Abstract
In the basic sciences, many researchers now use gap pre-pulse inhibition of the acoustic startle reflex (GPIAS) to determine if an animal has tinnitus after exposure to an ototoxic drug or intense noise. Tinnitus is assumed to be present if the silent gap in an ongoing narrow band noise (NBN) fails to suppress the startle reflex response evoked by an intense noise burst. The lack of gap pre-pulse inhibition presumably occurs because tinnitus fills in the silent intervals in the background noise. To test the perceptual aspects of this hypothesis, we asked hearing impaired subjects with tinnitus if they could perceive 50 ms silent intervals presented in a NBN, which was located above, below or at the subject’s tinnitus pitch. The same tests were performed on normal hearing subjects without tinnitus. All subjects, with and without tinnitus, could detect the 50 ms gaps. Thus, using the stimulus parameters similar to those employed in animal and human GPIAS studies, we found that the tinnitus percept does not fill in the silent interval in a perceptual gap detection task; however, these finding do not rule out the possibility that tinnitus interferes with pre-attentive filtering of sensory stimuli in the GPIAS sensorimotor gating paradigm.
Keywords: Acoustic startle reflex, gap detection, perception, pre-pulse inhibition, tinnitus
Introduction
Subjective tinnitus, the perception of a phantom sound in the absence of an external stimulus, affects 15–17% of the population and about 1% experience serious or disabling symptoms.[1,2] Subjective tinnitus is usually perceived as a ringing, humming, hissing, chirping or whistling sensation and is associated with a numerous hearing pathologies such as presbycusis, noise exposure, otitis media, Meniere’s disease, vestibular schwannoma and whiplash.[3] In most clinical settings, a detailed case history is taken to identify conditions that could have triggered the onset of tinnitus. Questionnaires such as the tinnitus handicap inventory, tinnitus handicap questionnaire and mini-tinnitus questionnaire are often administered to gauge its emotional severity, degree of annoyance, disability, sleep disturbance and social impact.[4,5] Psychoacoustic measurements are frequently obtained of the frequency and intensity of external sounds that match the pitch and loudness of the internal phantom sensation.[6–8] In addition, the minimum intensity of broad band or narrow band noise (NBN) needed to mask the tinnitus is often determined.[8]
Due to the subjective nature of tinnitus and legal and disability issues, there has been considerable interest in identifying reliable, efficient and cost-effective methods to objectively assess tinnitus in humans and animal models. Powerful, new structural and functional brain imaging techniques have proved useful in identifying regions of aberrant neural activity in the human central nervous system that appear to be linked to tinnitus.[9–14] However, most of these techniques are time consuming and expensive and in many cases the results have been variable or have not yet been replicated. While several other methods have been suggested as objective measures of tinnitus in humans,[15,16] none of these have gained widespread acceptance as objective measures that can be used to assess tinnitus in individual subjects.
Auditory neuroscientists have attempted to develop behavioral and electrophysiological methods for objectively assessing tinnitus in animals.[17–23] One very efficient and inexpensive paradigm that is being increasingly used to objectively assess tinnitus in animals is gap pre-pulse inhibition of the acoustic startle reflex (GPIAS).[24–30] The behavioral measure in this model is the startle reflex, a short latency, robust motoric response elicited by the abrupt onset of an acoustic startle stimulus, typically an intense, short duration noise burst presented at random inter-stimulus intervals.[31,32] In the GPIAS paradigm, the acoustic startle stimulus is presented on a background of continuous, low-intensity (60 dB sound pressure level [SPL]) NBN. The background noise remains on continuously for 50% of the trials in a session, but on the other half of the trials, a brief (50 ms) silent interval (gap) is inserted into the ongoing background noise 100 ms prior to the onset of the startle stimulus. The silent gap serves as a pre-pulse that in normal hearing animals inhibits the startle response, i.e., gap pre-pulse inhibition.[33,34] However, in animals with putative noise-induced or salicylate-induced tinnitus, the silent gap fails to suppress the startle response at certain frequencies of the background noise.[24,25,27,28,35] In these cases, absent or very weak GPIAS at specific frequencies of the background noise is interpreted as evidence that the animal is experiencing tinnitus with a pitch similar to the background noise.[24–30] We and others have hypothesized that when the pitch of tinnitus is similar to the frequency of the background noise, then the internal phantom sound “fills in” the silent gap in the NBN thereby reducing or eliminating gap pre-pulse inhibition.[24–30] The GPIAS paradigm was recently evaluated in humans using the eye blink response as the startle readout metric.[36] In subjects with mild, high frequency hearing loss (HL) (>8 kHz) and high-pitched tinnitus, gap inhibition was significantly less than in normal controls; however, gap inhibition was depressed with low-frequency background noise, where tinnitus was absent as well as high-frequency background noise where tinnitus was present. The fact that the gap deficit was present at the low frequencies raises questions about the frequency specific nature of the GPIAS paradigm and raises questions about whether the tinnitus is “filling in” the silent gap in the background noise.
If the hypothesis that tinnitus “fills in” the silent intervals in the background noise is correct, then subjects with tinnitus should show an impaired ability or should be unable to detect silent intervals in NBNs tuned to the pitch of their tinnitus. Conversely, these same subjects should be able to detect silent gaps in NBNs presented above or below the tinnitus pitch because the phantom sound of tinnitus is spectrally different from their tinnitus. To test this hypothesis, we evaluated 13 subjects with tinnitus (mean age 50 years) and varying degrees of HL and compared their results to 13 normal hearing subjects without tinnitus. Long duration silent gaps (50 ms) were employed to match previous animal and human studies and to minimize temporal acuity deficits as a source of impaired performance in the hearing impaired subjects.[37]
Methods
Subjects were recruited from flyers mailed to members of the University at Buffalo Tinnitus support group and from flyers posted in the University at Buffalo Speech and Hearing Clinic and in public areas at the university. The research project and recruitment notices were approved by the Institutional Review Board at the University at Buffalo and the research was carried out in accordance with the Declaration of Helsinki and National Institutes of Health (NIH) guidelines. Participants were informed about the nature of the study and signed a consent form prior to beginning the project. Participants were informed that they could elect to withdraw from this study at any point. Patients of both sexes above the age 18 were invited to participate in the study. Inclusion criteria for the tinnitus group included the presence of tinnitus for more than 3 months and the ability to perform the hearing tests. Exclusion criteria consisted of (1) tinnitus patients with intermittent and fluctuating tinnitus or (2) if the intensity of the NBN stimuli presented at 15 dB sensation level (SL) exceeded the limits of the audiometer or exceeded the subject’s loudness discomfort level. For the non-tinnitus subjects, inclusion criteria were thresholds of 20 dB HL or less from 0.25 to 8 kHz, 35 dB or less above 8 kHz and the ability to perform the hearing tasks.
