Abstract
Objectives
Standard sleep scoring criteria may be unreliable when applied to critically ill patients. We sought to quantify typical and atypical polysomnographic findings in critically ill patients and to begin development and reliability testing of methodology to characterize the atypical polysomnographic tracings that confound standard sleep scoring criteria.
Design
Prospective convenience sample.
Setting
Two academic, tertiary care medical centers.
Patients
Thirty-seven critically ill, mechanically ventilated, medical ICU patients.
Interventions
None.
Measurements and Main Results
Mechanically ventilated subjects were monitored by continuous polysomnography. After noting frequent atypical polysomnographic findings (i.e., lack of stage N2 markers, the presence of polymorphic delta, burst suppression, or isoelectric electroencephalography), attempts to use standard sleep scoring criteria alone were abandoned. Atypical polysomnographic findings were characterized and used to develop a modified scoring system. Polysomnographic data were scored manually via this revised scoring scheme. Of 37 medical ICU patients enrolled, 36 experienced atypical sleep, which accounted for 85% of all recorded data, with 5.1% normal sleep and 9.4% wake. Coupling observed patient arousal levels with polysomnographic characteristics revealed that standard polysomnographic staging criteria did not reliably determine the presence or absence of sleep. Rapid eye movement occurred in only five patients (14%). The revised scoring system incorporating frequently seen atypical characteristics yielded very high interrater reliability (weighted κ = 0.80; bootstrapped 95% CI, [0.48, 0.89]).
Conclusions
Analysis of polysomnographic data revealed profound deficiencies in standard scoring criteria due to a predominance of atypical polysomnographic findings in ventilated patients. The revised scoring scheme proved reliable in sleep staging and may serve as a building block in future work.
Keywords: analgesia, critical care, delirium, mechanical ventilation, polysomnography, sedation, sleep
Critically ill patients have severe sleep disruption with sleep fragmentation, abnormal circadian rhythms, increased N1 sleep, and decreased N3 (slow wave sleep [SWS]) and rapid eye movement (REM) sleep (1–3). Poor sleep quality has been hypothesized to contribute to ICU delirium (4–6), deranged immune function (7, 8), and prolonged mechanical ventilation (MV) (9). Though provocative, these data may be subject to errors due to the use of the American Association of Sleep Medicine (AASM) scoring criteria (10, 11), which have not been validated for use in critical illness, and difficulty in interpreting polysomnography (PSG) data from this patient population, in which atypical electroencephalography (EEG) findings are frequently present (1).
In ICU patients, medications and illness can alter brain activity and EEG tracings (1). Benzodiazepines and propofol, while mimicking a behavioral sleep state clinically, will induce a low amplitude and high-frequency EEG pattern at commonly used doses and even lead to burst suppression and isoelectric EEG (12, 13). Analgesics and antipsychotics as well as delirium cause EEG slowing (14–19). Since all of the above exposures are found in the ICU, not only must patients receiving such medications be included in clinical research efforts, but it is also critical that standard sleep staging criteria developed for non-ICU populations be subjected to modifications since they are fraught with hazards when used to classify sleep in critically ill patients. Previous studies characterizing sleep in ICU patients have often focused on less severely ill patients either off MV or on MV with light levels of sedation (5, 6, 20–24). Even in a group of MV patients not receiving sedation, researchers have demonstrated abnormal sleep EEG patterns in almost a third of patients and have proposed adding two new sleep states (pathologic wakefulness and atypical sleep) to more accurately classify sleep in ICU patients (24).
The aims of this investigation were 1) to describe the atypical PSG findings of sleep in critically ill MV patients and thus bring into focus the limitations (when applied in the ICU) of standard sleep scoring criteria and 2) to begin the process of developing and determining the reliability of a pilot scoring scheme for use in critically ill patients that would allow incorporation of atypical PSG findings and that could be validated in future work.
METHODS
Patients
We enrolled a convenience sample of 37 adult (≥18 yr), critically ill patients admitted to the medical ICUs at Vanderbilt University (21 patients) or the University of Chicago (16 patients) who were expected to require more than 24 hours of MV. The patients enrolled at the University of Chicago were also part of a local study and results have been previously reported (25). The scientific components presented here (i.e., the application of a sleep staging system incorporating additional EEG features) are unique to this analysis and presentation. Exclusion criteria were a history of psychosis, anoxic brain injury, stroke, subdural hematoma, neurotrauma, or Child-Pugh Class B or C cirrhosis. The institutional review board of both institutions reviewed and approved the study protocols. Informed consent was obtained from each patient or his/her surrogate decision maker.
ICU Management of Patients
All aspects of patient care in the ICU were managed by a team of critical care physicians per standardized protocols in place at the respective ICUs. Analgesic and sedative dosing was directed by the patient care team using the validated Richmond Agitation-Sedation Scale (RASS) (26, 27). Daily interruption of sedation was performed at the discretion of the ICU teams per their unit’s sedation protocol and was not mandated by study protocol. Analgesics and sedatives used included fentanyl, morphine, midazolam, lorazepam, and propofol. A number of patients received haloperidol or an atypical antipsychotic for attempted prevention or treatment of ICU delirium.
All patients received MV throughout the data collection period. Though the medical team dictated MV management, the standard practice in the participating ICUs consisted of low tidal volume ventilation using the assist control mode, along with daily screening for spontaneous breathing trial (SBT) readiness followed by an SBT if the patient passed the screen (28, 29).
Consciousness, Polysomnography, and Sleep Assessment
Arousal levels and delirium were monitored daily using the RASS (26, 27) and the Confusion Assessment Method for the ICU (30). Subjects were monitored by continuous PSG that typically began within 48 hours of the initiation of MV. PSG monitoring was terminated at the time of liberation from MV or a maximum of 5 days, whichever came first. PSG at Vanderbilt University and the University of Chicago was performed using portable systems with remote access monitoring (Nihon Kohden America, Foothills Ranch, CA).
Techniques for electrode placement conformed to the International 10–20 system (31). Electrode placement included EEG, bilateral electrooculography, and submental electromyography. EEG consisted of two central channels (C4-A1, C3-A2) and two occipital channels (O1-A2, O2-A1), with the addition, in some patients, of two frontal channels (F3-A2, F4-A1).
PSG epochs, each 30 seconds in duration, were scored manually by a registered PSG technologist and overseen by one of two sleep and critical care physicians (P.L.W., B.K.G.). If the PSG was consistent with standard sleep stages, it was scored using the AASM criteria (10, 11). Through the course of the study, investigators identified PSG findings that differed from normal sleep and could not be accurately staged using standard criteria. Examples of these atypical findings included polymorphic delta activity, burst suppression, and isoelectric activity. When atypical findings were noted on a PSG epoch, the epoch was scored as “atypical.” The time in each standard sleep stage and the time when atypical EEG epochs were present were determined.
