Abstract
Glucarpidase (Voraxaze) for methotrexate toxicity
INTRODUCTION
Methotrexate (MTX) is a folic acid antagonist used for its antiproliferative and anti-inflammatory activity, either as a stand-alone drug or in combination with other medications, in the treatment of neoplastic and autoimmune diseases (Table 1).1,2 Folic acid plays a crucial role in the synthesis of DNA and cell replication.2 In the dihydrofolate reductase (DHFR) pathway, tetrahydrofolate (THF) is the active form of folic acid.2 THF donates carbon groups that are eventually used for synthesizing deoxyribose purines (i.e., adenosine and guanosine) and pyrimidines (specifically thymidine) that constitute human DNA.2,3
Table 1.
FDA-Approved Indications for Methotrexate
Neoplastic diseases
Autoimmune diseases
|
Data from methotrexate prescribing information.15
Because MTX also affects noncancerous cells, treatment may lead to adverse drug effects (ADEs) and undesirable toxicities.4 MTX can crystallize and precipitate in the kidney, especially at higher systemic concentrations and in the presence of acidic urine.5 Neither hemodialysis (HD) nor peritoneal dialysis (PD) sufficiently removes the drug from circulation, whereas continuous venovenous hemodialysis(CVVHD) seems to be the most efficacious dialysis method.5,6
Leucovorin (LV), or folinic acid (citrovorum factor), is a rescue agent that exerts its effects via competitive cellular uptake, but it does not sufficiently reduce toxic levels of MTX.7 The standard-of-care regimen combines LV with continuous urinary alkalinization (with sodium bicarbonate) and rigorous hydration.8 Unfortunately, even with normal pretreatment renal function, this approach does not reverse nephrotoxicity in 2% to 10% of patients.9
HISTORY
Unlike other therapies used to treat MTX toxicity, glucarpidase (Voraxaze, BTG International, Inc.), also known as carboxypeptidase-G2 (CPDG2), is an enzyme that rapidly metabolizes circulating (not intracellular) MTX to two inactive metabolites: glutamate and 2,4-diamino-N-10-methylpteroic acid (DAMPA).10
Although patients have been receiving CPDG2 since 1993 through the FDA’s compassionate use criteria, glucarpidase was formally approved by the FDA in January 2012.10–12 The drug has demonstrated considerable safety and efficacy through case reports and clinical trials, and it is likely to be incorporated in future protocols for reversing toxic MTX levels (above 1 μmol/L) in adults and in pediatric patients with renal dysfunction.10
CHEMICAL COMPOSITION
Glucarpidase is a recombinant form of the CPDG2 enzyme, produced via modified Escherichia coli. It is a homodimeric protein composed of 390 amino acids. The molecular weight is 83 kilodaltons.10
INDICATIONS AND USAGE
Glucarpidase is approved for reversing MTX toxicity (plasma MTX concentration [CMTX] above 1 μmol/L) in patients with delayed MTX clearance resulting from renal dysfunction. This is most likely to occur when patients receive high-dose MTX (HDMTX) therapy for neoplastic diseases. Glucarpidase is not intended for patients who demonstrate the expected clearance of MTX, defined as a plasma MTX concentration within 2 standard deviations (SDs) of the mean MTX excretion curve specific for the dose of MTX administered. Patients with normal or mildly impaired renal function should not receive glucarpidase because of the potential for subtherapeutic MTX exposure.10
CLINICAL PHARMACOLOGY