Subjects
A total of 26 subjects participated in this study. The tinnitus group consisted of nine males and four females with a mean age of 50 years of age. The non-tinnitus group consisted of four males and nine females with a mean age of 24 years of age.
Audiometry
Air conduction and bone conduction thresholds were measured in an audiometric sound booth using a Grason Stadler (GSI)-61 audiometer and standard audiometric procedures. Air conducted thresholds were measured using pulsed tones from 0.25 to 8 kHz under TDH50P headphones; high frequency thresholds were measured at 10, 12.5 and 16 kHz using Sennheiser HDA 200 headphones. Bone conducted thresholds were measured using a Radio Ear B-71 bone oscillator.
Gap stimuli
One-third octave wide, NBN (one-third octave intervals, center frequencies from 1,000 to 16,000 Hz) of 90 s duration were generated digitally (NCH tone generator, V 2.00) and stored on the hard disk of a personal computer. Eighteen gaps (0.1 ms rise/fall time) of 50 ms duration were inserted into each NBN at randomly spaced intervals; these NBN gap stimuli were also stored on the disk of the computer. NBN stimuli and NBN gap stimuli were played out through the D/A converter (44 kHz sampling rate, 16 bit D/A) on the computer, routed to the GSI high frequency audiometer and presented to subjects through Sennheiser HDA 200 headphones.
For the tinnitus subjects, tinnitus pitch matching was conducted with the NBN stimuli. Subjects were first instructed to select the NBN that most closely matched the pitch of their tinnitus. Then the NBN was presented at a comfortable listening level to the ear contralateral to the subject’s tinnitus. Pairs of pseudorandomly selected frequencies of NBN were presented on each trial and the subject was asked to report, which one was closest to their tinnitus. After a series of iterative comparisons (15–20 pairs), the tinnitus pitch was defined as the center frequency of the NBN that most closely matched the tinnitus percept on three consecutive trials. After determining the tinnitus pitch, the intensity of the NBN was varied using standard audiometric (1 dB steps) procedures to determine the threshold of the NBN at the frequency of the tinnitus pitch match. Once threshold was determined at the tinnitus frequency, the subject was instructed to match the intensity of the NBN so that it was equal in loudness of their tinnitus. The NBN stimulus was presented to the tinnitus ear and gradually increased or decreased in 1 dB steps until the subject matched the intensity of the NBN to the loudness of their tinnitus. Tinnitus loudness was defined as the intensity that matched the loudness of the tinnitus on three consecutive trials. Thresholds were also determined for NBN presented one octave above the tinnitus pitch and one octave below the tinnitus pitch. The pitch matching data from the tinnitus subjects were evaluated to identify the most common pitch matching frequencies (modes) among the 13 subjects with tinnitus. The three most common frequencies for the pitch match were 1.2 kHz, 8 kHz and 12.6 kHz. These three frequencies were used to test the gap detection performance of the normal subjects without tinnitus.
The NBN gap stimuli were presented at a level 15 dB above the NBN threshold (i.e., 15 dB SL) for normal hearing subjects without tinnitus and the tinnitus subjects. For the subjects with tinnitus, the NBN gap stimuli were presented at the tinnitus frequency, one octave above the tinnitus frequency and one octave below the tinnitus frequency. NBN gap detection performance was evaluated when the NBN gaps were presented to the tinnitus ear as well as the contralateral ear. Because some tinnitus subjects had very high thresholds, the NBN gap stimuli could not be presented at 15 dB SL at the designated test frequencies. Consequently, in the right ear, NBN gap detection was assessed 1-octave below, 1-octave above and at the tinnitus frequency in 11, 11 and 12 subjects respectively. In the left ear, NBN gap detection was assessed 1-octave below, 1-octave above and at the tinnitus frequency in 11, 10 and 11 subjects respectively. In 13 normal hearing subjects without tinnitus, NBN gap detection was assessed at 1.2 kHz, 8 kHz and 12.6 kHz, three common frequencies that tinnitus subjects matched their tinnitus pitch to [Table 1].
Table 1.
Tinnitus Characteristics of each subject: Td: location of dominant tinnitus percept. Type: description of tinnitus. Pitch Match NBN: frequency of NBN noise matched to Td. Loudness Match: tinnitus loudness expressed in dB SL at Pitch Match NBN. Minimum Masking Level: dB SL of NBN that masked Td.
| Subject | Td | Type | Pitch Match NBN (Hz) |
Loudness Match dB SL |
Minimum Masking Level dB SL |
|---|---|---|---|---|---|
| 1 | R & L | Hissing | 4000 | 15 | 20 |
| 2 | R | Ringing | 1000 | 3 | 10 |
| 3 | C | Buzzing | 1260 | 1 | 25 |
| 4 | L | Buzzing | 10079 | 2 | 20 |
| 5 | L | Ringing | 8000 | 0 | 25 |
| 6 | L | Buzzing | 12699 | 4 | 10 |
| 7 | C | Ringing | 16000 | 32 | 55 |
| 8 | C | Ringing | 8000 | 3 | CNM |
| 9 | R & L | Ringing | 6350 | 5 | 15 |
| 10 | R | Buzzing | 12699 | 2 | 15 |
| 11 | R | Pulsing | 16000 | 28 | 25 |
| 12 | C | Hissing | 12699 | 18 | 25 |
| 13 | R & L | Ringing | 6350 | 4 | 5 |
TD: dominant pitch, R: right ear, L: left ear, R & L: right and left ear, C: central, CNM: could not mask
Prior to beginning the NBN gap testing, all subjects were instructed to quickly press the response button connected to the audiometer if they detected a gap and to refrain from pressing the button if they did not detect a gap. The experimenter monitored the presentation of the gap in the NBN on the computer monitor and recorded the participant’s responses to trials with a NBN gap (i.e., correct responses within ~2 s of the gap); other responses occurring outside this ~2 s response window during the remainder of the 90 s NBN gap presentations were considered false positive responses. The false positive response interval was ~54 s (90 s stimulus minus 18 2-s NBN response intervals).
