Summary
Background
Ceiba pentandra (L.) Gaertn, commonly called silk-cotton tree, has been extensively used by traditional medicine practitioners in Northern and Eastern Nigeria in the control and management of diabetes.
Objective
To evaluate the hypoglycaemic and anti-hyperglycaemic effect of ethanolic extract of Ceiba pentandra bark in normal and streptozotocin induced diabetic rats.
Method
Screening activity of the extract was carried out by OGTT. Diabetes mellitus was induced with streptozotocin and graded doses of the ethanolic bark extract (200 and 400 mg/kg, b.w.) were then administered to the experimentally diabetic rats. The blood glucose level was measured at different time intervals.
Results
The single dose study of C. pentandra extract at two different doses produced no significant hypoglycaemic effect in normal rats but C. pentandra (200 mg/kg) significantly decreased blood glucose level in diabetic rats. In OGTT, C. pentandra (200 mg/kg) significantly reduced elevated glucose level in normal and diabetic rats. In long term (21 days) study, C. pentandra (200 mg/kg) significantly decreased blood glucose level, total cholesterol and triglycerides level, prevented degeneration of liver and pancreas, and increased serum insulin level and liver glycogen content in diabetic rats. Acute toxicity study in rats did not show any signs of toxicity up to the dose of 2000 mg/kg b.w.
Conclusion
The results reveal that the extract improved glucose tolerance in normal and streptozotocin-induced diabetic rats. Thus the study suggests that the C. pentandra bark extract could be beneficial in the management of type I diabetes.
Keywords: Antihyperglycemic, Ceiba pentandra, glibenclamide, hypoglycaemic, Streptozotocin
Introduction
Diabetes mellitus is recognized as one of the leading causes of morbidity and mortality in the world.
About 2.5–3% of the world's population suffers from this disease, a proportion which, in some countries, can reach 7% or more. Hyperglycemia leads to metabolic disorders and various complications.1 According to W.H.O projections, the prevalence of diabetes is likely to increase by 35%. Currently there are over 150 million diabetic people worldwide and this is likely to increase to 300 million or more by the year 2025.
Statistical projections about India suggest that the number of diabetes will rise from 15 million in 1995 to 57 million in the year 2025 making it the country with the highest number of diabetic people in the world.2,3 There are more women than men with diabetes, especially in developed countries. In the future, diabetes will be increasingly concentrated in urban areas. In modern medicine, the beneficial effects on glycaemic levels are well documented; the preventing activity of the drugs against progressive nature of diabetes and its micro and macrovascular complications was modest and not always effective.
Insulin therapy affords effective glycaemic control, yet its short comings such as ineffectiveness on oral administration, short shelf life, requirement of constant refrigeration, and in the event of excess dosage fatal hypoglycaemia limits its usage. Treatment with sulfonylureas and biguanides is also associated with side effects.4 It is apparent that due to the side effects of the currently used drugs, there is a need for safe agents with minimal adverse effects, which can be taken for long duration. For various reasons in recent years, the popularity of complementary medicine has increased.
Dietary measures and traditional plant therapies as prescribed by Ayurvedic and other indigenous systems of medicine were used commonly in India.5
In the indigenous system of medicine (Ayurveda), a number of plants were in practice for the cure of diabetes or ‘Madhumeha’ and some of them have been experimentally evaluated and the active principles were isolated.6,9 However, search for newer antidiabetic drugs continues.
Ceiba pentandra (L.) Gaertn, commonly called white silk-cotton tree belongs to the family Bombacaceae. It is an elegant large tree, found throughout hotter parts of India, especially in southern and western India and the Andaman Islands. The tree is native to Mexico, Central America and the Caribbean, northern South America, and to tropical West Africa. It is widely used in traditional medicines as a diuretic and an emollient.