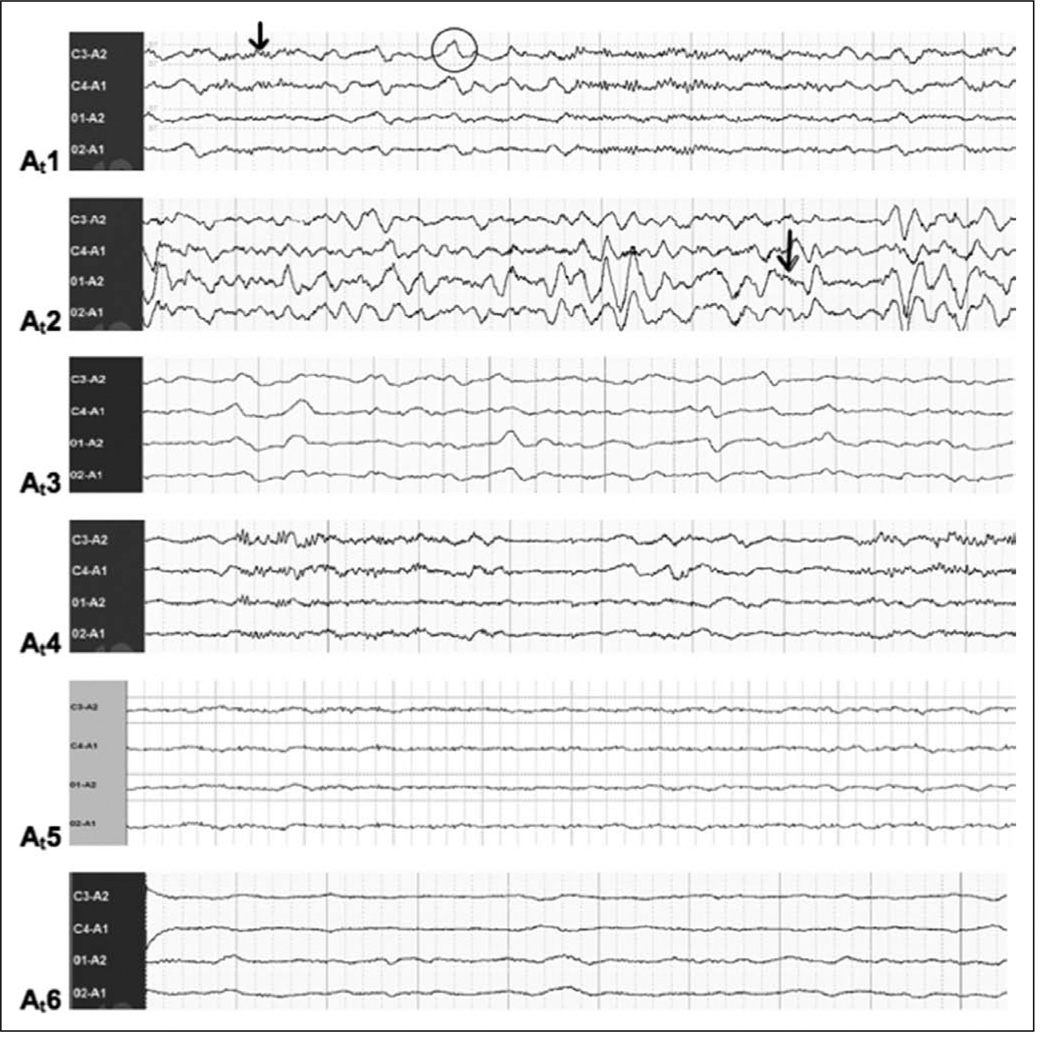
Atypical PSG tracings were further analyzed by visual characteristics and subdivided into six categories based on the EEG categories of encephalopathy previously described by Markand and Young et al (32, 33). These categories were then used to develop modified sleep scoring criteria for use in critically ill patients. The characteristics of these additional categories, designated as atypical sleep stage At1–At6, are outlined in Table 1 and demonstrated in Figure 1.
Table 1.
Description of Proposed Sleep Stages in Critically Ill Patients
| Sleep Stage (Current-Standard) | EE G Waveform Description |
| Wake | Alpha activity (8–13 Hz); relatively low voltage, mixed frequency EEG |
| N1 (NREM 1) | Relatively low voltage, mixed frequency EEG with >50% theta activity (4–7 Hz) |
| N2 (NREM 2) | Sleep spindles (12–14 Hz [>0.5 s]) and K-complexes (negative sharp wave followed immediately by slower positive component [>0.5 s]); on a background of relatively low voltage, mixed frequency EEG activity |
| N3 (NREM 3, slow wave sleep) | Delta activity, >20% high amplitude (>75 µV), slow frequency (0.5–2 Hz) |
| REM | Relatively low voltage, mixed frequency with intermittent REMs |
| Additional Sleep Stages Proposed for Atypical Sleep | |
| Sleep Stage (Proposed-Atypical)a | EE G Waveform Description |
| Pathologic wakefulness | Any EEG frequency other than alpha or beta with behavioral characteristics of wakefulness |
| At1 (Atypical 1) | Alpha and/or theta present on >10% of epoch, without sleep spindles or K-complexes in the preceding 3 min; may have polymorphic delta, FIRDA, or triphasic activity |
| At2 | Polymorphic delta, FIRDA, or triphasic activity with alpha or beta activity superimposed on delta waves, without sleep spindles or K-complexes in the preceding 3 min |
| At3 | Polymorphic delta, FIRDA, or triphasic activity without alpha or beta activity superimposed on delta waves |
| At4 | Burst-suppression pattern with EEG amplitude <5 µV for >0.5 s |
| At5 | Suppressed pattern with EEG amplitude <20 µV |
| At6 | Isoelectric activity (amplitude <5 µV) throughout epoch |
EEG = electroencephalography, NREM = non-rapid eye movement, REM = rapid eye movement, A = atypical, FIRDA = frontal intermittent rhythmic delta.
EEG examples for each of the proposed-atypical stages are shown in Figure 1.
Figure 1.
Electroencephalography (EEG) examples of the six proposed atypical sleep stages. Refer to Table 1 for full written descriptions for each of the proposed atypical stages shown in this figure. At1 (atypical stage 1), characterized by having at least 10% alpha and/or theta activity (indicated by arrow) but may also include delta activity (indicated by circle). At2 (atypical stage 2), characterized by the presence of polymorphic delta but with the presence of background beta, alpha, or theta activity (indicated by arrow). At3 (atypical stage 3), characterized by a polymorphic delta activity without the presence of background beta, alpha, or theta activity. At4 (atypical stage 4), defined by a burst-suppression pattern, intermittent EEG activity alternating with periods of isoelectric EEG activity. At5 (atypical stage 5), defined by a suppression pattern EEG, a very low-voltage EEG activity (< 20 µV amplitude). At6 (atypical stage 6), characterized by a complete lack of EEG/cortical activity as shown.
To assess interrater reliability of these modified sleep criteria, the records of 21 patients studied at Vanderbilt University were divided into 8-hour segments (10 pm–6 am, 6 am–2 pm, and 2 pm–10 pm). Ten epochs from each of these segments were randomly selected and manually scored by two PSG technicians. Each scorer was blinded to the results of the other as well as to clinical variables related to the patients’ hospital course. The Chicago patients were not included in the reliability testing data because we were only able to obtain one reading per patient.
