Abstract
BACKGROUND
Increased cost-sharing reduces utilization of prescription drugs, but there is little evidence about the exact mechanisms by which this reduction occurs or the factors associated with price-sensitivity.
METHODS
We conducted a retrospective cohort study of 272,474 elderly individuals with employer-provided drug coverage from 1997 to 2002 from 59 different health plans. We assessed the relationship between prescription drug cost-sharing and the time until the initiation of drug therapy after a new diagnosis of hypertension, hypercholesterolemia, or diabetes.
RESULTS
For all study conditions, higher copayments were associated with delayed initiation of therapy. In survival models, doubling copayments resulted in large reductions in the predicted proportion of patients initiating pharmacotherapy at one and five years after diagnosis (55.0 vs 40.1% at 1 year and 81.7% vs 66.3% at 5 years, p<0.000 for hypertension; 40.2% vs 31.1% at 1 year and 64.3% vs 53.8% at 5 years, p<0.002 for hypercholesterolemia; 45.8% vs 40.0% at 1 year and 69.3% vs 62.9% at 5 years, p<0.041 for diabetes). However, patients’ rate of initiation and sensitivity to copayments strongly depended upon their prior experience with prescription drugs. Those with a history of prior drug use initiated earlier and were less price-sensitive. These results were robust to a wide range of sensitivity analyses.
CONCLUSIONS
High cost-sharing delays the initiation of drug therapy for patients newly diagnosed with chronic disease. This effect is greater among patients who lack experience with prescription drugs. Policy makers and physicians should consider the effects of benefits design on patient behavior in order to encourage the adoption of necessary care.
INTRODUCTION
In the past decade, health plans have responded to rising prescription drug costs by implementing more restrictive insurance benefits, the hallmark of which has been increased cost-sharing (i.e., “copayments”), but has also included complex mechanisms such as sorting medications into “tiers” with varying copayments, mandatory generic substitution, and formularies.1 Several multi-year studies have demonstrated that these new arrangements reduce overall drug utilization and expenditures,2–4 and that the chronically ill are sensitive to out-of-pocket costs.5,6 However, detailed mechanisms by which these reductions occur have not been well investigated.
Medications are a crucial component of the therapeutic regimen for the chronically ill,7 and the interruption of drug therapy can have negative health consequences,8,9 particularly for the elderly, who have the highest rates of chronic disease and prescription drug use.12–15 Studies measuring the effect of pharmacy benefits designs on drug treatment for the chronically ill are inconsistent,3,5,6,16–21 but surveys of Medicare beneficiaries find cost to be the leading reason why patients don’t fill prescriptions, skip doses, or take smaller doses, followed by other causes, such as medication side-effects and beliefs about whether drugs improve health.22 Most empirical studies of cost-sharing have examined aggregate measures of utilization, such as total expenditures or days supplied, without explanations of how patients adjust their regimens. Although several studies suggest that sensitivity to cost-sharing depends upon a drug’s therapeutic class,5,21,23,24 and that increased cost-sharing may decrease “non-essential” drug use more than “essential” drug use,5,20,25–29 few studies have dissected the multiple mechanisms by which patients reduce their utilization in the face of higher cost sharing.
To fill this gap, this study examines whether cost-sharing affects the initiation of drug treatment for patients newly diagnosed with chronic disease. A sophisticated understanding of the effects of drug benefits is crucial for policy-makers, who, rather than applying blunt tools to control utilization, need to target those most at risk for the potentially harmful effects of utilization reductions.
METHODS
Data
We linked enrollment files, pharmacy claims, medical claims, and the salient features of health plan benefits for retirees of 15 large employers from 1997 to 2002. Each employer offered one or more health plans to its elderly retirees for a total of 23 health plans covering 399,034 retirees. All but two employers that offered multiple health plans provided a single drug benefit to their retirees, such that retirees had no choice of drug benefits. The content of the claims files have been described elsewhere.3,5 The Appendix details the construction of our longitudinal datasets, which included complete utilization data for our subjects over full calendar years. These datasets included thirty-one plan-year combinations, and these plans covered 272,474 unique persons, which translated to 688,620 person-year observations.
Study Sample
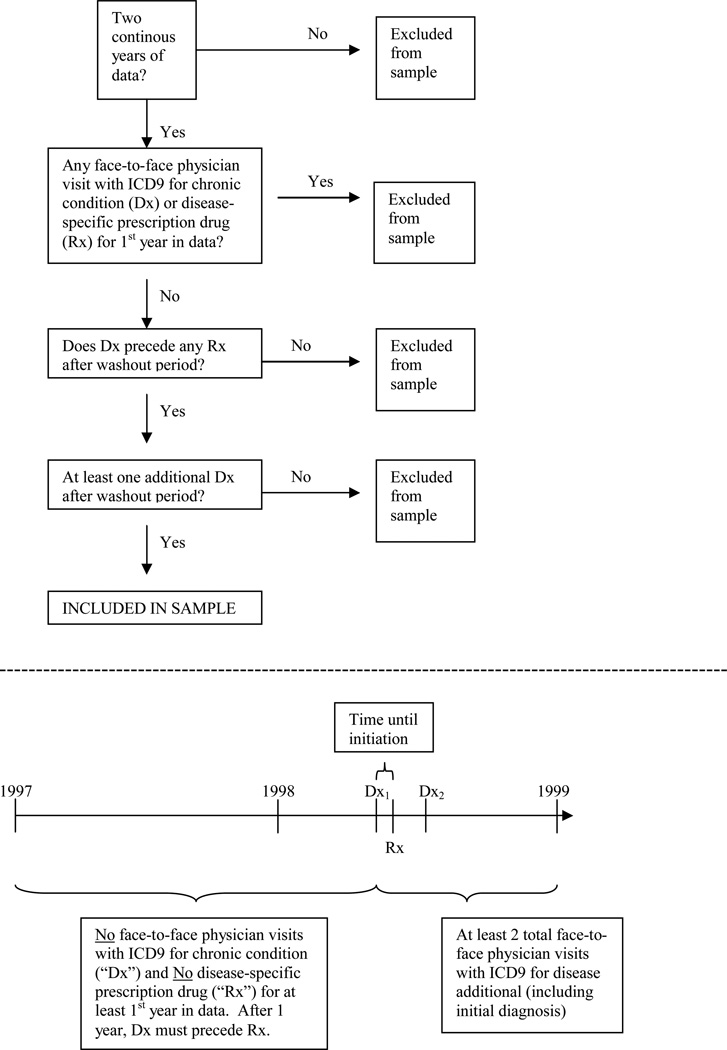
We created algorithms to identify patients with newly diagnosed hypertension, hypercholesterolemia, and diabetes (HTN, CHOL, and DIAB) using ICD-9-CM diagnosis codes (see Appendix) that were designed to ensure “rule-out” diagnoses were excluded from the sample. We required patients to be observed for at least their first year in the data without any outpatient or inpatient physician visits with an ICD-9-CM code for the chronic disease (hereafter, “diagnosis”) and without filling any disease-specific medications. Subsequent to this “washout” period, we required them to have the diagnosis of interest recorded during a physician visit on at least two occasions, the first of which must have occurred prior to or the same day as their first disease-specific medication. The first diagnosis was the “index date” on which the patient was considered newly diagnosed with the condition. (See Appendix for details.) Studies that examine the validity of using claims data to identify patients with chronic disease find that various factors affect the sensitivity and specificity of different claims-based algorithms, 30,31 and suggest our algorithm would yield specificity levels of 0.85 to 0.90.
