Abstract
Objective
Insulin resistance is the key feature of the “metabolic syndrome,” a cluster of risk factors for cardiovascular disease and diabetes that includes hypertension, dyslipidemia, obesity, and hyperglycemia. Existing treatments target individual metabolic syndrome components, and act non-specifically with respect to disease pathophysiology. Our goal is to understand the link between insulin resistance and the metabolic syndrome, and how to develop treatment approaches.
Methods
We present three cases of extreme, syndromic insulin resistance: lipodystrophy, autoantibodies to the insulin receptor, and mutations of the insulin receptor, with discussion of pathophysiology and treatment.
Results
In lipodystrophy, insulin resistance is a direct consequence of leptin deficiency, and thus leptin replacement reverses metabolic syndrome abnormalities, including diabetes and hypertriglyeridemia. The insulin “receptoropathies”, including autoantibodies to the insulin receptor and insulin receptor mutations, are characterized by extreme insulin resistance and ovarian hyperandrogenism, without dyslipidemia or fatty liver disease. Autoantibodies to the insulin receptor can be treated using an immunosuppressive paradigm adapted from treatment of other autoimmune and neoplastic conditions. Leptin treatment has shown some success in treating hyperglycemia in insulin receptor mutations. Treatment for this condition remains inadequate, and novel therapies that bypass insulin receptor signaling, such as enhancers of brown adipose tissue, are needed.
Conclusion
We presented a clinical approach to treatment of syndromic insulin resistance. The study of rare diseases that replicate the metabolic syndrome, with clear-cut pathophysiology, allows the opportunity to understand novel physiology, and develop targeted therapies that may be applicable to the broader population with obesity, insulin resistance, and diabetes.
Keywords: Insulin resistance, Metabolic syndrome, Lipodystrophy, Leptin, Type B insulin resistance, Insulin receptor mutation
Introduction
The prevalence of obesity has reached epidemic proportions and the incidence and economic burden of obesity-related complications are rapidly increasing 1-3. Adult obesity in the United States has more than doubled from 15% in the late 1970’s to 35.7% in 20104. Even more alarming, over the same period, the prevalence of adolescent obesity has tripled from 5% to 17%4. In parallel with the rise in obesity, the incidence of type 2 diabetes has risen, with a current prevalence rate of 8.3% of US adults5. The prevalence in children is less well established, but appears to be dramatically rising. The 1999-2002 NHANES survey estimated 39,005 US adolescents currently living with T2DM, or 4.1 per 10006.
Type 2 diabetes results from a combination of insulin resistance (usually associated with obesity) and relative insulin deficiency. Insulin resistance is the key pathophysiologic feature of the “metabolic syndrome,” a cluster of independent epidemiologic risk factors for cardiovascular disease and diabetes. The metabolic syndrome was first described by Reaven in 19887, and includes hypertension, dyslipidemia (elevated triglycerides and low HDL cholesterol), central adiposity, and elevated fasting glucose8. Specific etiologies for these risk factors are typically found in less than 10% of cases. For example, in hypertension, a minority of cases are due to monogenic ion channel mutations (e.g. Liddle syndrome), endocrine abnormalities (e.g. hyperaldosteronism), or vascular abnormalities (e.g. renal artery stenosis), while the majority is termed “essential,” with no clear pathophysiologic basis. Similarly, dyslipidemia is infrequently due to a defined cause, such as LDL receptor abnormalities in familial hypercholesterolemia. The same is true for diabetes, for which rare cases can be attributed to defined abnormalities of the insulin receptor, or defects in beta-cell transcription factors or machinery. The quest in the common metabolic syndrome is to find a unifying mechanism that joins these diverse risk factors, and could provide a unique therapeutic target.
The gold standard by which endocrinologists practice medicine
Therapies for most metabolic syndrome features exist, and have been proven effective using the gold standard of evidence-based medical practice: randomized, controlled trials. Pharmacologic therapies to treat hypertension arose first, with clinical trials from the 1960’s demonstrating reduced morbidity. In the 1980’s clinical trials of cholesterol lowering medications changed medical practice, demonstrating that cholesterol lowering, particularly of LDL using HMG-CoA reductase inhibitors (statins), reduced mortality and cardiovascular events. It wasn’t until 1993 that the Diabetes Control and Complications Trial (DCCT) definitively demonstrated benefit of blood glucose lowering to prevent microvascular complications of type 1 diabetes9. The follow-up study to the DCCT, the Epidemiology of Diabetes Interventions and Complications (EDIC) study, showed possible benefit of intensive insulin therapy on macrovascular disease, as well10. This paradigm shift was dependent on the development of several new technologies, including home blood glucose monitoring, the use of hemoglobin A1c as a measure of chronic glycemia control, and improved methods of insulin delivery11. Results of the United Kingdom Prospective Diabetes Study (UKPDS) conveyed a similar message for treatment of type 2 diabetes12, 13.
It is important to consider that, with the exception of insulin therapy for type 1 diabetes, all of the above treatments (antihypertensive medications, statins, and oral hypoglycemic agents) act in a non-specific manner with respect to disease pathophysiology. Our ultimate goal in medicine is to understand the pathophysiologic basis of disease, thus allowing targeted pharmacotherapy. The study of rare diseases that replicate the metabolic syndrome or its components, in the context of clear-cut pathophysiology, allows us the opportunity to both understand novel physiology, and develop targeted therapies that may be applicable to the broader population with prevalent obesity, insulin resistance, and diabetes. In the present commentary, we will describe patient examples where specific targeted therapeutic approaches have been used. The examples will include, (a) the development of new technology, (b) new use of existing technology, and (c) combining existing technology with proposed new technology.