Various parts of this plant has been used as demulcent, laxative, astringent, emollient, emetic, febrifuge, stimulant, tonic and antispasmodic. Ethno medically, C. pentandra bark decoction has been used as a diuretic, aphrodisiac, and to treat headache, as well as type II diabetes.10,11 The leaves, bark, shoots and roots of the plant have been used extensively by traditional medicine practitioners in Northern and Eastern Nigeria in the control and management of diabetes.12–13
There is scientific evidence to support the anti-diabetic effect of methylene chloride/methanol root extract of C. pentandra in normal and diabetic rats.14–16 The chronic hypoglycaemic activity of the stem bark aqueous extract of C. pentandra at high doses has been reported.17 A new isoflavone glycoside was isolated along with known isoflavones, vavain and vavain glucoside from the 80% ethanolic extract of the bark of C. pentandra.18
Hence the present study focused to evaluate the hypoglycemic and antihyperglycemic activity of ethanolic extract of C. pentandra bark at various doses in normal and streptozotocin (STZ) induced diabetic rats. Screening activity of the extract was carried out by type I antidiabetic models which includes Oral Glucose Tolerance Test (OGTT) and hypoglycemic effect on normal and streptozotocin induced diabetic rats.
Materials and Methods
Plant material and extract preparation
Bark of Ceiba pentandra (L.) Gaertn was collected in the month of June from the large tree grown in the Horticultural farm of Indian Institute of Horticulture Research (IIHR), Bangalore. It was identified and authenticated by Dr. T.N. Shivananda, Senior Scientist, IIHR, Bangalore, and Prof. Balakrishna Gowda, Head, Dept. of Botany, UAS, GKVK, Bangalore.
Shade-dried powdered bark (1000 g) of C. pentandra was extracted with alcohol (70% v/v) by soxhletting until there was no compound left over in solvent. The crude extract was evaporated to dryness at 60°C on a water bath (15.5% w/w).
Animals
Adult male Wistar rats weighing 175–200 g were used in the present study. Animals were obtained from Drugs Testing Laboratory, Palace Road, Bangalore. They were acclimatized to the laboratory conditions for 5 days before experimentation. The animals had free access to food and water and were housed under a natural light-dark cycle. The experiment was carried out with the approval of the Institutional Animal Ethics Committee (Proposal No. GCP/CPCSEA/2004-05).
Acute toxicity study
Acute toxicity study was conducted as per Centre for Drug Evaluation and Research (CDER), Guidance for Industry: Single Dose Acute Toxicity Testing for Pharmaceuticals.
To determine acute oral toxicity, different doses of the ethanolic bark extract (0.5, 1.0, 1.5, 2 g/kg) suspended in 0.5% sodium carboxy methylcellulose (CMC) were administered to different groups of rats (2 rats were used for each group, control rats received 0.5% sodium CMC). Mortality and general behaviour of the animals were observed periodically for 48 h. The animals were observed continuously for the initial 4 h and intermittently for the next 6 h and then again at 24 h and 48 h following drug administration. The parameters observed were grooming, hyperactivity, sedation, loss of righting reflex, respiratory rate and convulsion.
Induction of diabetes
Diabetes was induced by a single intraperitoneal injection of freshly prepared streptozotocin (STZ) (Sigma-Aldrich Co., St. Louis, MO, USA.) at the dose of 60 mg/kg in 0.01 M sodium citrate buffer (pH 4.5) to a group of overnight fasted rats.19 After 3 days of streptozotocin administration, depending on their blood glucose levels (BGLs) the severe diabetic animals were selected for the further study.
Experimental design
Initial screening of the ethanolic extract of C. pentandra bark for evaluating its glycemic potential was performed with two different doses (200 and 400 mg/kg) given orally by gavage in normal rats by conducting fasting blood glucose (FBG) test.
The OGTT and antidiabetic effect of the extract was also assessed in diabetic models with a dose of 200 mg/kg, which was identified as the most effective dose by initial screening.
Assessment of hypoglycemic activity in normal healthy rats (single dose study)
Overnight fasted male Wistar rats were divided into four groups of six animals each. Group I serving as control, received vehicle (0.5% w/v sodium CMC suspension). Group II & III received ethanolic extract orally in doses of 200 & 400 mg/kg b.w., respectively. Group IV served as reference standard and received glibenclamide (1 mg/kg). FBG was recorded initially and then blood samples (20 µL) were collected from the retroorbital sinus at 30, 60, 90 and 120 min after administering the extract. The blood glucose level (BGL) was measured by using Electronic Digital Glucometer (Ascensia Entrust Blood Glucose Meter, Bayer Diagnostics India Ltd).