As a zinc peptidase, glucarpidase hydrolyzes the amide linkage on circulating (not intracellular) MTX to form glutamate and DAMPA.10,13 The latter metabolite of MTX is inactive, less water-soluble than MTX, and seems to undergo hepatic metabolism (glucoronide conjugation) and nonrenal (biliary) elimination.14,15 DAMPA is eliminated more rapidly than MTX after glucarpidase administration.13,14
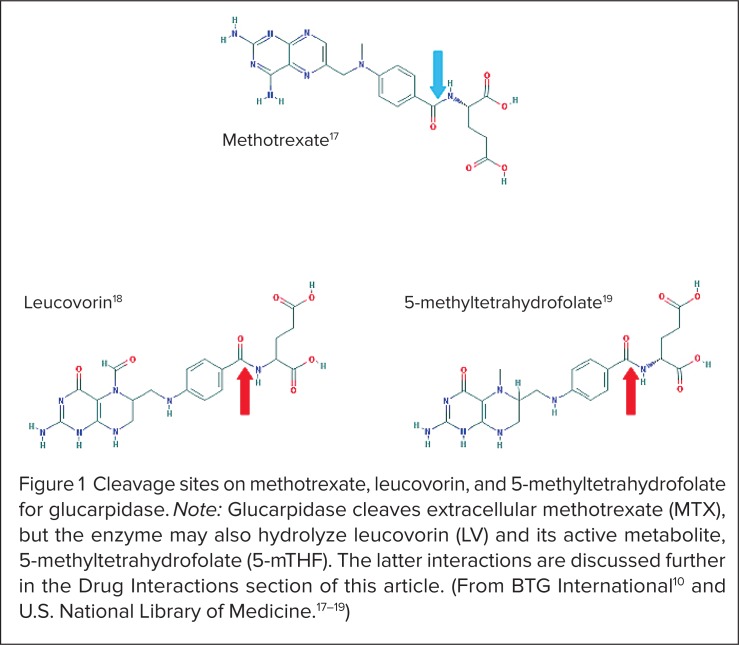
At normal human body temperature (37°C), each unit of glucarpidase exerts enzymatic activity to cleave 1 μmol/L of circulating MTX per minute.10 Although this is an undesirable effect, the drug may also cleave LV and its active metabolite, 5-methyltetrahydrofolate (5-mTHF), shown in Figure 1.10,15–19 Implications of this effect are discussed under Drug Interactions (see page 742).
Figure 1.
Cleavage sites on methotrexate, leucovorin, and 5-methyltetra hydro folate for glucarpidase. Note: Glucarpidase cleaves extracellular methotrexate (MTX), but the enzyme may also hydrolyze leucovorin (LV) and its active metabolite, 5-methyltetrahydrofolate (5-mTHF). The latter interactions are discussed further in the Drug Interactions section of this article. (From BTG International10 and U.S. National Library of Medicine.17–19)
PHARMACODYNAMICS AND PHARMACOKINETICS
In a study conducted by the manufacturer, glucarpidase decreased the CMTX by more than 97% within 15 minutes in 22 patients.10 Furthermore, in 20 of these patients (91%), the CMTX remained reduced by more than 95% for up to 8 days.10
Using enzymatic assays, Phillips et al. conducted a small pharmacokinetic study of glucarpidase (in the absence of MTX) in human subjects with normal and severely impaired renal function.20 After a single intravenous (IV) injection of glucarpidase 50 units/kg administered over a period of 5 minutes to eight healthy patients, the enzyme’s terminal half-life was 5.6 hours, the mean maximum concentration (Cmax) was 3.3 mcg/mL, the mean area-under-the-curve (AUC0→infinity) was 23.3 mcg • hour/mL, the mean systemic clearance was 7.5 mL/minute, and the mean volume of distribution (Vd) was 3.6 L.10,20 The Vd suggests that the drug is not widely distributed and is restricted mostly to plasma volume.20
In four patients with severe renal impairment, indicated by a creatinine clearance (CrCl) below 30 mL/minute, pharmacokinetic parameters were similar, except for a longer terminal half-life of 8.2 hours.10,20 Despite the slightly longer half-life, dosage adjustments are not required with glucarpidase in patients with renal or hepatic impairment.10,20
Because glucarpidase does not attain appreciable intracellular concentrations and does not cross the blood–brain barrier, Adamson et al. investigated the potential clinical utility of the enzyme when given via the intrathecal route.21 Although O’Marcaigh et al., Widemann et al., and Bradley et al. reported favorable outcomes with intrathecal glucarpidase, 22–24 the FDA approved glucarpidase for use only via the IV route.
EFFICACY AND SAFETY IN CLINICAL TRIALS
Glucarpidase is extremely effective in lowering MTX levels; clinical trials and compassionate use experiences from the previous two decades have generally shown that after the first dose of the enzyme, the CMTX was decreased by 71% to 99.6% within 5 to 15 minutes.11,25–30 Studies leading to the approval of glucarpidase are summarized in Table 2.10
Table 2.