Disease-specific medications were identified by matching National Drug Codes between the Redbook Database and the pharmaceutical claims. Manual verifications and edits were completed by the authors with clinical experience (JE, MS). HTN medications included angiotensin receptor blockers, angiotensin converting enzyme inhibitors, beta blockers, calcium channel, blockers, thiazide diuretics, osmotic diuretics, potassium sparing diuretics, carbonic anhydrase inhibitors, alpha-1 inhibitors, alpha-2 agonists, and vasodilators. DIAB medications included insulins, sulfonylureas, metformin, thiazolidinediones, and alpha glucosidase blockers. CHOL medications included statins, bile acid sequestrants, nicotinic acid, and fibric acid derivatives.
Outcome variable
The primary outcome measure was the time until initiation of prescription drug therapy, defined as the number of days between a patient’s first diagnosis and first disease-specific prescription. Because patients were observed in the data from two to six years and may have been diagnosed at any time after their first year in the data, the outcome is right-censored. It was possible that patients who did not initiate drug therapy during the observation window may have begun therapy after we ceased to observe them.
Explanatory variables
The main explanatory variable in our analysis was an index that measured the generosity of a plan’s prescription drug benefits. To capture the complexities of modern prescription drug plans, which base price upon not only its tier but also where it is dispensed, we developed a single index that summarized the average annual out-of-pocket (OOP) expense that members of a standard sample would have paid for their prescription drugs had they faced the cost-sharing requirements of each plan. This out-of-pocket index (OOP index) is similar to what would be calculated for the medical consumer price index, but it is specific to each plan. Details on the creation of the OOP index have been described elsewhere5 and are included in the Appendix. The OOP index ranked plans by their cost-sharing structure in a manner consistent with their absolute and relative co-payment levels. We also calculated separate out-of-pocket indices for disease-specific medications to measure the out-of-pocket burden for specific drug classes, but these indices were highly correlated with the overall OOP index and yielded the same results.
Covariates in the models included indicators for age categories; an indicator for sex; median household income in the ZIP code of residence; a categorical variable for urban residence; indicators for the year of the index date to control for secular time trends; selected outpatient medical benefits to include an exogenous measure of outpatient medical utilization; and indicators for 15 comorbid conditions as health status controls, identified by ICD-9-CM codes from physician visits in the year prior to a patient’s index date. Finally, we included an indicator variable for any prescription medication use in the year prior to the index date and, in some models, the interaction of this indicator and the OOP index, to assess whether prior use of prescription drugs affected time until initiation of drug therapy and price responsiveness.
Statistical Analysis
Because the data were structured in a time-to-event framework, we employed survival analysis techniques. For unadjusted analyses, we used Kaplan-Meier methods and log rank tests to compare survival functions. For adjusted analyses, we estimated six multivariate Cox proportional hazards models. For each of the three study conditions, we estimated a main effects and interacted model. The main effects models included the variables described above and the interacted models included an additional variable that interacted the OOP index with the indicator for patients who had any drug use in the year prior to the index date. To make the results easier to understand, we used our multivariate models to predict the effect of doubling copayments on the time to initiation of therapy for each study condition. For the predictions, we chose an OOP index value near the 25th percentile for the plans in our sample (OOP index = 205), which corresponded to a 1-tier $5-$5-$5 / $10-$10-$10 retail / mail-order copayment plan, to ensure that doubling copayments would yield an OOP index value that was within sample (OOP index = 410). Finally, because survival models require t > 0, the outcome variable was transformed by adding one day to all values.
RESULTS
Descriptive Results
Table 1 describes the characteristics of our three study samples, which included 7879, 6450 and 4486 patients with newly diagnosed HTN, CHOL, and DIAB, respectively. Little overlap existed between the three samples; together, the analyses included 15,613 unique patients (=6657 + 5277 + 3679). Within each sample, however, the study conditions were the most frequent comorbid conditions in each others’ sample, among preexisting conditions that contributed additional cardiovascular risk. Osteoarthritis and gastric acid disorder were the most frequent preexisting comorbid conditions not contributing additional cardiovascular risk. The mean length of the observation window after diagnosis was 877 days for HTN (S.D. 540 days), 930 days for CHOL (S.D. 544 days), and 799 days for DIAB (S.D. 533 days). Age was similar across samples; for all conditions, nearly half of patients were between the ages of 65 and 74 (46.3% for HTN, 54.1% for CHOL, 47.5% for DIAB). The samples included more females than males; as age increased, the proportion of men decreased. Most patients used at least 1 other medication in the year prior to the index date (73.9% for HTN, 89.6% for CHOL, 87.1% for DIAB).
Table 1.
Characteristics of Persons with Newly Diagnosed Chronic Disease, 1997–2002
| Characteristic | Hypertension | Hyperchol-esterolemia | Diabetes |
|---|---|---|---|
| Sample size, N | 7879 | 6450 | 4486 |
| Overlap between study samples, N | |||
| No. with a single newly diagnosed study condition | 6657 | 5277 | 3679 |
| No. with newly diagnosed hypertension & hypercholesterolemia | 763 | 763 | --- |
| No. with newly diagnosed hypertension & diabetes | 397 | --- | 397 |
| No. with newly diagnosed diabetes & hypercholesterolemia | --- | 348 | 348 |
| No. with new diagnosis of all three study conditions | 62 | 62 | 62 |
| Age, mean (SD), years | 75.8 (6.6) | 74.5 (5.8) | 75.5 (6.2) |
| Male gender, % | 38.9 | 40.6 | 48.8 |
| Median income in ZIP code, $ | 29145 | 29049 | 28943 |
| No. of unique medications in prior year, % | |||
| 0 | 26.1 | 10.4 | 12.9 |
| 1–3 | 26.1 | 21.6 | 14.1 |
| 4–6 | 21.1 | 24.7 | 19.0 |
| 7+ | 26.6 | 43.3 | 54.0 |
| Year of first diagnosis, % | |||
| 1998 | 23.5 | 25.3 | 19.8 |
| 1999 | 27.7 | 28.3 | 26.7 |
| 2000 | 18.0 | 17.3 | 17.4 |
| 2001 | 15.4 | 15.0 | 17.5 |
| 2002 | 15.4 | 14.1 | 18.6 |
| Plan Type, % | |||
| One-tier | 3.2 | 1.6 | 3.3 |
| Two-tier | 11.0 | 9.0 | 10.5 |
| Three-tier | 81.7 | 86.4 | 82.9 |
| Coinsurance | 4.0 | 2.9 | 3.2 |
| Comorbidities*, % | |||
| Conditions contributing cardiovascular risk | |||
| Hypertension | --- | 54.2 | 55.4 |
| Hypercholesterolemia | 20.3 | --- | 26.4 |
| Diabetes | 11.8 | 16.4 | --- |
| Congestive heart failure | 4.5 | 5.6 | 13.1 |
| Vascular disease | 3.5 | 4.1 | 5.7 |
| Coronary artery disease | 2.9 | 6.1 | 7.8 |
| Conditions not contributing cardiovascular risk | |||
| Osteoarthritis | 17.0 | 17.2 | 17.0 |
| Gastric acid disorder | 9.4 | 9.7 | 10.3 |
| Thyroid disorder | 8.2 | 9.5 | 7.5 |
| Depression | 7.1 | 5.3 | 7.1 |
| Glaucoma | 6.8 | 7.8 | 7.9 |
| Asthma/COPD | 5.3 | 5.6 | 8.7 |
| Allergic rhinitis | 4.7 | 5.5 | 4.8 |
| Chronic sinusitis | 2.2 | 2.8 | 2.8 |
| Inflammatory bowel disease | 2.0 | 1.8 | 1.8 |
| Ulcer | 1.2 | 1.1 | 1.9 |
Note: Comorbidities identified as >=1 physician visits with an ICD-9-CM code for the comorbid condition in the year prior to the new diagnosis of the study condition (i.e, the index date).
