SUMMARY
Purpose
Temsirolimus was combined with cixutumumab, a fully human IgG1 monoclonal antibody directed at the insulin growth factor-1 receptor (IGF-1R).
Experimental Design
Patients received cixutumumab, 6 mg/kg IV weekly, and temsirolimus, 25 mg-37.5 mg IV weekly (4-week cycles), with restaging after 8 weeks. Median follow up was 8.9 months.
Results
Twenty patients (17 with Ewing’s sarcoma [EWS], 3 with desmoplastic small-round-cell tumor [DSCRT]) were enrolled). Twelve patients (60%) were men; median age, 24 years; median number of prior systemic therapies in metastatic setting, 6. The most frequent toxicities were thrombocytopenia (85%), mucositis (80%), hypercholesterolemia (75%), hypertriglyceridemia (70%), and hyperglycemia (65%) (mostly grade 1–2). Seven of 20 patients (35%) achieved stable disease (SD) >5 months or complete/partial (CR/PR) responses. Tumor regression of over 20% (23%, 23%, 27%, 100%, 100%) occurred in 5/17 (29%) patients with EWS, and they remained on study for 8 to 27 months. One of six EWS patients who previously developed resistance to a different IGF-1R inhibitor antibody achieved a CR. Four of the seven best responders developed grade 3 mucositis, myelosuppression, or hyperglycemia, which was controlled while maintaining drug dose.
Conclusion
Cixutumumab combined with temsirolimus was well tolerated and showed preliminary evidence of durable antitumor activity in heavily-pretreated EWS family tumors.
Keywords: Phase I clinical trials, IGF-1R pathway, mTOR pathway, Ewing’s sarcoma, Desmoplastic small-round-cell tumor
INTRODUCTION
Upstream tyrosine kinases such as insulin and the insulin growth factor-1 receptor (IGF-1R) can regulate the PI3K/AKT/mTOR pathway.(1) In vitro, in vivo, and tumor biopsy studies demonstrate that mammalian target of rapamycin (mTOR) inhibitors activate a feedback loop resulting in upregulated AKT phosphorylation in tumor tissue, which occurs via an IGF-1R-dependent mechanism. This feedback and can be abrogated, or at least limited, by IGF-1R pathway inhibition.(2–4) These observations provide a rational basis for combining mTOR and IGF-1R inhibitors as a way to overcome resistance to the agents when given as monotherapy. This assumption is supported by preclinical evidence in in vivo Ewing’s sarcoma (EWS) and rhabdomyosarcoma models treated with the combination of an mTOR inhibitor and IGF-1R inhibitor, which demonstrated enhanced antitumor activity compared to treatment with each agent alone.(5, 6) Unfortunately, effective treatment for relapsed sarcoma has remained largely elusive despite the fact that sarcomas are among the most common cancers of childhood and early adolescence.(7, 8) Ewing’s sarcoma most frequently affects children and adolescents, and is characterized by a translocation between the EWS protein and various fusion proteins, most commonly FLI1.(9) Desmoplastic small-round-cell tumor (DSRCT) is a rare and aggressive soft tissue sarcoma, which primarily presents with abdominal masses, and is considered by some to be part of the EWS family of tumors. Despite this controversy, patients with DSRCT generally respond in the same manner to EWS-based chemotherapy regimens as those with EWS. Some would argue that responses in DSCRT tend to be much less predictable and of much reduced duration compared with responses in EWS and the prognosis is worse. DSRCT is associated with a unique chromosomal translocation, t(11;22)(p13:q12). This translocation results in a EWS-WT1 fusion transcript, and codes for a protein that acts as a transcriptional activator, which is implicated in tumor growth.(10) When tested in the treatment of the EWS family of tumors, single-agent IGF-1R inhibitors and the mTOR inhibitor, temsirolimus, have produced variable outcomes.(11–13)
Here we report a total of 20 patients with EWS and DSCRT who were treated as part of an expansion cohort from our phase I study of the IGF-1R inhibitor, cixutumumab, and the mTOR inhibitor, temsirolimus.(14)
PATIENTS AND METHODS
Eligibility Criteria
Eligible patients had advanced or metastatic, histologically proven malignant EWS or DSRCT. Further requirements were age 14 years or older, ECOG performance status of 0 or 1, and life expectancy greater than 12 weeks. Patients were required to have an absolute neutrophil count ≥ 1500/mL, platelets ≥ 100,000/mL, creatinine ≤ two times (2X) the upper limit of normal (ULN), bilirubin ≤ 1.5 X ULN; AST(SGOT) and/or ALT(SGPT) ≤ 5X ULN. There was no limit to number of prior treatment regimens permitted, and patients could have been previously treated with an IGF-1R or an mTOR inhibitor. Treatment with radiotherapy (except palliative), endocrine therapy, or chemotherapy must have ceased at least four weeks before starting treatment. Patients with well-controlled diabetes and hyperlipidemia were allowed. Patient exclusions were treatment with concurrent strong CYP3A modifiers, major surgery within four weeks, significant comorbidities, brain metastases and pregnant or breastfeeding females.