Assessment of hypoglycemic activity by Oral Glucose Tolerance Test in normal and STZ induced diabetic rats
The oral glucose tolerance test20 was performed in overnight fasted normal and diabetic rats. 36 Animals were divided into six groups. Group I, II & III were orally administered 0.5% w/v sodium CMC suspension, ethanolic extract of C. pentandra (200 mg/kg) and glibenclamide (1 mg/kg), respectively. Diabetic groups IV, V & VI were orally administered 0.5% w/v sodium CMC suspension, ethanolic extract of C. pentandra (200 mg/kg) and glibenclamide (1 mg/kg), respectively. FBG test was conducted initially and then BGL was recorded after 30 min of treatment considered as 0 min value. A dose of 10 g/kg of glucose was then given orally to all the groups. BGLs were further recorded, up to two hours at regular interval of 30 min, considered as 30, 60, 90 and 120 min values.
Assessment of antidiabetic activity in STZ induced diabetic rats (single dose study)
Overnight fasted diabetic male Wistar rats were divided into three groups of six animals each. Group I served as control received vehicle (0.5% w/v sodium CMC suspension). Group II received ethanolic extract (200 mg/kg). Group III served as reference standard received glibenclamide (1 mg/kg). FBG test was conducted initially and then blood samples were collected from the retroorbital sinus at 30, 60, 90 and 120 min after oral administration of various treatments.
Assessment of antidiabetic activity in STZ induced diabetic rats (repeated dose study)
Long term study of 21 days was conducted in diabetic rats. Overnight fasted diabetic male Wistar rats were divided into four groups of six animals each. Group I serving as normal control received vehicle (0.5% w/v sodium CMC suspension). Group II served as diabetic control and received vehicle (0.5% w/v sodium CMC suspension).
Animals of Group III received ethanolic bark extract (200 mg/kg). Group IV serving as a positive control received a dose of 1 mg/kg of a known antidiabetic drug, glibenclamide. Blood samples were collected from the retro orbital sinus on day 1 and day 21. BGL was measured initially and after the treatment period. Serum insulin was estimated by the method of radioimmunoassay (RIA) using Medicorp RIA kit. Total cholesterol and triglycerides were estimated from the serum samples through Semi auto analyzer of Glaxo Qualigens Co., using commercially available direct estimation kits from Team Diagnostics Pvt. Ltd. The animals were sacrificed by decapitation and the glycogen content of the liver was estimated.21 The histopathological studies were carried out on organs like liver and pancreas preserved in 10% formalin immediately after dissection.
Statistical analysis
Results were expressed as mean ± SEM. The data was evaluated by one-way ANOVA followed by Bonferroni's multiple comparison tests using GraphPad Prism 4.0 (GraphPad Software, San Diego, CA, USA). Values of p<0.05 were considered statistically significant.
Results
Acute toxicity study
No toxic effect was observed on treatment up to 2000 mg/kg of the extract as the behaviour of the treated rats appeared normal and no death occurred.
Effect on fasting blood glucose level of normal healthy rats (single dose study)
The effect of different doses of ethanolic extract of C. pentandra on fasting blood sugar level of normal rats were assessed at different time intervals and depicted in Table 1. The BGL at different time intervals (30, 60, 90 & 120 min) were compared with initial (0 min) BGL of their respective groups. In vehicle control group there was no significant change in BGL during the 2 h study period. There was significant (p<0.001) decrease in BGL at different time interval in glibenclamide group. The extract treatment groups at doses 200 & 400 mg/kg showed significant (p<0.001) increase in BGL at 30 min when compared to initial (0 min) BGL but at 120 min BGL was slightly decreased.
Table 1.
Effect of C. pentandra extract on normal rats (single dose study)
| Groups | Treatment & Dose (per os) |
Fasting Blood sugar level (mg/dL) at various time intervals | ||||
| 0 min | 30 min | 60 min | 90 min | 120 min | ||
| I | Vehicle control | 60.67 ± 1.64 | 63.33 ± 1.25 | 65.83 ± 1.64 | 62.83 ± 1.35 | 29.33 ± 0.66 |
| II |
C. pentandra extract (200 mg/kg) |
63.00 ± 1.21 | 69.00 ± 0.77*** | 70.83 ± 0.70*** | 67.83 ± 0.54** | 65.00 ± 1.41 |
| III |
C. pentandra extract (400 mg/kg) |
64.17 ± 1.10 | 73.83 ± 0.75*** | 71.50 ± 0.71*** | 65.67 ± 0.76 | 62.50 ± 0.99 |
| IV | Glibenclamide (1mg/kg) | 62.33 ± 0.73 | 45.00 ± 0.73*** | 42.33 ±0.49*** | 43.33 ±0.21*** | 41.17 ±0.60*** |
Values are mean ± SEM, (n = 6), ***p<0.001, **p<0.01.
