SUMMARY
The cytoplasmic pattern recognition receptor RIG-I is activated by viral RNA and induces type I IFN responses to control viral replication. The cellular dsRNA binding protein PACT can also activate RIG-I. To counteract innate antiviral responses, some viruses, including Ebola virus (EBOV), encode proteins that antagonize RIG-I signaling. Here, we show that EBOV VP35 inhibits PACT-induced RIG-I ATPase activity in a dose-dependent manner. The interaction of PACT with RIG-I is disrupted by wild-type VP35, but not by VP35 mutants that are unable to bind PACT. In addition, PACT-VP35 interaction impairs the association between VP35 and the viral polymerase, thereby diminishing viral RNA synthesis and modulating EBOV replication. PACT-deficient cells are defective in IFN induction and are insensitive to VP35 function. These data support a model in which the VP35-PACT interaction is mutually antagonistic and plays a fundamental role in determining the outcome of EBOV infection.
INTRODUCTION
Ebolaviruses (EBOVs) and marburgviruses (MARVs) are nonsegmented negative-sense RNA viruses of the Filoviridae family that cause severe hemorrhagic fever characterized by uncontrolled virus replication and excessive inflammation (Feldmann et al., 2003). The severity of these infections is likely aided by the potent suppression of innate antiviral immunity by filoviral gene products including the EBOV VP24 protein, the MARV VP40 protein, and the filoviral VP35 proteins (Basler and Amarasinghe, 2009).
The VP35 proteins are innate immune antagonists that target RIG-I-like receptors (RLRs), PKR, and RNA silencing activity (Bale et al., 2012; Basler et al., 2003; Basler et al., 2000; Cardenas et al., 2006; Fabozzi et al., 2011; Feng et al., 2007; Haasnoot et al., 2007; Ramanan et al., 2012; Schümann et al., 2009; Zhu et al., 2012). RLRs detect viral nucleic acids and signal to induce an interferon (IFN)-α/β response (Leung et al., 2012). That RLR inhibition is particularly important is supported by the observation that preactivation of RIG-I reduces EBOV titers in cell culture up to ~1000-fold (Spiropoulou et al., 2009). Additionally, EBOVs possessing mutated VP35s that are unable to disrupt RIG-I inhibition induce IFN-α/β responses during infection and are significantly attenuated in cell culture and in vivo (Cardenas et al., 2006; Hartman et al., 2006, 2008a, 2008b; Prins et al., 2010b). These observations suggest that the suppression of RIG-I is a critical determinant of virulence.
VP35 likely inhibits the RIG-I pathway at several levels, including by acting as a decoy substrate for the cellular kinases IKKε and TBK-1 and through interaction with the SUMOylation machinery (Chang et al., 2009; Prins et al., 2009). VP35 also binds to dsRNA (Cardenas et al., 2006; Leung et al., 2009, 2010). Biochemical, structural, and functional studies, including solved crystal structures EBOV VP35 IFN inhibitory domain (IID) and MARV IID alone and in complex with dsRNA, correlate VP35 dsRNA binding activity with its IFN-antagonist function (Cardenas et al., 2006; Leung et al., 2010; Ramanan et al., 2012). EBOV VP35 contacts both the dsRNA phosphodiester backbone and the ends of the dsRNA, forming an “endcap,” while MARV may not endcap the dsRNA (Bale et al., 2012; Kimberlin et al., 2010; Leung et al., 2010; Ramanan et al., 2012). Suppression of virus-induced IFN-α/β gene expression by VP35 proteins is disrupted by point mutations in the IID that abrogate dsRNA binding activity (Prins et al., 2010b). Despite these advances, the precise mechanisms by which VP35 dsRNA binding contributes to immune suppression and pathogenesis remain incompletely defined.
In addition to its immune antagonist functions, VP35 is also an essential component of the filovirus polymerase complex. Filoviral mRNA transcription and genome replication require the viral polymerase complex, which consists of viral proteins NP, VP35, VP30, and L (Mühlberger et al., 1998). The essential role of VP35 for viral polymerase activity reflects critical interactions with both NP and L (Becker et al., 1998; Theriault et al., 2004), including residues within the VP35 IID (Leung et al., 2010). Little is known regarding how the immune evasion and RNA replication functions of VP35 are integrated.
PACT (PKR activator; called PKR-associated protein X [RAX] in mice) is a double-stranded RNA binding domain (dsRBD)-containing protein that was initially identified as an interacting partner and non-RNA activator of PKR (Ito et al., 1999; Patel and Sen, 1998; Peters et al., 2001). PACT also interacts with transactivation response RNA-binding protein (TRBP) and together with TRBP is a binding partner of Dicer (Chendrimada et al., 2005; Haase et al., 2005; Kok et al., 2007; Lee et al., 2006, 2013). Previous studies proposed that VP35-PACT interaction may contribute to VP35 RNA silencing suppressor (RSS) activity (Fabozzi et al., 2011; Zhu et al., 2012). However, PACT also promotes IFN-α/β responses to viral infection and to dsRNA. This latter function is mediated through PACT interactions with the carboxy-terminal domain of RIG-I that stimulates RIG-I ATPase activity and RIG-I signaling (Iwamura et al., 2001; Kok et al., 2011). The physiological relevance of this PACT function is supported by the substantial reduction of IFN-β production in PACT-depleted cells (Kok et al., 2011).
Here, we demonstrate the functional consequences of VP35-PACT interaction. RIG-I activation by PACT is inhibited by EBOV VP35 interaction with PACT. Mutations in the highly conserved central basic patch within the IID that prevent VP35 binding to PACT also impair that ability of IID to inhibit RIG-I activation by PACT. Additionally, VP35-PACT interaction impairs the viral RNA replication complex, likely through binding to VP35. Finally, studies performed with recombinant EBOVs possessing wild-type (WT) or mutated VP35s provide evidence that PACT modulates both host innate response and virus replication in infected cells.