Study Design
Patients were enrolled across two dose cohorts. Seventeen patients with EWS were enrolled in the first dose cohort of cixutumumab 6 mg/kg IV weekly and temsirolimus 25 mg IV weekly. Three patients with DSRCT were enrolled in the second dose cohort of cixutumumab 6 mg/kg IV weekly and temsirolimus 37.5 mg IV weekly because the previous dose level was well tolerated. Treatment cycles were four weeks with restaging after approximately eight weeks. This study was performed according to the principles embodied in the Declaration of Helsinki and after approval by the institutional review boards of both study centers (MD Anderson Cancer Center and Barbara Ann Karmonos Cancer Institute). Informed consent was obtained from all patients enrolled on the study.
Dose-Limiting Toxicity
Dose-limiting toxicity (DLT) was defined as possibly/probably/definitely drug-related grade 3 to grade 4 non-hematologic toxicity (excluding grade 3 nausea or grade 3 to 4 vomiting or diarrhea in patients who had not received optimal prophylactic antiemetic and antidiarrheal treatment), grade 3 to 4 thrombocytopenia lasting seven days, or thrombocytopenia associated with active bleeding or requiring platelet transfusion, grade 3 anemia, grade 4 neutropenia, and drug-related death.
Evaluation of Safety
Adverse events were recorded for patients who received at least one dose of cixutumumab or temsirolimus. All patients were followed for a month after stopping treatment. Severity was assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), version 3.0. Temperature, blood pressure, and pulse were measured before each infusion. Hematology, blood chemistry and urinalysis, and physical examinations were also monitored regularly.
Evaluation of Efficacy
Treatment efficacy was evaluated by computed tomography (CT) or magnetic resonance imaging (MRI) per Response Evaluation Criteria in Solid Tumors (RECIST 1.0)(15) before treatment and approximately every eight weeks thereafter. Briefly, a complete response (CR) was disappearance of all lesions, partial response (PR) was a ≥ 30% reduction in the sum of the longest diameters of the lesions, SD was denoted in patients whose sum of longest diameters was not decreased more than 30% and not increased more than 20%, and progressive disease (PD) was a ≥ 20% increase in the sum of the longest diameters of the lesions. A response had to last for at least four weeks to be considered a PR or CR. Patients with SD≥ 5 months were considered to have durable SD. Patients who did not attain a PR but had a 20% tumor regression by RECIST and who felt well were also reported as having clinical benefit from the study treatment.
RESULTS
Patient Characteristics
A total of twenty patients (17 patients with EWS and 3 patients with DSCRT) were enrolled. All pathologic diagnoses were confirmed at MD Anderson Cancer Center. Patients’ demographic and clinical characteristics at study entry are summarized in Table 1. Ten patients with EWS had EWS/FLI1 fusion protein. Two of three patients with DSCRT were positive for EWS/WT1 fusion protein. The other patients were either not tested or were negative. Six patients received earlier IGF-1R treatment, and two patients had been previously treated with temsirolimus. Most patients had been heavily pretreated, with the median number of prior therapies being 6 (range 1–11).
Table 1.