Statistical Analysis
Continuous variables are described using medians and interquartile ranges (IQRs); categorical variables are described using frequencies and proportions. Amounts of time spent in each sleep stage (or in atypical sleep) are described in terms of minutes and in percentages of total sleep time scored.
To assess interrater reliability of the proposed sleep scoring classifications, we used the weighted kappa statistic to account for nearness of agreement (34). After calculating the kappa statistics, we used bootstrapping to obtain CIs for the weighted kappa statistic. Resampling was performed based on patient observations with replacement to generate 1,000 sets of samples with the same patients’ size to the original dataset, and the weighted kappa with a 95% CI was computed within each bootstrapped dataset.
RESULTS
Patients
The 37 patients studied provided 1,945.7 hours of PSG recording time with median (IQR) recording times per patient of 54.8 hours [40, 72]. Patient baseline demographic data are illustrated in Table 2. Median patient age was 63 years (49, 72), patients were severely ill with a median enrollment APACHE II score of 24 (18, 30), and 21 (57%) of the patients studied had sepsis.
Table 2.
Patient Baseline and Clinical Characteristics
| n = 37 | |
|---|---|
| Age, yr; median (IQR) | 63 (49; 72) |
| Race, n (%) | |
| White | 27 (73) |
| Sex, n (%) | |
| Female | 18 (49) |
| APACHE IIa, median (IQR) | 24 (18; 30) |
| Admission diagnosis, n (%) | |
| Sepsis/acute respiratory distress syndromeb | 21 (57) |
| Chronic obstructive pulmonary disease/asthma | 4 (11) |
| Pulmonary other | 5 (14) |
| Hepatic failure | 1 (3) |
| Renal failure | 1 (3) |
| Metabolic/endocrine | 1 (3) |
| Hemorrhage | 2 (5) |
| Neurologic disease | 1 (3) |
| Transplant | 1 (3) |
| Ever delirious | 30 (81) |
| Days of delirium, median (IQR) | 2 (1; 5) |
| Days of coma, median (IQR) | 1 (0; 4) |
| Days of delirium/coma, median (IQR) | 3 (3; 9) |
| First mental status in study, n (%) | |
| Normal | 8 (22) |
| Delirious | 12 (32) |
| Comatose | 17 (46) |
| Medications, by patient day, median (IQR) | |
| Benzodiazepines, mg (lorazepam equivalents) | 4.7 (2.8; 19.2) |
| Opiates, µg (fentanyl equivalent) | 1,200 (562; 2,719) |
| Propofol, µg | 2,980 (1,655; 3,883) |
| Days on ventilator, median (IQR) | 6 (4;12) |
| ICU length of stay, median (IQR) | 10 (6.1; 16.5) |
| Hospital length of stay, median (IQR) | 14.2 (9.8; 20.5) |
| Alive at hospital discharge, n (%) | 29 (78) |
IQR = interquartile range.
Acute Physiology and Chronic Health Evaluation (APACHE) II (42) is a severity of illness scoring system, and these data were calculated using the most abnormal variables during the first 24 hr following admission to the ICU. APACHE II scores range from 0 (best) to 71 (worst).
The diagnosis of sepsis was determined by the patients’ medical team.
Notable Differences in Polysomnographic Findings in Critically Ill Patients
EEG characteristics present in critically ill patients that drove efforts toward modification of traditional sleep scoring.
Dissociation of EEG Findings and the Sleep/Wake States
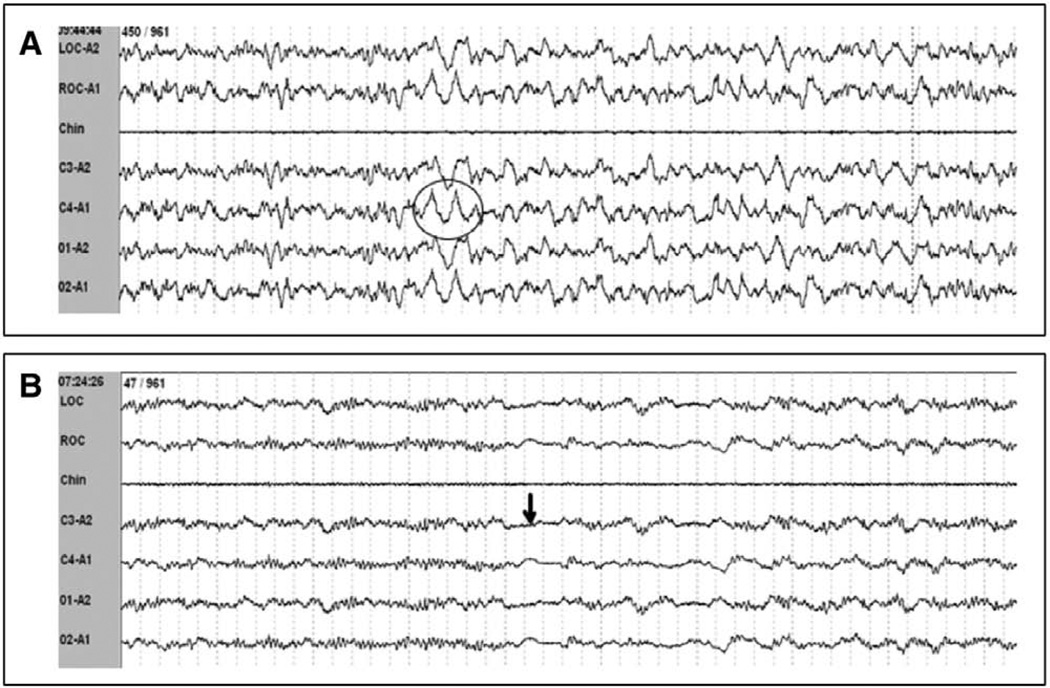
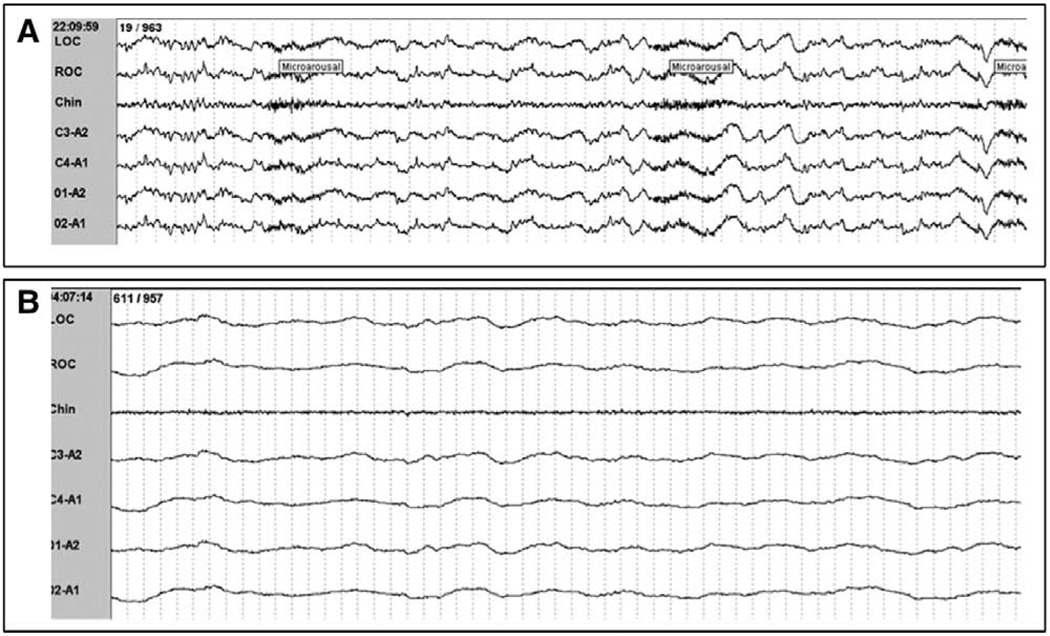
This cohort offers details regarding the dissociation of EEG and sleep/wake states due to our inclusion of selected bedside observations coupled to those patients’ PSG tracings. Such dissociations consisted of a) an abnormally slow EEG frequency in the theta range (3–7 Hz), a frequency normally indicative of sleep, or even delta range in some awake patients and b) low-amplitude, high-frequency beta EEG activity present during coma. Patients who were awake and interactive with research personnel were noted to exhibit predominately theta activity (3–7 Hz), a frequency normally indicative of sleep. One patient documented to be awake and able to follow simple instructions was documented to have >20% delta activity (>75 µV, <2 Hz), a finding normally associated with N3 (SWS) sleep (Fig. 2A). Conversely, unresponsive comatose patients were noted to have alpha activity present on PSG, an EEG frequency typically seen in the wake state (Fig. 2B). Therefore, wake could not be scored solely based on EEG criteria. Additionally, patients who were comatose, as defined by a RASS score of −4 or −5, had widely variable EEG findings ranging from EEG patterns indistinguishable from stage N1 with frequent arousals (Fig. 3A) to continuous isoelectric activity (Fig. 3B).