RESULTS
EBOV VP35 Blocks Activation of RIG-I by PACT
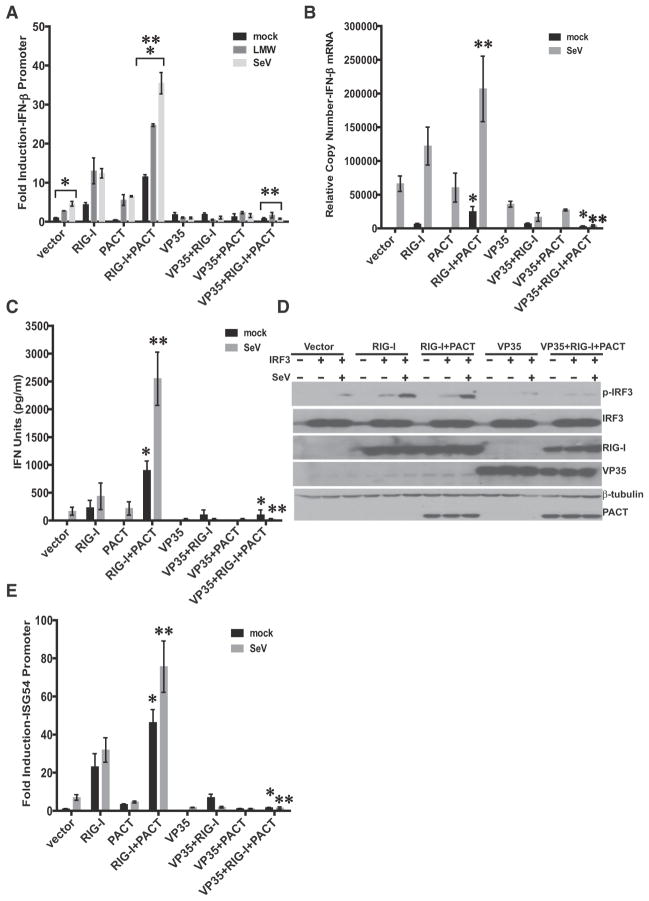
We sought to explore the functional implications of VP35-PACT interactions in the IFN-α/β response. PACT activation of RIG-I was confirmed by performing a reporter gene assay for IFN-β promoter activation; PACT modestly stimulated the IFN-β promoter when coexpressed with RIG-I in the absence of virus infection. Infection with Sendai virus (SeV) or transfection of poly(I:C) resulted in a significant stimulation of RIG-I-dependent responses, as measured by IFN-β promoter activation (Figure 1A). Expression of VP35 inhibited virus-, poly(I:C)-, and PACT-induced IFN-β promoter activation (Figure 1A). These results were confirmed by performing quantitative RT-PCR to measure the induction of endogenous IFN-β mRNA expression (Figure 1B) and by bioassay to quantify the release of IFN-α/β (Figure 1C).
Figure 1. VP35 Inhibits PACT Activation of RIG-I Signaling.
(A) IFN-β promoter reporter gene activity in the presence of RIG-I, PACT, or VP35 and following mock transfection, transfection with low-molecular-weight (LMW) poly(I:C), or infection with Sendai virus (SeV), as indicated. IFN-β-firefly luciferase activities were normalized to Renilla luciferase activity expressed from a cotransfected constitutive expression plasmid. Fold induction was determined by setting the vector (mock) transfection to a value of 1. The error bars indicate standard deviation of three independent replicates (*p < 0.001, **p < 0.001).
(B) Endogenous IFN-β mRNA levels as determined by quantitative RT-PCR. Transfections were performed as in (A) except that reporter plasmids were omitted. IFN-β mRNA levels were normalized to β-actin mRNA levels and are represented as relative copy number. The data are the average of three independent experiments, with error bars representing standard deviation (*p = 0.02, **p = 0.01 by Student’s t test).
(C) An IFN bioassay was performed to assess the amount of IFN-α/β in cell supernatants. The data in the chart represent the mean ± SD of three independent experiments; *p = 0.02, **p = 0.01, by Student’s t test.
(D) SeV-induced IRF-3 (S396) phosphorylation in the presence of transfected empty vector (vector), RIG-I, PACT, or VP35 expression plasmid with or without IRF-3 expression plasmid under mock-infected or SeV-infected conditions. Lysates were analyzed by western blotting for phospho-S396 IRF-3 (P-IRF-3), total IRF-3, VP35, HA-PACT, and Flag-RIG-I. Blots are representative of three independent experiments.
(E) ISG54 promoter luciferase reporter gene expression in the presence of RIG-I, PACT, or VP35. Methods were analogous to those described in (A), except a reporter plasmid expressing a firefly luciferase under the control of tandem ISRE elements from the ISG54 promoter was used. Fold luciferase activity was determined relative to the mock-treated, empty vector samples. The error bars indicate standard deviation from three independent replicates, *p = 0.007, **p = 0.01.
VP35 inhibits virus-induced IFN regulatory factor 3 (IRF-3) activation (Basler et al., 2000, 2003). As IRF-3 activation is associated with phosphorylation of serine/threonine residues near its carboxy terminus (Basler et al., 2003), we monitored the effect of VP35 on PACT activation of IRF-3 Ser396 phosphorylation. To facilitate its detection in HEK293T cells, IRF-3 was overexpressed. Transfection with RIG-I expression plasmid, or RIG-I and PACT expression plasmids, induced IRF-3 phosphorylation, and this phosphorylation was further enhanced by SeV infection (Figure 1D). VP35 coexpression resulted in a substantial decrease in IRF-3 phosphorylation in cells expressing both PACT and RIG-I (Figure 1D). VP35 also inhibited PACT-mediated activation of the ISG54 promoter (Figure 1E). Cumulatively, these data demonstrate that VP35 can counter induction of IFN-α/β by PACT.
The Impact of VP35 on IFN Responses Is Magnified in the Presence of PACT
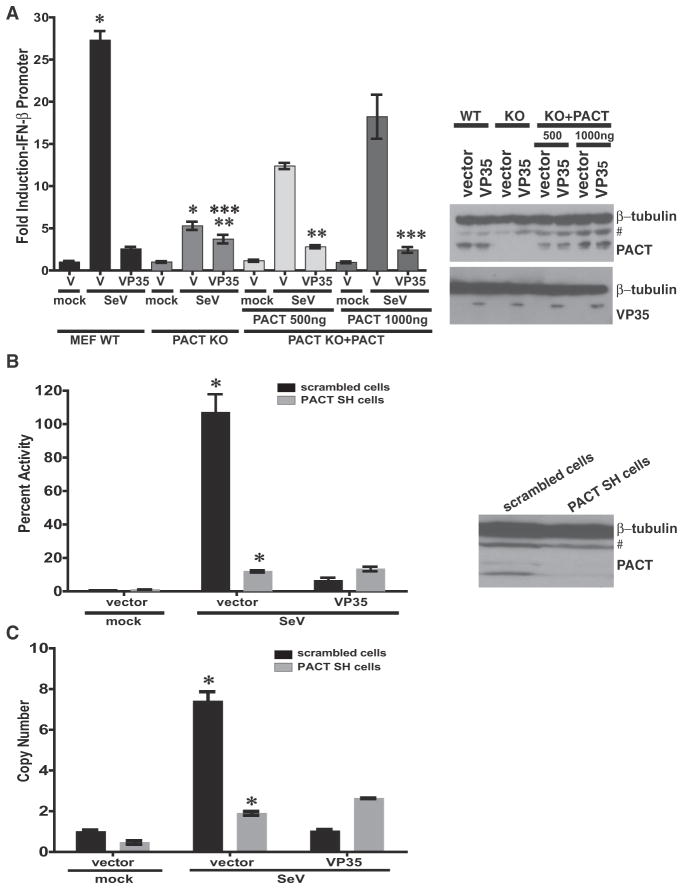
Knockdown of PACT decreases the SeV-induced activation of the IFN-β promoter and impairs cellular antiviral responses (Kok et al., 2011). Consistent with this, IFN-β promoter activation was decreased in PACT knockout (KO) MEFs, as compared to WT MEFs. However, overexpression of PACT augmented the IFN-β response in the knockout cells in a dose-dependent manner (Figure 2A). Interestingly, VP35 had little effect on the residual IFN response in the PACT knockout MEFs but effectively blocked IFN-β promoter activation in the WT cells. However, reconstituting PACT in the knockout MEFs restored VP35 suppression of IFN-β promoter activation (Figure 2A).
