from this crustacean is a rich source of phospholipids. The oil contains long-chain omega-3 polyunsaturated fatty acids as well as various anti-oxidants, including vitamin A, vitamin E, and astaxanthin (a carotenoid and antioxidant).70,71 Products include Neptune Krill Oil (NKO, Neptune Technologies & Bioressources, Quebec) and Superba Krill Oil (Aker BioMarine Antarctic AS).
The capsules may contain approximately 27 g/100 g of total omega-3 fatty acids and 1.5 g/100 g of total omega-6 fatty acids, with 14.2 g/100 g of EPA and 8.5 g/100 g of DHA.72 It is suggested that the association of omega-3 fatty acids with phospholipids in krill oil might provide greater intestinal absorption and bioavailability compared with fish oil.70 With daily administration of krill oil, plasma EPA and DHA levels have shown increases to a similar extent as with fish oil without any adverse effects.73,74
Daily doses of 1 to 3 g of krill oil can also reduce total cholesterol, LDL-C, and TG levels and increase HDL-C levels to a greater extent than 3 g/day of fish oil. In a study by Bunea and Deutsch, a maintenance dose of 0.5 g/day provided long-term benefits on blood lipids.71
A recently completed clinical trial (Study to Investigate the Effects of Krill Oil on Fasting Serum Triglycerides) recruited participants with borderline–high or high fasting serum TG levels. The study was conducted to assess the effects of 12 weeks of daily supplementation with Superba Krill Oil on changes from baseline in fasting serum TG levels and the Omega-3 Index (a biomarker of coronary heart disease). The index comprises the sum of EPA plus DHA in erythrocyte membranes, expressed as a percentage of total erythrocyte fatty acids.75
TRIFECTA is currently under way to determine whether a krill oil formulation (CaPre, Acasti Pharma/Neptune), given at doses of 1 or 2 g for 12 weeks, affects fasting plasma TG levels in patients with mild-to-high hypertriglyceridemia compared with placebo.76
THERAPY CONSIDERATIONS
Alternatives to omega-3 fatty acids for reducing non–HDL-C levels in patients with atherogenic dyslipidemia include fibrates and niacin.27 However, these agents are associated with adverse effects that may limit their long-term use. Niacin is associated with hepatotoxicity, hyperuricemia, gout, and hyperglycemia; it also causes flushing, which may be intolerable for some patients.22 Fibrates, although generally well tolerated, have the potential for an increased risk of gallstones and myopathy when combined with statins.22 By contrast, omega-3 fatty acids produce relatively fewer tolerability or safety problems compared with fibrates and niacin, although the potential long-term adverse effects of omega-3 fatty acids are not yet known.
Adherence to treatment is one of the main factors determining the effectiveness of drug therapy, particularly long-term therapy.54 A high pill burden, such as that encountered with omega-3 supplements, may lead to poor adherence, which in turn may lead to suboptimal disease control. The lack of a fishy taste and a low incidence of eructations with IPE (Vascepa) may also lead to better patient acceptance and adherence.
In a review of marketed fish oil supplements (110 non-liquid and 14 liquid), the median amount of EPA/DHA in non-liquid and liquid products was 0.216 g/0.2 g and 0.46 g/0.4 g, respectively.54 Therefore, in order to achieve a dose of 3.36 g/day omega-3 fatty acids, it was found that a median intake of 11.2 servings/day would be required at a median monthly cost of $63.49 for non-liquid formulations, and a median 3.6 teaspoons/day would be required at a median monthly cost of $13.60 for liquid formulations.54
As a result, an increased patient awareness of the differences between supplements and prescription formulations is an important clinical consideration. Patients who are prescribed omega-3 fatty acid therapy to reduce very high TG levels should be informed at the outset that substituting their prescription formulation with an omega-3 supplement may result in a diminished daily dose, hidden costs, and a potential for DHA-containing products to cause elevated LDL-C levels.
Another consideration for patients, pharmacists, and physicians is that some insurers might not cover omega-3 fatty acid prescription drugs. An insurance company might perceive that less expensive alternatives are available in the form of supplements; such a decision highlights the perceived lack of clarity regarding the differences between medications and supplements.