Patient Characteristics (N=20)
| Median age, yrs (range) | 24 (14–41) |
| Median # prior systemic therapies in the metastatic setting (range) | 6 (1–11) |
| Men/Women | 12/8 (60%/40%) |
| Prior IGF-1R inhibitor | N=6 |
| Prior mTOR inhibitor | N=2 |
| Diagnosis | |
| Ewing’s sarcoma | 17 (85%) |
| Desmoplastic small-round-cell tumors | 3 (15%) |
Toxicities
The current study represents an expansion of a previous phase I dose escalation study.(14) In the original study, 29 patients were treated with cixutumumab 6 mg/kg IV weekly and temsirolimus 25 mg IV weekly. One patient experienced a DLT of grade 3 mucositis at this dose level. Six patients were treated with cixutumumab 6 mg/kg IV weekly and temsirolimus 37.5 mg IV weekly. One patient experienced grade 4 thrombocytopenia and another patient had febrile neutropenia. As a result, cixutumumab 6 mg/kg IV weekly and temsirolimus 25 mg IV weekly was the recommended phase II dose for the combination.(14)
The twenty patients reported in this study with EWS and DSCRT (only three of whom were part of the previous dose escalation study) had the following toxicities, that were at least possibly drug-related at both dose levels, although most instances of them were grade 1 or 2 (Table 2): thrombocytopenia (85%), mucositis (80%), hypercholesterolemia (75%), hypertriglyceridemia (70%), and hyperglycemia (65%) (Table 2). Hyperglycemia was managed in collaboration with an endocrinologist and included the use of insulin together with metformin (n=1). One of 20 patients developed diabetes mellitus on study. No patient was diabetic at baseline. Mucositis was managed with xyloxylin (1:1:1 ratio of diphenhydramine, antacid, lidocaine; 10 mL swish/swallow every 6 hours as needed), Caphasol solution (sodium phosphate; 15 mL swish/spit every 4 hours as needed), valacyclovir (500 mg po TID), biotene mouth wash (every 4 hours as needed), and sucralfate, (1 gm/10 mL; 10 mL swish/swallow or spit QID as needed).
Table 2.
Treatment-Related Toxicities
| Dose Cixutumumab, Temsirolimus | 6 mg/kg, 25mg N=17 |
6 mg/kg, 37.5mg N=3 |
||
|---|---|---|---|---|
| NCI CTCAE Grade | 1–2 | 3–4 | 1–2 | 3–4 |
|
| ||||
| Endocrine | N/(%) | |||
|
| ||||
| Hypercholesterolemia | 12 (71%) | __ | 3 (100%) | __ |
| Hypertriglyceridemia | 10 (59%) | 2 (12%) | 1 (33%) | 1 (33%) |
| Hyperglycemia | 10 (59%) | 1 (6%) | 2 (67%) | __ |
|
| ||||
| Hematologic | ||||
|
| ||||
| Thrombocytopenia | 8 (47%) | 6 (35%) | 2 (67%) | 1 (33%)* |
| Neutropenia | 4 (24%) | 6 (35%) | 2 (67%) | 1 (33%)* |
| Anemia | 2 (12%) | __ | __ | __ |
|
| ||||
| Non-hematologic | ||||
|
| ||||
| Mucositis | 10 (59%) | 3 (18%) | 3 (100%) | __ |
| Fatigue | 9 (53%) | __ | 2 (67%) | __ |
| Rash/itching | 9 (53%) | __ | 2 (67%) | __ |
| Elevated AST/ALT | 8 (47%) | __ | __ | __ |
| Elevated Creatinine | 6 (35%) | __ | __ | __ |
| Diarrhea | 3 (18%) | __ | 2 (67%) | __ |
| Anorexia/Weight loss | 3 (18%) | __ | 1 (33%) | __ |
| Nausea/Vomiting | 2 (12%) | __ | 1 (33%) | __ |
febrile neutropenia;
NCI, National Cancer Institute; ALT= alanine aminotransferase; AST= aspartate aminotransferase
In one patient, temsirolimus was reduced to 15 mg IV weekly (from 25 mg IV weekly). And in two patients both drugs were reduced to 15 mg IV weekly of temsirolimus and 5 mg /kg IV weekly of cixutumumab. The reasons for dose reductions were mucositis, thrombocytopenia and neutropenia. These patients were re-escalated to either full dose (for cixutumumab and/or temsirolimus) or, in one case, to temsirolimus 20 mg IV weekly, without recurrence of clinically limiting toxicities.