Figure 2. PACT Augments the VP35 Inhibition of IFN-β.
(A) IFN-β promoter activation by SeV infection was assessed by reporter gene assay in WT MEFs, in PACT knockout (KO) MEFs, or in PACT KO MEFs transiently transfected with PACT expression plasmid. The cells were cotransfected with a constitutive Renilla luciferase reporter gene and either empty vector (vector) or VP35 expression plasmid. The firefly luciferase activities were normalized to Renilla luciferase activities, and the fold inductions were determined relative to the mock-treated, empty vector (V) samples. Western blots for PACT and VP35 are to the right of the graph. “#” denotes nonspecific band. The bars depict the standard deviation from three independent replicates, *p = 0.0003, **p = 0.04, ***p = 0.006 as determined by Student’s t test.
(B) HEK293T cell lines stably transduced with either scrambled shRNA (scrambled SH cells) or PACT shRNA (PACT SH cells) were used for an IFN-β reporter assay in the presence of transfected empty vector or VP35, following mock infection or SeV infection. Error bars represent standard deviation from three independent replicates (*p = 0.004). A western blot for endogenous PACT is on the right. “#” denotes a nonspecific band.
(C) The stable HEK293T cell lines expressing either scrambled shRNA or PACT shRNA were transfected with either empty vector (vector) or VP35 expression plasmid. Eighteen hours post-transfection, cells were mock infected or infected with SeV. Relative levels of endogenous IFN-β mRNA were determined by qRT-PCR. The error bars represent the standard deviation from three independent replicates (*p = 0.002).
We also generated cell lines that stably express a scrambled, nontargeting shRNA or a shRNA that targets PACT (Figure 2B). Cells transfected with the shRNA to PACT mRNA had reduced PACT expression levels as compared to the scrambled shRNA cells (Figure 2B). Following SeV infection, a significant decrease in IFN-β promoter activation was observed in the absence of PACT in cells transfected with either VP35 plasmid or empty vector, while IFN-β responses were readily detected in the control knockdown cells (Figure 2B). VP35 readily suppressed IFN-β promoter activation in the control cells but did not reduce the residual IFN-β promoter response in PACT knockdown cells (Figure 2C). These data suggest that PACT is a critical component of the pathway antagonized by VP35 and that the impact of VP35 on IFN responses is magnified when PACT is present.
Expression of VP35 Disrupts the Association of PACT with RIG-I
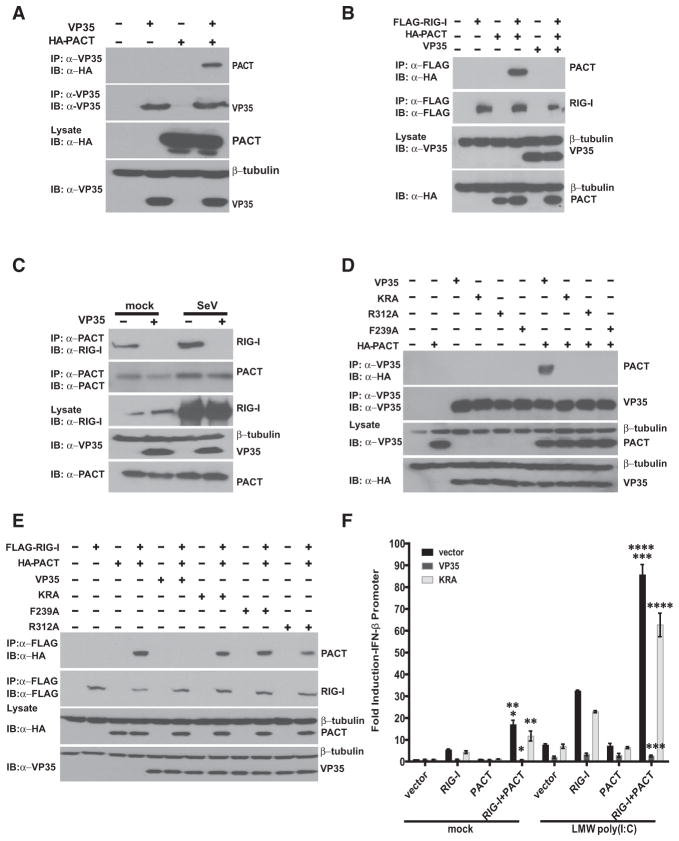
PACT interacts with RIG-I to augment RIG-I signaling (Kok et al., 2011). To understand how VP35 interferes with PACT-mediated RIG-I signaling, we first confirmed VP35-PACT and RIG-I-PACT interaction by coimmunoprecipitation assay (Figures 3A and 3B). We further demonstrated that VP35 expression is sufficient to disrupt the interaction between overexpressed RIG-I and PACT (Figure 3B). To determine if VP35 expression also affects the interaction of endogenous PACT with endogenous RIG-I, we utilized control 293T cells and 293T cells stably expressing VP35. With anti-PACT antibody in control cells, RIG-I was coprecipitated with PACT in the presence or absence of SeV infection. The strong interaction observed in virus-infected cells correlates with an increase in RIG-I levels following infection which occurs even in the VP35-expressing cells. Despite this increase, PACT and RIG-I did not coprecipitate in the VP35-expressing cells (Figure 3C), suggesting that VP35 can disrupt the RIG-I-PACT complex.
Figure 3. Expression of VP35 Disrupts RIG-I-PACT Interaction.
(A) Coimmunoprecipitation of VP35 and PACT. Cells were transfected PACT and VP35 plasmids, as indicated. Transfected cell lysates were immunoprecipitated (IP) with anti-VP35 antibody, and VP35, PACT, and β-tubulin were detected by western blotting.
(B) RIG-I and PACT interaction in the absence or presence of VP35. A coimmunoprecipitation (coIP) experiment was performed. VP35, RIG-I, and PACT were detected by western blotting as indicated.
(C) IP with anti-PACT antibody was performed on mock or SeV-infected control HEK293T cells or HEK293T cells stably expressing VP35 (293T-VP35). The expression of RIG-I and VP35 was detected by western blot.
(D) WT or mutant VP35s, RIG-I, and PACT were expressed individually or in combination, by transient transfection, as indicated. A coIP experiment was performed as above.
(E) WT or mutant VP35s, RIG-I, and PACT were expressed individually or in combination, as indicated. A coIP experiment was performed as above.