Development of new technology: Leptin treatment in lipodystrophy
Clinical vignette
A fourteen year old Caucasian female presented with generalized lack of subcutaneous adipose tissue, insulin resistance and diabetes, severe hypertriglyceridemia (10,000-12,000 mg/dL), eruptive xanthomas, and recurrent pancreatitis (Figure 1). She required three times weekly plasmapheresis for management of hypertriglyceridemia. She was also found to have steatohepatitis, amenorrhea, and hypertrophic cardiomyopathy. After the diagnosis of acquired generalized lipodystrophy was established at National Institute of Health, she became the first lipodystrophic patient to be treated with leptin replacement. Over the past 11 years she has been on leptin therapy (Metreleptin, the pharmaceutical form of leptin, which is composed of 146 amino acids of mature human leptin with an additional methionyl residue at the N-terminal end of the recombinant protein) and her metabolic profile has significantly improved, with marked reduction in her insulin requirements, hypertriglyceridemia, and hepatic steatosis, resolution of the eruptive xanthomas and recurrent pancreatitis. She is now 28 years old, married and is living an active, productive life.
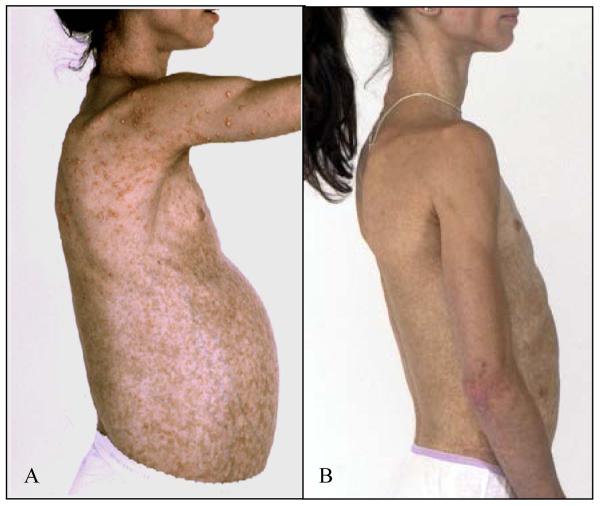
Figure 1.
A) Patient with acquired generalized lipodystrophy prior to treatment, demonstrating generalized paucity of subcutaneous fat, eruptive xanthomas, and protuberant abdomen due to enlarged fatty liver. Additional presenting features included insulin resistance and diabetes, severe hypertriglyceridemia, and recurrent pancreatitis. B) The same patient after one year of leptin therapy, demonstrating resolution of eruptive xanthomas, and a significant decrease in liver volume. She also had significant improvement in diabetes and hypertriglyceridemia, with resolution of pancreatitis.
Leptin: From hormone to pharmaceutical
Leptin was the first adipokine, or adipocyte-derived hormone, to be discovered. This discovery heralded a new understanding of adipose tissue as not merely a passive energy storage depot, but an active endocrine organ. The discovery of leptin derived from a spontaneously occurring mouse model of obesity, the ob/ob mouse. Through positional cloning, the laboratory of Jeffry Friedman identified the causative gene responsible for obesity in this animal14. The ob gene product was christened leptin, derived from the Greek word “leptos,” meaning thin. When ob/ob mice were given leptin replacement, they became lean, primarily through reduction in appetite2, 6. Unfortunately, leptin did not prove to be the hoped-for “magic bullet” treatment for obesity, because obese individuals have high endogenous leptin levels15 as a function of increased body fat, and do not respond to exogenously administered leptin16. This is analogous to the hyperinsulinemia observed in insulin resistant states. After its failure in common obesity, leptin therapy was tried in humans with obesity secondary to leptin gene mutations, an extremely rare disorder that is essentially the human equivalent of the ob/ob mouse17. Like the mice, these leptin-deficient individuals proved to be extremely sensitive to leptin’s appetite-suppressing effects, experiencing dramatic weight loss, coupled with normalization of other endocrine and non-endocrine abnormalities18.
Leptin treatment was subsequently tested in another condition associated with leptin deficiency: lipodystrophy. The term lipodystrophy refers to a heterogeneous group of disorders characterized by partial or complete loss of adipose tissue. Table 1 summarizes the clinical features common to the various forms of lipodystrophy, and Table 2 distinguishes among lipodystrophy subtypes. Because leptin is made in adipocytes, lipodystrophic patients, who have deficient subcutaneous adipose tissue, have very low leptin levels. Analogous to the mouse model of congenital leptin deficiency, mouse models of lipodystrophy proved to be responsive to leptin treatment19, 20. The first patient with lipodystrophy was treated with leptin beginning in 2000, with the dramatic clinical results described in the vignette, above. In the 12 years since, leptin replacement in lipodystrophy has been demonstrated to reduce food intake by half21, 22 and ameliorate most metabolic abnormalities, including insulin resistance22-24 and diabetes23, 25, 26. Leptin replacement in lipodystrophy reduces hypertriglyceridemia24-26 and lowers LDL cholesterol, but does not change HDL25. In addition, it reduces ectopic lipid storage, including reductions in muscle and liver triglyceride27, as well as improved non-alcoholic steatohepatitis (NASH) pathologic scores22, 28. Leptin also normalizes reproductive status both by reducing the ovarian hyperandrogenism caused by insulin resistance29, and by restoring the ultradian rhythm of luteinizing hormone secretion (demonstrated in congenital leptin deficiency and hypothalamic amenorrhea)18, 30. These effects are consistent in all variants of lipodystrophy studied25, and have been sustained throughout long-term leptin treatment31.