Summary of Clinical Trials Leading to the Approval of Glucarpidase (Voraxaze)*
| Study 1 | Study 1 Subset | Study 2 | |
|---|---|---|---|
| Number of patients | 149 | 22 | 141 |
| Patient age (years) | <1 to 85 (median: 18) | 5 to 84 (median: 15.5) | <1 to 85 (median: 17) |
| Underlying disease state(s) |
|
|
|
| Glucarpidase dose (units/kg) | 18 to 98 (median: 49) | 50 | 6 to 189 (median: 50) |
| Total number of glucarpidase doses provided |
|
|
|
Patients also received intravenous hydration, urinary alkalinization, and leucovorin (not administered 2 hours before or after glucarpidase) in both studies. Data from glucarpidase (Voraxaze) prescribing information.10
As glucarpidase does not cleave intracellular MTX, patients needed to receive LV in addition to supportive care.11,25–30 In several studies, additional doses of glucarpidase did not significantly hydrolyze more MTX.29,30 ADEs were minimal (e.g., paresthesias, flushing, shaking, and headache), which usually resolved without additional interventions (Table 3).25–30 Patients with additional risk factors for nephrotoxicity (such as older age, the use of certain concomitant drugs, and prolonged exposure of MTX) had increased morbidity and mortality rates, but deaths were not directly attributed to glucarpidase.25–30
Table 3.
Adverse Effects of Glucarpidase From Select Clinical Trials and Compassionate Use Experiences*
| Study | Patients (n) | Reported Adverse Drug Effects (n, %) |
|---|---|---|
| DeAngelis LM, et al. (1996)25 | 4 | Rash (n = 1, 25%) |
| Widemann BC, et al. (1997)26 | 20 | Flushing (n = 2, 10%) Head pressure (n = 1, 5%) Minimal burning of face/extremities (n = 1, 5%) Shaking (n = 1, 5%) Tingling fingers (n = 1, 5%) Warmth (n = 2, 10%) |
| Buchen S, et al. (2005)28 | 58 | Flushing (n = 2, 3.4%) Shaking (n = 1, 1.7%) |
| Widemann BC, et al. (2010)31 | 100 | Erythema and pruritus of face and legs (n = 1, 1%) Flushing (n = 2, 2%) Head pressure (n = 1, 1%) Minimal burning of face and extremities (n = 1, 1%) Neck rash within a day after glucarpidase (n = 1, 1%) Numbness/burning sensation around penis (n = 1, 1%) Shaking (n = 1, 1%) Tingling fingers (n = 1, 1%) Warmth (n = 2, 2%) |
| Manufacturer’s Studies 1 and 210 | 290 | Blurred vision (n = 1, <1%) Diarrhea (n = 1, <1%) Flushing (n = 5, 2%) Headache (n = 2, 1%) Hypersensitivity (n = 1, <1%) Hypertension (n = 1, <1%) Hypotension (n = 2, 1%) Nausea/vomiting (n = 5, 2%) Paresthesia (n = 7, 2%) Rash (n = 1, <1%) Throat irritation and throat tightness (n = 1, <1%) Tremor (n = 1, <1%) |
| Widemann BC, et al. (2012)11 | 429 | Flushing (1.8%) Headache (1%) Paresthesia (2%) |
In the pivotal studies leading to the approval of glucarpidase, the primar y outcome of interest was the proportion of patients who achieved a rapid and sustained clinically important reduction (RSCIR) in CMTX.10 The investigators defined this parameter as a CMTX of 1 μmol/L or below within 15 minutes, sustained for up to 8 days following the initial injection.10
In a subset of 22 patients, 10 (45%) achieved RSCIRs, whereas five patients (23%) attained a transient CMTX of 1 μmol/L or below.10 The likelihood of attaining a RSCIR following the first dose of glucarpidase correlated with the pre-glucarpidase CMTX.10 Although all nine patients with a CMTX greater than 50 μmol/L did not achieve a RSCIR, they had more than a 95% reduction in CMTX for up to 8 days after a single dose of glucarpidase.10
Of the seven patients with a CMTX exceeding 100 μmol/L, six (86%) received a second dose of glucarpidase (50 units/kg) within 48 hours after the first dose.10 Four patients (67%) failed to achieve a RSCIR; the other two patients already had a CMTX of 1 μmol/L or below within 48 hours.10 According to the manufacturer, no controlled trials have compared glucarpidase plus supportive care with supportive care alone.10 In addition, glucarpidase did not prevent fatal MTX toxicity in approximately 3% of patients in the safety population.10