Results
Hearing levels
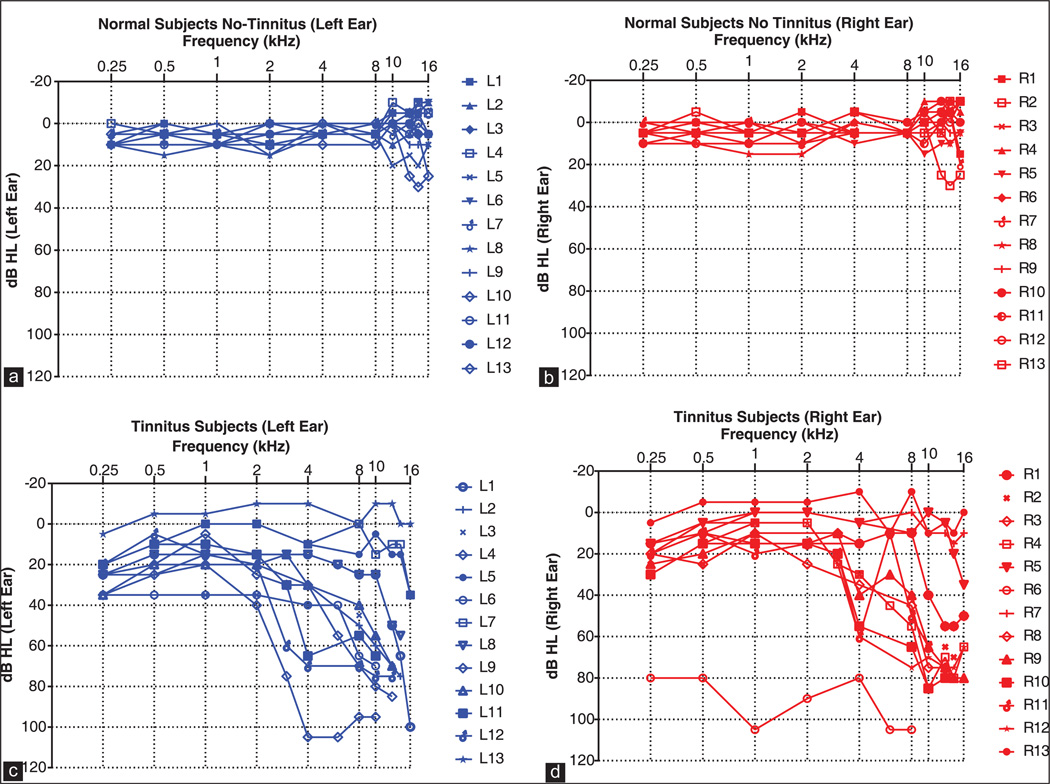
Figure 1a and b shows the audiograms from 13 normal hearing subjects without tinnitus in the left and right ears. Hearing thresholds in the left and right ears were <20 dB HL between 0.25 and 16 kHz except for one subject whose thresholds ranged from 20 to 30 dB HL between 10 and 16 kHz. Hearing thresholds in the subjects with tinnitus were typically higher than non-tinnitus subjects. Hearing thresholds in the left ear were typically <40 dB HL at 2 kHz or below except for one subject that had thresholds between 80 and 100 dB HL in the right ear. Thresholds above 2 kHz generally increased with frequency rising to 60–80 dB HL for most subjects. However, a few subjects had thresholds <20 dB HL at most frequencies while one had thresholds between 80 and 100 dB HL. For each tinnitus subject, Table 1 shows the location (right ear, left ear, right and left ear or centrally located in the head) of the dominant tinnitus (Td), the subjects description of the tinnitus (e.g., buzzing, ringing or hissing etc.), the NBN pitch matched to Td, the intensity of the NBN that matched the loudness of Td and the NBN minimum masking level of Td.
Figure 1.