Table 1.
Phenotypic features of patients with lipodystrophy
| Paucity of fat |
| Deficiency of adipocyte hormones (e.g. leptin) |
| Insulin resistance |
| Acanthosis nigricans |
| Severe hypertriglyceridemia and resultant recurrent pancreatitis |
| Fatty infiltration of the liver and Non-alcoholic Steatohepatitis |
| Features of Polycystic Ovarian Syndrome |
| Low High-Density Lipoprotein Cholesterol |
Table 2.
| Inheritance (Genes) |
Leptin level range (ng/mL) |
Phenotype and distinct features | |
|---|---|---|---|
| Congenital Generalized Lipodystrophy (CGL) |
Autosomal recessive (AGPAT2 Seipin) |
0.31-3.32 | Near-total lack of body fat and muscular appearance at birth, acanthosis nigricans, umbilical prominence/hernia, primary amenorrhea in females, hirsutism, hypertrophic cardiomyopathy, and polycystic ovaries. Absence of mechanical fat in palms and soles in seipin mutations. Focal lytic lesions in the appendicular bones in AGPAT2 mutations. |
| Familial and Acquired Partial Lipodystrophy (FPL) |
Autosomal dominant (LMNA, PPARγ, Unknown) |
0.95-14.1 | Normal fat distribution at birth and during early childhood. Development of abnormal subcutaneous fat distribution during late childhood and adolescence; usually absence of subcutaneous fat in limbs but abundance of fat around the neck and face, occasionally over pubic area or axillae and intra- abdominal region. |
| Acquired Generalized Lipodystrophy (AGL) |
_ | 0.36-3.95 | Born with normal fat, start to lose subcutaneous fat in childhood. May initially present with localized panniculitis which then becomes generalized. Often associated with other autoimmune diseases. |
| Mandibuloacral Dysplasia (MAD)- associated lipodystrophy |
Autosomal recessive (LMNA) |
0.33-1.8 | Progeroid features, skeletal abnormalities (hypoplasia of mandibles and clavicles) |
Lipodystrophy as model for human metabolic syndrome
Eighty percent of patients with lipodystrophy meet clinical criteria for the metabolic syndrome32. A comparison of features of lipodystrophy versus obesity is shown in Table 3. In the common form of the metabolic syndrome, the basis for the cluster of insulin resistance, diabetes, and dyslipidemia is unclear, and therapy is directed at each component. By contrast, in patients with lipodystrophy, leptin deficiency represents a common etiologic factor that explains the metabolic defects of insulin resistance and dyslipidemia. The logical corollary to this is that leptin replacement in this population represents a common therapeutic target that can correct these defects.
Table 3.
Comparison of the phenotype of obesity with lipodystrophy.
| Obesity | Lipodystrophy | |
|---|---|---|
| Total fat | High | Low |
| Leptin level | High | Low |
| Energy intake | High | High |
| Ectopic fat | Moderate | High |
| Insulin resistance | Moderate | Extreme |
| Insulin receptor expression |
Regulated1 | Regulated1 |
Down-regulation of insulin receptor expression in hyperinsulinemic conditions (i.e. excess energy storage and leptin deficiency) and up-regulation in conditions of reduced insulin concentrations (i.e. fasting)63.
The mechanism by which leptin deficiency causes insulin resistance has not been well established, but likely shares many common pathways with obesity-associated insulin resistance. Ectopic lipid accumulation within the liver is clearly important in both obesity and lipodystrophy, and is associated with hepatic insulin resistance. Accumulation of intramyocellular diacylglycerol (also present in both obesity and lipodystrophy) can cause activation of atypical protein kinase C isoforms, leading to muscle insulin resistance33. Additional mechanisms include activation of the unfolded protein response (aka, endoplasmic reticulum stress), inflammation, and mitochondrial dysfunction33.
Just as there exist common mechanisms for insulin resistance in obesity and lipodystrophy, there exists a common treatment modality for insulin resistance in these two conditions. This treatment is reduction of energy storage. This is clearly very challenging to accomplish, and may be achieved by reducing energy intake (e.g. via dieting or bariatric surgery), and/or by increasing energy expenditure via exercise. In lipodystrophy, treatment with leptin reduces caloric intake by ~50%, and this caloric restriction is likely a major mediator of leptin’s clinical benefits. However, leptin-induced improvements in insulin resistance and glycemia occurring independent of food intake have been demonstrated in rodents19, 34-37, and, to some extent, in humans26.
New use of existing technology: Autoantibodies to the insulin receptor
Clinical Vignette
A twenty year-old African-American woman presented to National Institutes of Health with extreme insulin resistance and diabetes, with an average fasting blood glucose of 371 mg/dL and glycated hemoglobin levels ranging between 12-20% despite 18,000 units of insulin daily. She had a history of a 35 lbs weight loss on a 3500 kcal /day diet, massive polyuria (up to 15 liters of urine daily), and severe acanthosis nigricans. She was the first patient who was treated for autoantibodies to the insulin receptor (also called Type B insulin resistance) using a 3-pronged approach with rituximab, cyclophosphamide, and pulsed steroids. She has been in remission for the past 4 years.