Figure 2.
Dissociation between observed wakefulness or level of consciousness and electroencephalography (EEG) patterns. A, EEG demonstrating delta waves (indicated by circle) suggestive of slow wave sleep in a patient with evidence of wakefulness by following simple commands. This epoch would be scored as pathologic wakefulness by the proposed scoring criteria. B, EEG demonstrating theta frequency EEG activity, normally seen in light (stage N1) sleep, in a patient who was unresponsive. Short periods of isoelectric activity can be seen (indicated by arrow) in this example, which can alert the polysomnography reader that this is not normal stage N1. This epoch would be scored as At4 by the proposed scoring criteria..
Figure 3.
Variability in electroencephalography (EEG) characteristics in comatose patients. A, Polysomnography (PSG) from a patient who had a Richmond Agitation-Sedation Scale (RASS) score of −5 (unresponsive to verbal and physical stimulus) but with theta activity resembling N1 sleep with frequent arousals. B, PSG from a patient who had a RASS score of −5 demonstrating isoelectric EEG activity. This epoch would be scored as At6 by the proposed scoring criteria..
Markedly Abnormal Sleep Architecture
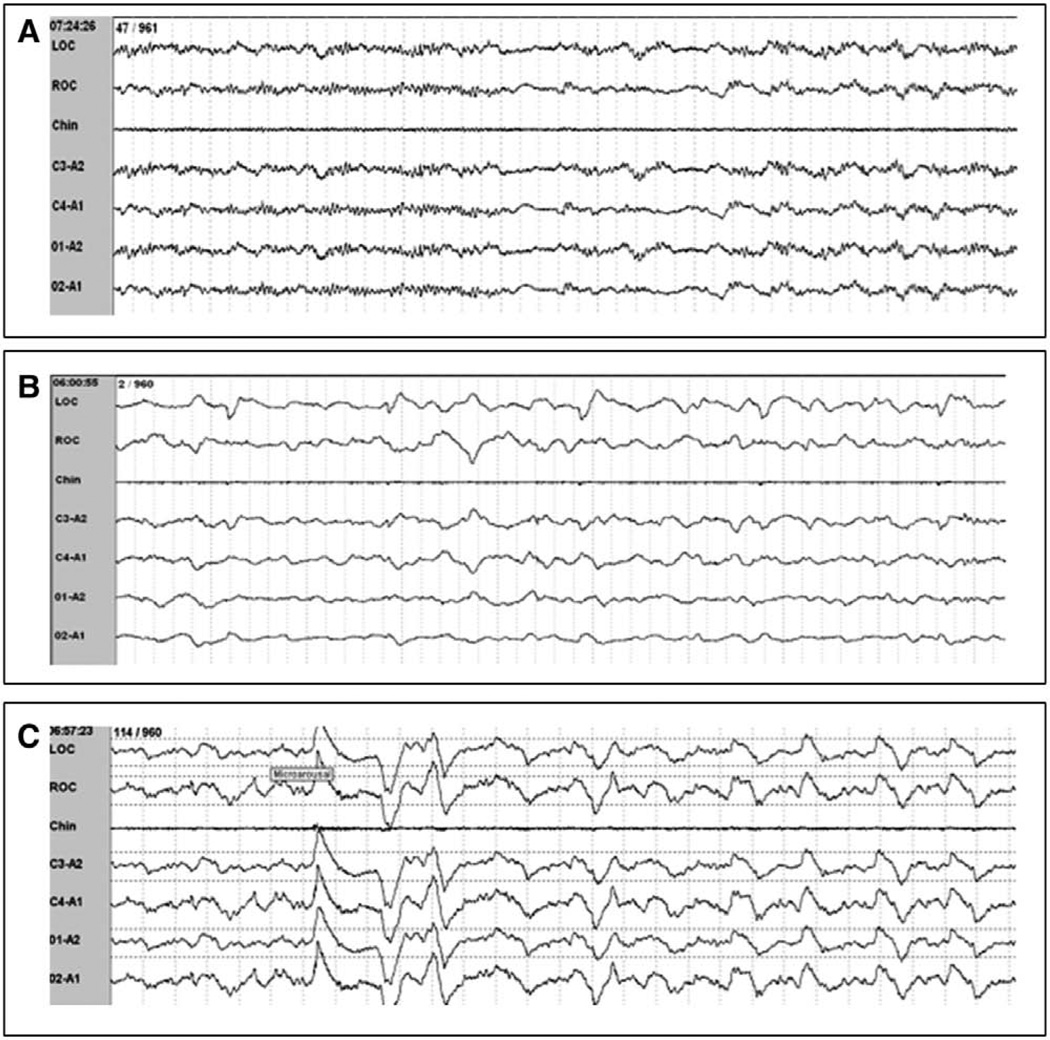
This abnormality consisted of: 1) lack of variability in the PSG, 2) lack of normal non-rapid eye movement (NREM) sleep patterns, and 3) rare episodes of REM sleep. A common characteristic observed in ICU patients was that of prolonged episodes of monotonous, nonvariable EEG patterns. Whereas normal sleep is characterized by frequent shifts between sleep stages, most patients had PSG characteristics that were relatively unchanged for hours. Patients commonly lacked normal NREM sleep patterns with K-complexes and spindles absent throughout the PSG tracing. REM sleep occurred rarely; REM was observed in five patients for a mean period of 8.8 ± 35.8 minutes (Table 3). Only one patient experienced >6% REM sleep during any 24-hour period. 1) Theta activity occurring in an epoch with a brief period of isoelectric activity (Fig. 4A), 2) polymorphic delta (0.5–4 Hz), slow frequency waveforms with a lack of the normal background activity (Fig. 4B), 3) triphasic waves, and 4) arousal induced delta activity (Fig. 4C).