Left ear audiograms and right ear audiograms shown for 13 normal subjects (a and b) and 13 tinnitus subjects (c and d)
NBN gap detection in tinnitus subjects
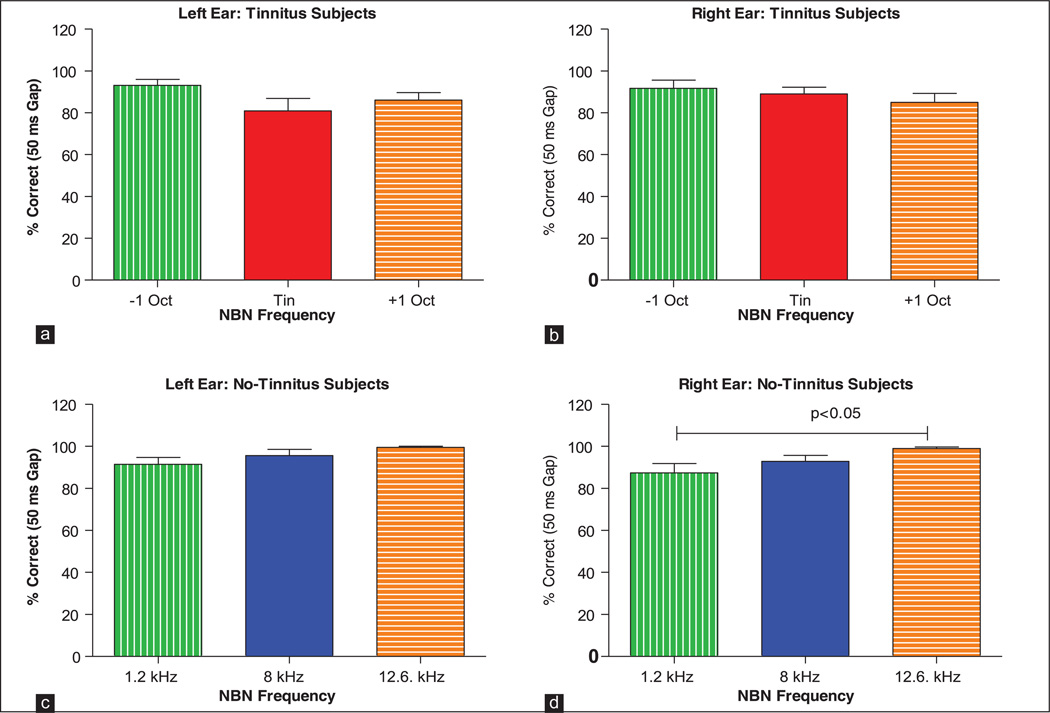
Figure 2a and b shows the percentages of correct responses of tinnitus subjects on the NBN gap detection tasks. (Note: Since the mean numbers of false positive responses were extremely low in tinnitus and no-tinnitus subjects, typically 1–2 false positives in 90 s, we did not attempt to correct for false positive responses.) The 50 ms silent gaps were embedded in one-third octave bands of noise located 1-octave below, 1-octave above and at the pitch of the subject’s tinnitus. Despite the fact that the NBN were only 15 dB above threshold, mean (+standard error of mean [SEM]) percentages of gaps detected in the NBN were quite high in the subjects with tinnitus ranging from 81% to 94% in the left ear and 85–92% in the right ear. A Kruskal-Wallis one-way analysis of variance on ranks performed on the data from the left ears (H = 3.636, 2 df) and right ears (H = 1.469, 2 df) of tinnitus subjects indicated that there were no significant differences (P > 0.05) in the percentage of correct responses across the three NBN frequencies (1-octave below, 1-octave above and at the tinnitus frequency). Importantly, tinnitus subjects were able to detect the silent gaps located in NBN located at the tinnitus frequency with the same precision as when the NBN was located above or below the tinnitus frequency.
Figure 2.
Mean (standard error of mean [SEM], n = 13) data for tinnitus subjects showing the percent correct detection of 50 ms gaps embedded in narrow band noise (NBN) located 1-octave below, 1-octave above and at the subject’s tinnitus frequency. Results shown for NBN presented to the left ear (a) and right ear (b) of tinnitus subjects and left ear (c) and right ear (d) of no-tinnitus subjects. Mean (SEM, n = 13) data for no-tinnitus subjects showing the percent correct detection of 50 ms gaps embedded in NBN located at 1.2, 8 and 12.6 kHz
NBN gap detection in no-tinnitus subjects
Since HL and tinnitus could potentially affect performance on the NBN gap detection task, we tested the ability of normal hearing subjects without tinnitus to detect 50 ms gaps embedded in NBN located at 1.2, 8 and 12.6 kHz; frequencies at which the tinnitus pitch was often present in out tinnitus subjects. Figure 2c and d shows the percentages of 50 ms gaps that were detected in the left ear and right ear of normal hearing subjects without tinnitus. Mean (SEM, n = 13) gap performance ranged from 91% to 99% in the left ear and from 87% to 99% in the right ear. There was no significant difference in performance in the left ear across the three frequencies (Kruskal-Wallis one-way analysis of variance on ranks, H = 5.559, 2 df, P > 0.05). However, in the right ear, there was a small, but significant difference in performance across the three frequencies with performance at 12.6 kHz being significantly better than that at 1 kHz (Kruskal-Wallis one-way analysis of variance on ranks, H = 7.683, 2 df, Tukey post-hoc analysis, P < 0.05).
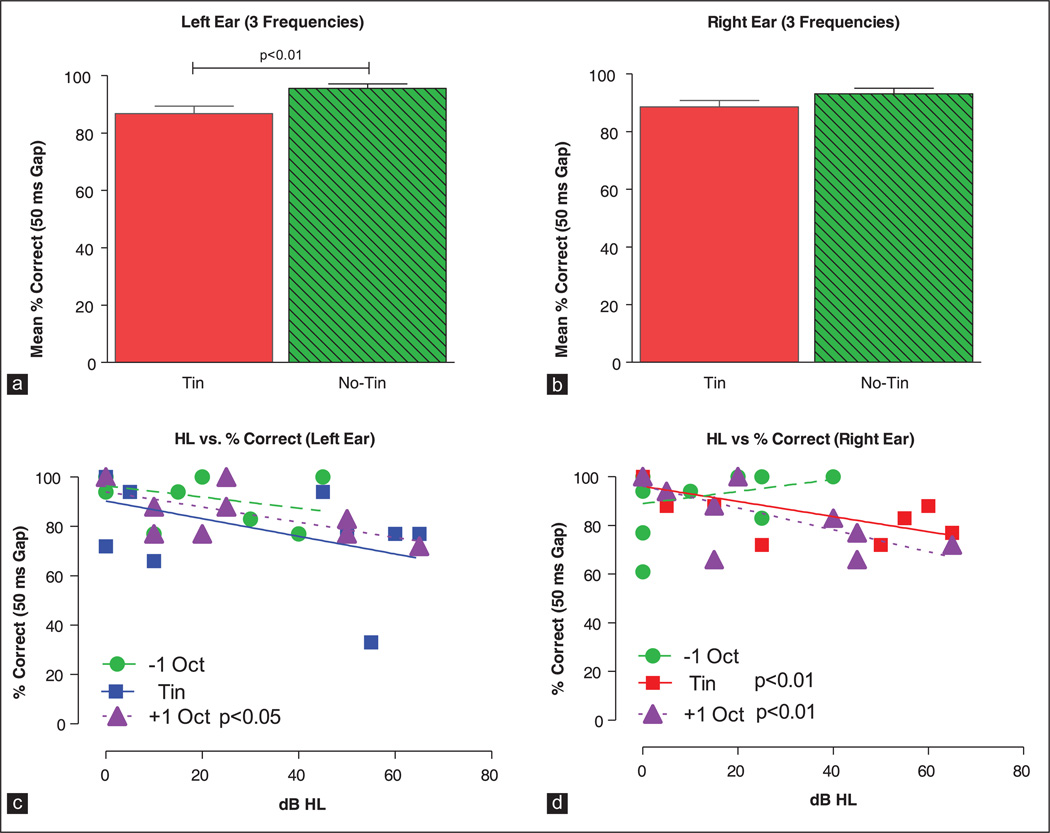
Since the gap detection data from no-tinnitus and tinnitus subjects were collected at different frequencies, an exact comparison of their results is not possible. To overcome this limitation, we compared the mean performance of tinnitus subjects (above, below and at the tinnitus frequency) with the mean performance of no-tinnitus subjects at 1, 8 and 12.6 kHz. The mean percent correct in the left ears of no-tinnitus subjects was 95.5% (standard deviation [SD]: 9.2, n = 36) versus 86.8% (SD: 14.9, n = 32) in the left ears of tinnitus subjects; this difference was statistically significant [Figure 3a; Mann-Whitney rank sum test, U = 800, P < 0.01]. In the right ears, the mean percent correct in no-tinnitus subjects was 93% (SD: 12.1, n = 36) versus 88.6% (SD: 12.8, n = 34) in the tinnitus subjects; this difference was not significant [Figure 3b; Mann-Whitney rank sum test, U = 796, P = 0.114].