Autoantibodies to the Insulin Receptor
Type B insulin resistance is caused by circulating autoantobodies to the insulin receptor. The resultant lack of insulin signaling causes extreme insulin resistance and hyperglycemia that is refractory to very large doses of exogenous insulin (up to 18,000 units daily, as described in the vignette). Hypoglycemia is an occasional symptom, thought to be associated with low-titer antibodies with partial agonist effects. Other major presenting features of this disease are acanthosis nigricans, severe hyperandrogenism, and weight loss; additional laboratory features include low triglycerides and high adiponectin levels. This condition is frequently associated with autoimmune and rheumatologic diseases, such as lupus and Sjogren syndrome, or it can be part of a paraneoplastic syndrome. Although some patients may undergo spontaneous remission, the mortality rate is generally high and treatment has been challenging38.
Immunologic Therapy for Autoantibodies to the Insulin Receptor
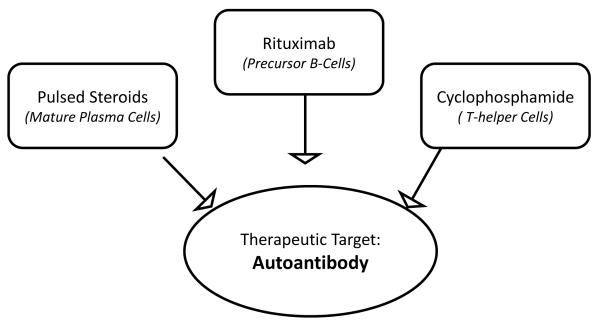
While high doses of immunosuppressive drugs used to treat conditions such as lupus nephritis can also resolve autoantibodies to the insulin receptor39, these treatments are also extremely toxic. In 2010, our group reported successful treatment of seven patients using a three-pronged approach (Figure 2), with the goal of eliminating the autoantibody to the insulin receptor40. First, rituximab, an antibody against CD-20, a cell surface molecule expressed by B-cell progenitors, was used to target antibody-producing B lymphocytes. Second, pulsed steroids were used to target the pre-existing antibody-producing plasma cells. Third, a low dose of an immunosuppressive drug, (either cyclophosphamide or cyclosporine for neutropenic patients) was used to achieve non-specific B- and T-cell function suppression. This treatment protocol evolved out of available treatments for other autoimmune or neoplastic conditions.
Figure 2.
Therapeutic strategy for Type B insulin resistance caused by autoantibodies to the insulin receptor. Elimination of the autoantibody is the goal for this three-pronged approach. Rituximab, an antibody against CD-20, a cell surface molecule expressed by B-cell progenitors, targets antibody-producing B lymphocytes. Pulsed steroids target the pre-existing antibody-producing plasma cells. Non-specific B- and T-cell function suppression is achieved by a low dose immunosuppressive drug, either cyclophosphamide or cyclosporine.
Autoantibodies to the insulin receptor are representative of a class of disorders caused by pathogenic antibodies to cell-surface receptors, and thus the concept of multifaceted immunosuppression may be applicable to other endocrine and non-endocrine conditions with similar pathophysiology. Examples of such conditions are Graves disease (TSH receptor antibodies), myasthenia gravis (acetylcholine receptor antibodies), ovarian failure (gonadotropin receptor antibodies), iron deficiency anemia (transferrin receptor antibodies), hypoparathyroidism (antibodies against the parathyroid hormone or calcium sensing receptors), and even occasional forms of obesity due to melanocortin 4 receptor antibodies41-45. In addition, this treatment approach might be broadened to target autoantibodies against circulating hormones, such as insulin antibodies in the insulin autoimmune syndrome. In this syndrome, autoantibodies against circulating insulin produce hypoglycemia46, 47. Preliminary studies suggest that the general therapeutic protocol described above may be applicable to the insulin autoimmune syndrome. Depending on the severity of the patient’s condition, all three arms of this treatment regimen may not be necessary; in less severe cases, only one or two components may be adequate.
Combining old and new technologies: Insulin receptor mutations
Clinical Vignette
Two siblings with the Rabson-Mendenhall syndrome due to homozygous insulin receptor mutations initially presented at age 3 to 4 years with a history of hypoglycemia, growth retardation, and acanthosis nigricans. By age 7 and 9 years they were found to have severe hyperinsulinemia and diabetes. The diabetes was initially managed by oral hypoglycemic agents (metformin and rosiglitazone, and insulin was later added to the male patient’s regimen at age 12 years. Severe hyperandrogenism and hirsutism were also present in the female patient. These two patients were the first patients with insulin receptor mutations to be treated with leptin, beginning at age 11 and 13 years. After 10 months of leptin treatment, the fasting blood glucose decreased by 60 % in the female and by 40 % in the male. The male’s insulin requirement was reduced from 300 to 200 units daily. Both patients showed a reduction in hemoglobin A1C and fasting insulin. After withdrawal of leptin for three months, all glycemic parameters returned to, or were worse than, the baseline values48.