Table 3.
Descriptive Statistics of All Sleep Stages by Patient
| Total polysomnographic data, min; median (IQR) | 3,285 (2,400; 4,321) |
| Ever experienced wake, n (%) | 22 (59) |
| Wake time, min; median (IQR) | 246 (116; 653) |
| Wake time, overall %; median (IQR) | 7 (2.2; 34.5) |
| Ever experienced normal sleep, n (%) | 11 (30) |
| Normal sleep, min; median (IQR) | 303 (135; 475) |
| Normal sleep, overall %; median (IQR) | 8 (5; 28) |
| Ever experienced N1 sleep, n (%) | 8 (22) |
| Stage N1 sleep, min; median (IQR) | 93 (32; 282) |
| N1 sleep, overall %; median (IQR) | 5.5 (1; 9.2) |
| Ever experienced N2 sleep, n (%) | 8 (22) |
| Stage N2 sleep, min; median (IQR) | 148 (27; 495) |
| N2 sleep, overall %; median (IQR) | 4.5 (1; 29) |
| Ever experienced N3 sleep, n (%) | 5 (14) |
| Stage N3 sleep, min; median (IQR) | 101 (19; 126) |
| N3 sleep, overall %; median (IQR) | 3 (1; 4) |
| Ever experienced REM sleep, n (%) | 5 (14) |
| Stage REM sleep, min; median (IQR) | 19 (14; 58) |
| REM sleep, overall %; median (IQR) | 0 (0; 2) |
| Experienced > 6% REM sleep any day, n (%) | 1 (3) |
| Ever experienced atypical sleep, n (%) | 36 (97) |
| Atypical sleep, min; median (IQR) | 2,943 (1,775; 3,709) |
| Atypical sleep, overall %; median (IQR) | 96 (79; 100) |
IQR = interquartile range, REM = rapid eye movement.
Figure 4.
Examples of atypical electroencephalography (EEG) findings which if scored using standard sleep staging criteria would cause misleading results. A, Polysomnography (PSG) demonstrating burst suppression, which if standard scoring criteria were used, would be misleadingly scored as stage N1 sleep. This epoch would be scored as stage At4 by the proposed scoring criteria. B, PSG demonstrating EEG waveforms with lack of background activity are only seen in pathologic states. This epoch would be scored as At3 by the proposed criteria. C, PSG demonstrating onset of rhythmic delta activity after stimulations of patient (suctioning), which would be misleadingly scored as stage N3 sleep if standard scoring criteria were used. Using the proposed criteria, this epoch would be scored as At2.
Presence of Atypical Sleep
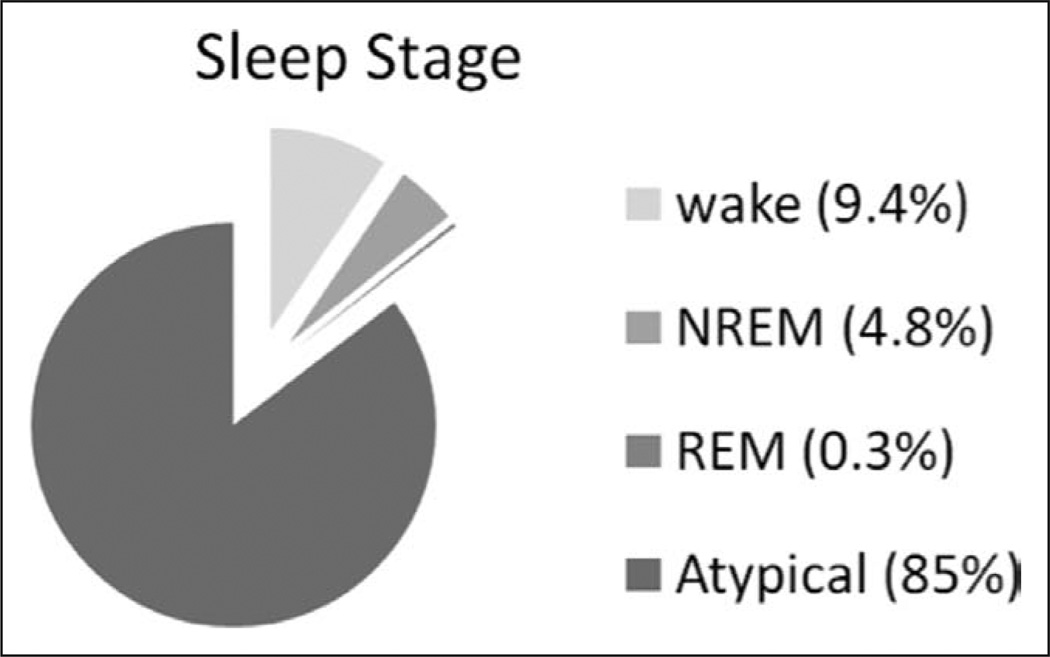
Thirty-six of 37 patients had atypical PSG findings. Only 11 patients (30%) were observed to have normal sleep at any time during the study. SWS and stage REM sleep were observed in only five patients each. Further details of sleep stages per patient are provided in Table 3, and cumulative patient data are shown in Figure 5.
Figure 5.
Cumulative sleep stage analysis of all patient data. This pie chart demonstrates the percentage of time that the population spent in each sleep stage as determined by the analysis of 1,945.7 hr of polysomnographic data in all 37 patients. The majority of the collected data (85%) was atypical in character and could not be scored using standard scoring criteria. REM = rapid eye movement, NREM = non-REM.
Development and Reliability of Critical Care Sleep Scoring Criteria
A modified scoring system for sleep in the critically ill was developed as reported in Methods, outlined in Table 1, and demonstrated in Figure 1. A total of 1,745 epochs from 21 study patients were randomly selected and tested for interrater reliability. Weighted kappa showed high/very substantial interrater reliability (κ = 0.80; bootstrapped 95% CI, [0.48, 0.89]).