Figure 3.
Mean (standard error of mean [SEM], n = 13) data for tinnitus subjects (3 frequencies, 1-octave below, 1-octave above and at the tinnitus frequency) and no-tinnitus subjects (3 frequencies, 1.2, 8 and 12.6 kHz) for the left ear (a) and right ear (b). Mean scores of tinnitus subjects were significantly less (P < 0.01) than no-tinnitus subjects in the left ear. Scatterplot showing the percent correct detection of 50 ms gaps versus dB HL in the left ear (c) and right ear (d) of tinnitus subjects. Data and linear regression lines are shown for frequencies 1-octave below, 1-octave above and at the tinnitus frequency. significant values for the regression analysis are shown in the legend of each panel
NBN detection and HL
HL is known to impair gap detection performance; however, the effects of HL are predominantly seen with very short duration gaps and presumably exert little or no impact on long duration gaps such as those employed here and in animal studies utilizing GPIAS to test for tinnitus.[37–39] To evaluate this possibility, gap detection performance was plotted as a function of HL in the left and right ear of tinnitus subjects. Data obtained above, below and at the tinnitus frequency of the left ear and right ear were subjected to linear regression analysis as shown in Figure 3c and d. In the left ear [Figure 3c], percent correct scores decreased slightly with increasing HL, but only the results 1-octave above the tinnitus frequency showed a statistically significant decrease (slope −0.31%/dB, F = 5.72, 1, 8 df, P < 0.05). In the right ear [Figure 3d], percent correct scores again decreased slightly, but significantly with HL 1-octave above (slope −0.45%/dB, F = 10.21, 1, 9 df, P < 0.01) and at the tinnitus frequency (slope −0.31%/dB, F = 12.8, 1, 10 df, P < 0.01).
Discussion
Does tinnitus fill in” silence in the gap?
The GPIAS paradigm has been frequently used in auditory neuroscience to obtain evidence of tinnitus in animals[24,25,28,30] and more recently a modified gap-startle paradigm was used to test for tinnitus in humans.[36] The underlying hypothesis of GPIAS is that tinnitus “fills in” the silent gap that precedes the acoustic startle stimulus. Evidence from some animal studies has suggested that the “filling in” of the gap often spans several test frequencies, whereas others have shown that GPIAS impairment is limited to a narrow range of frequencies associated with noise induce tinnitus. Presumably, if the spectrum of the background noise containing the gap matches the pitch of the tinnitus, then the ability to hear the silent interval (gap) is impaired. Moreover, gaps in NBN that no longer inhibit the startle response are used to index the pitch of the subject’s tinnitus. In contrast, a silent gap embedded in a NBN located above or below the tinnitus pitch should not inhibit the startle response as has been reported in some of the animal studies. Here, we tested this hypothesis by asking subjects if they could detect silent gaps embedded in NBN located above, below or at the tinnitus pitch. The gaps employed were 50 ms in order to match previous animal and human studies and to eliminate temporal acuity deficits as a confounding effect of HL, i.e., gap thresholds greater than 40 ms are seldom if ever seen in subjects with sensorineural HL.[37] Our results indicate that tinnitus subjects are able to detect the silent gaps on 81–89% of the trials [Figure 2a and b] when the spectrum of the NBN matches the pitch of the tinnitus. Moreover, gap detection performance at the tinnitus frequency was similar to performance one octave below or one octave above the tinnitus frequency, i.e., there was no significant difference between gap detection performance at the tinnitus frequency versus frequencies 1 octave above or below the tinnitus frequency. These results indicate that even when the spectrum of the NBN was near the tinnitus pitch and the patient was experiencing tinnitus the “gaps” were readily detected. Taken together, these results suggest that with the gap stimuli employed in our study, which were similar to those used in previous human and animals studies, the phantom sound of tinnitus did not simply fill in the silent interval as previously hypothesized by ourselves and others.[24,25,28,30] However, this interpretation needs to be tempered by the fact that the stimulus parameters employed in our study produced gap detection scores close to 100%. This near-ceiling effect in gap performance may have made the detection task insensitive to slight differences in performance between tinnitus and no-tinnitus subjects. The ceiling effect in gap performance could be minimized by reducing the duration of the gap. However, this would shift the nature of the task to one emphasizing temporal resolution. In contrast, advocates of GPIAS emphasize the use of long duration gaps in order to rule out impaired temporal resolution as a confounding factor in GPIAS tests of tinnitus. A final cautionary note is that our moderate samples sizes (n = 13) may have been underpowered to detect slight differences in performance between tinnitus and non-tinnitus subjects or performance differences at the tinnitus frequency versus NBN located 1-octave above or 1-octave below the tinnitus frequency.