Insulin Receptor Mutations
Insulin receptor mutations are characterized by extreme forms of insulin resistance. Like type 2 diabetes, the initial hyperinsulinism becomes attenuated over time through loss of beta cell function, and overt diabetes ensues. Patients with insulin receptor mutations are subject to all of the attendant microvascular complications of diabetes, and early mortality may occur in late childhood or young adulthood49. In its most severe form, called Donohue syndrome or leprechaunism, death occurs prior to age two years49. Prior attempts at treatment have involved the use of concentrated (U-500) insulin in an attempt to ameliorate the degree of hyperglycemia, but this has met with limited success. Oral agents such as metformin have some added benefit, but overall, treatment for this condition is inadequate.
New Use of an Existing Technology: Leptin treatment in Insulin Receptor Mutations
Because treatment of patients with insulin receptor mutations with high-dose insulin has only limited efficacy, it is critical to consider treatment strategies that bypass the insulin receptor. Leptin treatment for patients with insulin receptor mutations was initiated based on the observation that the signal transduction cascades downstream of the insulin and the leptin receptors overlap at the level of phosphoinositide (PI) 3-kinase. It was therefore hypothesized that treatment with exogenous leptin might increase post-receptor insulin signaling downstream of PI 3-kinase. Although some efficacy of leptin in lowering blood glucose has been observed in these patients, it has not been possible to demonstrate whether this improvement was, indeed, mediated via increased post-insulin-receptor signaling, as hypothesized.
Potential new approaches to treatment of insulin receptor mutations
Even with leptin therapy, management of diabetes in patients with insulin receptor mutations remains inadequate, and additional, novel treatments for this condition are needed. One potential target is activation of brown adipose tissue. One well known activator of brown adipose tissue is thyroid hormone, and a single patient observation suggests that this mechanism may be relevant for patients with insulin receptor mutations50: A patient with homozygous mutation of the insulin receptor was started on suppressive doses of levothyroxine for treatment of thyroid cancer. Over the subsequent 30 months, she had remarkable improvement in her glycemia control, with a decrease in hemoglobin A1c from 9.9 to 5.5%, despite tapering of insulin from 3000 to 0 units per day, and discontinuation of metformin. Positron emission tomography demonstrated the presence of brown adipose tissue, which was confirmed on biopsy. During withdrawal of levothyroxine, the brown adipose tissue diminished, and glycemia control worsened, supporting the hypothesis that high-dose levothyroxine mediated the improvements in blood glucose via brown adipose tissue. Importantly, the patient’s insulin sensitivity (assessed via the gold-standard hyperinsulinemic, euglycemic clamp technique) did not change in the presence or absence of suppressive levothyroxine, suggesting that the improvements in glycemia occurred independent of insulin signaling through its receptor.
Additional activators of brown adipose tissue have been suggested based on rodent data. Fibroblast growth factor 21 (FGF21) mediates direct activation of brown fat thermogenesis during the fetal-to neonatal transition51. Mice that are deficient in FGF21 have diminished “browning” of white adipose tissue and have an impaired ability to adapt to chronic cold exposure52. Transcriptional activators, like PPAR-α in the liver and PPAR-γ in adipocytes control expression of FGF21. Transgenic mice overexpressing FGF21 in the liver have increased insulin sensitivity, glucose clearance, decreased plasma triglyceride, increased fat utilization and energy expenditure 53, 54. The protein irisin is another activator of brown adipose tissue in rodents55. Production of this protein is enhanced in response to the exercise-induced rise in the transcriptional co-activator PPAR-γ co-activator-1 α (PGC1-α). Mice that overexpress irisin develop browning of white adipose tissue, and have improved glucose tolerance after high-fat diet feeding. Neither FGF-21 nor irisin have been studied in humans, but, because they improve glucose homeostasis independent of insulin-receptor signal transduction, they may have potential therapeutic roles for management of diabetes in patients with insulin receptor mutations.
Lessons Learned from Syndromic Insulin Resistance as Models for the Metabolic Syndrome
Several lessons can be derived from these clinical studies of rare, syndromic forms of insulin resistance. It is clear that the common metabolic syndrome, lipodystrophy, and insulin receptor mutations are all characterized by varying degrees of insulin resistance (Table 4). Furthermore, the hyperinsulinemia in these conditions is sufficient to cause polycystic ovarian syndrome and hyperandrogenism56. However, the other phenotypic features of these conditions cannot be derived from insulin resistance, per se. For example, in the common metabolic syndrome and lipodystrophy, triglycerides are elevated, while in insulin receptoropathies, triglyceride levels are low57. This demonstrates that, in order to stimulate lipid synthetic pathways in the liver, the insulin receptor is required. Thus, ectopic fat can only be generated in the common metabolic syndrome and in lipodystrophy, and does not occur in insulin receptoropathies despite extreme insulin resistance. In addition, and paradoxically, the insulin receptoropathies are characterized by high adiponectin58, 59, as contrasted to the low adiponectin levels observed in the common metabolic syndrome and lipodystrophy. Again, this suggests that intact signaling through the insulin receptor is required for the low adiponectin levels typically seen with insulin resistant states.
Table 4.