Statistically significant p<0.001, p<0.01 compared to 0 min of their respective group.
Effect on oral glucose tolerance of normal and STZ induced diabetic rats
Table 2 demonstrates the effect of ethanolic extract of C. pentandra on BGL of normal and STZ induced diabetic rats during OGTT studies.
Table 2.
Effect of C. pentandra on OGTT of normal healthy and STZ induced diabetic rats
| Groups | Treatment & Dose (p.o.) |
Fasting Blood sugar level (mg/dL) at various time intervals | ||||
| 0 min | 30 min | 60 min | 90 min | 120 min | ||
| I | Vehicle control | 82.00 ± 1.21 | 181.20 ± 0.60 | 175.50 ± 1.40 | 155.70 ± 1.40 | 129.3 ± 0.49 |
| II |
C. pentandra extract (200 mg/kg) |
84.00 ± 0.89 | 177.50 ± 1.23 | 165.30 ± 2.12** | 140.70 ±1.54*** | 119.00 ± 1.25*** |
| III | Glibenclamide (1 mg/kg) |
84.00 ± 0.77 | 141.2±1.19*** | 134.00 ±1.63*** | 128.20 ± 1.81*** | 97.33 ± 2.216*** |
| IV | Diabetic control | 171.70 ±12.40 | 472.80 ± 7.43 | 550.00 ± 0.00 | 550.00 ± 0.00 | 550.00 ± 0.00 |
| V | STZ + C. pentandra extract (200 mg/kg) |
153.20 ± 6.84 | 269.30 ± 7.88*** | 340.30 ± 9.38*** | 393.50 ± 4.22*** | 497.00 ± 3.62** |
| VI | STZ + Glibenclamide (1 mg/kg) |
169.20 ± 9.69 | 289.20 ± 7.51*** | 388.50 ± 10.50*** | 460.00 ± 9.76*** | 455.20 ± 10.82*** |
Values are mean ± SEM, (n = 6), ***p<0.001, **p<0.01.
Statistically significant p<0.001, p<0.01 compared to their respective control group
After 2h of glucose administration the significant (p<0.001) decrease in BGL was observed with the extract treatment (200 mg/kg) and glibenclamide group of normal rats when compared to control.
In STZ induced diabetic rats, the extract treatment and glibenclamide group showed significant decrease (p<0.01, p<0.001) in BGL respectively, when compared to diabetic control.
Effect on fasting blood glucose level of STZ induced diabetic rats (single dose study)
The effect of ethanolic extract of C. pentandra on fasting blood sugar level in STZ induced diabetic rats were assessed at different time intervals and depicted in Table 3. The BGL at different time intervals (30, 60, 90 & 120 min) were compared with initial (0 min) BGL of their respective groups. In diabetic control group there was no significant change in BGL during the entire study period. The oral administration of Glibenclamide to diabetic rats significantly (p<0.001) decreased the BGL at different time interval. The extract treatment group in STZ induced diabetic rats showed significant (p<0.01) decrease in BGL at 90 min when compared to initial (0 min) BGL.
Table 3.
Effect of C. pentandra extract on STZ induced diabetic rats (single dose study)
| Groups | Treatment & Dose (p.o.) |
Fasting Blood sugar level (mg/dL) at various time intervals | ||||
| 0 min | 30 min | 60 min | 90 min | 120 min | ||
| I | Diabetic control | 340.70±12.23 | 354.00 ±13.90 | 357.50 ± 14.06 | 349.70 ± 11.47 | 341.00 ± 11.22 |
| II | STZ + C. pentandra extract (200 mg/kg) |
348.50 ± 6.05 | 355.20 ± 5.91 | 325.70 ± 7.66* | 321.20 ± 3.12** | 327.30 ± 3.27* |
| III | STZ + Glibenclamide (1 mg/kg) |
342.30 ±1.52 | 329.20 ±2.38** |
315.80±3.02*** | 303.00±2.49*** | 295.00±2.67*** |
Values are mean ± SEM, (n = 6), ***p<0.001, **p<0.01, *p<0.05
Statistically significant p<0.001, p<0.01, p<0.05 compared to 0 min of their respective group.