Widemann et al. recognized that in patients with osteosarcoma and a very high CMTX, glucarpidase was far more effective than hemodialysis in reversing MTX toxicity.31 Although the enzyme did not seem to normalize renal function as quickly as the dialysis methods, there were fewer repeated CMTX elevations.31
Christensen et al. evaluated 1,141 patients who had received glucarpidase for MTX toxicity in order to determine the safety and efficacy of rechallenging patients with MTX after rescue.32 In 20 of these patients (median CMTX, 29.1 μM/L), glucarpidase was administered 22 times. The results were promising; 13 patients (65%) completed 39 courses of HDMTX, and renal function returned to baseline in all studied patients. The elimination time for MTX plus glucarpidase decreased from a median of 355 hours during the first course, to 90 hours during the second course, and to a median of 72 hours in later courses. This finding reassured clinicians that MTX therapy could be continued in patients who previously received rescue therapy with glucarpidase.32
ADVERSE DRUG REACTIONS
Patients did not experience major adverse effects from glucarpidase and rarely required additional interventions. A summary of reported adverse drug reactions from clinical trials and compassionate use experiences is presented in Table 3.10,11,25–30
DRUG INTERACTIONS
Reduced Folate Levels
Leucovorin (LV) and its active metabolite, 5-mTHF, are substrates for glucarpidase because of an amide linkage in each molecule (see Figure 1).10,17–19 In a study of cancer patients who received 1 g/m2 or more of MTX with LV rescue, a single dose of glucarpidase 50 units/kg IV 2 hours before LV reduced (6S)-LV AUC0→3h by 33% and the Cmax by 52%.10 The enzyme also reduced (6S)-5-mTHF, AUC0→3h by 92% and the Cmax by 93%.10
Widemann et al. measured LV and 5-mTHF levels in 16 patients.31 Similar to the effect on MTX, glucarpidase reduced 5-mTHF levels by 75% to 99.9% (median, 98.6%) and LV levels up to 62.4% (median, 17.7%) within 15 minutes.31
Therefore, to minimize a clinically significant drug–drug interaction, patients should receive LV 2 hours before or after a dose of glucarpidase.10 There is no recommended dosage adjustment for LV because the dose is contingent on patients’ pre-glucarpidase CMTX.10
To restore intracellular folate levels, some researchers have advised continuing high doses of LV (250 mg/m2 every 6 hours) for 48 hours after glucarpidase administration.33
Antifolate Medications
In addition to MTX, glucarpidase may cleave other antifolate medications, including aminopterin (formerly made by Lederle), pemetrexed (Alimta, Eli Lilly), pralatrexate (Folotyn, Allos/Spectrum), and raltitrexed (Tomudex, AstraZeneca).10
Drugs That Decrease Urinary pH Or Renal Elimination
McBride et al. noted that although many interacting drugs are not listed in the product information for glucarpidase, they may complicate recovery after high-dose MTX therapy.34 Examples include nonsteroidal anti-inflammatory drugs (NSAIDs), antibiotics, proton pump inhibitors (PPIs), immunosuppressants, contrast dyes, and vitamins.34 NSAIDs, such as aspirin, ibuprofen, indomethacin, and naproxen (Naprosyn, Roche), could displace MTX from protein-binding sites and decrease renal clearance.34
Antibiotics, such as beta-lactams and vancomycin (Vancocin, ViroPharma), and PPIs, such as omeprazole (Prilosec) and esomeprazole (Nexium, both Astra-Zeneca), pantoprazole (Protonix, Pfizer), and lansoprazole (Prevacid, Takeda), decrease renal elimination of MTX.34,35 Vitamin C and glutamine decrease the urinary pH and promote the precipitation of MTX in the renal tubules.34,36,37