Pharmacoeconomic investigations of omega-3 fatty acids therapy include a decision-analytic model, which predicted fewer myocardial infarction (MIs) and cardiovascular deaths in the short and long term, with a reduction in direct and total medical costs.77
Another decision-analytic (Markov) model, based on the outcomes of the GISSI–HF trial, found that the addition of omega-3 fatty acids to the therapy of patients with chronic heart failure was likely to be cost-effective.78 In an analysis of the 3.5-year follow-up period of the GISSI–Prevenzione study, the cost-effectiveness of long-term omega-3 fatty acid treatment (involving direct costs only) was comparable to other drugs recently introduced in the routine secondary prevention of MI, such as statins.79
Finally, the cost-effectiveness of TG lowering was assessed in an analysis that included two large U.S. health care claims databases. Nichols et al. observed a reduced risk of pancreatitis, cardiovascular events, diabetes-related events, and kidney disease, along with a decrease in health care utilization and all-cause and cardiovascular-related costs, in patients with initial severe hypertriglyceridemia and follow-up TG levels below 500 mg/dL.80
Additional studies demonstrated significantly greater costs for patients with TG levels of 500 mg/dL or higher than in patients with TG levels below 150 mg/dL81 and a trend in lower use of hospital resources and lower medical care costs following TG reductions.82 From a managed care perspective, these results suggest that omega-3 fatty acids and TG-lowering therapy have the potential to have a positive impact on resource utilization and to reduce costs.
PLACE OF OMEGA-3 FATTY ACIDS IN CLINICAL THERAPY
Both OM-3-A EE (Lovaza) and IPE (Vascepa) are approved by the FDA for patients with very high TG levels. In clinical trials, both agents reduced TG levels and other atherogenic lipids in statin-treated patients with residual high TG levels, but IPE did not raise LDL-C levels. Although add-on therapies such as niacin and fibrates are recommended by the NCEP ATP III and the American Heart Association,22,27 these recommendations were made before more recent studies showed that an improvement in cardiovascular outcomes was not confirmed following the addition of fibrates or niacin to statin therapy.
In the ACCORD study, the annual rate of cardiovascular outcomes over a period of 4.7 years was similar in simvastatin-treated patients with type-2 diabetes receiving fenofibrate (2.2%) or placebo (2.4%).83
The HPS2–THRIVE study set out to examine the long-term clinical effects of increasing HDL-C levels with extended-release niacin/laropiprant (e.g., Tredaptive, Merck) in simvastatin-treated patients with a history of CHD. The results indicated no further significant reductions in the risk of the combination of coronary deaths, nonfatal heart attacks, strokes, or revascularizations, compared with statin therapy alone,84 and a safety analysis revealed an increased risk of myopathy when niacin/laropiprant was added to simvastatin.85
Similarly, in the AIM–HIGH trial, the addition of extended-release niacin to ongoing simvastatin therapy in patients with established cardiovascular disease did not reduce rates of the primary composite cardiovascular endpoint (16.4% with niacin vs. 16.2% with placebo).86
An event-driven cardiovascular outcomes trial, REDUCE–IT, is evaluating the ability of IPE 4 g/day to reduce cardiovascular events in high-risk statin-treated patients with hypertriglyceridemia.88 The primary outcome measure is the composite endpoint of cardiovascular death, MI, stroke, coronary revascularization, and hospitalization for unstable angina. Secondary outcome measures include the incidence of additional cardiovascular events, lipid and lipoprotein levels, and subgroup analyses in diabetic patients.87 The lack of recent positive outcomes data, in particular in studies involving interventions in addition to statin therapy, coupled with the known excess residual risk in patients with elevated TG or decreased HDL-C levels during statin therapy, highlights the importance of the REDUCE–IT study. The results are highly anticipated.
CONCLUSION
The very-long-chain omega-3 fatty acids EPA and DHA represent viable treatment options for patients with elevated TG levels. The FDA has approved two prescription omega-3 fatty acid agents—OM-3-A EE (Lovaza) and IPE (Vascepa). Although there are no data that suggest the LDL-C increases associated with some omega-3 fatty acid formulations lead to adverse outcomes, these increases in LDL-C may compromise the achievement of lipid targets; thus, there is a need for agents that can lower TG levels without increasing LDL-C levels. IPE, a high-purity form of EPA, either alone or in combination with statin therapy, may have the potential to meet this need. The ongoing REDUCE–IT study should help to address the need for additional outcomes data for EPA-only therapy.