Overall, the mean OOP index value at the plan level was 305 (S.D. 118) with an interquartile range of 220 to 370. Three-tier plans were the most prevalent in the sample and included the largest share of each sample’s patients (Table 2). In addition, three-tier plans were, on average, the most generous plans, as measured by the OOP index. This was due to two factors. First, three-tier plans had lower copayments for generic drugs (vs. 1- and 2-tier plans) and preferred brand drugs (vs. 2-tier plans) at retail pharmacies, and lower copayments at mail-order pharmacies across all tiers (vs. 1- and 2-tier plans). Second, mail-order copayments were higher in 1- and 2-tier plans versus 3-tier plans. The 5 coinsurance plans were the least generous plans, and had flat coinsurance rates of 25% (N=2), or 2-tier rates of 20%−45% (N=1) or 35%−60% (N=2).
Table 2.
Mean Prescription Drug Benefits by Type of Rx Plan*
| Plan Type | ||||
|---|---|---|---|---|
| 1-tier (N=5) |
2-tier (N=7) |
3-tier (N=14) |
Coinsurance (N=5) |
|
| Average copayment, $ (SD) | ||||
| Retail | ||||
| Generics | 6.6 (0.9) | 5.3 (0.8) | 4.5 (0.2) | 28% (6.7) |
| Preferred brands | 6.6 (0.9) | 12.7 (1.8) | 9.5 (1.6) | 43% (17.5) |
| Nonpreferred brands | 6.6 (0.9) | 12.7 (1.8) | 13.6 (2.9) | 43% (17.5) |
| Mail order | ||||
| Generics | 14 (2.2) | 7.7 (3.5) | 3.6 (3.3) | 28% (6.7) |
| Preferred brands | 14 (2.2) | 17.1 (6.9) | 8.7 (5.8) | 43% (17.5) |
| Nonpreferred brands | 14 (2.2) | 17.1 (6.9) | 13.5 (6.5) | 43% (17.5) |
| Percent of Rx Mail order, % (SD) | 5 (0.3) | 39 (0.1) | 36 (0.1) | 51 (0.1) |
| OOP index, $ (SD) | 259 (30) | 323 (71) | 238 (67) | 516 (78) |
All copayment and coinsurance means are computed at the plan-level. The HTN, CHOL, DIAB samples included 317 (4.0%), 186 (2.9%), and 147 (3.2%) patients in coinsurance plans; 253 (3.2%), 103 (1.6%), and 149 (3.3%) patients in one-tier plans, 870 (11.0%), 584 (9.0%), and 471 (10.5%) patients in two-tier plans, and 6439 (81.7%), 5577 (86.4%), and 3719 (82.9%) patients in three-tier copayment plans. Mail-order copayments are generally higher than retail because they cover up to a 90-day supply, whereas retail covers a 30-day supply. Several three-tier plans in our sample offered mail-order copayments that were equal to or less than their retail equivalent.
Table 3 lists examples of pharmacy benefits and OOP index values for plans in the lower, median, and upper percentiles of the OOP index our sample. Several formulations of plans, including 1-tier, 2-tier and 3-tier plans, yielded OOP index values near those used for our predictions (OOP index=205 and 410). As noted, the OOP index depended upon the magnitude of both retail pharmacy and mail-order cost-sharing arrangements.
Table 3.
Mapping the Out-of-Pocket Index and Pharmacy Benefits Design
| Out-of-pocket IndexValue |
Plan Type | Retail Copayments |
Mail-Order Copayments |
Coinsurance* |
|---|---|---|---|---|
| 15th-25th Percentile | ||||
| 179 | 2-tier | $5-$10 | $1-$5 | --- |
| 207 | 1-tier | $5 | $10 | --- |
| 220 | 3-tier | $4.50-$9-$12 | $4.50-$9-$12 | --- |
| 50th Percentile | ||||
| 272 | 1-tier | $7 | $15 | --- |
| 75th-85th Percentile | ||||
| 370 | 2-tier | $5-$12 | $10-$24 | --- |
| 393 | 2-tier | --- | --- | 20%–45% |
| 426 | 3-tier | $5-$15-$20 | $10-$20-$30 | --- |
Coinsurance plans in our sample did not differ in their retail and mail-order cost-sharing levels.
Figure 1 displays the Kaplan-Meier survival estimates for the number of days until a patient’s first prescription for their newly diagnosed HTN, CHOL, or DIAB. The figure separates survival functions for patients in plans above and below the median OOP index value. For all conditions, the rate of initiation was high in the first several months after diagnosis; subsequently, the rate of initiation slowed. Log-rank tests showed that, for each condition, survival functions for patients in high- and low-OOP index groups were significantly different (p<0.000 HTN, p<0.000 CHOL, p<0.036 DIAB). Thus, in the unadjusted data, higher cost-sharing was associated with delayed initiation of drug therapy. At five years after diagnosis, the percentage of patients remaining untreated with medications in our sample was 21.4% [19.8%−23.1%] for HTN, 36.0% [34.3%−37.8%] for CHOL, and 32.5% [30.1%–34.9%] for DIAB.
Figure 1. Unadjusted Kaplan-Meier Estimates of Time Until First Medication for Patients with Newly Diagnosed Chronic Disease, Above and Below Median Copay Levels 1997–2002*.
*Note: “Below median” includes patients in plans below the median OOP index value; “Above median” includes patients in plans above the median OOP index value.
Multivariate Results
(Note: Figure 1.5 presents the regression results from our six models for reviewers – not to be included in final proofs). After adjusting for covariates, doubling copayments resulted in large, statistically significant differences in predicted time until initiation for all study conditions (Figure 2). The predicted percentage of newly diagnosed patients initiating pharmacotherapy at one and five years after diagnosis was largest for patients with newly diagnosed HTN or CHOL (55.0 vs 40.1% at one year and 81.7% vs 66.3% at five years, p<0.000 for HTN; 40.2% vs 31.1% at one year and 64.3% vs 53.8% at five years, p<0.002 for CHOL; 45.8% vs 40.0% at one year and 69.3% vs 62.9% at five years, p<0.041 for DIAB). The difference in the median number of days until pharmacotherapy that resulted from doubling copayments was substantial for all study conditions (242 vs 774 days for HTN; 766 vs 1382 days for CHOL; 527 vs 813 days for DIAB).