Santucci et al. reported a 27% decrease in the clearance of MTX and a 39% decrease in the clearance of 7-hydroxy-MTX after a combination of PPI treatment and HDMTX.35 In this setting, dose-adjusted histamine-2 receptor antagonists (H2RAs), including ranitidine (Zantac, GlaxoSmithKline) and famotidine (Pepcid, Merck), were recommended as substitutes for PPIs.35
DRUG–LABORATORY INTERACTIONS
As one of the inactive metabolites of MTX resulting from treatment with glucarpidase, DAMPA interferes with or cross-reacts with immunoassay measurements, such as the fluorescence polarization immunoassay (FPIA). DAMPA has a long half-life (9 hours), and these immunoassays are likely to overestimate the actual CMTX. Therefore, a chromatographic method, such as high-pressure liquid chromatography (HPLC), is preferred for measuring the CMTX within 48 hours after glucarpidase administration.10
CONTRAINDICATIONS
No contraindications are listed in the prescribing information for glucarpidase.10
DOSAGE AND ADMINISTRATION
The standard dose of glucarpidase is a single IV bolus injection of 50 units/kg administered over a period of 5 minutes.10 The drug is sold as a sterile, preservative-free white lyophilized powder with 1,000 units per single-use vial. Each vial also contains lactose monohydrate (10 mg), Tris-HCl (0.6 mg), and zinc acetate dihydrate (0.002 mg).10
Glucarpidase is reconstituted with 1 mL of sterile saline (0.9% sodium chloride) for injection, USP. The vial should be rolled and tilted gently (not shaken) to mix the contents. The vial must be discarded if the reconstituted solution is not clear, colorless, or free of particulate matter. Glucarpidase is administered immediately upon reconstitution, or it may be stored under refrigeration (but not frozen) at 36° to 46°F (2°–8°C) for up to 4 hours.10
Patients should receive hydration and alkalinization of the urine as required. In addition, LV therapy should be provided 2 hours before or after a dose of glucarpidase. The spacing in administration is necessary because LV and its active metabolite (5-mTHF) are substrates for the enzyme.
Within the first 48 hours after glucarpidase is given, the same LV dose provided before glucarpidase should be continued afterward. After 48 hours, LV doses should be adjusted based on chromatographic (not assay) measurements of the CMTX and continued for a minimum of 3 days.10
P&T COMMITTEE CONSIDERATIONS
Although no pharmacoeconomic studies of glucarpidase are available, the Centers for Medicare & Medicaid Services (CMS) granted the drug’s manufacturer (BTG International, Inc.) a temporary, new technology add-on payment, effective October 1, 2012, in which the federal government will pay hospitals up to 50% of the cost of glucarpidase in addition to the standard Diagnosis-Related Group (DRG) reimbursement payment. This add-on payment for glucarpidase is expected to last for 2 to 3 years until the standard DRGs are recalibrated to include this new technology. CMS aims to provide a maximum add-on payment of $45,000 per patient for glucarpidase.38
CONCLUSION
Among its many adverse effects, HDMTX therapy may lead to toxic levels (above 1 μmol/L) and elevated serum creatinine in adult and pediatric patients with underlying risks of renal dysfunction. Several clinical trials and compassionate use experiences have demonstrated the considerable safety and efficacy of glucarpidase for lowering the CMTX. Although the majority of patients benefit from the standard-of-care regimen (LV, urinary alkalinization, and hydration), the decreases in CMTX are not always satisfactory. Glucarpidase should be considered as an addition to protocols for select patients who experience renal dysfunction and toxic MTX levels.
Footnotes
Disclosure: The authors report that they have no commercial or financial relationships in regard to this article.
REFERENCES
- 1.Genome Alberta and Genome Canada Folate Metabolism. Available at: http://path-man.smpdb.ca/pathways/SMP00053/pathway?level=2. Accessed January 20, 2013.