Proposed Approach to PSG Scoring in the Critically Ill
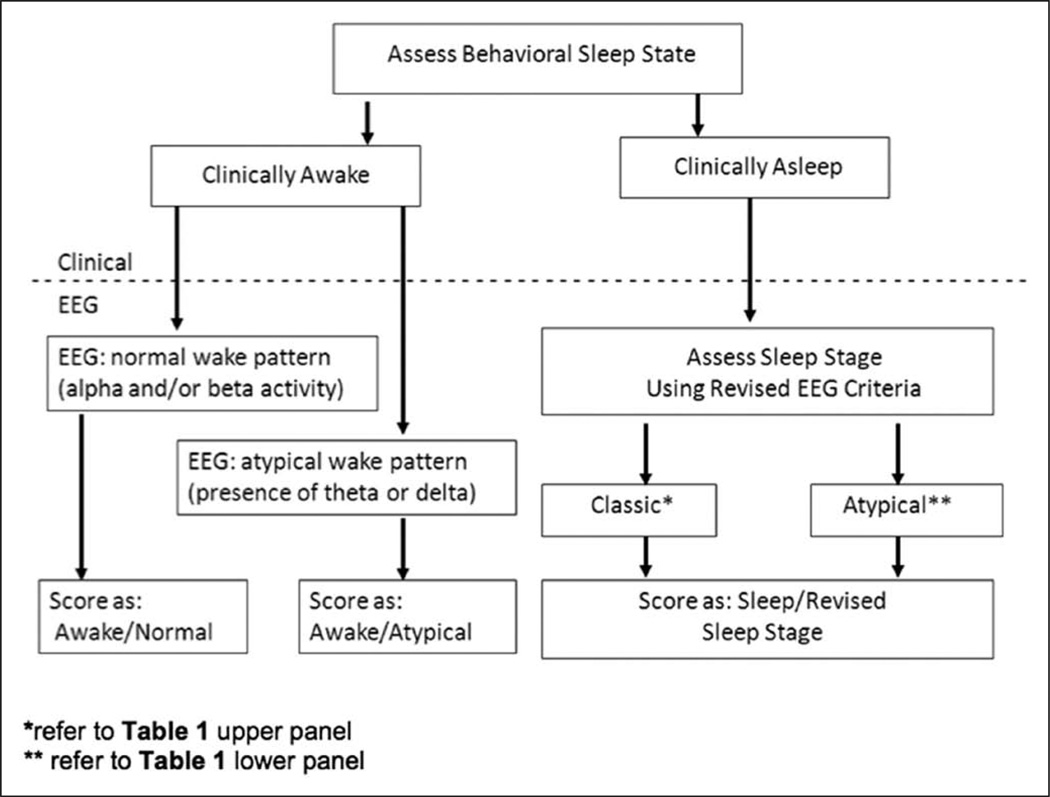
Based on the PSG characteristics of the study subjects, and in order to capture more accurately both the pathological brain states and the sleep stage in critically ill patients, we developed the following stepwise approach to scoring sleep in this population as a template for future investigations (Fig. 6):
Step 1. Assess the patient for behavioral evidence of wake versus sleep. Behavioral characteristics defining wakefulness include opening eyes to verbal stimuli, making eye contact, or following simple commands. EEG reactivity to verbal and physical stimuli should be assessed.
Step 2. If the patient is determined to be awake by behavioral characteristics, the EEG should be assessed to see if it shows alpha and/or beta activity (normal in awake individuals) or slower frequencies, such as theta and/or delta (atypical; not usually present in awake individuals). Both the behavioral wake/sleep state and whether the EEG is in the normal range should be noted. For example, a patient who is awake but whose PSG reveals theta waves would be scored as wake/atypical (also known as pathologic wakefulness) or, more specifically, could be scored as wake/atypical/theta.
Step 3. If the patient exhibits behavioral characteristics consistent with sleep or sedation, the sleep stage should be scored based on PSG characteristics as outlined in Table 1. Epochs that meet standard (wake, N1–N3, or REM) criteria should be classified as such. Epochs deemed atypical should be defined as atypical stages At1–At6 depending on the EEG characteristics as proposed in Table 1.
Figure 6.
Proposed approach to scoring sleep in critically ill patients, which can be incorporated into future investigations. EEG = electroencephalography.
DISCUSSION
This investigation describes a cohort of ICU patients with widespread sleep dysregulation and provides evidence of the complexities of measuring sleep in the critically ill. We characterized the atypical EEG findings in this population and began development and reliability testing of a system by which to characterize atypical PSG findings that confound standard sleep scoring criteria. Given the prevalence of disordered sleep among ICU patients and the potential impact of sleep deprivation on cognitive function and clinical outcomes, the development of a combined typical/atypical sleep measurement system will be an important advance in monitoring of and prognostication for ICU patients.
Several atypical PSG characteristics were identified, including the dissociation of EEG findings and the sleep/wake state as well as the presence of atypical EEG findings not consistent with the sleep stages of normal individuals. These atypical PSG characteristics were prevalent in our patients and accounted for 85% of all PSG epochs evaluated. The cyclic progression of sleep stages and ultradian rhythm that is characteristic of sleep in healthy individuals were absent in our patients. These atypical findings were likely multifactorial and may occur secondary to factors, such as sepsis-associated encephalopathy and effects of potent psychoactive medications, such as sedatives and analgesics.
One of the primary findings of this study was the inability to determine the sleep/wake states solely on EEG criteria. In contrast to healthy individuals, no EEG frequency reliably predicted the presence or absence of behavioral sleep in our patient population. The EEG frequencies of beta, alpha, theta, and delta were seen in both the behavioral wake and sleep states. For example, a patient with delirium had delta frequency activity present during the wake state, whereas unresponsive, comatose patients were noted to have high-frequency alpha/beta activity. These frequencies, normally seen as the predominant rhythm only during wakefulness, are suspected to have occurred in our patients as a result of benzodiazepine exposure. In some epochs where the EEG frequency was predominantly in the theta range, the reader could be alerted to the presence of a deep sedation state (rather than stage N1 sleep) by the presence of brief periods of isoelectric activity.
The Rechtschaffen and Kales criteria for scoring sleep were regarded for many years as the gold standard for sleep stage scoring (35). Only recently, these criteria have been modified (though still not specifically for ICU patients) (10, 11). Although these criteria are applicable to the majority of individuals, their appropriateness in specialized populations has been challenged (36–39). These standard criteria were developed as a reference method in healthy individuals without neuropathology and who were not receiving potent psychoactive medications such as those seen in ICU patients. Even at the time of their development, it was recognized that “some individuals or groups whose polygraph recordings may require further description or elaboration than that provided by the stages proposed here (35).” Critically ill patients are a group in whom there is not a validated method at this time for scoring sleep.
Previous research using PSG to measure sleep in the ICU have noted the presence of atypical EEG activity (1, 6, 38). It has been shown that such atypical PSG findings limit the applicability of standard sleep scoring criteria in this population. Ambrogio et al (38) showed that the overall interobserver reliability of the standard scoring methodology when used in MV, critically ill patients was poor (κ = 0.19), demonstrating the need for new guidelines for manual sleep assessment in these patients. Our work is in direct response to this unmet need in the crossover fields of sleep and critical care medicine. Just recently, Drouot et al (24) published a proposed new classification for sleep analysis in critically ill patients, suggesting the addition of two new stages (atypical and pathologic wakefulness) to the standard scoring criteria.
Our study builds on this above-mentioned proposed classification scheme of Drouot et al (24) by further defining the characteristics of atypical sleep in patients with a higher severity of illness and also on the EEG classification for coma developed by Young et al (33) which has been shown to have a near perfect interrater reliability (κ = 0.90). This pilot scoring system may provide a standardized method by which to track the EEG evidence of pathological brain states, effects of sedative and analgesics, as well as the reemergence of classic sleep/wake characteristics. Using the proposed scoring criteria, we found a high interrater reliability, weighted κ = 0.80 [0.48, 0.89].