GPIAS and gap detection
The present results highlight a discrepancy between gap detection and GPIAS performance. Several factors could contribute to this disparity. One possible explanation for the lack of correspondence between gap detection and GPIAS is that the former is a perceptual task involving attention,[40] whereas the latter is a sensorimotor gating phenomenon that presumably involves pre-attentive filtering of sensory stimuli.[41] Thus, it is conceivable that tinnitus could interfere with the GPIAS, but not prevent the subject from perceiving the gap altogether. Further studies are needed to determine if humans with tinnitus show impaired GPIAS performance when tested with acoustic stimuli similar to those employed here (i.e., NBN at 15 dB SL).
Differences in the acoustic stimuli used in GPIAS testing and our gap detection study need to be considered. Most animal studies of GPIAS present the noise containing the gap at 60 dB SPL. In the case of a rat, a 60 dB SPL signal would be approximately 55 dB SL near 16 kHz and 40–45 dB SL an octave below or above 16 kHz. Detecting a 50 ms gap presented at 40–55 dB SL would be substantially easier than detecting a silent gap presented at 15 dB SL.[42,43] However, in many of our subjects with HL [Figure 1c and d], the 15 dB SL NBN could conceivably provide strong loudness cues due to recruitment,[44,45] but this caveat would not apply to a few of our tinnitus subjects that had nearly normal hearing. In either case, gap detection was not impaired near the tinnitus frequency.
Another acoustic factor to consider is the bandwidth of the NBN. The one-third octave band NBN used in our human study are identical to those used in GPIAS studies of tinnitus in mice[46] and similar in bandwidth to the low frequency NBN used for GPIAS testing in rats.[24,25] Thus, stimulus bandwidth is unlikely to explain why our human subjects were proficient in detecting the gaps in our NBN. Although our subjects were able to match the NBN to the pitch of the tinnitus, the perceptual characteristics of the NBN could be still be different enough from the perceptual characteristics of the subject’s tinnitus to make it easily detectable [Table 1] (hissing, buzzing, ringing). These subtle perceptual features may be unavailable to animal subjects during the GPIAS testing. Finally, the NBN stimuli used in our human studies had abrupt rise/ fall times (~0.1 ms), which could generate spectral cues to aid detection. Because the NBN used in this study were presented at a very low intensity (15 dB SL), spectral cues would likely be of little or no value because they would be at or below the threshold. However, spectral cues might play a more important role in GPIAS because the NBN are presented at higher SL than those used here.
HL and gap detection
HL has long been known to impair temporal resolution and the detection of very short duration gaps at low SL levels. However, HL has little or no effect on detecting long duration gaps (>30 ms) except in cases of significant (>60 dB) hearing impairment.[37–39,42,47] Our results are generally consistent with previous results. Detection of the 50 ms gaps decreased slightly with increasing HL indicating a slight effect of HL on gap performance [Figure 3c and d]. Gap detection was slightly poorer (9%) in the left ear of tinnitus subjects with HL than normal hearing subjects [Figure 3a], but no differences were seen when comparing results from the right ear [Figure 3b]. While a small left ear difference was seen between tinnitus and no-tinnitus subjects, this difference could be due to HL [Figure 3c and d] and the fact that these Tinnitus subject were tested at different frequencies than the no-tinnitus subjects [Figure 2a and b vs. 2c and d]. The key point of Figure 2a and b is that tinnitus subjects were able to detect the gaps the vast majority of the time at the tinnitus frequency and there was no significant performance difference between the tinnitus frequency and frequencies above or below this frequency. These results are consistent with previous data showing that salicylate and noise induced HL do not significantly interfere with the detection of long duration gaps such as the 50 ms gap used in this study.[47–49]
One limitation of the current study design was the lack of subject matching when comparing 50 ms gap performance of tinnitus and no-tinnitus groups. A more robust experimental design would be to match each tinnitus subject with a no-tinnitus subject in terms of HL and then to test gap performance in the no-tinnitus subject at the same frequencies used for the tinnitus subject. Such a comparison would avoid the confounding effects of HL and frequency mismatch as occurred in Figure 2a and b versus Figure 2c and d.
Summary
An underlying assumption of the GPIAS paradigm is that the phantom sound of tinnitus “fills in” the silent gap in the background noise and prevents the silent gap from suppressing the startle reflex. We tested the “fill in” hypothesis in human subjects with tinnitus using gap detection stimulus parameters similar to those employed in previous animal and human studies and found that our tinnitus subjects were able to detect the silent gaps regardless of whether the NBN was above, below or near the tinnitus frequency. Thus, with stimulus parameters similar to those used in animal and human studies, we found that tinnitus does not simply “fill in” the silent intervals embedded in the background noise. This raises the possibility that the underlying perceptual processes that mediate the detection of gaps in NBN may be different from those involved with gap-inhibition of the startle response in GPIAS paradigms.
Acknowledgment
Research supported in part by grants from NIH (R01DC009091-05; R01DC009219-05) and ONR (N000141210731).
Footnotes
Conflict of Interest: None declared.