Phenotypic features of syndromic forms of insulin resistance versus the common metabolic syndrome.
| Insulin Resistance |
Triglycerides | Adiponectin | Ectopic fat | PCOS features | |
|---|---|---|---|---|---|
| Common Metabolic Syndrome |
Yes ↑↑ | Elevated ↑ | Low ↓ | Yes ↑↑ | Yes ↑ |
| Lipodystrophy | Yes ↑↑↑ | Elevated ↑↑ | Low ↓↓ | Yes ↑↑↑ | Yes ↑↑ |
| Receptoropathies | Yes ↑↑↑↑ | Low ↓ | Elevated ↑↑ | No | Yes ↑↑↑ |
PCOS, Polycystic Ovarian Syndrome
In the insulin receptoropathies, the mechanism of insulin resistance is clear – the failure of insulin to activate its receptor, and hence, downstream signal transduction cascades. The mechanism of insulin resistance in lipodystrophy clearly involves leptin deficiency, but the downstream effectors of leptin deficiency are complex, and likely overlap with common, obesity-associated insulin resistance. There is a common conundrum in lipodystrophy and obesity about the inciting event leading to insulin resistance. For instance, mitochondrial dysfunction has been proposed as a mechanism leading to insulin resistance, but it may also be a consequence of insulin resistance, as shown in patients with insulin receptor mutations60. Thus, we continue to face the conundrum of the initial event leading to insulin resistance in the common metabolic syndrome.
Conclusions
The clinical endocrinologist has responsibility for several of the world’s most prevalent and debilitating chronic diseases. The public cost of these diseases in both fiscal and social terms is enormous. All available technologies, including genomics, epidemiology, developmental science, and model systems will be needed in the pursuit of effective prevention and treatment. We have offered a clinical approach using disease models that have been informative for both developing therapeutic and conceptual approaches to certain rare diseases, but which may also be informative for the serious common diseases associated with metabolic dysfunction.
Acknowledgements
This work was supported by the intramural research program of the National Institute of Diabetes and Digestive and Kidney Diseases. We are indebted to many fellows of the NIH Inter-institute Endocrine Training Program, the nursing staff of the NIH Clinical Center, other professionals, and patients, who have participated in these studies. We would like to thank Amylin Pharmaceuticals for Metreleptin used in these studies. We are grateful to the NIH Clinical Center, winner of the 2011 Lasker award, as a resource for these studies. We are also grateful to many collaborators worldwide, who have enriched these studies.
Footnotes
Conflict of Interest: Metreleptin was provided by Amylin Pharmaceuticals under a Cooperative Research and Development Agreement. The authors declare that there is no conflict of interest associated with this manuscript.
References
- 1.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The Disease Burden Associated With Overweight and Obesity. JAMA. 1999;282:1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 2.Allison DB, Zannolli R, Narayan KMV. The direct health care costs of obesity in the United States. Am J Public Health. 1999;89:1194–1199. doi: 10.2105/ajph.89.8.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab. 2004;89:2583–2589. doi: 10.1210/jc.2004-0535. [DOI] [PubMed] [Google Scholar]
- 4.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009-2010. National Center for Health Statistics; Hyattsville, MD: 2012. NCHS data brief, no 82. [Google Scholar]
- 5.Centers for Disease Control and Prevention . National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 2011. [Google Scholar]
- 6.Duncan GE. Prevalence of diabetes and impaired fasting glucose levels among US adolescents: National Health and Nutrition Examination Survey, 1999-2002. Arch Pediatr Adolesc Med. 2006;160:523–528. doi: 10.1001/archpedi.160.5.523. [DOI] [PubMed] [Google Scholar]
- 7.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 8.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC., Jr. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 9.The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. New England Journal of Medicine. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 10.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorden P, Musso C. The impact of evidence-based medicine on diabetes therapy. Curr Diab Rep. 2005;5:157–159. doi: 10.1007/s11892-005-0001-8. [DOI] [PubMed] [Google Scholar]
- 12.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 13.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 14.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 15.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 16.Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, Lubina JA, Patane J, Self B, Hunt P, McCamish M. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA : the journal of the American Medical Association. 1999;282:1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 17.Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O’Rahilly S. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341:879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 18.Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C, Sanna V, Jebb SA, Perna F, Fontana S, Lechler RI, DePaoli AM, O’Rahilly S. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–1103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401:73–76. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- 20.Ebihara K, Ogawa Y, Masuzaki H, Shintani M, Miyanaga F, Aizawa-Abe M, Hayashi T, Hosoda K, Inoue G, Yoshimasa Y, Gavrilova O, Reitman ML, Nakao K. Transgenic overexpression of leptin rescues insulin resistance and diabetes in a mouse model of lipoatrophic diabetes. Diabetes. 2001;50:1440–1448. doi: 10.2337/diabetes.50.6.1440. [DOI] [PubMed] [Google Scholar]