(F) An IFN-β promoter firefly luciferase reporter assay was performed. The empty vector (vector) or expression plasmids for RIG-I, PACT, and WT or mutant VP35 were transfected as indicated. The cultures were subsequently mock transfected or transfected with low-molecular-weight (LMW) poly(I:C). A dual luciferase assay was performed, and IFN-β firefly luciferase expression activity was quantified and normalized to control Renilla lucif-erase activity. Fold induction for the IFN-β promoter was determined by setting the mock-treated, empty vector samples to 1. Error bars indicate standard deviation of three independent replicates. *p = 0.004, **p = 0.123, ***p = 0.001, ****p = 0.06 (not significant). See also Figure S1.
To identify the region of VP35 sufficient for interaction with PACT, we performed domain mapping. We expressed the VP35 amino terminus (1–218 amino acids) and the carboxy terminal IFN inhibitory domain (IID, 215–340 amino acids) (see Figure S1). By coimmunoprecipitation assay, the IID domain was sufficient for interaction with PACT (Figure S1).
To determine whether residues that are important for dsRNA binding are also required to inhibit the activation of RIG-I by PACT, we tested the activity of K319A/R322A (KRA), R312A, and F239A mutant VP35 proteins (Cardenas et al., 2006; Leung et al., 2010). Each mutant failed to coimmunoprecipitate with PACT (Figure 3D), which correlated with an inability to block RIG-I-PACT interaction (Figure 3E) and a greatly reduced ability to inhibit PACT-stimulated activation of the IFN-β promoter (Figure 3F and Figure S1). Therefore, the ability of VP35 to inhibit PACT activation of RIG-I signaling requires an intact central basic patch and endcap residues, which are also important for dsRNA binding.
VP35 IID Can Inhibit Both PACT- and dsRNA-Mediated RIG-I ATPase Activation
To test the role of PACT in RIG-I activation, we performed in vitro ATPase assays, which report on RIG-I interactions with activators such as dsRNA and PACT or inhibitors. In the absence of activators, RIG-I proteins had negligible intrinsic ATPase activity. In contrast, the presence of activating ligands such as 8bp dsRNA or PACT resulted in the activation of RIG-I ATPase activity of a RIG-I construct lacking the CARD domains and full-length RIG-I (Figures S2A and S2B).
To test if the RIG-I-activating function localizes to a particular domain in PACT, we generated PACT truncation mutants consisting of the first two dsRNA binding domains (dsRBD) (PACT 1-2), the third dsRBD (PACT 3), and full-length (PACT 1-2-3) PACT. In ATPase assays, PACT 1-2 and PACT 3 (Figure S2C) show minimal activation of RIG-I, even when used at 5- to10-fold molar excess compared to PACT 1-2-3 (Figure S2C). Of note, addition of PACT 1-2 and PACT 3 in trans also did not result in activation of RIG-I (Figure S2C), suggesting that full-length PACT, including its linkers, is required for RIG-I activation. Addition of increasing amounts of PACT 1-2 to full-length PACT showed a very low level of inhibition at the highest concentration levels (Figure S2C), suggesting that there may be intermolecular interactions between the PACT 1-2 and full-length PACT constructs, as previously shown (Peters et al., 2001).
VP35 Can Bind to dsRNA and to PACT
To address the relevance of VP35-dsRNA and VP35-PACT interactions for inhibition of RIG-I, we performed ATPase assays in the presence and absence of dsRNA and in the presence of increasing concentrations of VP35 IID. The results revealed a dose-dependent inhibition of RIG-I activation by VP35 (Figures 4A and 4B). This is consistent with our previous studies, which have shown that VP35 IID can bind to dsRNA and sequester dsRNA from being recognized by RIG-I (Cardenas et al., 2006; Leung et al., 2009, 2010). Our data also suggest that PACT can directly interact with RIG-I, and that the presence of VP35 IID disrupts this interaction (Figures 3B and 3C). Therefore, we tested the ability of VP35 IID to inhibit PACT-mediated activation of RIG-I. Consistent with our pull-down data, VP35 IID also inhibited PACT-mediated activation of RIG-I ATPase activity in a dose-dependent manner (Figures 4C and 4D). We tested whether PACT activation of RIG-I was an intrinsic property of PACT or whether trace levels of dsRNA bound to PACT were responsible for this activity, as PACT also binds dsRNA. As shown in Figures 4E and 4F, 8 bp dsRNA or PACT can activate RIG-I ATPase activity. When the reactions were preincubated with RNase A, the dsRNA-mediated ATPase activation was completely abrogated, as expected. However, RNase A addition had no effect on PACT-mediated activation of RIG-I. Similarly, addition of RNase III did not affect the ability of PACT to activate RIG-I ATPase activity and had very little effect on the ability of VP35 IID to inhibit PACT-mediated activation of RIG-I (Figure S3D) (the difference between lanes 5 and 7 is not statistically significant: p value is 0.223).
Figure 4. VP35 Inhibition of RIG-I ATPase Activation Is dsRNA Independent.
(A and C) VP35 inhibits activation of RIG-I ATPase activity by dsRNA (A) and PACT (C) in a concentration-dependent manner.
(B and D) Quantitation of ATPase activity, represented by the amount of inorganic phosphate in each lane, was normalized to lane 2. The data represent the mean, and error bars indicate the standard deviation. The difference between the indicated groups was found to significant as indicated by *p = 0.0015 (B) and p = 0.0007 (D).
(E) Inhibition of PACT-mediated RIG-I activation by VP35 is RNA independent. Addition of RNase A abolishes RIG-I activation by dsRNA (lane 4 versus lane 5), but RNase A has no impact on the VP35 inhibition of PACT (compare lane 8 versus lane 9). Free inorganic phosphate levels were quantified for the region highlighted by the red dotted box.
(F) Quantitation of relative ATPase activity for lanes 6–9 normalized to ATPase activity of lane 6. The difference between the indicated groups was found to be significant, *p = 0.0054. The data represent the mean of three independent experiments with the error bars indicating the standard deviation. See also Figure S2.
Next, we assessed the ability of mutant VP35 IID proteins which lack the ability to bind dsRNA to inhibit PACT-mediated activation of RIG-I. While WT VP35 IID can inhibit PACT-mediated RIG-I activation to almost background levels, the mutant VP35 IID proteins, such as R312A, F239A, and K319A/R322A, show diminished ability to inhibit PACT-mediated RIG-I activation (Figure S3E). Together, these data clarify the requirements for PACT-mediated activation of RIG-I and demonstrate that VP35 can inhibit the activation of RIG-I by dsRNA and PACT.