Antitumor Activity
The best responses for the 20 study patients are shown in the waterfall plot in Figure 1. Median follow up was 8.9 months. Seven of 20 patients (35%) had SD >5 months or CR/PR. Median overall survival was 12.3 months (95%CI: 6.6 – 20+ months). One of 17 EWS patients (6%) who had previously developed resistance to a different IGF-1R inhibitor antibody achieved a CR, with time to treatment failure lasting 27 months. Tumor regression was seen in 5/17 (29%) EWS patients (23%, 23%, 27%, 100%, 100%,) and each of these patients has remained on study for 8 to 27 months. Two of three DSCRT patients had SD lasting longer than 5 months. (The EWS patient who had the best response of a 27% tumor regression remained on study for 15 months until financial/personal issues related to living in another country prevented him from continuing on study.)
Figure 1.
Best response by RECIST in 20 patients treated. Patients with early clinical progression or with new lesions or clinical progression are indicated on the graph as a 21% increase (*). The number on the graph indicates the number of months on treatment. Plus sign after the number of months indicates that patients continue on treatment. The dotted horizontal line represents 30% regression (PR).
Relationship Between Toxicity and Response
There were no statistically significant differences in the degree of toxicity between patients with response versus those who progressed. However, four of the seven patients with the best and most durable responses had grade 3 toxicities (hyperglycemia necessitating insulin and metformin initiation, mucositis and neutropenia/thrombocytopenia [albeit without infection or bleeding]). These patients were maintained on study, either without dose reduction or with transient dose reduction and re-escalation, and with sponsor and IRB notification. The patient who had a 27% regression lasting 15 months developed diabetes mellitus requiring insulin and metformin. He also had mucositis, which resolved with temsirolimus dose reduction and did not recur upon re-escalation. The patient with tumor regression of 23% lasting 18 months had a decrease in ANC to 0.8 103/mm3. Growth factor support was given and ANC is maintained above 1.0 103/mm3. One of the two patients who achieved a CR dropped his neutrophil count to 0.99 103/mm3. The other patient who attained a CR had platelet counts between 29 and 50 103/mm3 (grade 3) with absolute neutrophil counts (grade 3) that dropped as low as 0.79 103/mm3. No infection or bleeding was noted and, performance status remained at 0. Growth factor support maintained ANC above 1.0 103/mm3 and platelet counts remained stable despite ongoing treatment. These results suggest that, for responding patients, less stringent toxicity criteria and adequate supportive care should be applied to maintain the necessary dose levels to maintain an ongoing response.
DISCUSSION
Weekly administration of cixutumumab, a fully human IgG1 monoclonal antibody directed against the IGF-1R, combined with temsirolimus, an mTOR inhibitor, was well tolerated. The recommended phase II dose was established as cixutumumab 6 mg/kg IV weekly and temsirolimus 25 mg IV weekly, which is consistent with the results of the dose escalation portion of the phase I study.(14)
The most prevalent side effects were metabolic, hyperglycemia, hyperlipidemia and thrombocytopenia. Most patients felt well on the study agents and their performance status was generally stable or improved on drug, unless progressive disease intervened. Diabetic and hyperlipidemic patients were included in the study if they were well controlled prior to enrollment. We worked closely with an endocrinologist to observe and carefully treat these patients when they developed associated adverse events. Of the 20 study patients, one (5%) developed grade 3 hyperglycemia (diabetes mellitus), which was rapidly controlled with metformin and insulin, even though the patient stayed on the same dose of drug. Twelve patients had grade 1–2 hyperglycemia, which was watched with home glucose monitoring, without further worsening. Only three of 20 patients (15%) developed grade 3 hypercholesterolemia or hypertriglyceridemia. These sequelae were abrogated with appropriate treatment, including statins, fibrates and lifestyle changes. These observations suggest that the side effects of this regimen can be well controlled with medication and the assistance of an endocrinologist, when necessary.