- 2.Visentin M, Zhao R, Goldman ID. The antifolates. Hematol Oncol Clin North Am. 2012;26(3):629–648. ix. doi: 10.1016/j.hoc.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalhan SC, Marczewski SE. Methionine, homocysteine, one carbon metabolism, and fetal growth. Rev Endocr Metab Disord. 2012;13(2):109–119. doi: 10.1007/s11154-012-9215-7. [DOI] [PubMed] [Google Scholar]
- 4.Sioka C, Kyritsis AP. Central and peripheral nervous system toxicity of common chemotherapeutic agents. Cancer Chemother Pharmacol. 2009;63(5):761–767. doi: 10.1007/s00280-008-0876-6. [DOI] [PubMed] [Google Scholar]
- 5.Morgacheva O, Furst DE. Use of MTX in the elderly and in patients with compromised renal function. Clin Exp Rheumatol. 2010;28(5 Suppl 61):S85–S94. [PubMed] [Google Scholar]
- 6.Vilay AM, Mueller BA, Haines H, et al. Treatment of methotrexate intoxication with various modalities of continuous extracorporeal therapy and glucarpidase. Pharmacotherapy. 2010;30(1):111. doi: 10.1592/phco.30.1.111. [DOI] [PubMed] [Google Scholar]
- 7.Sadée W. Antineoplastic agents: High-dose methotrexate and citrovorum factor rescue. Ther Drug Monit. 1980;2(2):177–185. [PubMed] [Google Scholar]
- 8.Fleisher M. Antifolate analogs: Mechanism of action, analytical methodology, and clinical efficacy. Ther Drug Monit. 1993;15(6):521–526. [PubMed] [Google Scholar]
- 9.Holmboe L, Andersen AM, Mørkrid L, et al. High dose methotrexate chemotherapy: Pharmacokinetics, folate, and toxicity in osteosarcoma patients. Br J Clin Pharmacol. 2012;73(1):106–114. doi: 10.1111/j.1365-2125.2011.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voraxaze (glucarpidase) intravenous injection, product information. West Conshohocken, Pa.: BTG International, Inc.; Mar, 2013. [Google Scholar]
- 11.Widemann BC, Jayaprakash N, Howard SC, et al. Clinical trial and compassionate use experience with glucarpidase for methotrexate toxicity. J Clin Oncol. 2012;30(Suppl):6530. [Google Scholar]
- 12.FDA Availability of Investigational Drugs for Compassionate Use. Jul 24, 2009. Available at: www.fda.gov/NewsEvents/Testimony/ucm115209.htm. Accessed January 20, 2013.
- 13.Widemann BC, Sung E, Anderson L, et al. Pharmacokinetics and metabolism of the methotrexate metabolite 2, 4-diamino-N(10)-methylpteroic acid. J Pharmacol Exp Ther. 2000;294(3):894–901. [PubMed] [Google Scholar]
- 14.Buchen S, Ngampolo D, Melton RG, et al. Carboxypeptidase G2 rescue in patients with methotrexate intoxication and renal failure. Br J Cancer. 2005;92(3):480–487. doi: 10.1038/sj.bjc.6602337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Methotrexate intramuscular, intravenous, intra-arterial, intrathecal injection, product information. New York: Pfizer; Mar, 2012. [Google Scholar]
- 16.Leucovorin calcium for injection, product information. Irvine, Calif.: Teva Parenteral Medicines, Inc.; Sep, 2007. [Google Scholar]
- 17.National Center for Biotechnology Information, U.S. National Library of Medicine. Methotrexate—Compound Summary. Available at: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=126941. Accessed January 20, 2013.
- 18.National Center for Biotechnology Information, U.S. National Library of Medicine Leucovorin—Compound Summary. Available at: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=143. Accessed January 20, 2013.
- 19.National Center for Biotechnology Information, U.S. National Library of Medicine 5-Methyltetrahydrofolate—Compound Summary. Available at: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=439234. Accessed January 20, 2013.
- 20.Phillips M, Smith W, Balan G, et al. Pharmacokinetics of glucarpidase in subjects with normal and impaired renal function. J Clin Pharmacol. 2008;48(3):279–284. doi: 10.1177/0091270007311571. [DOI] [PubMed] [Google Scholar]
- 21.Adamson PC, Balis FM, McCully CL, et al. Rescue of experimental intrathecal methotrexate overdose with carboxypeptidase-G2. J Clin Oncol. 1991;9(4):670–674. doi: 10.1200/JCO.1991.9.4.670. [DOI] [PubMed] [Google Scholar]