PACT Inhibits EBOV RNA Polymerase Activity
VP35 also plays a critical role in viral RNA synthesis (Basler and Amarasinghe, 2009). VP35 IID contributes to VP35 polymerase cofactor function, as select mutations in the IID impair viral polymerase function (Prins et al., 2010a). We therefore asked whether PACT affects the participation of VP35 in viral RNA synthesis. PACT was coexpressed with the components of a reconstituted viral polymerase complex, and viral RNA synthesis was assessed by measuring expression of a Renilla luciferase reporter gene expressed from a noninfectious, minigenome RNA. PACT expression inhibited the EBOV polymerase complex as indicated by a dose-dependent reduction in minigenome reporter gene expression (Figure 5A, top panel). Representative western blots indicate that expression levels of NP and VP35 were not affected by PACT overexpression (Figure 5A, bottom panel). This suggested that PACT has the ability to function as a restriction factor for EBOV replication, at least when expressed to sufficient levels. The VP35 central basic patch and endcap mutants (KRA, R312A, and F239A), which are functional in the viral minigenome system (Leung et al., 2010), were also tested for their sensitivity to PACT. In contrast to the results obtained with WT VP35, these mutants were refractory to inhibition by PACT (Figure 5B), correlating inhibition with PACT binding to VP35.
Figure 5. PACT Inhibits EBOV RNA Polymerase Activity.
(A) PACT inhibits EBOV RNA synthesis in a dose-dependent manner. Increasing amounts of HA-tagged PACT plasmid (20, 100, 200, 400 ng) were cotransfected with the plasmids required for minigenome replication and transcription. Also cotransfected was a plasmid that expresses firefly luciferase from an RNA polymerase II promoter. Renilla luciferase activity was normalized firefly luciferase activity. The error bars indicate standard deviation of three independent replicates, *p = 0.0008. The western blot shows expression of NP, VP35, and PACT (HA antibody).
(B) A minigenome experiment similar to that described for (A) was performed except that WT VP35 or the indicated VP35 mutants were included and the PACT plasmid amount was kept constant at 400 ng. The error bars indicate standard deviation of three independent replicates (*p = 0.0009).
(C) Effect of PACT on NP-VP35 association. Cells were transfected with empty vector or plasmids expressing FLAG-VP35 (VP35), NP, or HA-PACT, alone or in the indicated combinations. The lysates were immunoprecipitated with anti-Flag antibody (VP35) and western blotted for Flag and NP.
(D) A coIP assay was performed to analyze the effect of PACT on L-VP35 association. Cells were transfected with empty vector or plasmids for VP35, Flag-L1-505 (Flag-L505), or HA-PACT, as indicated. For immunoprecipitation (IP), anti-VP35 antibody was used. IP and cell lysates (lysates) were analyzed by western blotting to detect Flag-L505, HA-PACT, and VP35. See also Figure S3.
VP35 is an essential component of the EBOV RNA-dependent RNA polymerase (RdRp) complex because it bridges the enzymatic component of the viral RdRp complex, the L protein, and NP, which associates with the template viral RNA (Becker et al., 1998). We hypothesized that PACT would disrupt assembly of the RdRp complex through its interaction with VP35. To determine if this is the case, VP35 and NP were expressed in presence or absence of PACT. Immunoprecipitation with an anti-VP35 monoclonal antibody pulled down equivalent amounts of VP35 in each sample (Figure 5C), and PACT did not detectably affect the amount of NP that coprecipitated with VP35. To explore the effect of PACT on association with L, we utilized a plasmid encoding amino acids 1–505 of L (L505), a region which was previously shown to be sufficient for interaction with VP35 (Prins et al., 2010a; Trunschke et al., 2013). PACT overexpression reduced L-VP35 interaction (Figure 5D); however, L interaction with a representative central patch mutant (KRA) was not affected (Figure S3). Therefore, PACT disruption of VP35-L interaction likely accounts for its inhibition of EBOV RNA synthesis as measured by the minigenome system.
PACT Expression Modulates EBOV Replication and Host Response
To determine whether PACT exerts its effects in the context of replicating EBOV, we generated A549 cells stably transduced with a PACT-expressing lentivirus or a control, empty vector lentivirus (Figure 6A). Both cell lines were infected (moi = 0.5) with a recombinant WT EBOV (zEBOV-wt) or a recombinant EBOV possessing mutations K319A, R322A, and F239A within the VP35 IID (zEBOV-VP35 mut). Virus replication was monitored by plaque assay (Figure 6B). Overexpression of PACT reduced infectious zEBOV-wt titers released into the cell culture medium by 15-fold (from 4.5 × 105 to 3 × 104 PFU/mL) by 24 hr postinfection (Figure 6B). While the titer of zEBOV-wt did not increase further by 72 hr postinfection in the control cells, virus replication eventually rose in the PACT cells and reached titers comparable to that in the control cells. Interestingly, zEBOV VP35 mut grew in control cells to 100-fold lower levels, as assessed by plaque assay and qRT-PCR, than did zEBOV-wt (Figure 6B), but the replication of this mutant virus was not further impaired by PACT overexpression (Figure 6B). This is in agreement with the minigenome assays performed with mutant VP35s, where viral RNA synthesis was not impaired by PACT (Figure 5B). These data support the hypothesis that PACT can inhibit EBOV RNA synthesis in a manner that depends upon the presence of an intact central basic patch to mediate PACT-VP35 interaction.
Figure 6. PACT Expression Inhibits Replication and Modulates Innate Immune Responses during EBOV Infection.
(A) Western blot for PACT in the control and stable PACT expressing (PACT) cell lines. # denotes a nonspecific band.
(B) The cells were infected with zEBOV-wt or zE-BOV-VP35 mut at moi = 0.5. The supernatants from infected cells were collected. The virus titers in 24 and 72 hr postinfection supernatants were determined by plaque assay. (*p = 0.014).
(C and D) Quantitative RT-PCR for endogenous (C) IFN-β and (D) ISG54 mRNA levels normalized to β-actin mRNA at 24 hr postinfection. The error bars indicate the standard deviation of three replicates of the RT-PCR reaction. The induction of IFN-β and ISG54 was found to be significant in PACT cells compared to control cells for virus infection, where *p = 0.01 and **p = 0.03 for (C) and *p = 0.003 and **p = 0.03 for (D). See also Figure S4.
To determine whether PACT overexpression also modulates innate immune responses to EBOV infection, we analyzed the levels of IFN-β and ISG54 by qRT-PCR. IFN-β and ISG54 levels remained suppressed in zEBOV-wt-infected control cells at 24 hpi (Figures 6C and 6D). However, in PACT cells this inhibition was overcome (Figures 6C and 6D), resulting in about 8-fold higher levels of IFN-β induction and 20-fold higher ISG54 induction. By comparison, zEBOV VP35 mut infection resulted in significant increase in IFN-β and ISG54 mRNA accumulation in the PACT-expressing cells, consistent with the RIG-I-activating nature of PACT and the inability of mutant VP35 to block this activation (Figures 6C and 6D). These data further support the functional relevance of VP35-PACT interaction in the context of EBOV infection.
We also infected the PACT knockdown 293T cells and the corresponding control stable cell line (Figure S4A) with zEBOV-wt and zEBOV-VP35 mut at moi = 1 and moi = 0.01. The partial knockdown did not significantly impact zEBOV-wt growth, as assessed by measuring infectious viral titers in infected cell medium (moi = 1) (Figure S4B), as might be expected of a virus encoding an effective inhibitor of PACT-induced IFN responses. Interestingly, we also did not see significant restriction of ZE-BOV-VP35 mut growth, even in the control cells. Nonetheless, the knockdown cells did display a significant increase viral RNA synthesis (Figures S4C and S4D) and significantly decreased IFN-β and ISG54 expression upon zEBOV VP35-mut infection (Figures S4E and S4F).