References
- 1.Cooper JC., Jr. Health and nutrition examination survey of 1971–75: Part II. Tinnitus, subjective hearing loss, and well-being. J Am Acad Audiol. 1994;5:37–43. [PubMed] [Google Scholar]
- 2.Axelsson A, Ringdahl A. Tinnitus – A study of its prevalence and characteristics. Br J Audiol. 1989;23:53–62. doi: 10.3109/03005368909077819. [DOI] [PubMed] [Google Scholar]
- 3.Chan Y. Tinnitus: Etiology, classification, characteristics, and treatment. Discov Med. 2009;8:133–136. [PubMed] [Google Scholar]
- 4.Hiller W, Goebel G. Factors influencing tinnitus loudness and annoyance. Arch Otolaryngol Head Neck Surg. 2006;132:1323–1330. doi: 10.1001/archotol.132.12.1323. [DOI] [PubMed] [Google Scholar]
- 5.Figueiredo RR, Rates MA, Azevedo AA, Oliveira PM, Navarro PB. Correlation analysis of hearing thresholds, validated questionnaires and psychoacoustic measurements in tinnitus patients. Braz J Otorhinolaryngol. 2010;76:522–526. doi: 10.1590/S1808-86942010000400018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terry AM, Jones DM, Davis BR, Slater R. Parametric studies of tinnitus masking and residual inhibition. Br J Audiol. 1983;17:245–256. doi: 10.3109/03005368309081485. [DOI] [PubMed] [Google Scholar]
- 7.Henry JA, Flick CL, Gilbert A, Ellingson RM, Fausti SA. Comparison of manual and computer-automated procedures for tinnitus pitch-matching. J Rehabil Res Dev. 2004;41:121–138. doi: 10.1682/jrrd.2004.02.0121. [DOI] [PubMed] [Google Scholar]
- 8.Tyler RS, Conrad-Armes D. Masking of tinnitus compared to masking of pure tones. J Speech Hear Res. 1984;27:106–111. doi: 10.1044/jshr.2701.106. [DOI] [PubMed] [Google Scholar]
- 9.Pantev C, Hoke M, Lütkenhöner B, Lehnertz K, Kumpf W. Tinnitus remission objectified by neuromagnetic measurements. Hear Res. 1989;40:261–264. doi: 10.1016/0378-5955(89)90167-6. [DOI] [PubMed] [Google Scholar]
- 10.Melcher JR, Sigalovsky IS, Guinan JJ, Jr, Levine RA. Lateralized tinnitus studied with functional magnetic resonance imaging: Abnormal inferior colliculus activation. J Neurophysiol. 2000;83:1058–1072. doi: 10.1152/jn.2000.83.2.1058. [DOI] [PubMed] [Google Scholar]
- 11.Schlee W, Hartmann T, Langguth B, Weisz N. Abnormal resting-state cortical coupling in chronic tinnitus. BMC Neurosci. 2009;10:11. doi: 10.1186/1471-2202-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorenz I, Müller N, Schlee W, Hartmann T, Weisz N. Loss of alpha power is related to increased gamma synchronization-A marker of reduced inhibition in tinnitus? Neurosci Lett. 2009;453:225–228. doi: 10.1016/j.neulet.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 13.Reyes SA, Salvi RJ, Burkard RF, Coad ML, Wack DS, Galantowicz PJ, et al. Brain imaging of the effects of lidocaine on tinnitus. Hear Res. 2002;171:43–50. doi: 10.1016/s0378-5955(02)00346-5. [DOI] [PubMed] [Google Scholar]
- 14.Lockwood AH, Salvi RJ, Coad ML, Towsley ML, Wack DS, Murphy BW. The functional neuroanatomy of tinnitus: Evidence for limbic system links and neural plasticity. Neurology. 1998;50:114–120. doi: 10.1212/wnl.50.1.114. [DOI] [PubMed] [Google Scholar]
- 15.Das SK, Wineland A, Kallogjeri D, Piccirillo JF. Cognitive speed as an objective measure of tinnitus. Laryngoscope. 2012;122:2533–2538. doi: 10.1002/lary.23555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodwin PE, Johnson RM. A comparison of reaction times to tinnitus and nontinnitus frequencies. Ear Hear. 1980;1:148–155. doi: 10.1097/00003446-198005000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Heffner HE. A two-choice sound localization procedure for detecting lateralized tinnitus in animals. Behav Res Methods. 2011;43:577–589. doi: 10.3758/s13428-011-0061-4. [DOI] [PubMed] [Google Scholar]
- 18.Jastreboff PJ, Brennan JF, Sasaki CT. An animal model for tinnitus. Laryngoscope. 1988;98:280–286. doi: 10.1288/00005537-198803000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Rüttiger L, Ciuffani J, Zenner HP, Knipper M. A behavioral paradigm to judge acute sodium salicylate-induced sound experience in rats: A new approach for an animal model on tinnitus. Hear Res. 2003;180:39–50. doi: 10.1016/s0378-5955(03)00075-3. [DOI] [PubMed] [Google Scholar]
- 20.Lobarinas E, Sun W, Cushing R, Salvi R. A novel behavioral paradigm for assessing tinnitus using schedule-induced polydipsia avoidance conditioning (SIP-AC) Hear Res. 2004;190:109–114. doi: 10.1016/S0378-5955(04)00019-X. [DOI] [PubMed] [Google Scholar]
- 21.Cazals Y, Huang ZW. Average spectrum of cochlear activity: A possible synchronized firing, its olivo-cochlear feedback and alterations under anesthesia. Hear Res. 1996;101:81–92. doi: 10.1016/s0378-5955(96)00135-9. [DOI] [PubMed] [Google Scholar]
- 22.Martin WH, Schwegler JW, Scheibelhoffer J, Ronis ML. Salicylate-induced changes in cat auditory nerve activity. Laryngoscope. 1993;103:600–604. doi: 10.1288/00005537-199306000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Liu XP, Chen L. Auditory brainstem response as a possible objective indicator for salicylate-induced tinnitus in rats. Brain Res. 2012;1485:88–94. doi: 10.1016/j.brainres.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 24.Turner JG, Brozoski TJ, Bauer CA, Parrish JL, Myers K, Hughes LF, et al. Gap detection deficits in rats with tinnitus: A potential novel screening tool. Behav Neurosci. 2006;120:188–195. doi: 10.1037/0735-7044.120.1.188. [DOI] [PubMed] [Google Scholar]