Table 1.5.
Regression Results from Six Models of Time to Initiation of Drug Therapy*
| Hypertension |
Hypercholesterolemia |
Diabetes |
||||
|---|---|---|---|---|---|---|
| Main Effects | Interactions | Main Effects | Interactions | Main Effects | Interactions | |
| Coeff. | Coeff. | Coeff. | Coeff. | Coeff. | Coeff. | |
| Potential predictors | ||||||
| Average copay index | −.002175** | −.005085** | −.001404** | −.004226** | −.000891* | −.004746** |
| [.0003902] | [.0009459] | [.0004607] | [.001378] | [.000437] | [.001449] | |
| History of prior Rx use | .6823** | −0.2756 | .916** | 0.1587 | .7396** | −0.3594 |
| [.08408] | [.193] | [.1353] | [.3672] | [.1091] | [.2896] | |
| Interaction of Rx use & copay | --- | .004237** | --- | .003299* | --- | .004673** |
| --- | [.0009328] | --- | [.001392] | --- | [.001417] | |
| Demographics | ||||||
| Age | ||||||
| 65–70 | .186** | .1885** | .2787** | .2791** | .3721** | .3781** |
| [.02916] | [.02802] | [.0645] | [.06445] | [.06606] | [.06346] | |
| 71–75 | .1509** | .1485** | .2286** | .2332** | .2864** | .2957** |
| [.02186] | [.02251] | [.0565] | [.05547] | [.06287] | [.06268] | |
| 76–80 | .08289* | .08917** | .1713** | .1739** | .1476* | .1524** |
| [.0342] | [.03329] | [.04564] | [.04538] | [.05862] | [.05775] | |
| 81+ | --- | --- | --- | --- | --- | --- |
| --- | --- | --- | --- | --- | --- | |
| Male gender | −.05749* | −.05629** | .06821** | .06965** | 0.05673 | 0.05601 |
| [.02262] | [.01939] | [.02072] | [.021] | [.03862] | [.03844] | |
| Urban residence | −0.01646 | −0.02605 | 0.1817 | 0.1729 | 0.1907 | 0.1641 |
| [.1288] | [.1314] | [.1182] | [.1208] | [.2938] | [.2965] | |
| Median HH income | 0.000003848 | 0.000003695 | 0.000001751 | 0.000002576 | 0.000004427 | 0.00000559 |
| [2.781e-06] | [2.573e-06] | [2.659e-06] | [2.540e-06] | [2.994e-06] | [3.031e-06] | |
Note: Standard errors in brackets;
significant at 5% level;
significant at 1% level.
Figure 2. Effect of Doubling Copayments on the Initiation of Drug Therapy for Patients with Newly Diagnosed Chronic Disease*.
*Note: An OOP index level of 205 roughly corresponded to a 1-tier $5-$5-$5 / $10-$10-$10 retail / mail-order copayment plan (actual OOP index value = 206.7), and an OOP index value of 410 roughly corresponded to a 3-tier $5-$15-$20 / $10-$20-$30 retail / mail-order copayment plan (actual OOP index value = 425.7). Both values were well within the range of OOP index values observed in the sample.
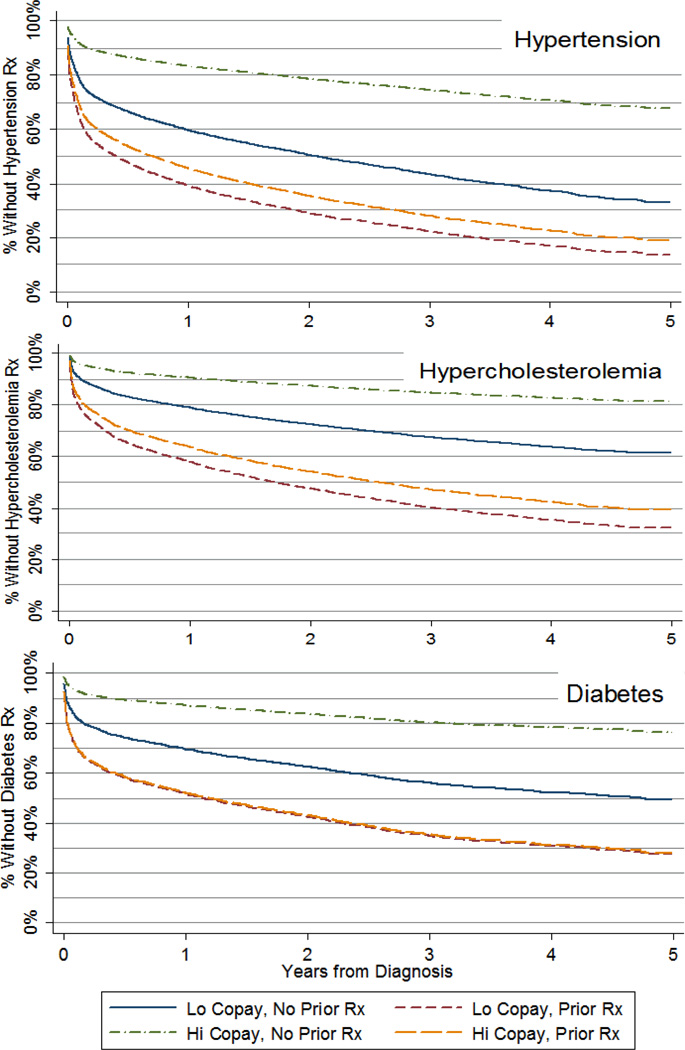
Figure 3 demonstrates that the rate of initiation of drug therapy and the effect of doubling copayments depended upon a patient’s prior history of prescription drug use. Compared to patients with no drug use in the year prior to the index date, patients with any drug use in that period initiated pharmacotherapy earlier and were much less price-sensitive. For example, holding cost-sharing levels constant at the lower of our two predicted levels (OOP index = 205), the percent of patients initiating drug therapy by one year after diagnosis was much larger among patients with a history of prior drug use for all study conditions (60.7% vs. 40.3% for HTN, p< 0.000; 41.9% vs. 21.0% for CHOL, p< 0.000; 48.4% vs. 30.4% for DIAB, p< 0.000).
Figure 3. Effect of Doubling Copayments on the Initiation of Drug Therapy for Patients with Newly Diagnosed Chronic Disease With and Without Prior Drug Use*.
*Note: An OOP index level of 205 roughly corresponded to a 1-tier $5-$5-$5 / $10-$10-$10 retail / mail-order copayment plan (actual OOP index value = 206.7), and an OOP index value of 410 roughly corresponded to a 3-tier $5-$15-$20 / $10-$20-$30 retail / mail-order copayment plan (actual OOP index value = 425.7). Both values were well within the range of OOP index values observed in the sample.