Responses in EWS have been reported with other IGF-1R antagonists, such as AMG479 and R1507,(16–18) suggesting that a subset of patients with heavily pretreated EWS is particularly sensitive to IGF-1R antagonists. EWS/FLI can bind the IGFBP3 promoter and repress its activity, further supporting the role of IGF-1R signaling in this malignancy.(19) However, many patients with EWS are resistant to IGF-1R antagonists, suggesting that signaling pathways in addition to IGF-1R are activated in these tumors.(20)
The combination of an IGF-1R inhibitor, ganitumab (AMG 479), with the mTORC1 inhibitor, rapamycin, showed activity in EWS and osteogenic sarcoma models.(21) When figitumumab, another IGF-1R inhibitor, was combined with the mTOR inhibitor everolimus, the combination was well tolerated and one of 18 evaluable patients achieved a PR; one patient with EWS was treated and that patient had SD for 8 cycles.(22)
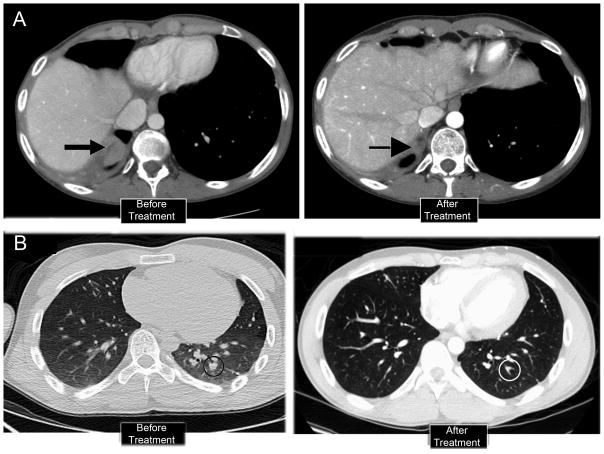
Median follow up in our study was 8.9 months. Best overall response rate (SD ≥5 months) was 35%. Median overall survival was 12.3 months (95%CI: 6.6 – 20+). In the present study, of the 17 EWS patients, two (12%) achieved a CR [Figure 2) and three (18%) had a best response of SD lasting 8, 15, and 18 months. Of interest, although initial reduction in tumor size was rapid in patients who attained CR, they remained in PR for 5 to 16 months before a CR was established.
Figure 2.
Two patients with CR showing response to treatment with cixutumumab and temsirolimus. CT scans of the chest are shown before and after treatment. Both patients had PET FDG avid disease at baseline and PET scans normalized with treatment. Patient in upper panel had had two prior open lung resection, creating some anatomical distortions.
Importantly, four patients with significant durable responses had ≥3 grade toxicities by CTCAE criteria, including neutropenia, thrombocytopenia, mucositis, and hyperglycemic requiring metformin and insulin, but these toxicities were not life threatening. The patients with neutropenia and thrombocytopenia did not experience infection or bleeding. Furthermore, maintaining the dose did not result in worsening of these effects, which could be managed with supportive care and, in the case of hyperglycemia, the help of an endocrinologist. All responding patients maintained a near 100% performance status. Thus, it appears that the presence of metabolic or myelosuppressive toxicity may reflect a targeted agent effect, and whether or not these side effects correlate with response merits further investigation. Future studies should therefore address this question and permit a greater range of such side effects in responding patients while on study.
Eight patients received prior treatment with an IGF-1R inhibitor (n=6) or mTOR inhibitor (n=2). The two patients who had previously not responded to treatment with an mTOR inhibitor did not respond to the combination of cixutumumab and temsirolimus. One patient who had previously received a different IGF-1R inhibitor (R1507) with response and then resistance achieved a CR on the current study.(16) In biopsy samples taken at the time of emergence of resistance to the IGF-1R inhibitor, this patient’s tumor demonstrated upregulation of mTOR pathway proteins, as determined by morphoproteomic analysis of the resistant tumor.(23) This patient was treated durably on our study within 45 days of developing resistance to R1507. These results suggest that, in selected patients with EWS family tumors, upregulation of the mTOR pathway serves as a resistance mechanism to IGF-1R inhibitors and demonstrates that treatment with combined IGF-1R and mTOR inhibitors can re-induce response.
Interestingly, a second patient with EWS had a PR with prior IGR-1R treatment and then had a mixed, remarkable regression in three lung modules after cixutumumab and temsirolimus were initiated, but had disease progression in a fourth lesion. Morphoproteomic analysis of this patient’s resistant tumor demonstrated that in addition to mTOR upregulation, ERK/MEK signals were increased.23 The latter finding suggests the possibility that a combination of IGFR/mTOR and MEK inhibitors might warrant investigation in order to reverse resistance.