The approach to scoring outlined in this document has several strengths. First, it combines behavioral assessments necessary for determining wakefulness with EEG analysis, a key requirement of any scoring system in this patient population. Second, the various stages outlined in our approach have a firm basis in the existing neurophysiological literature (32, 33). Third, because our system is built upon the 30-second epoch, it can be used in a variety of contexts for which detailed, time-sensitive analyses are required (e.g., emergence from sedation and the correlation of EEG phenomena with serum drug levels).
The use of a visual scoring methodology based on 30-second epochs has definite “resource demand” limitations. This approach is costly in terms of both time and personnel demands. However, we feel that this degree of detailed analysis is beneficial at this juncture for the eventual application of this emerging research. Detailed analyses, such as those we present here, will be necessary to explore the etiologies of atypical sleep patterns and the effects of “atypical sleep” on important clinical outcomes, such as delirium, long-term neurocognitive dysfunction, and mortality. Burst (At4) in combination with At5 and At6, has been shown to be both a risk factor for mortality in patients and is associated with an increased likelihood of postcoma delirium (40, 41, 43). Once fully developed and validated, a scoring system such as the one we have described would allow incorporation into outcome studies, interventional trials, and routine clinical practice.
Several limitations of this pilot investigation should be discussed. Because of the existence of many different levels of consciousness in critically ill patients, it is artificial and inaccurate to classify patients in a binary fashion as either “awake” or “asleep,” as if only these two states of consciousness are possible. Instead, we elected to characterize patients as awake when they exhibited unambiguous behavioral correlates of wakefulness (opening eyes to verbal stimuli, making eye contact, or following simple commands). As a result, our system likely underestimated total time spent awake and thus, if anything, was a conservative approach to determination of sleep deprivation. We understand that the subjective measurement of the sleep/wake state introduces variability to sleep scoring yet felt that this approach was preferable to an approach that either made no effort to link patient behavior and the accompanying EEG, as in a purely mathematical analysis of the EEG, or attempted to force artificial dichotomous categorization of all recording time into periods of “wakefulness” or “sleep.” Our current study used the RASS to define level of consciousness, which though imperfect is one of the most robustly validated and widely used approaches in clinical practice (thus lending face validity and easier acceptance of future work using a similar approach). The addition of six atypical sleep stages may be unnecessarily burdensome. This amount of detailed subdivision may not be necessary and future studies may elect to combine the atypical stages 1 and 2 as well as combining atypical stages 5 and 6 for simplicity’s sake, leaving only four stages of atypical categorization. After further modifying these proposed criteria and in concert with other cohorts and validation studies, future investigations with a larger sample size and perhaps with more streamlined exposures (e.g., one or maybe even two psychoactive medication exposures) should retest the interrater reliability and incorporate more detailed modeling to incorporate patient-related comorbidities and risk factors for atypical EEG findings, such as metabolic disturbances and plasma levels of the sedatives and analgesics present in ICU patients.
CONCLUSIONS
Our results demonstrate the presence of multiple atypical PSG findings (indeed 85% of epochs were atypical) in MV, critically ill patients that limit the applicability of standard sleep scoring criteria in this population. This led us to the development and preliminary testing of a scoring system for the critically ill, which concomitantly tracks pathologic brain states as well as sleep. These criteria should not be viewed as definitive but as a starting point for further research and discussion. These criteria must be honed and validated in future studies that include more explicit measures of level of consciousness, electrolyte, and liver function abnormalities as well as drug levels. With the eventual development of a reliable and valid method to measure sleep among patients in the ICU during the periods of exposure to psychoactive medications, the impact of sleep deprivation on cognitive function and clinical outcomes can be more clearly defined.
Acknowledgments
Dr. Watson received support from the National Institutes of Health (MO1 RR-00095) and the Vanderbilt CTSA grant UL1 RR024975-01 from NCRR/NIH. Dr. Gehlbach received support from the National Institutes of Health (K23 HL088020), the Brain Research Foundation, the Institute for Translational Medicine (CTSA grant number UL1 RR024999), and the Department of Medicine at the University of Chicago. Dr. Pandharipande is supported by a VA Career Development Award (CSRD). Dr. Bernard is supported by NIH/NCRR 3UL1RR024975-03S5. Dr. Malow is supported by the Vanderbilt CTSA grant UL1 RR024975-01 from NCRR/NIH. Dr. Ely is supported by the National Institutes of Health (R01AG027472 and R01AG035117) and the VA Clinical Science Research and Developmental (VA Merit Review Award). Dr. Watson has received an unrestricted research grant for an investigator-initiated study from Aspect Medical Systems, Inc. and honorarium from Hospira Inc. Dr. Pandharipande has received a research grant from Hospira Inc. and honoraria from Hospira Inc. and Orion Pharma. Dr. Ely has received grants from Aspect Medical Systems, Eli Lily, and honoraria from Hospira, Abbott, and Masimo. Dr. Shintani received grant support from NIH. Dr. Bernard received grant support from NCATS and CTSA (CTSA supports the Sleep Center research projects and I am PI of the CTSA. Dr. Malow received grant support from Autism Speaks and HRSA. Drs. Dittus, Ely, and Bernard received funding support from NIH. Dr. Watson received support for travel from SCCM. Dr. Ely received support for travel from Hospira. Dr. Gehlbach received support for the development of educational presentations from the ACCP Critical Care Board Review Course). Dr. Malow lectured for Autism Speaks. Dr. Ely lectured for Hospira. Dr. Ely consulted for Cumberland and Masimo. Dr. Watson consulted for Hospira (speaker's fee for The First International Meeting of Critical Care, Bogota, Columbia). Dr. Watson served as speaker at the Critical Care Congress.
Footnotes
This work was performed in Vanderbilt University Medical Center and University of Chicago.
Dr. Thompson has disclosed that she does not have any potential conflicts of interest.