- 21.Moran SA, Patten N, Young JR, Cochran E, Sebring N, Reynolds J, Premkumar A, Depaoli AM, Skarulis MC, Oral EA, Gorden P. Changes in body composition in patients with severe lipodystrophy after leptin replacement therapy. Metabolism. 2004;53:513–519. doi: 10.1016/j.metabol.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 22.Petersen KF, Oral EA, Dufour S, Befroy D, Ariyan C, Yu C, Cline GW, DePaoli AM, Taylor SI, Gorden P, Shulman GI. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002;109:1345–1350. doi: 10.1172/JCI15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Javor ED, Cochran EK, Musso C, Young JR, Depaoli AM, Gorden P. Long-term efficacy of leptin replacement in patients with generalized lipodystrophy. Diabetes. 2005;54:1994–2002. doi: 10.2337/diabetes.54.7.1994. [DOI] [PubMed] [Google Scholar]
- 24.Park JY, Javor ED, Cochran EK, DePaoli AM, Gorden P. Long-term efficacy of leptin replacement in patients with Dunnigan-type familial partial lipodystrophy. Metabolism. 2007;56:508–516. doi: 10.1016/j.metabol.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chong AY, Lupsa BC, Cochran EK, Gorden P. Efficacy of leptin therapy in the different forms of human lipodystrophy. Diabetologia. 2010;53:27–35. doi: 10.1007/s00125-009-1502-9. [DOI] [PubMed] [Google Scholar]
- 26.Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML, Taylor SI, Gorden P, Garg A. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 27.Petersen KF, Oral EA, Dufour S, Befroy D, Ariyan C, Yu C, Cline GW, DePaoli AM, Taylor SI, Gorden P, Shulman GI. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. The Journal of clinical investigation. 2002;109:1345–1350. doi: 10.1172/JCI15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Javor ED, Ghany MG, Cochran EK, Oral EA, DePaoli AM, Premkumar A, Kleiner DE, Gorden P. Leptin reverses nonalcoholic steatohepatitis in patients with severe lipodystrophy. Hepatology. 2005;41:753–760. doi: 10.1002/hep.20672. [DOI] [PubMed] [Google Scholar]
- 29.Lungu AO, Zadeh ES, Goodling A, Cochran E, Gorden P. Insulin resistance is a sufficient basis for hyperandrogenism in lipodystrophic women with polycystic ovarian syndrome. The Journal of clinical endocrinology and metabolism. 2012;97:563–567. doi: 10.1210/jc.2011-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004;351:987–997. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- 31.Chan JL, Lutz K, Cochran E, Huang W, Peters Y, Weyer C, Gorden P. Clinical effects of long-term metreleptin treatment in patients with lipodystrophy. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2011;17:922–932. doi: 10.4158/EP11229.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorden P, Lupsa BC, Chong AY, Lungu AO. Is there a human model for the ‘metabolic syndrome’ with a defined aetiology? Diabetologia. 2010;53:1534–1536. doi: 10.1007/s00125-010-1719-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 35.Hedbacker K, Birsoy K, Wysocki RW, Asilmaz E, Ahima RS, Farooqi IS, Friedman JM. Antidiabetic effects of IGFBP2, a leptin-regulated gene. Cell Metab. 2010;11:11–22. doi: 10.1016/j.cmet.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz MW, Baskin DG, Bukowski TR, Kuijper JL, Foster D, Lasser G, Prunkard DE, Porte D, Jr., Woods SC, Seeley RJ, Weigle DS. Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes. 1996;45:531–535. doi: 10.2337/diab.45.4.531. [DOI] [PubMed] [Google Scholar]
- 37.Hidaka S, Yoshimatsu H, Kondou S, Tsuruta Y, Oka K, Noguchi H, Okamoto K, Sakino H, Teshima Y, Okeda T, Sakata T. Chronic central leptin infusion restores hyperglycemia independent of food intake and insulin level in streptozotocin-induced diabetic rats. FASEB J. 2002;16:509–518. doi: 10.1096/fj.01-0164com. [DOI] [PubMed] [Google Scholar]
- 38.Arioglu E, Andewelt A, Diabo C, Bell M, Taylor SI, Gorden P. Clinical course of the syndrome of autoantibodies to the insulin receptor (type B insulin resistance): a 28-year perspective. Medicine (Baltimore) 2002;81:87–100. doi: 10.1097/00005792-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Coll AP, Morganstein D, Jayne D, Soos MA, O’Rahilly S, Burke J. Successful treatment of Type B insulin resistance in a patient with otherwise quiescent systemic lupus erythematosus. Diabet Med. 2005;22:814–815. doi: 10.1111/j.1464-5491.2005.01529.x. [DOI] [PubMed] [Google Scholar]
- 40.Malek R, Chong AY, Lupsa BC, Lungu AO, Cochran EK, Soos MA, Semple RK, Balow JE, Gorden P. Treatment of type B insulin resistance: a novel approach to reduce insulin receptor autoantibodies. J Clin Endocrinol Metab. 2010;95:3641–3647. doi: 10.1210/jc.2010-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gorden P, Collier E, Roach P. Autoimmune mechanisms of insulin resistance and hypoglycemia. In: Moley DE, editor. Insulin Resistance. John Wiley; London: 1993. pp. 123–124. [Google Scholar]