Effect on blood glucose level and other parameters of STZ induced diabetic rats (repeated dose study)
Table 4 describes the effect of 21 days long treatment with ethanolic extract of C. pentandra on blood glucose level and other parameters of diabetic rats. BGL of diabetic control animals was significantly increased by 18.62% when compared to normal control animals.
Table 4.
Effect of C. pentandra extract on STZ induced diabetic rats after 21 days treatment
| Groups | Treatment & Dose (p.o.) |
Blood glucose level (mg/dL) |
Serum insulin level (pmol/L) |
Liver glycogen (mg/g liver) |
Total cholesterol (mg/dL) |
Triglycerides (mg/dL) |
| I | Normal control | 64.17 ± 1.759 | 244.20 ± 5.74 | 4.80 ± 0.19 | 148.70 ± 2.06 | 105.7 ± 3.16 |
| II | Diabetic control | 502.80 ±19.62*** a |
158.60 ± 19.86**a | 2.50 ± 0.13* a | 241.30±7.8*** a | 212.50 ± 5.26** a |
| III | STZ + C. pentandra extract (200 mg/kg) |
318.50 ± 8.7*** b | 248.80 ± 4.36** b | 3.98 ± 0.57 | 200.70±3.8*** b | 189.30 ± 3.38 |
| IV | STZ + Glibenclamide (1 mg/kg) |
357.80±28.5*** b | 179.80 ± 16.75 | 10.29 ± 0.66*** b | 195.10±4.16*** b | 181.40 ± 17.77 |
Values are mean ± SEM, (n = 6), ***p<0.001, **p<0.01, *p<0.05
statistically significant versus Normal control,
statistically significant versus Diabetic control.
The extract treatment (200 mg/kg) and glibenclamide group of diabetic rats showed significant decrease in BGL by 31.45% and 28.34%, respectively when compared to diabetic control. Serum insulin level of diabetic control group was significantly decreased by 36.26% when compared to normal control group.
The extract treatment and Glibenclamide group of diabetic rats increased the serum insulin level by 6.44% and 23.70%, respectively. Hepatic glycogen content was significantly reduced in diabetic control compared to normal control group. The extract treatment and glibenclamide group of diabetic rats increased the hepatic glycogen content (3.98 and 10.29 mg/g, respectively) when compared to diabetic control group (2.50 mg/g). The total cholesterol level of extract treated group was significantly decreased when compared to diabetic group whereas the decrease in triglycerides level was not significant.
Histopathological studies
Figures 1 and 2 depict the effect of ethanolic extract of C. pentandra bark on STZ-induced liver and pancreas damage in rats.
Figure 1.
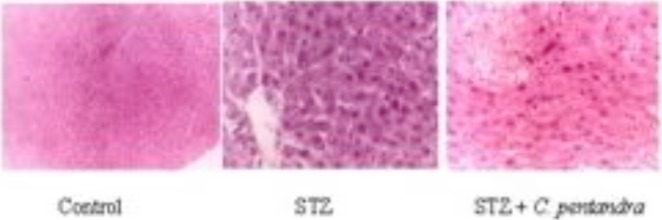
Effect of ethanolic bark extract of C. pentandra on STZ-induced liver damage in rats
Figure 2.
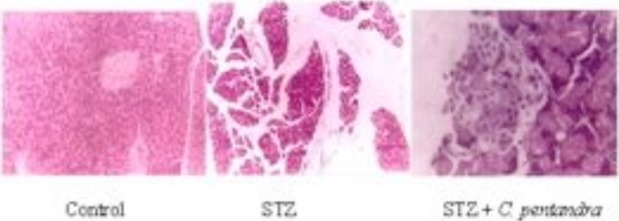
The effect of ethanolic bark extract of C. pentandra on STZ-induced pancreatic damage in rats
In the hepatocytes of diabetic group (Figure 1), excess vacuolization and granular appearance in the cytoplasm and periphery of the nucleus, picnotic nuclei, increased sinusoidal spaces, and damage to the central vein with moderate infiltration of inflammatory cells were observed.
The pancreas of diabetic group (Figure 2) showed reduction in size, scarcity of cells along with degeneration and necrosis, large decrease in endocrine tissue. Partly decrease of degenerative cells and the protective effect of tissues were noticed in the C. pentandra extract treated groups.