Doubling copayments among patients with a history of prior drug use resulted in differences in the rate of initiation that were small in magnitude and statistically significant only for patients with newly diagnosed HTN or CHOL (60.7% vs. 54.4% at one year for HTN, p< 0.021; 42.0% vs. 36.3% at one year for CHOL, p< 0.027; 48.5% vs. 48.0% at one year for DIAB, p< 0.853). By contrast, among patients without a prior history of drug use, the effect of doubling copayments resulted in large, statistically significant differences in the survival function for all study conditions (40.3% vs. 16.6% at one year for HTN, p< 0.000; 21.1% vs. 9.5% at one year for CHOL, p< 0.002; 30.5% vs. 12.9% at one year for DIAB, p< 0.001).
Sensitivity Analyses
We conducted multiple sensitivity analyses to assess the robustness of our results. First, to ensure that “rule-out” diagnoses were not affecting our findings, we tested the model using restrictive inclusion criteria designed to produce samples with more homogenous and severe disease, including samples that required patients to have at least three outpatient physician visits for the disease condition after diagnosis, and requiring the 2nd and 3rd visits be at least 30 days apart. Second, we examined whether the type of drug used prior to initial diagnosis changed the observed effect of prior drug use and its interaction with cost-sharing. Specifically, we separated the effect of prior medications used to treat conditions that contributed additional cardiovascular risk (defined as drugs for HTN, CHOL, DIAB, CAD, CHF, and vascular disease) versus other medications. Third, we examined patients who used a small supply of medications in the prior year – as little as thirty days worth of medications – as well as patients who took only antibiotics in the prior year. Fourth, we estimated models that included controls for physician visits. Fifth, we examined alternative definitions for comorbid conditions. Finally, we excluded the oldest-old (age>80 yrs) and excluded plans requiring coinsurance, the least generous plans in our sample. None of these sensitivity analyses appreciably changed our findings.
DISCUSSION
Previous work has established that the chronically ill are sensitive to the cost of prescription drugs. Our study looked at one component of utilization: the initiation of drug therapy after diagnosis. We found that increased cost-sharing delays the initiation of medications to treat newly diagnosed chronic disease, suggesting that out-of-pocket costs may prevent patients from initiating medically necessary care.
In addition, we found that the initiation of drug therapy and sensitivity to prices depends on a patient's "experience" with prescription drug use. Relative to those without experience, patients with experience using prescription drugs were less price-sensitive and adopted therapy earlier, suggesting that patients differ in their willingness to initiate prescription drug therapy. In some patients, an initial resistance against treatment may be reduced once experience using prescription drugs is established. We found no threshold effect for the number of prior or concurrent medications at which the results of our models changed. Thus, our data suggest that out-of-pocket costs may prevent patients from initiating treatment – which could negatively impact health outcomes – but the magnitude of this effect strongly depends whether patients have experience used drugs in the past.
Our survival estimates were consistent with epidemiological studies from NHANES and other sources that estimate the proportion of patients who are aware they have a medical condition but remain untreated.32–54 In our study, the proportion of newly diagnosed patients who had not initiated anti-hypertensive, anti-cholesterol, or anti-diabetes drug therapy by five years was 21.4%, 36.0%, and 32.5%, respectively. Consistent with our data, a variety of studies indicate that the proportion of patients aware of their hypertension but without drug treatment ranges from 8% – 68%.32–39,53 In the Framingham Heart Study, 68.3% of patients with newly diagnosed hypertension had not initiated antihypertensive therapy by four years, including 53.9% of those with Stage II hypertension at baseline,53 and recent estimates range from 8% in a VA population34 to 38–55% in a community population.35
Untreated hypercholesterolemia among those aware of their condition is a well documented and chronic problem. Our estimate of the proportion diagnosed but untreated by five years is at the lower end of most population-based estimates, which range from 25% to 66%.32,36,41,42,46,51
Among diagnosed diabetics, estimates of the proportion without drug treatment range from 8% to 47%.45,50,56 Recent analyses of NHANES yield estimates ranging from 19%–28.6%,32,36,47–49 and even 23.2% of diabetics who survived a myocardial infraction or stroke, a group likely to be hyper-vigilant about controlling cardiovascular risk factors, did not use antidiabetic medications.47 Although our estimate of the proportion of new diabetics who remained untreated after five years was slightly higher than NHANES estimates, NHANES subjects carried their diagnosis for two to three times longer than our five-year follow up period,32,57 and the proportion of untreated diabetics increases with age.45,55
There are several limitations to our study. First, our sample may not be generalizable to a younger population. However, Medicare Part D has increased the proportion of elderly retirees who have prescription drug insurance, and CMS has control over the basics of benefits design. Thus, our results may be particularly relevant for federal policy-makers setting standards for Medicare Part D insurance packages. Second, we could not completely control for selection of drug benefits. However, in all but two employers in the sample, employees had no choice of drug benefits, minimizing the possibility that employees selected plans suited to their anticipated needs, and patients in these two employers accounted for less than 2.5% of the sample. Excluding these patients did not change our results. Third, despite controls for comorbidities, disease severity may differ between patients with and without prior drug experience. Although administrative data do contain detailed clinical information contained in medical charts, our sensitivity analyses examining inclusion criteria designed to produce samples with more homogenous and severe disease did not change our findings. One initial treatment option for patients newly diagnosed with less severe disease is to initiate non-pharmaceutical therapy, such as diet modification and exercise. However, there was no a priori reason that disease severity was correlated with benefits generosity, since almost no patients in our study had a choice of drug benefits plans. Further, analysis of the NHANES has shown that patients diagnosed with hypertension without pharmacologic treatment have, for example, disease severe enough to warrant treatment (SBP>140).52
Despite these limitations, our results suggest a novel distinction between groups of patients, some of whom are price-sensitive to prescription drugs and others who are not. Although the majority of patients in our sample did have experience using prescription drugs, the large impact of cost-sharing on those without experience make this population a prime target for interventions to encourage appropriate treatment of chronic disease, particularly diseases that contribute to cardiovascular risk, such as those included in our study. Future research should explore the mechanisms underlying our results, such as the factors that may influence the effect of cost-sharing within specific patient populations, and should examine the health outcomes of varying times to initiation of drug therapy for chronic disease.
Our findings have implications for policy makers designing insurance benefits and for physicians treating patients with chronic disease. First, these results raise concerns about high cost-sharing levels for elderly, insured patients without experience using prescription drugs. Based on our findings, high cost-sharing levels could be a barrier to treatment for this population, and possibly result in poor health outcomes. Physicians should also heed these findings when treating patients with a new diagnosis of hypertension, hypercholesterolemia, or diabetes; those who do have experience with pharmacologic therapy may be much less likely to initiate prescribed treatments and may be very sensitive to cost-sharing levels.
More broadly, these results add to the growing chorus that our reliance on blunt instruments to influence prescription drug utilization, such as formularies and tiered copayments, which are primarily used to manage cost, need to be updated by more sophisticated tools that take into account therapeutic need as well as patients' complex response to insurance benefits.58,59 For example, recent evidence indicates that among people who have initiated medications for chronic disease, patients are less likely to adhere to their regimen if they begin with high copayments when compared to patients that begin with lower copayments that gradually increase.60 This suggests that new users are likely to be more price sensitive than continuing users, and is congruent with our finding that patients with prescription drug experience are less price-sensitive. Lessons such as these need to be incorporated into benefits design to ensure that patients who require medical therapy are not discouraged from initiating treatment.