Footnotes
Disclosure: The authors report no commercial or financial relationships in regard to this article. Editorial assistance was provided by Peloton Advantage, LLC, in Parsippany, New Jersey, and was funded by Amarin Pharma, Inc., in Bedminster, New Jersey.
REFERENCES
- 1.Nettleton JA. Omega-3 Fatty Acids and Health. New York: Springer (formerly Chapman & Hall); 1995. [Google Scholar]
- 2.James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. 2000;71:343S–348S. doi: 10.1093/ajcn/71.1.343s. [DOI] [PubMed] [Google Scholar]
- 3.Koski RR. Omega-3-acid ethyl esters (Lovaza) for severe hyper-triglyceridemia. P&T. 2008;33:271–281. 303. [Google Scholar]
- 4.Surette ME. The science behind dietary omega-3 fatty acids. Can Med Assoc J. 2008;178:177–180. doi: 10.1503/cmaj.071356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tur JA, Bibiloni MM, Sureda A, Pons A. Dietary sources of omega 3 fatty acids: Public health risks and benefits. Br J Nutr. 2012;107(Suppl 2):S23–S52. doi: 10.1017/S0007114512001456. [DOI] [PubMed] [Google Scholar]
- 6.Kris-Etherton PM, Harris WS, Appel LJ. Omega-3 fatty acids and cardiovascular disease: New recommendations from the American Heart Association. Arterioscler Thromb Vasc Biol. 2003;23:151–152. doi: 10.1161/01.atv.0000057393.97337.ae. [DOI] [PubMed] [Google Scholar]
- 7.Delgado-Lista J, Perez-Martinez P, Lopez-Miranda J, Perez-Jimenez F. Long chain omega-3 fatty acids and cardiovascular disease: A systematic review. Br J Nutr. 2012;107(Suppl 2):S201–S213. doi: 10.1017/S0007114512001596. [DOI] [PubMed] [Google Scholar]
- 8.Lavie CJ, Milani RV, Mehra MR, Ventura HO. Omega-3 polyunsatu-rated fatty acids and cardiovascular diseases. J Am Coll Cardiol. 2009;54:585–594. doi: 10.1016/j.jacc.2009.02.084. [DOI] [PubMed] [Google Scholar]
- 9.Bays H. Fish oils in the treatment of dyslipidemia and cardiovascular disease. In: Kwiterovich PO Jr, editor. The Johns Hopkins Textbook of Dyslipidemia. Philadelphia: Lippincott Williams & Wilkins/Wolters Kluwer Health; 2010. pp. 245–257. [Google Scholar]
- 10.FDA announces qualified health claims for omega-3 fatty acids. Available at: www.fda.gov/SiteIndex/ucm108351.htm. Accessed June 27, 2013.
- 11.Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSI–Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 12.Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 13.Kromhout D, Giltay EJ, Geleijnse JM. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363:2015–2026. doi: 10.1056/NEJMoa1003603. [DOI] [PubMed] [Google Scholar]
- 14.Rauch B, Schiele R, Schneider S, et al. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation. 2010;122:2152–2159. doi: 10.1161/CIRCULATIONAHA.110.948562. [DOI] [PubMed] [Google Scholar]
- 15.Kromhout D, Geleijnse JM, de GJ, et al. n-3 fatty acids, ventricular arrhythmia-related events, and fatal myocardial infarction in postmyocardial infarction patients with diabetes. Diabetes Care. 2011;34:2515–2520. doi: 10.2337/dc11-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ORIGIN Trial Investigators n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med. 2012;367:309–318. doi: 10.1056/NEJMoa1203859. [DOI] [PubMed] [Google Scholar]
- 17.Galan P, Kesse-Guyot E, Czernichow S, et al. Effects of B vitamins and omega-3 fatty acids on cardiovascular diseases: A randomised placebo controlled trial. BMJ. 2010;341:c6273. doi: 10.1136/bmj.c6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blacher J, Czernichow S, Paillard F, et al. Cardiovascular effects of B-vitamins and/or n-3 fatty acids: The Su.Fol.Om3 trial. Int J Cardiol. 2013;167:508–513. doi: 10.1016/j.ijcard.2012.01.060. [DOI] [PubMed] [Google Scholar]
- 19.GISSI–HF Investigators Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI–HF trial): A randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 20.Rizos EC, Ntzani EE, Bika E, et al. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: A systematic review and meta-analysis. JAMA. 2012;308:1024–1033. doi: 10.1001/2012.jama.11374. [DOI] [PubMed] [Google Scholar]