DISCUSSION
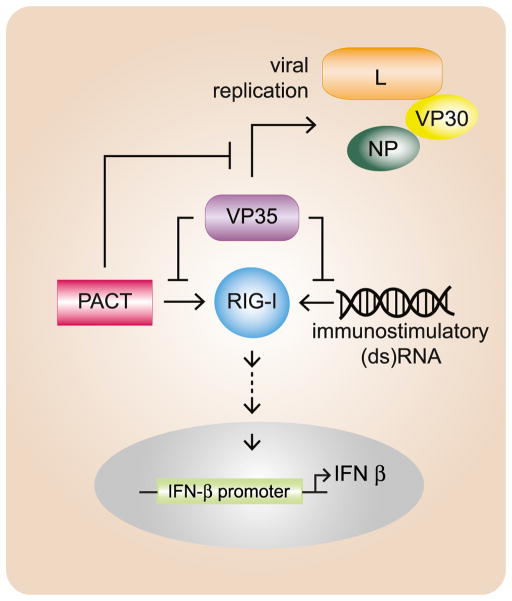
These data represent a unique mechanism of IFN-antagonism by a viral protein through direct inhibition of PACT as well as a role for PACT in inhibition of viral replication. It is notable that VP35 carries out several functions critical to EBOV pathogenesis, including suppression of IFN-α/β production and assembly of a functional viral RNA polymerase complex. Although the VP35 C-terminal IID comprises only one-third of VP35, this domain contributes to several functionally important interactions including with dsRNA, with NP, and, as shown here, with PACT and L. Thus, our observations in this study provide a rationale for the coexistence of IFN-antagonist and RNA replication functions within the same domain of VP35. In this model, filovirus RNA synthesis, a source of immunostimulatory RNAs, is modulated by VP35 engagement by the RIG-I activator PACT (Figure 7).
Figure 7. Working Model for Mutual Antagonism between VP35 and PACT.
EBOV VP35 functions as an inhibitor of RIG-I signaling and as an essential component of the viral RNA replication complex. VP35 can block either the RNA- or the PACT-mediated activation of RIG-I, but PACT can inhibit the RNA replication complex through interaction with VP35. Under conditions in which VP35 levels may be limited, PACT interaction with VP35 may slow virus replication, resulting in decreased production of immunostimulatory viral replication products. This would allow VP35 to sense the immune status through PACT availability and thereby regulate replication accordingly.
Although PACT interacts with the C terminus of RIG-I, the structural and biochemical basis by which it modulates RIG-I activation remains incompletely understood. Our data suggest that VP35 can interact directly with PACT and thereby prevent PACT interaction with and activation of RIG-I. Notably, PACT interacts in a context-dependent fashion with multiple cellular binding partners, reflecting its multifunctional nature. Among these, PACT interacts with TRBP and Dicer, components of the RNA-induced silencing complex (RISC), and stimulates the function of Dicer (Kok et al., 2007; Lee et al., 2006). PACT is also a dsRNA-binding protein but can activate PKR through protein-protein interactions between the dsRBDs present in each protein (Li et al., 2006; Patel and Sen, 1998; Peters et al., 2001). Evidence suggests that PACT can be phosphorylated and activated in response to cell stresses, relieving it from interaction with TRBP and allowing it to bind to and activate PKR (Patel et al., 2000; Peters et al., 2006; Singh et al., 2011). Given that PACT activates PKR and that VP35 inhibits PKR, it will be of interest to determine whether VP35-PACT interaction also contributes to VP35-PKR inhibition (Fabozzi et al., 2011; Feng et al., 2007; Schümann et al., 2009; Zhu et al., 2012).
Previous biochemical and structural studies demonstrated that VP35 interacts with dsRNA via the IID (Kimberlin et al., 2010; Leung et al., 2009, 2010). Within the VP35 IID structure, central basic patch residues contact the dsRNA phosphodiester backbone, while a hydrophobic pocket, which includes residue F239, binds the ends of the dsRNA, forming an endcap (Kimberlin et al., 2010; Leung et al., 2010). Central basic patch residues also made inter-IID contacts in the structure, highlighting their additional role in protein-protein interactions (Kimberlin et al., 2010; Leung et al., 2010). Central basic patch mutations abrogated the interaction with PACT in transfected cells and inhibited its RIG-I stimulatory activity. These data, however, cannot distinguish whether loss of interaction and inhibition is dependent on or independent of dsRNA. In vitro assays, in which purified IID, PACT, and RIG-I were employed, demonstrated that IID-mediated inhibition occurs in the absence of added dsRNA and that it is not affected by addition of RNase A, suggesting that inhibition is RNA independent and that IID-PACT interaction does not require RNA. It remains possible that dsRNA can augment the VP35-PACT interaction.
The IID contributes to interactions necessary for functioning of the EBOV RNA polymerase (Leung et al., 2010; Prins et al., 2010a). In our studies, PACT clearly inhibits WT EBOV RNA synthesis. This was evidenced by the fact that PACT is able to inhibit minigenome activity in a dose-dependent manner and by the fact that inhibition was seen when using WT but not mutant VP35s. Similarly, we detected reduced accumulation of viral mRNA and genomic RNA in cells infected with WT recombinant EBOV, but not with an EBOV encoding a mutant VP35. These data strongly suggest that PACT mediates its inhibitory effects through interaction with the VP35 IID. Residues of the IID first basic patch were previously shown to affect VP35 interaction with NP and thereby disrupt function in the minigenome assay (Prins et al., 2010a), and interaction of VP35 with NP can be affected by VP35 interaction with another cellular protein that binds dsRNA, DRBP76 (Shabman et al., 2011). In contrast, our coprecipitation assays did not demonstrate any effect of PACT on VP35-NP interaction. Instead, we demonstrated a dose-dependent disruption of VP35-L interaction. Although previous studies on MARV VP35 demonstrated a critical role for a coiled-coil domain located within the VP35 N-terminal half for interaction with L (Becker et al., 1998), our data demonstrate that the VP35 IID is also involved in interaction with L.
The data above support the view that the levels of PACT present in EBOV-infected cells can influence both virus replication and host IFN responses. While the impact of PACT observed here on viral RNA synthesis and production of infectious viral particles is modest, this may not be surprising, given that filoviruses can replicate with high efficiency in human and nonhuman primate cells and encode multiple mechanisms to counteract IFN responses. Nonetheless, our observations support a model in which PACT can impair viral RNA synthesis. This represents a cell-intrinsic antiviral mechanism for PACT. The combined antiviral effects of many IFN-induced genes/host factors are often greater than the individual impact, and therefore it is likely that the cumulative effect of multiple IFN-induced and cell intrinsic host factors, including those identified here for PACT, are likely to produce a potent antiviral response (Schoggins and Rice, 2011). Therefore we expect that while PACT itself may not effectively control virus replication, the antiviral impact of PACT might be greater in combination with other factors or in the absence of effective PACT inhibitors. Understanding the exact role of PACT, its interaction(s) with other cellular factors, and its interference with viral replication processes may lead to antiviral strategies.