In conclusion, this mechanism-based molecular approach shows preliminary evidence of activity in heavily pretreated patients with EWS family tumors. Further studies in larger numbers of patients with EWS and DSCRT as well as additional investigation into underlying resistance mechanisms in individual patients, are needed.
Translational Relevance.
This dose expansion phase I study explored further the activities of the drug combination reported in our previously published manuscript entitled, “Phase I trial of cixutumumab combined with temsirolimus in patients with advanced cancer” (Clin Cancer Res. 2011 Sep 15;17(18):6052–60. Epub 2011 Jul 12). We now report the outcomes of 20 patients with refractory Ewing’s sarcoma (EWS) family tumors after being treated with cixutumumab combined with temsirolimus. This molecular mechanism-based targeted therapy approach demonstrated the activity of the combination in some patients with advanced EWS. Our results further suggest that for responding patients, less stringent toxicity criteria and adequate supportive care should be applied to maintain the dose levels necessary to maintain response in this otherwise highly refractory disease.
Acknowledgments
This study was supported by R21CA13763301A1 (Aung Naing), U01CA62461 (Razelle Kurzrock), and U01CA62487 (Patricia LoRusso)
The authors acknowledge Joann Aaron, MA, for scientific review of and editing the paper.
Footnotes
Presented in part at the American Association for Cancer Research-National Cancer Institute-European Organization for Research and Treatment of Cancer Molecular Targets and Cancer Therapeutics, November 15–19 2009, Boston, MA; the National Cancer Institute/Cancer Therapy Evaluation Program Early Drug Development Meeting, October, 2009, Bethesda MD; the 46th Annual Meeting of the American Society of Clinical Oncology, June 4–8 2011, Chicago, IL, the 47th Annual Meeting of the American Society of Clinical Oncology, June 3–7 2011, Chicago, IL
References
- 1.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–44. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi Y, Yan H, Frost P, Gera J, Lichtenstein A. Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol Cancer Ther. 2005;4:1533–40. doi: 10.1158/1535-7163.MCT-05-0068. [DOI] [PubMed] [Google Scholar]
- 3.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–40. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 4.O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–8. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurmasheva RT, Dudkin L, Billups C, Debelenko LV, Morton CL, Houghton PJ. The insulin-like growth factor-1 receptor-targeting antibody, CP-751,871, suppresses tumor-derived VEGF and synergizes with rapamycin in models of childhood sarcoma. Cancer Res. 2009;69:7662–71. doi: 10.1158/0008-5472.CAN-09-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao L, Yu Y, Bilke S, Walker RL, Mayeenuddin LH, Azorsa DO, et al. Genome-wide identification of PAX3-FKHR binding sites in rhabdomyosarcoma reveals candidate target genes important for development and cancer. Cancer Res. 2010;70:6497–508. doi: 10.1158/0008-5472.CAN-10-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes DP. Novel agents in development for pediatric sarcomas. Curr Opin Oncol. 2009;21:332–7. doi: 10.1097/CCO.0b013e32832c94e2. [DOI] [PubMed] [Google Scholar]
- 8.Howlander N, Noone A, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review 1975–2008. National Cancer Institute; Bethesda, MD: based on November 2010 SEER data submission, posted to the SEER web site, 2011. Available from: http://seer.cancer.gov/csr/1975_2008/ [Google Scholar]
- 9.Balamuth NJ, Womer RB. Ewing’s sarcoma. Lancet Oncol. 2010;11:184–92. doi: 10.1016/S1470-2045(09)70286-4. [DOI] [PubMed] [Google Scholar]