- 22.O’Marcaigh AS, Johnson CM, Smithson WA, et al. Successful treatment of intrathecal methotrexate overdose by using ventriculolumbar perfusion and intrathecal instillation of carboxypeptidase G2. Mayo Clin Proc. 1996;71(2):161–165. doi: 10.4065/71.2.161. [DOI] [PubMed] [Google Scholar]
- 23.Widemann BC, Balis FM, Shalabi A, et al. Treatment of accidental intrathecal methotrexate overdose with intrathecal carboxypeptidase G2. J Natl Cancer Inst. 2004;20(20):1557–1559. doi: 10.1093/jnci/djh270. 96. [DOI] [PubMed] [Google Scholar]
- 24.Bradley AM, Buie LW, Kuykendal A, et al. Successful use of intrathecal carboxypeptidase G2 for intrathecal methotrexate overdose: A case study and review of the literature. Clin Lymphoma Myeloma Leuk. 2013;13(2):166–170. doi: 10.1016/j.clml.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 25.DeAngelis LM, Tong WP, Lin S, et al. Carboxypeptidase G2 rescue after high-dose methotrexate. J Clin Oncol. 1996;14(7):2145–2149. doi: 10.1200/JCO.1996.14.7.2145. [DOI] [PubMed] [Google Scholar]
- 26.Widemann BC, Balis FM, Murphy RF, et al. Carboxypeptidase-G2, thymidine, and leucovorin rescue in cancer patients with methotrexate-induced renal dysfunction. J Clin Oncol. 1997;15(5):2125–2134. doi: 10.1200/JCO.1997.15.5.2125. [DOI] [PubMed] [Google Scholar]
- 27.Krause AS, Weihrauch MR, Bode U, et al. Carboxypeptidase-G2 rescue in cancer patients with delayed methotrexate elimination after high-dose methotrexate therapy. Leuk Lymphoma. 2002;43(11):2139–2143. doi: 10.1080/1042819021000032953. [DOI] [PubMed] [Google Scholar]
- 28.Buchen S, Ngampolo D, Melton RG, et al. Carboxypeptidase G2 rescue in patients with methotrexate intoxication and renal failure. Br J Cancer. 2005;92(3):480–487. doi: 10.1038/sj.bjc.6602337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartz S, Borner K, Müller K, et al. Glucarpidase (carboxypeptidase g2) intervention in adult and elderly cancer patients with renal dysfunction and delayed methotrexate elimination after high-dose methotrexate therapy. Oncologist. 2007;12(11):1299–1308. doi: 10.1634/theoncologist.12-11-1299. [DOI] [PubMed] [Google Scholar]
- 30.Widemann BC, Balis FM, Kim A, et al. Glucarpidase, leucovorin, and thymidine for high-dose methotrexate-induced renal dysfunction: Clinical and pharmacologic factors affecting outcome. J Clin Oncol. 2010;28(25):3979–3986. doi: 10.1200/JCO.2009.25.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Widemann BC, Balis FM, Kempf-Bielack B, et al. High-dose methotrexate-induced nephrotoxicity in patients with osteosarcoma. Cancer. 2004;100(10):2222–2232. doi: 10.1002/cncr.20255. [DOI] [PubMed] [Google Scholar]
- 32.Christensen AM, Pauley JL, Molinelli AR, et al. Resumption of high-dose methotrexate after acute kidney injury and glucarpidase use in pediatric oncology patients. Cancer. 2012;118(17):4321–4330. doi: 10.1002/cncr.27378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11(6):694–703. doi: 10.1634/theoncologist.11-6-694. [DOI] [PubMed] [Google Scholar]
- 34.McBride A, Antonia SJ, Haura EB, et al. Suspected methotrexate toxicity from omeprazole: A case review of carboxypeptidase G2 use in a methotrexate-experienced patient with methotrexate toxicity and a review of the literature. J Pharm Pract. 2012;25(4):477–485. doi: 10.1177/0897190012442717. [DOI] [PubMed] [Google Scholar]
- 35.Santucci R, Leveque D, Kemmel V, et al. Severe intoxication with methotrexate possibly associated with concomitant use of proton pump inhibitors. Anticancer Res. 2010;30:963–966. [PubMed] [Google Scholar]
- 36.Cózar Olmo JA, Martínez Colmenero C, Peláez Pleguezuelos I, et al. Carboxypeptidase-G2 administration after high-dose methotrexate: Treatment and drug interactions. An Pediatr (Barc) 2009;71(3):230–234. doi: 10.1016/j.anpedi.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 37.Marín Pozo JF, García JA, Muriel AC, et al. Hazards of concomitant administration of methotrexate and glutamine. Am J Health Syst Pharm. 2010;67(8):601–602. doi: 10.2146/ajhp090173. [DOI] [PubMed] [Google Scholar]
- 38.CMS grants new prospective reimbursement for Voraxaze. West Conshohocken, Pa.: BTG International, Inc.; Sep 5, 2012. Available at: www.btgplc.com/document/570. Accessed October 23, 2013. [Google Scholar]