- 21.Fruchart JC, Sacks FM, Hermans MP, et al. The Residual Risk Reduction Initiative: A call to action to reduce residual vascular risk in dyslipidaemic patients. Diabetes Vasc Dis Res. 2008;5:319–335. doi: 10.3132/dvdr.2008.046. [DOI] [PubMed] [Google Scholar]
- 22.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III), Final Report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 23.Ford ES, Li C, Zhao G, Pearson WS, Mokdad AH. Hypertriglyceridemia and its pharmacologic treatment among U.S. adults. Arch Intern Med. 2009;169:572–578. doi: 10.1001/archinternmed.2008.599. [DOI] [PubMed] [Google Scholar]
- 24.Miller M, Stone NJ, Ballantyne C, et al. Triglycerides and cardiovascular disease: A scientific statement from the American Heart Association. Circulation. 2011;123:2292–2333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- 25.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 26.Kones R. Primary prevention of coronary heart disease: Integration of new data, evolving views, revised goals, and role of rosuvastatin in management. A comprehensive survey. Drug Design Devel Ther. 2011;5:325–380. doi: 10.2147/DDDT.S14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fletcher B, Berra K, Ades P, et al. Managing abnormal blood lipids: A collaborative approach. Circulation. 2005;112:3184–3209. doi: 10.1161/CIRCULATIONAHA.105.169180. [DOI] [PubMed] [Google Scholar]
- 28.Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:2969–2989. doi: 10.1210/jc.2011-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris WS. n-3 fatty acids and serum lipoproteins: Human studies. Am J Clin Nutr. 1997;65:1645S–1654S. doi: 10.1093/ajcn/65.5.1645S. [DOI] [PubMed] [Google Scholar]
- 30.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 31.Nordoy A, Barstad L, Connor WE, Hatcher L. Absorption of the n-3 eicosapentaenoic and docosahexaenoic acids as ethyl esters and triglycerides by humans. Am J Clin Nutr. 1991;53:1185–1190. doi: 10.1093/ajcn/53.5.1185. [DOI] [PubMed] [Google Scholar]
- 32.Krokan HE, Bjerve KS, Mork E. The enteral bioavailability of eicosapentaenoic acid and docosahexaenoic acid is as good from ethyl esters as from glyceryl esters in spite of lower hydrolytic rates by pancreatic lipase in vitro. Biochim Biophys Acta. 1993;1168:59–67. doi: 10.1016/0005-2760(93)90266-c. [DOI] [PubMed] [Google Scholar]
- 33.Hansen JB, Olsen JO, Wilsgard L, et al. Comparative effects of prolonged intake of highly purified fish oils as ethyl ester or triglyceride on lipids, haemostasis, and platelet function in normolipaemic men. Eur J Clin Nutr. 1993;47:497–507. [PubMed] [Google Scholar]
- 34.Balk EM, Lichtenstein AH, Chung M, et al. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: A systematic review. Atherosclerosis. 2006;189:19–30. doi: 10.1016/j.atherosclerosis.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 35.Pownall HJ, Brauchi D, Kilinc C, et al. Correlation of serum triglyceride and its reduction by omega-3 fatty acids with lipid transfer activity and the neutral lipid compositions of high-density and low-density lipoproteins. Atherosclerosis. 1999;143:285–297. doi: 10.1016/s0021-9150(98)00301-3. [DOI] [PubMed] [Google Scholar]
- 36.Bays HE, Tighe AP, Sadovsky R, Davidson MH. Prescription omega-3 fatty acids and their lipid effects: Physiologic mechanisms of action and clinical implications. Exp Rev Cardiovasc Ther. 2008;6:391–409. doi: 10.1586/14779072.6.3.391. [DOI] [PubMed] [Google Scholar]
- 37.Davidson MH, Stein EA, Bays HE, et al. Efficacy and tolerability of adding prescription omega-3 fatty acids 4 g/d to simvastatin 40 mg/d in hypertriglyceridemic patients: An 8-week, randomized, double-blind, placebo-controlled study. Clin Ther. 2007;29:1354–1367. doi: 10.1016/j.clinthera.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 38.Jacobson TA, Glickstein SB, Rowe JD, Soni PN. Effects of eicosapentaenoic acid and docosahexaenoic acid on low-density lipoprotein cholesterol and other lipids: A review. J Clin Lipidol. 2012;6:5–18. doi: 10.1016/j.jacl.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 39.Egert S, Kannenberg F, Somoza V, et al. Dietary alpha-linolenic acid, EPA, and DHA have differential effects on LDL fatty acid composition but similar effects on serum lipid profiles in normolipidemic humans. J Nutr. 2009;139:861–868. doi: 10.3945/jn.108.103861. [DOI] [PubMed] [Google Scholar]