EXPERIMENTAL PROCEDURES
Cell Lines and Viruses
Vero, HEK293T, A549, and mouse embryo fibroblast cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (FBS), 1% penicillin, and streptomycin (100 unit/ml) (Invitrogen) and were grown at 37°C and 5% CO2. BSRT7 cells were grown in the same medium supplemented with 1% of G418 (50 mg/ml). The PACT WT and knockout mouse embryo fibroblasts were kindly provided by Dr. Ganes Sen (Lerner Research Institute, Cleveland Clinic). Sendai virus (SeV) strain Cantell stocks were prepared by growth in 10-day-old embryonated chicken eggs for 2 days at 37°C.
Stable cell lines overexpressing PACT or VP35 were generated using replication-defective lentiviruses derived from the plasmid pCSIGW. This plasmid was a gift from Drs. Lubbertus Mudler and Viviana Simon (Icahn School of Medicine at Mount Sinai).
Short hairpin (sh) PACT knockdown cell lines were generated by using constructs based on the retroviral silencing plasmid (pGFP-V-RS shRNA vector) (OriGene) which contains a CMV promoter-driven tGFP gene, a puromycin resistance selection, and 29-mer shRNA construct to target PACT (CACCTT CAGAGTAACCGTTGGTGACATAA) or a control shRNA that consists of an on-silencing scrambled sequence (5′-GCACTACCAGAGCTAACTCAGATAG TACT-3′). The PACT knockdown and control cells were selected following transfection with puromycin.
Generation of Recombinant EBOV
Starting with a full-length cDNA clone encoding WT Zaire EBOV strain Mayinga-76, we introduced nucleotide substitutions resulting in three amino acid changes at VP35 amino acids 239, 319, and 322 (F239A/K319A/R322A) to generate the virus referred to as zEBOV-VP35 mut. Established procedures were used to recover the recombinant virus (see the Supplemental Experimental Procedures for details) (Ebihara et al., 2006). After transfection supernatants were passaged on Vero cells, the first-passage supernatants were collected at 12 days postinfection, and RNA was extracted using the QIAamp Viral RNA mini kit (QIAGEN). Complete sequencing of the rescued EBOV was performed. Virus infectivity titers (focus-forming units, FFUs) were determined by counting the number of infected cell foci using an indirect immunofluorescent antibody assay (Ebihara et al., 2006). Generation of recombinant zEBOV was carried out in the biosafety level 4 (BSL-4) facilities at Rocky Mountain Laboratories (NIAID, NIH), and sample inactivation/removal was performed according to standard operating protocols approved by the local Institutional Biosafety Committee.
EBOV Infections and RNA Isolation
The following infections were performed under BSL-4 conditions at the Galveston National Laboratory. A549 cells expressing GFP (control) or PACT were infected (moi = 0.5), and also stable 293T cells, with scrambled or PACT knockdown, were infected (moi = 0.1 and 1) with either WT Zaire EBOV (zEBOV-wt) or zEBOV-VP35 mut viruses. At 24 and 72 hr postinfection (hpi), viral supernatants were collected and clarified by centrifugation, and Trizol (Life Technologies) cell extracts were collected. The resulting viral RNAs were quantified by real-time PCR (RT-PCR) using OneStep RT-PCR kits (QIAGEN) (see the Supplemental Experimental Procedures for details).
EBOV Minigenome Assay
The EBOV minigenome assay was based on a previously described system and was performed in BSRT7 cells (Mühlberger et al., 1999). Details are provided in the Supplemental Experimental Procedures.
IFN-β and ISG54 Promoter Reporter Gene Assays
HEK293T cells were transfected by using Lipofectamine 2000 (Invitrogen) with the indicated expression plasmids, an ISG54-promoter or an IFN-β-promoter firefly luciferase reporter plasmid (100 ng), and a constitutively expressed Renilla luciferase reporter plasmid, pRLTK (10 ng). At 18 hr posttransfection (hpt), IFN responses were induced as described in the Results. Sixteen hours later, the cell lysates were assayed with the Dual Luciferase reporter assay (Promega), and firefly luciferase activity was normalized to Renilla luciferase activity.
IRF-3 Activation Assay
293T cells were transfected with empty vector or with plasmids expressing RIG-I, PACT or both, with or without plasmids encoding VP35 and/or IRF-3. Eighteen hours posttransfection, the cells were either mock infected or infected with SeV for 1 hr. Sixteen hours later, cells were lysed with NP-40 lysis buffer (10 mM Tris-HCL [pH 7.4], 100 mM sodium chloride, 1 mM EGTA, 1 mM EDTA, glycerol, 0.5% NP-40). The lysates were analyzed by western blotting with anti-phospho-IRF-3 (S396), anti-IRF-3 (total IRF-3), anti-FLAG, anti-HA, and anti-VP35 antibodies.
Quantitative RT-PCR
Viral RNAs were extracted from infected cells with TRIZOL reagent (Sigma) and quantified by RT-PCR (see the Supplemental Experimental Procedures for details).
IFN Bioassay
IFN bioassays were performed as previously described (Leung et al., 2011).
Immunoprecipitation and Western Blotting
Immunoprecipitations were performed in NP-40 lysis buffer using antibodies described in the Supplemental Experimental Procedures.
Cloning, Protein Expression, and Purification of Proteins for Biochemical Assays
The coding regions for EBOV VP35 IID (residues 215–340), full-length human RIG-I (residues 1–925), RIG-I ΔCARDs (residues 230–925) and human PACT (residues 1–313), PACT 1-2 (residues 1–193), and PACT 3 (residues 194–313) were subcloned into a modified pET15b vector and expressed as previously described (Ramanan et al., 2012).
ATPase Assays
Thin-layer chromatography assays were performed to assess the ATPase activity of RIG-I constructs in a reaction containing 10 mM HEPES (pH 7.0), 150 mM NaCl, 1 mM MgCl2, 2 mM TCEP, 100 μM ATP, 5 μCi γ-32P-ATP, and 100 nM RIG-I ΔCARDs or 2 μM MBP-RIG-I full-length. Sixty nanomolar 8 bp dsRNA (IDT) with a palindromic sequence (rCrGrCrArUrGrCrG) or 0.7 μM of MBP-PACT full-length was used to activate RIG-I ATPase activity. VP35 IID constructs (WT or mutants) were added at various concentrations ranging from 5 to 100 μM. ATPase activity was monitored as previously described (Ramanan et al., 2012). Some assays were carried out in the presence of RNase III or RNase A in order to test RNA-independent PACT-VP35 interactions.