- 10.Leuschner I, Radig K, Harms D. Desmoplastic small round cell tumor. Semin Diagn Pathol. 1996;13:204–12. [PubMed] [Google Scholar]
- 11.Olmos D, Tan DS, Jones RL, Judson IR. Biological rationale and current clinical experience with anti-insulin-like growth factor 1 receptor monoclonal antibodies in treating sarcoma: twenty years from the bench to the bedside. Cancer J. 2010;16:183–94. doi: 10.1097/PPO.0b013e3181dbebf9. [DOI] [PubMed] [Google Scholar]
- 12.Blay JY. Updating progress in sarcoma therapy with mTOR inhibitors. Ann Oncol. 2010;22:280–7. doi: 10.1093/annonc/mdq307. [DOI] [PubMed] [Google Scholar]
- 13.Schoffski P, Adkins D, Blay J, Gill T, Elias AD, Rutkowski P, et al. Phase II Trial of anti-IGF-1R antibody cixutumumab in patients with advanced or metastatic soft-tissue sarcoma and Ewing family of tumors. J Clin Oncol. 29(suppl):abst 10004. doi: 10.1016/j.ejca.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Naing A, Kurzrock R, Burger A, Gupta S, Lei X, Busaidy N, et al. Phase I trial of cixutumumab combined with temsirolimus in patients with advanced cancer. Clin Cancer Res. 2011;17:6052–60. doi: 10.1158/1078-0432.CCR-10-2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 16.Kurzrock R, Patnaik A, Aisner J, Warren T, Leong S, Benjamin R, et al. A phase I study of weekly R1507, a human monoclonal antibody insulin-like growth factor-I receptor antagonist, in patients with advanced solid tumors. Clin Cancer Res. 2010;16:2458–65. doi: 10.1158/1078-0432.CCR-09-3220. [DOI] [PubMed] [Google Scholar]
- 17.Olmos D, Postel-Vinay S, Molife LR, Okuno SH, Schuetze SM, Paccagnella ML, et al. Safety, pharmacokinetics, and preliminary activity of the anti-IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing’s sarcoma: a phase 1 expansion cohort study. Lancet Oncol. 2009;11:129–35. doi: 10.1016/S1470-2045(09)70354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tolcher AW, Sarantopoulos J, Patnaik A, Papadopoulos K, Lin CC, Rodon J, et al. Phase I, pharmacokinetic, and pharmacodynamic study of AMG 479, a fully human monoclonal antibody to insulin-like growth factor receptor 1. J Clin Oncol. 2009;27:5800–7. doi: 10.1200/JCO.2009.23.6745. [DOI] [PubMed] [Google Scholar]
- 19.Prieur A, Tirode F, Cohen P, Delattre O. EWS/FLI-1 silencing and gene profiling of Ewing cells reveal downstream oncogenic pathways and a crucial role for repression of insulin-like growth factor binding protein 3. Mol Cell Biol. 2004;24:7275–83. doi: 10.1128/MCB.24.16.7275-7283.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garofalo C, Manara MC, Nicoletti G, Marino MT, Lollini PL, Astolfi A, et al. Efficacy of and resistance to anti-IGF-1R therapies in Ewing’s sarcoma is dependent on insulin receptor signaling. Oncogene. 2011;30:2730–40. doi: 10.1038/onc.2010.640. [DOI] [PubMed] [Google Scholar]
- 21.Beltran PJ, Chung YA, Moody G, Mitchell P, Cajulis E, Vonderfecht S, et al. Efficacy of ganitumab (AMG 479), alone and in combination with rapamycin, in Ewing’s and osteogenic sarcoma models. J Pharmacol Exp Ther. 2011;337:644–54. doi: 10.1124/jpet.110.178400. [DOI] [PubMed] [Google Scholar]
- 22.Quek R, Wang Q, Morgan JA, Shapiro GI, Butrynski JE, Ramaiya N, et al. Combination mTOR and IGF-1R inhibition: phase I trial of everolimus and figitumumab in patients with advanced sarcomas and other solid tumors. Clin Cancer Res. 2010;17:871–9. doi: 10.1158/1078-0432.CCR-10-2621. [DOI] [PubMed] [Google Scholar]
- 23.Subbiah V, Naing A, Brown RE, Chen H, Doyle L, LoRusso P, et al. Targeted morphoproteomic profiling of Ewing’s sarcoma treated with insulin-like growth factor 1 receptor (IGF1R) inhibitors: response/resistance signatures. PLoS One. 2011;6:e18424. doi: 10.1371/journal.pone.0018424. [DOI] [PMC free article] [PubMed] [Google Scholar]