- 40.Grimsgaard S, Bonaa KH, Hansen JB, Nordoy A. Highly purified eicosapentaenoic acid and docosahexaenoic acid in humans have similar triacylglycerol-lowering effects but divergent effects on serum fatty acids. Am J Clin Nutr. 1997;66:649–659. doi: 10.1093/ajcn/66.3.649. [DOI] [PubMed] [Google Scholar]
- 41.Woodman RJ, Mori TA, Burke V, et al. Effects of purified eicosapentaenoic and docosahexaenoic acids on glycemic control, blood pressure, and serum lipids in type 2 diabetic patients with treated hypertension. Am J Clin Nutr. 2002;76:1007–1015. doi: 10.1093/ajcn/76.5.1007. [DOI] [PubMed] [Google Scholar]
- 42.Mori TA, Burke V, Puddey IB, et al. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. Am J Clin Nutr. 2000;71:1085–1094. doi: 10.1093/ajcn/71.5.1085. [DOI] [PubMed] [Google Scholar]
- 43.Wei MY, Jacobson TA. Effects of eicosapentaenoic acid versus docosahexaenoic acid on serum lipids: A systematic review and meta-analysis. Curr Atheroscler Rep. 2011;13:474–483. doi: 10.1007/s11883-011-0210-3. [DOI] [PubMed] [Google Scholar]
- 44.Tatsuno I, Saito Y, Kudou K, Ootake J. Efficacy and safety of TAK-085 compared with eicosapentaenoic acid in Japanese subjects with hypertriglyceridemia undergoing lifestyle modification: The Omega-3 fatty acids Randomized Double-blind (ORD) study. J Clin Lipidol. 2013;7:199–207. doi: 10.1016/j.jacl.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 45.Nestel P, Shige H, Pomeroy S, et al. The n-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid increase systemic arterial compliance in humans. Am J Clin Nutr. 2002;76:326–330. doi: 10.1093/ajcn/76.2.326. [DOI] [PubMed] [Google Scholar]
- 46.Ando M, Sanaka T, Nihei H. Eicosapentanoic acid reduces plasma levels of remnant lipoproteins and prevents in vivo peroxidation of LDL in dialysis patients. J Am Soc Nephrol. 1999;10:2177–2184. doi: 10.1681/ASN.V10102177. [DOI] [PubMed] [Google Scholar]
- 47.Kurabayashi T, Okada M, Tanaka K, The Niigata Epadel Study Group Eicosapentaenoic acid effect on hyperlipidemia in menopausal Japanese women. Obstet Gynecol. 2000;96:521–528. doi: 10.1016/s0029-7844(00)00988-1. [DOI] [PubMed] [Google Scholar]
- 48.Satoh N, Shimatsu A, Kotani K, et al. Purified eicosapentaenoic acid reduces small dense LDL, remnant lipoprotein particles, and C-reactive protein in metabolic syndrome. Diabetes Care. 2007;30:144–146. doi: 10.2337/dc06-1179. [DOI] [PubMed] [Google Scholar]
- 49.Bays HE, Ballantyne CM, Kastelein JJ, et al. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multicenter, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] trial) Am J Cardiol. 2011;108:682–690. doi: 10.1016/j.amjcard.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 50.Ballantyne CM, Bays HE, Kastelein JJ, et al. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin-treated patients with persistent high triglycerides (from the ANCHOR study) Am J Cardiol. 2012;110:984–992. doi: 10.1016/j.amjcard.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 51.Brunton S, Collins N. Differentiating prescription omega-3-acid ethyl esters (P-OM3) from dietary-supplement omega-3 fatty acids. Curr Med Res Opin. 2007;23:1139–1145. doi: 10.1185/030079907x188017. [DOI] [PubMed] [Google Scholar]
- 52.Collins N, Tighe AP, Brunton SA, Kris-Etherton PM. Differences between dietary supplement and prescription drug omega-3 fatty acid formulations: A legislative and regulatory perspective. J Am Coll Nutr. 2008;27:659–666. doi: 10.1080/07315724.2008.10719743. [DOI] [PubMed] [Google Scholar]
- 53.FDA. Regulatory information: Dietary supplement health and education act of 1994. Available at: www.fda.gov/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/ucm148003.htm. Accessed June 27, 2013.