REFERENCES
- 1.Cooper AB, Thornley KS, Young GB, et al. Sleep in critically ill patients requiring mechanical ventilation. Chest. 2000;117:809–818. doi: 10.1378/chest.117.3.809. [DOI] [PubMed] [Google Scholar]
- 2.Aurell J, Elmqvist D. Sleep in the surgical intensive care unit: Continuous polygraphic recording of sleep in nine patients receiving postoperative care. Br Med J (Clin Res Ed) 1985;290:1029–1032. doi: 10.1136/bmj.290.6474.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freedman NS, Gazendam J, Levan L, et al. Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. Am J Respir Crit Care Med. 2001;163:451–457. doi: 10.1164/ajrccm.163.2.9912128. [DOI] [PubMed] [Google Scholar]
- 4.Helton MC, Gordon SH, Nunnery SL. The correlation between sleep deprivation and the intensive care unit syndrome. Heart Lung. 1980;9:464–468. [PubMed] [Google Scholar]
- 5.Trompeo AC, Vidi Y, Locane MD, et al. Sleep disturbances in the critically ill patients: Role of delirium and sedative agents. Minerva Anestesiol. 2011;77:604–612. [PubMed] [Google Scholar]
- 6.Roche Campo F, Drouot X, Thille AW, et al. Poor sleep quality is associated with late noninvasive ventilation failure in patients with acute hypercapnic respiratory failure. Crit Care Med. 2010;38:477–485. doi: 10.1097/CCM.0b013e3181bc8243. [DOI] [PubMed] [Google Scholar]
- 7.Shearer WT, Reuben JM, Mullington JM, et al. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 2001;107:165–170. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
- 8.Benca RM, Quintas J. Sleep and host defenses: A review. Sleep. 1997;20:1027–1037. [PubMed] [Google Scholar]
- 9.White DP, Douglas NJ, Pickett CK, et al. Sleep deprivation and the control of ventilation. Am Rev Respir Dis. 1983;128:984–986. doi: 10.1164/arrd.1983.128.6.984. [DOI] [PubMed] [Google Scholar]
- 10.Iber C, Ancoli-Israel S, Chesson A, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. First Edition. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 11.Silber MH, Ancoli-Israel S, Bonnet MH, et al. The visual scoring of sleep in adults. J Clin Sleep Med. 2007;3:121–131. [PubMed] [Google Scholar]
- 12.Herkes GK, Wszolek ZK, Westmoreland BF, et al. Effects of midazolam on electroencephalograms of seriously ill patients. Mayo Clin Proc. 1992;67:334–338. doi: 10.1016/s0025-6196(12)61548-1. [DOI] [PubMed] [Google Scholar]
- 13.Banoczi W. How some drugs affect the electroencephalogram (EEG) Am J Electroneurodiagnostic Technol. 2005;45:118–129. [PubMed] [Google Scholar]
- 14.Blume WT. Drug effects on EEG. J Clin Neurophysiol. 2006;23:306–311. doi: 10.1097/01.wnp.0000229137.94384.fa. [DOI] [PubMed] [Google Scholar]
- 15.Niedermeyer E, Lopes Da Silva F. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. Fourth Edition. Baltimore: Williams & Wilkins; 1999. [Google Scholar]
- 16.Young GB, Bolton CF, Archibald YM, et al. The electroencephalogram in sepsis-associated encephalopathy. J Clin Neurophysiol. 1992;9:145–152. doi: 10.1097/00004691-199201000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Brenner RP. The interpretation of the EEG in stupor and coma. Neurologist. 2005;11:271–284. doi: 10.1097/01.nrl.0000178756.44055.f6. [DOI] [PubMed] [Google Scholar]
- 18.Young GB. The EEG in coma. J Clin Neurophysiol. 2000;17:473–485. doi: 10.1097/00004691-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Engel GL, Romano J. Delirium, a syndrome of cerebral insufficiency. J Chronic Dis. 1959;9:260–277. doi: 10.1016/0021-9681(59)90165-1. [DOI] [PubMed] [Google Scholar]
- 20.Edwards GB, Schuring LM. Pilot study: Validating staff nurses’ observations of sleep and wake states among critically ill patients, using polysomnography. Am J Crit Care. 1993;2:125–131. [PubMed] [Google Scholar]
- 21.Bosma K, Ferreyra G, Ambrogio C, et al. Patient-ventilator interaction and sleep in mechanically ventilated patients: Pressure support versus proportional assist ventilation. Crit Care Med. 2007;35:1048–1054. doi: 10.1097/01.CCM.0000260055.64235.7C. [DOI] [PubMed] [Google Scholar]
- 22.Toublanc B, Rose D, Glérant JC, et al. Assist-control ventilation vs. low levels of pressure support ventilation on sleep quality in intubated ICU patients. Intensive Care Med. 2007;33:1148–1154. doi: 10.1007/s00134-007-0659-2. [DOI] [PubMed] [Google Scholar]
- 23.Cabello B, Thille AW, Drouot X, et al. Sleep quality in mechanically ventilated patients: Comparison of three ventilatory modes. Crit Care Med. 2008;36:1749–1755. doi: 10.1097/CCM.0b013e3181743f41. [DOI] [PubMed] [Google Scholar]
- 24.Drouot X, Roche-Campo F, Thille AW, et al. A new classification for sleep analysis in critically ill patients. Sleep Med. 2012;13:7–14. doi: 10.1016/j.sleep.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 25.Gehlbach BK, Chapotot F, Leproult R, et al. Temporal disorganization of circadian rhythmicity and sleep-wake regulation in mechanically ventilated patients receiving continuous intravenous sedation. Sleep. 2012;35:1105–1114. doi: 10.5665/sleep.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: Reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289:2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 27.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: Validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 28.Ely EW, Baker AM, Dunagan DP, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med. 1996;335:1864–1869. doi: 10.1056/NEJM199612193352502. [DOI] [PubMed] [Google Scholar]
- 29.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): A randomised controlled trial. Lancet. 2008;371:126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 30.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: Validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 31.Jasper HH. The ten twenty electrode system of the International Federations. Electroencephalogr Clin Neurophysiol. 1958;10:371–375. [PubMed] [Google Scholar]
- 32.Markand ON. Pearls, perils, and pitfalls in the use of the electroencephalogram. Semin Neurol. 2003;23:7–46. doi: 10.1055/s-2003-40750. [DOI] [PubMed] [Google Scholar]
- 33.Young GB, McLachlan RS, Kreeft JH, et al. An electroencephalographic classification for coma. Can J Neurol Sci. 1997;24:320–325. doi: 10.1017/s0317167100032996. [DOI] [PubMed] [Google Scholar]
- 34.Cohen J. Weighted kappa: Nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull. 1968;70:213–220. doi: 10.1037/h0026256. [DOI] [PubMed] [Google Scholar]
- 35.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Washington, DC: U.S. Department of Health, Education, and Welfare Public Health Service; 1968. [Google Scholar]
- 36.Marzec ML, Malow BA. Approaches to staging sleep in polysomnographic studies with epileptic activity. Sleep Med. 2003;4:409–417. doi: 10.1016/s1389-9457(03)00112-6. [DOI] [PubMed] [Google Scholar]
- 37.Himanen SL, Hasan J. Limitations of Rechtschaffen and Kales. Sleep Med Rev. 2000;4:149–167. doi: 10.1053/smrv.1999.0086. [DOI] [PubMed] [Google Scholar]
- 38.Ambrogio C, Koebnick J, Quan SF, et al. Assessment of sleep in ventilator-supported critically Ill patients. Sleep. 2008;31:1559–1568. doi: 10.1093/sleep/31.11.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santamaria J, Högl B, Trenkwalder C, et al. Scoring sleep in neurological patients: The need for specific considerations. Sleep. 2011;34:1283–1284. doi: 10.5665/SLEEP.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watson PL, Shintani AK, Tyson R, et al. Presence of electroencephalogram burst suppression in sedated, critically ill patients is associated with increased mortality. Crit Care Med. 2008;36:3171–3177. doi: 10.1097/CCM.0b013e318186b9ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andresen J, King MS, Davidson MA, et al. Deeper sedation during coma as measured by bispectral index monitoring is associated with delirium upon emergence from coma. Intensive Care Med. 2010;36:S222. [Google Scholar]
- 42.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 43.Kamdar BB, King LM, Collop NA, et al. The effect of a quality improvement intervention on perceived sleep quality and cognition in a medical ICU. Crit Care Med. 2013;41:800–809. doi: 10.1097/CCM.0b013e3182746442. [DOI] [PMC free article] [PubMed] [Google Scholar]