ACKNOWLEDGMENT
This research was supported by the Agency for Healthcare Research and Quality (R03 HS013869-01) with additional funding from the California HealthCare Foundation. Data were provided by Ingenix, Inc. Dr. Goldman reports honoraria and consulting income from Amgen, Genentech, and UnitedHealth
ROLE OF THE SPONSOR
RAND is solely responsible for the manuscript's content. Neither the Agency for Healthcare Research and Quality, nor California HealthCare Foundation, had any authority over the design and conduct of the study; the collection, analysis, preparation, and interpretation of the data; and preparation of the manuscript.
Appendix 1: ICD-9-CM Codes Used to Identify Chronic Conditions
| Disease | ICD-9-CM |
|---|---|
| Essential hypertension | 401 |
| Hypercholesterolemia | 272.0, 272.1, 272.2, 272.4 |
| Diabetes | 250, excluding [250.1, 250.x1, 250.x3] |
| Congestive heart failure | 428 |
| Vascular disease | 440, 443 |
| Coronary artery disease | 410, 412, 413 |
| Osteoarthritis | 715 |
| Gastric acid disorder | 530.1, 530.2, 530.81, 534, 535.5, 535.6, |
| Mental health disorders | 293, 294, 295, 296.2, 300.0, 311 |
| Thyroid disorder | 240, 241, 242, 243, 244, 245, 246 |
| Asthma/COPD | 491, 492, 493 |
| Glaucoma | 365 |
| Allergic Rhinitis | 477 |
| Inflammatory bowel disease | 555, 556, 564.1 |
| Chronic sinusitis | 473 |
| Ulcer | 531, 532, 533 |
Appendix 2: Algorithm for Study Sample Eligibility
Appendix 4: Calculation of Out-of-Pocket Index to Measure Plan Generosity
To capture plan generosity with a single variable, we developed an index that simulated the average annual out-of-pocket expenses that members of a standard sample would have paid had they faced the cost-sharing requirements and restrictions of each plan. To create the standard sample, one hundred people from each plan-year were randomly sampled and their drug claims pooled, creating a “market basket” of drugs. Each drug claim in the market basket was standardized into 30-day supply equivalents. Thus, a 90-day mail-order claim was converted to three 30-day claims. A plan’s average out-of-pocket cost for a 30-day supply of each drug was calculated and assigned to each corresponding 30-day drug claim in the market basket. Total costs were calculated for each individual (person-year), and the average total cost across individuals computed as the plan’s OOP index value.
Formally, if we define D as the universe of drug claims in the market basket, let d be a specific 30-day equivalent drug claim in the market basket, let p be a plan in a given year, let N be the number of persons in the standard sample, and let denote the average out-of-pocket payment for drug d in plan-year p, the value of the OOP index for each plan-year can be written as:
Appendix 5: Additional information on eligibility criteria
We established several rules for including plans and beneficiaries in the study. Plans whose average pharmaceutical and medical utilization were significantly lower than the entire sample’s plan-level averages were excluded to ensure that we had complete utilization data for our subjects. We also excluded plans with enrollment of less than 1,000 members, to ensure ample variation in individual characteristics within plans. We examined only primary beneficiaries, and excluded dependents, to maximize the likelihood that our plans would be the main source of prescription drug insurance coverage for the study population. To ensure that a patient’s course of drug therapy could be followed longitudinally, only plans with at least two consecutive years of data were considered, and only patients who were enrolled continuously in each year were eligible.
Footnotes
Dr. Solomon had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Solomon, Goldman, Joyce, Escarce
Acquisition of data: Solomon, Goldman, Joyce
Analysis and interpretation of data: Solomon, Goldman, Joyce, Escarce
Drafting of the manuscript: Solomon
Critical revision of the manuscript for important intellectual content: Solomon, Goldman, Joyce, Escarce
Statistical expertise: Solomon, Goldman, Joyce, Escarce
Obtained funding: Solomon, Goldman, Escarce
Administrative, technical, or material support: Solomon, Goldman, Joyce, Escarce
Supervision: Solomon, Goldman, Joyce, Escarce
References
- 1.Smith C, Cowan C, Heffler S, Caitlin A. National Health Spending in 2004: Recent Slowdown Led By Prescription Drug Spending. Health Aff. 2006 Jan-Feb;25(1):186–196. doi: 10.1377/hlthaff.25.1.186. [DOI] [PubMed] [Google Scholar]
- 2.Motheral BR, Fairman KA. Effect of a three-tier prescription copay on pharmaceutical and other medical utilization. Med Care. 2001;39(12):1293–1304. doi: 10.1097/00005650-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Joyce GF, Goldman DP, Solomon MD, Escarce JJ. Impact of multi-tier pharmacy benefits and mandatory generic substitution on prescription drug spending. JAMA. 2002;288(14):1733–1739. doi: 10.1001/jama.288.14.1733. [DOI] [PubMed] [Google Scholar]
- 4.Huskamp HA, Deverka PA, Epstein AM, Epstein RS, McGuigan KA, Frank RG. The effect of incentive-based formularies on prescription drug utilization and spending. New Engl J Med. 2003;349:2224–2232. doi: 10.1056/NEJMsa030954. [DOI] [PubMed] [Google Scholar]
- 5.Goldman DP, Joyce GF, Escarce JJ, Pace JE, Solomon MD, Laouri M, Landsman PB, Teutsch SM. Pharmacy benefits and the use of drugs by the chronically ill. JAMA. 2004;291(19):2344–2350. doi: 10.1001/jama.291.19.2344. [DOI] [PubMed] [Google Scholar]
- 6.Hsu J, Price M, Huang J, Brand R, Fung V, Hui R, Fireman B, Newhouse JP, Selby JV. Unintended consequences of caps on Medicare drug benefits. N Engl J Med. 2006 Jun 1;354(22):2349–2359. doi: 10.1056/NEJMsa054436. [DOI] [PubMed] [Google Scholar]
- 7.Mueller C, Schur C, O’Connell J. Prescription Drug Spending: the impact of age and chronic disease status. Am J Public Health. 1997 Oct 87;(10):1626–1629. doi: 10.2105/ajph.87.10.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCombs JS, Nichol MB, Newman CM, Sclar DA. The costs of interrupting antihypertensive drug therapy in a Medicaid population. Med Care. 1994;32(3):214–226. doi: 10.1097/00005650-199403000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Anderson F, Cline C, Ryden-Bergsten T, Erhardt L. Angiotensin Converting Enzyme (ACE) Inhibitors and Heart Failure. The Consequences of Underprescribing. Pharmacoeconomics. 1999 Jun;15(6):535–550. doi: 10.2165/00019053-199915060-00002. [DOI] [PubMed] [Google Scholar]