- 54.Zargar A, Ito MK. Long chain omega-3 dietary supplements: A review of the National Library of Medicine Herbal Supplement Database. Metab Syndr Relat Disord. 2011;9:255–271. doi: 10.1089/met.2011.0004. [DOI] [PubMed] [Google Scholar]
- 55.Omega-3 oil: Fish or pills? Consumer Rep. 2003:30–32. [PubMed] [Google Scholar]
- 56.Fish oil and omega-3 fatty acid supplements (EPA and DHA from fish, algae, and krill). Consumer Lab Available at: https://www.consumerlab.com/reviews/fish_oil_supplements_review/omega3/. Accessed June 27, 2013.
- 57.Bays HE. Safety considerations with omega-3 fatty acid therapy. Am J Cardiol. 2007;99:35C–43C. doi: 10.1016/j.amjcard.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 58.The name Omacor (Omega-3-acid ethyl esters) will be Lovaza. Reliant Pharmaceuticals, Inc. Available at: www.ncbop.org/PDF/OmacorBecomesLovazaJuly2007.pdf. Accessed June 27, 2013.
- 59.Lovaza, prescribing information. Research Triangle Park, N.C.: GlaxoSmithKline; 2012. [Google Scholar]
- 60.Harris WS, Ginsberg HN, Arunakul N, et al. Safety and efficacy of Omacor in severe hypertriglyceridemia. J Cardiovasc Risk. 1997;4:385–391. [PubMed] [Google Scholar]
- 61.Ridker PM, Rifai N, Clearfield M, et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. 2001;344:1959–1965. doi: 10.1056/NEJM200106283442601. [DOI] [PubMed] [Google Scholar]
- 62.Davidson MH, Maki KC, Bays H, et al. Effects of prescription omega-3 ethyl esters on lipoprotein particle concentrations, apolipoproteins AI and CIII, and lipoprotein-associated phospholipase A2 mass in statin-treated subjects with hypertriglyceridemia. J Clin Lipidol. 2009;3:332–340. doi: 10.1016/j.jacl.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 63.Vascepa, prescribing information. Bedminster, N.J.: Amarin Pharma, Inc.; 2012. [Google Scholar]
- 64.Davidson MH, Kling D, Maki KC. Novel developments in omega-3 fatty acid-based strategies. Curr Opin Lipidol. 2011;22:437–444. doi: 10.1097/MOL.0b013e32834bd642. [DOI] [PubMed] [Google Scholar]
- 65.Epanova overview. Omthera Pharmaceuticals. Available at: www.omthera.com/epanova_overview.html. Accessed June 27, 2013.
- 66.Davidson MH, Johnson J, Rooney MW, et al. A novel omega-3 free fatty acid formulation has dramatically improved bioavailability during a low-fat diet compared with omega-3-acid ethyl esters: The ECLIPSE (Epanova Compared to Lovaza In a Pharmacokinetic Single-dose Evaluation) study. J Clin Lipidol. 2012;6:573–584. doi: 10.1016/j.jacl.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 67.Kastelein JJ, Maki KC, Susekov A, et al. Dose reponse of a novel free-fatty acid formulation of omega-3 for the management of dyslipidemia in patients with severe hypertriglyceridemia: EpanoVa fOr Lowering Very High TriglycErides (the EVOLVE trial) (Abstract 16374) Circulation. 2012:126. [Google Scholar]
- 68.Maki KC, Orloff DG, Nicholls SJ, et al. A highly bioavailable omega-3 free fatty acid formulation improves the cardiovascular risk profile in high-risk, statin-treated patients with residual hypertriglyceridemia (the ESPRIT trial) Clin Ther. 2013;35(9):1400–1411. e3. doi: 10.1016/j.clinthera.2013.07.420. [DOI] [PubMed] [Google Scholar]
- 69.The effect of multiple doses of Epanova® on the multiple-dose pharmacokinetics of simvastatin in healthy normal subjects. Available at: www.clinicaltrials.gov/ct2/show/record/NCT01486433?term=NCT01486433&rank=1. Accessed June 27, 2013.