- 42.Sluss PM, Schneyer AL. Low molecular weight follicle-stimulating hormone receptor binding inhibitor in sera from premature ovarian failure patients. J Clin Endocrinol Metab. 1992;74:1242–1246. doi: 10.1210/jcem.74.6.1592865. [DOI] [PubMed] [Google Scholar]
- 43.Peter JC, Bekel A, Lecourt AC, Zipfel G, Eftekhari P, Nesslinger M, Breidert M, Muller S, Kessler L, Hofbauer KG. Anti-melanocortin-4 receptor autoantibodies in obesity. J Clin Endocrinol Metab. 2009;94:793–800. doi: 10.1210/jc.2008-1749. [DOI] [PubMed] [Google Scholar]
- 44.Juppner H, Bialasiewicz AA, Hesch RD. Autoantibodies to parathyroid hormone receptor. Lancet. 1978;2:1222–1224. doi: 10.1016/s0140-6736(78)92099-8. [DOI] [PubMed] [Google Scholar]
- 45.Kifor O, McElduff A, LeBoff MS, Moore FD, Jr., Butters R, Gao P, Cantor TL, Kifor I, Brown EM. Activating antibodies to the calcium-sensing receptor in two patients with autoimmune hypoparathyroidism. J Clin Endocrinol Metab. 2004;89:548–556. doi: 10.1210/jc.2003-031054. [DOI] [PubMed] [Google Scholar]
- 46.Taylor SI, Barbetti F, Accili D, Roth J, Gorden P. Syndromes of autoimmunity and hypoglycemia. Autoantibodies directed against insulin and its receptor. Endocrinol Metab Clin North Am. 1989;18:123–143. [PubMed] [Google Scholar]
- 47.Lupsa BC, Chong AY, Cochran EK, Soos MA, Semple RK, Gorden P. Autoimmune forms of hypoglycemia. Medicine (Baltimore) 2009;88:141–153. doi: 10.1097/MD.0b013e3181a5b42e. [DOI] [PubMed] [Google Scholar]
- 48.Cochran E, Young JR, Sebring N, DePaoli A, Oral EA, Gorden P. Efficacy of recombinant methionyl human leptin therapy for the extreme insulin resistance of the Rabson-Mendenhall syndrome. J Clin Endocrinol Metab. 2004;89:1548–1554. doi: 10.1210/jc.2003-031952. [DOI] [PubMed] [Google Scholar]
- 49.Musso C, Cochran E, Moran SA, Skarulis MC, Oral EA, Taylor S, Gorden P. Clinical course of genetic diseases of the insulin receptor (type A and Rabson-Mendenhall syndromes): a 30-year prospective. Medicine (Baltimore) 2004;83:209–222. doi: 10.1097/01.md.0000133625.73570.54. [DOI] [PubMed] [Google Scholar]
- 50.Skarulis MC, Celi FS, Mueller E, Zemskova M, Malek R, Hugendubler L, Cochran C, Solomon J, Chen C, Gorden P. Thyroid hormone induced brown adipose tissue and amelioration of diabetes in a patient with extreme insulin resistance. J Clin Endocrinol Metab. 2010;95:256–262. doi: 10.1210/jc.2009-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hondares E, Rosell M, Gonzalez FJ, Giralt M, Iglesias R, Villarroya F. Hepatic FGF21 expression is induced at birth via PPARalpha in response to milk intake and contributes to thermogenic activation of neonatal brown fat. Cell Metab. 2010;11:206–212. doi: 10.1016/j.cmet.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, Spiegelman BM. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149:6018–6027. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- 55.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP, Spiegelman BM. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lungu AO, Zadeh ES, Goodling A, Cochran E, Gorden P. Insulin resistance is a sufficient basis for hyperandrogenism in lipodystrophic women with polycystic ovarian syndrome. J Clin Endocrinol Metab. 2012;97:563–567. doi: 10.1210/jc.2011-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Semple RK, Savage DB, Cochran EK, Gorden P, O’Rahilly S. Genetic Syndromes of Severe Insulin Resistance. Endocr Rev. 2011 doi: 10.1210/er.2010-0020. [DOI] [PubMed] [Google Scholar]
- 58.Semple RK, Halberg NH, Burling K, Soos MA, Schraw T, Luan J, Cochran EK, Dunger DB, Wareham NJ, Scherer PE, Gorden P, O’Rahilly S. Paradoxical elevation of high-molecular weight adiponectin in acquired extreme insulin resistance due to insulin receptor antibodies. Diabetes. 2007;56:1712–1717. doi: 10.2337/db06-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Semple RK, Soos MA, Luan J, Mitchell CS, Wilson JC, Gurnell M, Cochran EK, Gorden P, Chatterjee VK, Wareham NJ, O’Rahilly S. Elevated plasma adiponectin in humans with genetically defective insulin receptors. J Clin Endocrinol Metab. 2006;91:3219–3223. doi: 10.1210/jc.2006-0166. [DOI] [PubMed] [Google Scholar]
- 60.Sleigh A, Raymond-Barker P, Thackray K, Porter D, Hatunic M, Vottero A, Burren C, Mitchell C, McIntyre M, Brage S, Carpenter TA, Murgatroyd PR, Brindle KM, Kemp GJ, O’Rahilly S, Semple RK, Savage DB. Mitochondrial dysfunction in patients with primary congenital insulin resistance. J Clin Invest. 2011;121:2457–2461. doi: 10.1172/JCI46405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garg A. Acquired and inherited lipodystrophies. N Engl J Med. 2004;350:1220–1234. doi: 10.1056/NEJMra025261. [DOI] [PubMed] [Google Scholar]
- 62.Garg A. Clinical review#: Lipodystrophies: genetic and acquired body fat disorders. J Clin Endocrinol Metab. 2011;96:3313–3325. doi: 10.1210/jc.2011-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bar RS, Gorden P, Roth J, Kahn CR, de Meyts P. Fluctuations in the affinity and concentration of insulin receptors on circulating monocytes of obese patients: effects of starvation, refeeding, and dieting. Journal of Clinical Investigation. 1976;58:1123–1135. doi: 10.1172/JCI108565. [DOI] [PMC free article] [PubMed] [Google Scholar]