Discussion
Diabetes mellitus of long duration is associated with several complications such as atherosclerosis, myocardial infarction, neuropathy, nephropathy, etc.
These complications have long been assumed to be related to chronically elevated blood glucose levels. Diabetes mellitus causes disturbances in the uptake of glucose as well as glucose metabolism. STZ-induced hyperglycemia has been described as a useful experimental model to study the activity of hypoglycemic agents.22
It is known that treatment of rats with high-dose STZ is an established model for type 1 diabetes, high-dose of STZ severely impairs insulin secretion leading to hyperglycemia in rats.
Besides hyperglycemia, STZ-induced diabetic rats also shows an important lipolytic activity, due to the insulinopenic state which contributes to maintain the abnormally elevated plasma triglycerides and cholesterol levels.23 The purpose of the present study was to assess the effect of ethanolic bark extract of C. pentandra therapy on normal and STZ-induced diabetic rats.
The scientific literatures have shown that the root extracts of C. pentandra possessed hypoglycaemic effect and is capable of ameliorating hyperglycaemic in STZ-induced diabetic rats.14–16 An aqueous bark extract of C. pentandra also exhibited hypoglycaemic effect in STZ-induced diabetic rats.17 From the dry bark of C. pentandra, a new vavain glycoside was isolated in addition to known isoflavones, vavain and its glucoside showed no activity against α-glucosidase enzyme.18 Our results demonstrated that the oral administration of single dose of ethanolic extract of C. pentandra bark at two different doses (200 and 400 mg/kg) did not significantly affect the blood glucose levels in normal rats.
Treatment with bark extract (200 mg/kg) in STZ-induced diabetic rats significantly reduced BGL when compared to diabetic control. C. pentandra bark extract (200 mg/kg) also prevented the rise of blood glucose after oral administration of glucose. This could be due to an improvement of insulin response to glucose levels because the main cause of hyperglycaemia in STZ-induced diabetic rats appeared to be the lack of response of beta-cells to a glucose stimulus.
Thus the extract enhanced glucose utilization and improves glucose tolerance in glucose-loaded rats. The long term (21 days) study of ethanolic extract of C. pentandra (200 mg/kg) lowered the increased levels of blood glucose in STZ-induced diabetic rats. The anti-hyperglycemic effect of C. pentandra may result from the potentiation of insulin from existing □-cells of the islets of Langerhans. The blood glucose lowering effect was compared with Glibenclamide, a standard hypoglycemic drug. Glibenclamide has been used for many years to treat diabetes and stimulates insulin secretion from pancreatic □-cells.24
The serum insulin level was increased in diabetic rats administered Gymnema sylvestre suggesting the insulinotropic activity of Gymnema sylvestre leaf extract.25, 26 This result agrees well with those of the present study, when the diabetic animals treated with the ethanolic extract of C. pentandra, increased the serum insulin level compared with the diabetic controls. The decreased level of tissue glycogen content in diabetic rats is probably due to the lack of insulin in the diabetic state which results in the inactivation of the glycogen synthetase system.27 Administration of C. pentandra extract increased the liver glycogen content. Thus prevention of glycogen depletion in the liver might be possibly due to stimulation of insulin release from □-cells, which in turn reactivate the glycogen synthetase system. These results are in agreement with other studies which prevented the depletion in the liver glycogen content in alloxan-induced diabetic rabbits by Eugenia jambolana.28
High levels of total cholesterol and more importantly LDL cholesterol in the blood are major coronary risk factors.29 The abnormally high concentration of serum lipids in the diabetic condition is due to mainly the increase in the mobilization of free fatty acids from the peripheral fat depots. Insulin deficiency or insulin resistance may be responsible for dyslipidemia. Oral administration of C. pentandra bark extract lowered total cholesterol and triglycerides level in diabetic rats when compared to diabetic controls. Histopathological studies have shown that the diabetogenic action of STZ is due to specific cytotoxicity actions against hepatocytes and pancreatic □-cells. The long term (21 days) treatment of ethanolic bark extract of C. pentandra results in partly decrease of degenerative cells and the protective effect of liver and pancreas against STZ action in diabetic rats.
It is concluded that the long term (21 days) administration of ethanolic extract of C. pentandra bark was effective in decreasing the blood glucose level and normalizing the other biochemical parameters in diabetic rats. The single dose study of the extract has no hypoglycemic effect on normal rats. These evidences suggest that the bark of C. pentandra could be beneficial for the protection and alleviation of diabetic complications. Further studies need to be carried out to define the active principle(s) present in the ethanolic crude extract.