- 70.Krill oil Monograph. Altern Med Rev. 2010;15:84–86. [PubMed] [Google Scholar]
- 71.Bunea R, El FK, Deutsch L. Evaluation of the effects of Neptune Krill Oil on the clinical course of hyperlipidemia. Altern Med Rev. 2004;9:420–428. [PubMed] [Google Scholar]
- 72.Neptune Krill Oil specifications. Neptune Technologies & Bioressources. Available at: www.neptunebiotech.com/sites/default/files/Neptune_Krill_Oil_Specifications_Sheet.pdf. Accessed June 27, 2013.
- 73.Maki KC, Reeves MS, Farmer M, et al. Krill oil supplementation increases plasma concentrations of eicosapentaenoic and docosahexaenoic acids in overweight and obese men and women. Nutr Res. 2009;29:609–615. doi: 10.1016/j.nutres.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 74.Ulven SM, Kirkhus B, Lamglait A, et al. Metabolic effects of krill oil are essentially similar to those of fish oil but at lower dose of EPA and DHA in healthy volunteers. Lipids. 2011;46:37–46. doi: 10.1007/s11745-010-3490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harris WS, von SC. The Omega-3 Index: A new risk factor for death from coronary heart disease? Prev Med. 2004;39:212–220. doi: 10.1016/j.ypmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 76.TRIal For Efficacy of CaPre on hyperTriglyceridemiA (TRIFECTA) Available at: http://clinicaltrials.gov/ct2/show/NCT01455844?term=%28TRIFECTA%29.&rank=1. Accessed June 27, 2013.
- 77.Schmier JK, Rachman NJ, Halpern MT. The cost-effectiveness of omega-3 supplements for prevention of secondary coronary events. Manag Care. 2006;15:43–50. [PubMed] [Google Scholar]
- 78.Cowie MR, Cure S, Bianic F, et al. Cost-effectiveness of highly purified omega-3 polyunsaturated fatty acid ethyl esters in the treatment of chronic heart failure: Results of Markov modelling in a U.K. setting. Eur J Heart Fail. 2011;13:681–689. doi: 10.1093/eurjhf/hfr023. [DOI] [PubMed] [Google Scholar]
- 79.Franzosi MG, Brunetti M, Marchioli R, et al. Cost-effectiveness analysis of n-3 polyunsaturated fatty acids (PUFA) after myocar-dial infarction: Results from Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto (GISSI)–Prevenzione Trial. Pharmacoeconomics. 2001;19:411–420. doi: 10.2165/00019053-200119040-00008. [DOI] [PubMed] [Google Scholar]
- 80.Christian JB, Arondekar B, Buysman E, et al. Clinical and economic benefits observed when follow-up triglyceride levels are less than 500 mg/dL in patients with severe hypertriglyceridemia. J Clin Lipidol. 2012;6:450–461. doi: 10.1016/j.jacl.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 81.Nichols GA, Arondekar B, Garrison LP., Jr Patient characteristics and medical care costs associated with hypertriglyceridemia. Am J Cardiol. 2011;107:225–229. doi: 10.1016/j.amjcard.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 82.Nichols GA, Arondekar B, Jacobson TA. Hospital use and medical care costs up to 5 years after triglyceride lowering among patients with severe hypertriglyceridemia. J Clin Lipidol. 2012;6:443–449. doi: 10.1016/j.jacl.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 83.Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Merck announces HPS2–THRIVE study of Tredaptive™ (extended-release niacin/laropiprant) did not achieve primary endpoint, December 20, 2012. Available at: www.mercknewsroom.com. Accessed June 27, 2013.
- 85.HPS2–THRIVE randomized placebo-controlled trial in 25,673 high-risk patients of ER niacin/laropiprant: Trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J. 2013;34:1279–1291. doi: 10.1093/eurheartj/eht055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 87.A study of AMR101 to evaluate its ability to reduce cardiovascular events in high risk patients with hypertriglyceridemia and on statin (REDUCE–IT). Available at: http://clinicaltrials.gov/show/NCT01492361. Accessed June 27, 2013.