- 10.Franklin SS, Jacobs MJ, Wong ND, Italien GJ, Lapuerta P. Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: analysis based on national health and nutrition examination survey (NHANES) III. Hypertension. 2001;37:869–874. doi: 10.1161/01.hyp.37.3.869. [DOI] [PubMed] [Google Scholar]
- 11.Grant RW, Cagliero E, Murphy-Sheehy P, Singer DE, Nathan DM, Meigs JB. Comparison of hyperglycemia, hypertension, and hypercholesterolemia management in patients with type 2 diabetes. Am J Med. 2002;112:603–609. doi: 10.1016/s0002-9343(02)01103-8. [DOI] [PubMed] [Google Scholar]
- 12.Burt VL, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adults population: results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25 doi: 10.1161/01.hyp.25.3.305. 305-131. [DOI] [PubMed] [Google Scholar]
- 13.Burt VL, Culter JA, Higgins M, et al. Trends in the prevalence, awareness, treatment, and control of hypertension in the adult US population. Data from the health examination surveys, 1960 to 1991. Hypertension. 1995;26:60–69. doi: 10.1161/01.hyp.26.1.60. [DOI] [PubMed] [Google Scholar]
- 14.Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in US adults: the Third National health and Nutrition Examination Survey, 1988–1994. Diabetes Care. 1998;21:518–524. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 15.Hwang W, Weller W, Ireys H, Anderson G. Out-of-Pocket Medical Spending for Care of Chronic Conditions. Health Aff. 2001;20(6):267–276. doi: 10.1377/hlthaff.20.6.267. [DOI] [PubMed] [Google Scholar]
- 16.Kane NM. Pharmaceutical cost containment and innovation in the United States. Health Policy. 1997 Sep;41(Suppl):S71–S89. doi: 10.1016/s0168-8510(97)00048-1. [DOI] [PubMed] [Google Scholar]
- 17.Lipton HL, Gross DJ, Stebbins MR, Syed LH. Managing the pharmacy benefit in Medicare HMOs: what do we really know? Health Aff. 2000;19(2):42–58. doi: 10.1377/hlthaff.19.2.42. [DOI] [PubMed] [Google Scholar]
- 18.Hong SH, Shepherd MD. Outpatient prescription drug use by children enrolled in five drug benefit plans. Clin Ther. 1996 May-Jun;18(3):528–545. doi: 10.1016/s0149-2918(96)80035-x. [DOI] [PubMed] [Google Scholar]
- 19.Smith DG, Kirking DM. Impact of consumer fees on drug utilization. Pharmacoeconomics. 1992;2:335–342. doi: 10.2165/00019053-199202040-00008. [DOI] [PubMed] [Google Scholar]
- 20.Harris BL, Stergachis A, Reid LD. The effect of pharmaceutical copayments on utilization and costs of pharmaceuticals in a health maintenance organization. Med Care. 1990;28:907–917. doi: 10.1097/00005650-199010000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Johnson R, et al. The impact of increasing patient prescription drug cost-sharing on therapeutic classes of drugs received and on the health status of elderly HMO patients. Health Serv Res. 1997;32(1):103–122. [PMC free article] [PubMed] [Google Scholar]
- 22.Safran DG, Neuman P, Schoen C, Kitchman MS, Wilson IB, Cooper B, Li A, Chang H, Rogers WH. Prescription Drug Coverage And Seniors: Findings From A 2003 National Survey. Health Aff. 2005 Apr 19; doi: 10.1377/hlthaff.w5.152. [DOI] [PubMed] [Google Scholar]
- 23.Reeder CD, Nelson AA. The differential impact of copayment on drug use in a Medicaid population. Inquiry. 22:396–403. [PubMed] [Google Scholar]
- 24.Federman AD, Adams AS, Ross-Degnan D, Soumerai SB, Ayanian JZ. Supplemental insurance and use of effective cardiovascular drugs among elderly medicare beneficiaries with coronary heart disease. JAMA. 2001 Oct 10;286(14):1732–1739. doi: 10.1001/jama.286.14.1732. [DOI] [PubMed] [Google Scholar]
- 25.Lohr KN, Brook RH, Kamberg CJ, Goldberg GA, Leibowitz A, Keesey J, Reboussin D, Newhouse JP. Effect of cost-sharing on use of medically effective and less effective care. Med Care. 1986;24:S31–S38. [PubMed] [Google Scholar]
- 26.Soumerai SB, et al. Payment restrictions for prescription drugs under Medicaid: Effects on therapy, cost and equity. New Engl J Med. 1987;317:550–556. doi: 10.1056/NEJM198708273170906. [DOI] [PubMed] [Google Scholar]
- 27.Soumerai SB, et al. Effects of limiting Medicaid drug reimbursement benefits on the use of psychotropic agens and acute mental health services by patients with schizophrenia. New Engl J Med. 1994;331:650–655. doi: 10.1056/NEJM199409083311006. [DOI] [PubMed] [Google Scholar]
- 28.Martin BC, McMillan JA. The Impact of Implementing a More Restrictive Prescription Limit on Medicaid Recipients. Effects on Cost, Therapy, and Out-of-Pocket Expenditures. Med Care. 1996;34(7):686–701. doi: 10.1097/00005650-199607000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Fortress EE, Soumerai SB, McLaughlin TJ, Ross-Degnan D. Utilization of Essential Medications by Vulnerable Older People After a Drug Benefit Cap: importance of mental disorders, chronic pain, and practice setting. J Am Geriatr Soc. 2001 Jun;49(6):793–797. doi: 10.1046/j.1532-5415.2001.49158.x. [DOI] [PubMed] [Google Scholar]
- 30.Quam L, et al. Using Claims Data for Epidemiological Research: The concordance of claims-based criteria with the medical record and patient survey for identifying a hypertensive population. Med Care. 1993;31(6):498–507. [PubMed] [Google Scholar]
- 31.Rector TS, Wickstrom SL, Shah M, et al. Specificity and Sensitivity of claims-based algorithms for identifying members of Medicare+Choice Health Plans that have chronic medical conditions. Health Serv Res. 2004 Dec;39(6):1839–1857. doi: 10.1111/j.1475-6773.2004.00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saydah SH, Fradkin J, Cowie CC. Poor Control of Risk Factors for Vascular Disease Among Adults With Previously Diagnosed Diabetes. JAMA. 2004;291:335–342. doi: 10.1001/jama.291.3.335. [DOI] [PubMed] [Google Scholar]
- 33.Hajjar I, Kotchen T. Trends in the prevalence, treatment, and control of Hypertension in the US, 1988–2000. JAMA. 2003;290:199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 34.Berlowitz DR, Ash AS, Hickey EC, et al. Inadequate management of blood pressure in a hypertensive population. New Engl J Med. 1998;339:1957–1963. doi: 10.1056/NEJM199812313392701. [DOI] [PubMed] [Google Scholar]
- 35.Raji MA, Kuo YF, Salazar JA, et al. Ethnic differences in antihypertensive medication use in the elderly. Ann Pharmacother. 2004;38:209–214. doi: 10.1345/aph.1D224. [DOI] [PubMed] [Google Scholar]