References
- 1.Seghrouchni I, Drai J, Bannier E, Riviere J, Calmard P, Garcia I, Orgiazzi J, Revol A. Oxidative stress parameters in type I, type II and insulin-treated type 2 diabetes mellitus; insulin treatment efficiency. Clin Chim Acta. 2002;321:89–96. doi: 10.1016/s0009-8981(02)00099-2. [DOI] [PubMed] [Google Scholar]
- 2.King H, Aubert RE, Herman WH. Global Burden of Diabetes 1995–2025: Prevalence, Numerical Estimates and Projections. Diabetes Care. 1998;21:1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 3.Boyle JP, Honeycutt AA, Narayan KM, Hoerger TJ, Geiss LS, Chen H, Thompson TJ. Projection of Diabetes Burden through 2050: Impact of Changing Demography and Disease Prevalence in the U.S. Diabetes Care. 2001;24:1936–1940. doi: 10.2337/diacare.24.11.1936. [DOI] [PubMed] [Google Scholar]
- 4.Rang HP, Dale MM. Pharmacology. 2nd edn. New York: Longman, Churchill Livingstone; 1991. The Endocrine System; pp. 504–508. [Google Scholar]
- 5.Warrier PK, Nambiar VPK, Ramankutty C. Indian Medicinal Plants: A. Compendium of 500 species. Madras: Orient Longman; 1995. [Google Scholar]
- 6.Chopra RN, Nayar SL, Chopra IC. Glossary of Indian Medicinal Plants. New Delhi: CSIR; 1958. [Google Scholar]
- 7.Grover JK, Yadav S, Vats V. Medicinal plants of India with anti-diabetic potential. J Ethnopharmacol. 2002;81:81–100. doi: 10.1016/s0378-8741(02)00059-4. [DOI] [PubMed] [Google Scholar]
- 8.Pickup J, Williams G. Textbook of Diabetes. 2nd edn. Oxford: Blackwell Scientific Publications; 1991. pp. 467–469. [Google Scholar]
- 9.Ivorra MD, Paya M, Villar A. A review of natural products and plants as potential antidiabetic drugs. J Ethnopharmacol. 1989;27:243–275. doi: 10.1016/0378-8741(89)90001-9. [DOI] [PubMed] [Google Scholar]
- 10.Gupta AK, Sharma Madhu. Reviews on Indian Medicinal Plants. New Delhi: ICMR; 2007. [Google Scholar]
- 11.Dunstan CA, Noreen Y, Serrano G, Cox PA, Perera P, Bohlin L. Evaluation of some Samoan and Peruvian medicinal plants by prostaglandin biosynthesis and rat ear oedema assays. J Ethnopharmacol. 1997;57:35–56. doi: 10.1016/s0378-8741(97)00043-3. [DOI] [PubMed] [Google Scholar]
- 12.Dalziel JM. The useful plants of West Tropical Africa. London, UK: Crown Agents for Overseas Governments and Administrations; 1955. p. 297. [Google Scholar]
- 13.Kafaru E. Nature's Workshop Publ. Nigeria: Elikat Health Services Ltd.; 1994. [Google Scholar]
- 14.Djomeni Dzeufiet PD, Tedong L, Asongalem EA, Dimo T, Sokeng SD, Kamtchouing P. Hypoglycaemic effect of methylene chloride/methanol root extract of Ceiba pentandra in normal and diabetic rats. Indian J Pharmacol. 2006;38:194–197. [Google Scholar]
- 15.Djomeni Dzeufiet PD, Ohandja DY, Tedong L, Asongalem EA, Dimo T, Sokeng SD, Kamtchouing P. Antidiabetic effect of Ceiba pentandra extract on streptozotocin-induced non-insulin-dependent diabetic (NIDDM) rats. Afr J Trad CAM. 2007;4:47–54. [PMC free article] [PubMed] [Google Scholar]
- 16.Djomeni Dzeufiet PD, Tedong L, Asongalem EA, Dimo T, Sokeng SD, Kamtchouing P. Hypoglycaemic and antidiabetic effect of root extracts of Ceiba pentandra in normal and diabetic rats. Afr J Trad CAM. 2006;3:129–136. [PMC free article] [PubMed] [Google Scholar]