- 25.Yang G, Lobarinas E, Zhang L, Turner J, Stolzberg D, Salvi R, et al. Salicylate induced tinnitus: Behavioral measures and neural activity in auditory cortex of awake rats. Hear Res. 2007;226:244–253. doi: 10.1016/j.heares.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Turner JG, Parrish J. Gap detection methods for assessing salicylate-induced tinnitus and hyperacusis in rats. Am J Audiol. 2008;17:S185–S192. doi: 10.1044/1059-0889(2008/08-0006). [DOI] [PubMed] [Google Scholar]
- 27.Luo H, Zhang X, Nation J, Pace E, Lepczyk L, Zhang J. Tinnitus suppression by electrical stimulation of the rat dorsal cochlear nucleus. Neurosci Lett. 2012;522:16–20. doi: 10.1016/j.neulet.2012.05.072. [DOI] [PubMed] [Google Scholar]
- 28.Dehmel S, Eisinger D, Shore SE. Gap prepulse inhibition and auditory brainstem-evoked potentials as objective measures for tinnitus in guinea pigs. Front Syst Neurosci. 2012;6:42. doi: 10.3389/fnsys.2012.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norman M, Tomscha K, Wehr M. Isoflurane blocks temporary tinnitus. Hear Res. 2012;290:64–71. doi: 10.1016/j.heares.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 30.Longenecker RJ, Galazyuk AV. Methodological optimization of tinnitus assessment using prepulse inhibition of the acoustic startle reflex. Brain Res. 2012;1485:54–62. doi: 10.1016/j.brainres.2012.02.067. [DOI] [PubMed] [Google Scholar]
- 31.Davis M, Gendelman DS, Tischler MD, Gendelman PM. A primary acoustic startle circuit: Lesion and stimulation studies. J Neurosci. 1982;2:791–805. doi: 10.1523/JNEUROSCI.02-06-00791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis M, Parisi T, Gendelman DS, Tischler M, Kehne JH. Habituation and sensitization of startle reflexes elicited electrically from the brainstem. Science. 1982;218:688–690. doi: 10.1126/science.7134967. [DOI] [PubMed] [Google Scholar]
- 33.Ison JR, Taylor MK, Bowen GP, Schwarzkopf SB. Facilitation and inhibition of the acoustic startle reflex in the rat after a momentary increase in background noise level. Behav Neurosci. 1997;111:1335–1352. doi: 10.1037//0735-7044.111.6.1335. [DOI] [PubMed] [Google Scholar]
- 34.Ison JR, Agrawal P, Pak J, Vaughn WJ. Changes in temporal acuity with age and with hearing impairment in the mouse: A study of the acoustic startle reflex and its inhibition by brief decrements in noise level. J Acoust Soc Am. 1998;104:1696–1704. doi: 10.1121/1.424382. [DOI] [PubMed] [Google Scholar]
- 35.Ralli M, Lobarinas E, Fetoni AR, Stolzberg D, Paludetti G, Salvi R. Comparison of salicylate-and quinine-induced tinnitus in rats: Development, time course, and evaluation of audiologic correlates. Otol Neurotol. 2010;31:823–831. doi: 10.1097/MAO.0b013e3181de4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fournier P, Hébert S. Gap detection deficits in humans with tinnitus as assessed with the acoustic startle paradigm: Does tinnitus fill in the gap? Hear Res. 2013;295:16–23. doi: 10.1016/j.heares.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 37.Giraudi-Perry DM, Salvi RJ, Henderson D. Gap detection in hearing-impaired chinchillas. J Acoust Soc Am. 1982;72:1387–1393. doi: 10.1121/1.388444. [DOI] [PubMed] [Google Scholar]
- 38.Buus S, Florentine M. Gap detection in normal and impaired listeners: The effect of level and frequency. In: Michelson A, editor. Time Resolution in Auditory Systems. Berlin: Springer-Verlag; 1985. pp. 159–179. [Google Scholar]
- 39.Fitzgibbons PJ, Wightman FL. Gap detection in normal and hearing-impaired listeners. J Acoust Soc Am. 1982;72:761–765. doi: 10.1121/1.388256. [DOI] [PubMed] [Google Scholar]
- 40.Cromwell HC, Mears RP, Wan L, Boutros NN. Sensory gating: A translational effort from basic to clinical science. Clin EEG Neurosci. 2008;39:69–72. doi: 10.1177/155005940803900209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geyer MA. The family of sensorimotor gating disorders: Comorbidities or diagnostic overlaps? Neurotox Res. 2006;10:211–220. doi: 10.1007/BF03033358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salvi RJ, Arehole S. Gap detection in chinchillas with temporary high-frequency hearing loss. J Acoust Soc Am. 1985;77:1173–1177. doi: 10.1121/1.392181. [DOI] [PubMed] [Google Scholar]
- 43.Syka J, Rybalko N, Mazelová J, Druga R. Gap detection threshold in the rat before and after auditory cortex ablation. Hear Res. 2002;172:151–159. doi: 10.1016/s0378-5955(02)00578-6. [DOI] [PubMed] [Google Scholar]
- 44.Moore BC. Psychoacoustics of normal and impaired hearing. Br Med Bull. 2002;63:121–134. doi: 10.1093/bmb/63.1.121. [DOI] [PubMed] [Google Scholar]
- 45.Reger SN. Differences in loudness response of normal and hard-of-hearing ears at intensity levels slightly above threshold. Ann Otol Rhinol Laryngol. 1936;45:1029–1039. [Google Scholar]
- 46.Longenecker RJ, Galazyuk AV. Development of tinnitus in CBA/CaJ mice following sound exposure. J Assoc Res Otolaryngol. 2011;12:647–658. doi: 10.1007/s10162-011-0276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rybalko N, Syka J. Effect of noise exposure on gap detection in rats. Hear Res. 2005;200:63–72. doi: 10.1016/j.heares.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 48.McFadden D, Plattsmier HS, Pasanen EG. Aspirin-induced hearing loss as a model of sensorineural hearing loss. Hear Res. 1984;16:251–260. doi: 10.1016/0378-5955(84)90114-x. [DOI] [PubMed] [Google Scholar]
- 49.McFadden D. Tinnitus: Facts, Theories, and Treatments. Washington, DC: National Academy Press; 1982. [PubMed] [Google Scholar]