- 36.Hertz RP, Unger AN, Ferrario CM. Diabetes, Hypertension, and Dyslipidemia in Mexican Americans and Non-Hispanic Whites. Am J Prev Med. 2006;30(2):103–110. doi: 10.1016/j.amepre.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 37.Ong, et al. Prevalence, Awareness, Treatment, and Control of Hypertension Among United States Adults 1999–2004. Hypertension. 2007;49:69–75. doi: 10.1161/01.HYP.0000252676.46043.18. [DOI] [PubMed] [Google Scholar]
- 38.Psaty BM, Manolio TA, Smith NL, Heckbert SR, Gottdiener JS, Burke GL, Weissfeld J, Enright P, Lumley T, Powe N, Furberg CD. Cardiovascular Health Study. Time trends in high blood pressure control and the use of antihypertensive medications in older adults: the Cardiovascular Health Study. Arch Intern Med. 2002 Nov 11;162(20):2325–2332. doi: 10.1001/archinte.162.20.2325. [DOI] [PubMed] [Google Scholar]
- 39.Natarajan S, Nietert PJ. National trends in screening, prevalence, and treatment of cardiovascular risk factors. Prev Med. 2003 Apr;36(4):389–397. doi: 10.1016/s0091-7435(02)00057-9. [DOI] [PubMed] [Google Scholar]
- 40.Barker WH, Mullooly JP, Linton KL. Trends in hypertension prevalence, treatment, and control: in a well-defined older population. Hypertension. 1998;31:552–559. doi: 10.1161/01.hyp.31.1.552. [DOI] [PubMed] [Google Scholar]
- 41.Ford ES, Mokdad AH, Giles WH, Mensah GA. Serum total cholesterol concentrations and awareness, treatment, and control of hypercholesterolemia among US adults: findings from the National Health and Nutrition Examination Survey, 1999 to 2000. Circulation. 2003;107:2185–2189. doi: 10.1161/01.CIR.0000066320.27195.B4. [DOI] [PubMed] [Google Scholar]
- 42.Arnett DK, Jacobs DR, Luepker RV, Blackburn H, Armstrong C, Claas SA. Twenty-Year Trends in Serum Cholesterol, Hypercholesterolemia, and Cholesterol Medication Use: The Minnesota Heart Survey, 1980–1982 to 2000–2002. Circulation. 2005;112:3884–3891. doi: 10.1161/CIRCULATIONAHA.105.549857. [DOI] [PubMed] [Google Scholar]
- 43.Burt V, Cutler J, Higgins M, Horan M, Labarthe D, Whelton P, Brown C, Roccella E. Trends in the prevalence, awareness, treatment, and control of hypertension in the adult US population: data from the health examination surveys, 1960 to 1991. Hypertension. 1995;26:60–69. doi: 10.1161/01.hyp.26.1.60. [DOI] [PubMed] [Google Scholar]
- 44.Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the US adult population. Results from the third national health and nutrition examination survey, 1988–1991. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 45.Spooner JJ, et al. Pharmacologic treatment of diabetes in long-term care. J Clin Epidemiol. 2001;54:525–530. doi: 10.1016/s0895-4356(00)00326-7. [DOI] [PubMed] [Google Scholar]
- 46.Jacobs MJ, et al. Prevalence and control of dyslipidemia among persons with diabetes in the United States. Diabetes Res Clin Pract. 2005;70:263–269. doi: 10.1016/j.diabres.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 47.Qureshi AI, Suri FK, Guterman LR, Hopkins LN. Ineffective secondary prevention in survivors of cardiovascular events in the US population. Arch Intern Med. 2001;161:1621–1628. doi: 10.1001/archinte.161.13.1621. [DOI] [PubMed] [Google Scholar]
- 48.Harris MI. Health Care and Health Status and Outcomes for Patients With Type 2 Diabetes. Diabetes Care. 2000;23:754–758. doi: 10.2337/diacare.23.6.754. [DOI] [PubMed] [Google Scholar]
- 49.Harris MI. Racial and Ethnic Differences in Health Care Access and Health Outcomes for Adults With Type 2 Diabetes. Diabetes Care. 2001;24:454–459. doi: 10.2337/diacare.24.3.454. [DOI] [PubMed] [Google Scholar]
- 50.Grant RW, Buse JB, Meigs JB. Quality of Diabetes Care in US Academic Medical Centers. Diabetes Care. 2005;28:337–442. doi: 10.2337/diacare.28.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nelson K, Norris K, Mangione CM. Disparities in the diagnosis and pharmacologic treatment of high serum cholesterol by race and ethnicity: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2002;162:929–935. doi: 10.1001/archinte.162.8.929. [DOI] [PubMed] [Google Scholar]
- 52.Franklin SS, Jacobs MJ, Wong ND, Italien GJ, Lapuerta P. Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: analysis based on national health and nutrition examination survey (NHANES) III. Hypertension. 2001;37:869–874. doi: 10.1161/01.hyp.37.3.869. [DOI] [PubMed] [Google Scholar]
- 53.Lloyd-Jones DM, Evans JC, Larson MG, Levy D. Treatment and Control of Hypertension in the Community: A Prospective Analysis. Hypertension. 2002;40:640–646. doi: 10.1161/01.hyp.0000035855.44620.da. [DOI] [PubMed] [Google Scholar]
- 54.Most population based estimates of treatment patterns for chronic disease report three measures: the proportion of patients who are aware of their disease, the proportion who are treated, and the proportion who have their disease controlled. Few report the specific proportion of those who are aware and treated. To construct an estimate of this measure to validate our findings, we assumed that all patients who report being treated are also aware of their disease [Google Scholar]
- 55.Glynn RJ, Monane M, Gurwitz JH. Choodnovskiy I, Avorn J. Aging, comorbidity, and reduced rates of drug treatment for diabetes mellitus. J Clin Epidemiol. 1999;52:781–790. doi: 10.1016/s0895-4356(99)00055-4. [DOI] [PubMed] [Google Scholar]
- 56.Roblin DW, Platt R, Goodman MJ, Hsu J, Nelson WW, Smith DH, Andrade SE, Soumerai SB. Effect of increased cost-sharing on oral hypoglycemic use in five managed care organizations: how much is too much? Med Care. 2005 Oct;43(10):951–959. doi: 10.1097/01.mlr.0000178216.23514.b7. [DOI] [PubMed] [Google Scholar]
- 57.Author’s own estimates [Google Scholar]
- 58.Fendrick MA, Smith DG, Chernew ME, Shah SN. A Benefit-Based Copay for Prescription Drugs: Patient Contribution Based on Total Benefits, Not Drug Acquisition Cost. Am J Manag Care. 2001;7:861–867. [PubMed] [Google Scholar]
- 59.Goldman DP, Joyce GF, Karaca-Mandic P. Varying pharmacy benefits with clinical status: the case of cholesterol-lowering therapy. Am J Manag Care. 2006;12:21–28. [PubMed] [Google Scholar]
- 60.Gibson TB, Mark TL, McGuigan KA, Axelsen K, Wang S. The Effects of Prescription Drug Copayments on Statin Adherence. Am J Manag Care. 2006;12:509–517. [PubMed] [Google Scholar]