- 17.Ladeji O, Omekarah I, Solomon M. Hypoglycemic properties of aqueous bark extract of Ceiba pentandra in streptozotocin induced diabetic rats. J Ethnopharmacol. 2003;84:139–142. doi: 10.1016/s0378-8741(02)00321-5. [DOI] [PubMed] [Google Scholar]
- 18.Ueda H, Kaneda N, Kawanishi K, Alves SM, Moriyasu M. A new isoflavone glycoside from Ceiba pentandra (L). Gaertner. Chem Pharm Bull. 2002;50:403–404. doi: 10.1248/cpb.50.403. [DOI] [PubMed] [Google Scholar]
- 19.Shanmugasundaram ER, Gopinath KL, Shanmugasundaram KR, Rajendaran VM. Possible regeneration of the islets of Langerhans in streptozotocin diabetic rats given Gymnema sylvestre leaf extracts. J Ethnopharmacol. 1990;30:265–279. doi: 10.1016/0378-8741(90)90106-4. [DOI] [PubMed] [Google Scholar]
- 20.Babu V, Gangadevi T, Subramoniam A. Antidiabetic activity of ethanol extract of Cassia kleinii leaf in streptozotocin-induced diabetic rats and isolation of an active fraction and toxicity evaluation of the extract. Indian J Pharmacol. 2003;35:290–296. [Google Scholar]
- 21.Caroll NV, Longley RW, Roe JH. The determination of glycogen in liver and muscle by use of an-throne reagent. J Biol Chem. 1956;220:583–593. [PubMed] [Google Scholar]
- 22.Junod A, Lambert AE, Stauffacher W, Renold AE. Diabetogenic action of streptozotocin: relationship of dose to metabolic response. J Clin Invest. 1969;48:2129–2139. doi: 10.1172/JCI106180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lemhadri A, Hajji L, Michel JB, Eddouks M. Cholesterol and triglycerides lowering activity of caraway fruits in normal and streptozotocin diabetic rats. J Ethnopharmacol. 2006;106:321–326. doi: 10.1016/j.jep.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 24.Tian YM, Johnson G, Ashcroft JH. Sulfonylureas enhance exocytosis from pancreatic □-cells by a mechanism that does not involve direct activation of protein kinase C. Diabetes. 1998;47:1722–1726. doi: 10.2337/diabetes.47.11.1722. [DOI] [PubMed] [Google Scholar]
- 25.Shanmugasundaram KR, Paneerselvam C, Samudram P, Shanmugasundaram ERB. The insulinotropic activity of Gymnema sylvestre R. Br. an Indian medicinal herb used in controlling diabetes mellitus. Pharmacol Res. 1981;13:475–486. doi: 10.1016/s0031-6989(81)80074-4. [DOI] [PubMed] [Google Scholar]
- 26.Shanmugasundaram KR, Paneerselvam C, Samudram P, Shanmugasundaram ERB. Enzyme changes and glucose utilization in diabetic rabbits, the effect of Gymnema sylvestre. J Ethnopharmacol. 1983;7:205–234. doi: 10.1016/0378-8741(83)90021-1. [DOI] [PubMed] [Google Scholar]
- 27.Perfumi M, Arnold N, Tacconi R. Hypoglycemic activity of Salvia fruticosa Mill from Cyprus. J Ethnopharmacol. 1991;34:135–140. doi: 10.1016/0378-8741(91)90030-h. [DOI] [PubMed] [Google Scholar]
- 28.Sharma SB, Nasir A, Prabhu KM, Murthy PS, Dev G. Hypoglycemic and hypolipidemic effect of ethanolic extract of seeds of Eugenia jambolana in alloxan induced diabetic rabbits. J Ethnopharmacol. 2003;85:201–206. doi: 10.1016/s0378-8741(02)00366-5. [DOI] [PubMed] [Google Scholar]
- 29.Hannan JMA, Rockeya B, Faruque O, Nahar N, Mosihuzzaman M, Khan AKA, Ali L. Effect of soluble dietary fibre fraction of Trigonella foenum graecum on glycemic, insulinemic, lipedimic and platelet aggregation status of type 2 diabetic model rats. J Ethnopharmacol. 2003;88:73–77. doi: 10.1016/s0378-8741(03)00190-9. [DOI] [PubMed] [Google Scholar]