Supplementary Material
Acknowledgments
The work was supported in part by NIH grants AI059536 and AI093786 to C.F.B.; NIH grant AI081914 to G.K.A.; and the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA. Monoclonal antibodies against EBOV VP35 and EBOV NP were generated in collaboration with the Mount Sinai Hybridoma Shared Research Facility. We thank Ganes Sen (Lerner Research Institute, Cleveland Clinic) for providing PACT knockout MEFs, Qinghua Liu (UT Southwestern, Dallas) for PACT cDNA, and Adolfo García-Sastré (Mount Sinai School of Medicine) for providing the RIG-I expression plasmid. We thank Reed S. Shabman for assistance in setting up the minigenome assays and Juyoung Huh (WUSM), Krystle Agans (UTMB), and Kwabena Bonsu (MSSM) for excellent technical support.
Footnotes
Supplemental Information includes four figures, Supplemental Experimental Procedures, and Supplemental References and can be found with this article at http://dx.doi.org/10.1016/j.chom.2013.06.010.
References
- Bale S, Julien JP, Bornholdt ZA, Kimberlin CR, Halfmann P, Zandonatti MA, Kunert J, Kroon GJ, Kawaoka Y, MacRae IJ, et al. Marburg virus VP35 can both fully coat the backbone and cap the ends of dsRNA for interferon antagonism. PLoS Pathog. 2012;8:e1002916. doi: 10.1371/journal.ppat.1002916. http://dx.doi.org/10.1371/journal.ppat.1002916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basler CF, Amarasinghe GK. Evasion of interferon responses by Ebola and Marburg viruses. J Interferon Cytokine Res. 2009;29:511–520. doi: 10.1089/jir.2009.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basler CF, Wang X, Mühlberger E, Volchkov V, Paragas J, Klenk HD, García-Sastre A, Palese P. The Ebola virus VP35 protein functions as a type I IFN antagonist. Proc Natl Acad Sci USA. 2000;97:12289–12294. doi: 10.1073/pnas.220398297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basler CF, Mikulasova A, Martinez-Sobrido L, Paragas J, Mühlberger E, Bray M, Klenk HD, Palese P, García-Sastre A. The Ebola virus VP35 protein inhibits activation of interferon regulatory factor 3. J Virol. 2003;77:7945–7956. doi: 10.1128/JVI.77.14.7945-7956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S, Rinne C, Hofsäss U, Klenk HD, Mühlberger E. Interactions of Marburg virus nucleocapsid proteins. Virology. 1998;249:406–417. doi: 10.1006/viro.1998.9328. [DOI] [PubMed] [Google Scholar]
- Cardenas WB, Loo YM, Gale M, Jr, Hartman AL, Kimberlin CR, Martínez-Sobrido L, Saphire EO, Basler CF. Ebola virus VP35 protein binds double-stranded RNA and inhibits alpha/beta interferon production induced by RIG-I signaling. J Virol. 2006;80:5168–5178. doi: 10.1128/JVI.02199-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TH, Kubota T, Matsuoka M, Jones S, Bradfute SB, Bray M, Ozato K. Ebola Zaire virus blocks type I interferon production by exploiting the host SUMO modification machinery. PLoS Pathog. 2009;5:e1000493. doi: 10.1371/journal.ppat.1000493. http://dx.doi.org/10.1371/journal.ppat.1000493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chendrimada TP, Gregory RI, Kumaraswamy E, Norman J, Cooch N, Nishikura K, Shiekhattar R. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature. 2005;436:740–744. doi: 10.1038/nature03868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara H, Takada A, Kobasa D, Jones S, Neumann G, Theriault S, Bray M, Feldmann H, Kawaoka Y. Molecular determinants of Ebola virus virulence in mice. PLoS Pathog. 2006;2:e73. doi: 10.1371/journal.ppat.0020073. http://dx.doi.org/10.1371/journal.ppat.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabozzi G, Nabel CS, Dolan MA, Sullivan NJ. Ebolavirus proteins suppress the effects of small interfering RNA by direct interaction with the mammalian RNA interference pathway. J Virol. 2011;85:2512–2523. doi: 10.1128/JVI.01160-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann H, Jones S, Klenk HD, Schnittler HJ. Ebola virus: from discovery to vaccine. Nat Rev Immunol. 2003;3:677–685. doi: 10.1038/nri1154. [DOI] [PubMed] [Google Scholar]
- Feng Z, Cerveny M, Yan Z, He B. The VP35 protein of Ebola virus inhibits the antiviral effect mediated by double-stranded RNA-dependent protein kinase PKR. J Virol. 2007;81:182–192. doi: 10.1128/JVI.01006-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase AD, Jaskiewicz L, Zhang H, Lainé S, Sack R, Gatignol A, Filipowicz W. TRBP, a regulator of cellular PKR and HIV-1 virus expression, interacts with Dicer and functions in RNA silencing. EMBO Rep. 2005;6:961–967. doi: 10.1038/sj.embor.7400509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haasnoot J, de Vries W, Geutjes EJ, Prins M, de Haan P, Berkhout B. The Ebola virus VP35 protein is a suppressor of RNA silencing. PLoS Pathog. 2007;3:e86. doi: 10.1371/journal.ppat.0030086. http://dx.doi.org/10.1371/journal.ppat.0030086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman AL, Dover JE, Towner JS, Nichol ST. Reverse genetic generation of recombinant Zaire Ebola viruses containing disrupted IRF-3 inhibitory domains results in attenuated virus growth in vitro and higher levels of IRF-3 activation without inhibiting viral transcription or replication. J Virol. 2006;80:6430–6440. doi: 10.1128/JVI.00044-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman AL, Bird BH, Towner JS, Antoniadou ZA, Zaki SR, Nichol ST. Inhibition of IRF-3 activation by VP35 is critical for the high level of virulence of ebola virus. J Virol. 2008a;82:2699–2704. doi: 10.1128/JVI.02344-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman AL, Ling L, Nichol ST, Hibberd ML. Whole-genome expression profiling reveals that inhibition of host innate immune response pathways by Ebola virus can be reversed by a single amino acid change in the VP35 protein. J Virol. 2008b;82:5348–5358. doi: 10.1128/JVI.00215-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Yang M, May WS. RAX, a cellular activator for double-stranded RNA-dependent protein kinase during stress signaling. J Biol Chem. 1999;274:15427–15432. doi: 10.1074/jbc.274.22.15427. [DOI] [PubMed] [Google Scholar]
- Iwamura T, Yoneyama M, Koizumi N, Okabe Y, Namiki H, Samuel CE, Fujita T. PACT, a double-stranded RNA binding protein acts as a positive regulator for type I interferon gene induced by Newcastle disease virus. Biochem Biophys Res Commun. 2001;282:515–523. doi: 10.1006/bbrc.2001.4606. [DOI] [PubMed] [Google Scholar]
- Kimberlin CR, Bornholdt ZA, Li S, Woods VL, Jr, MacRae IJ, Saphire EO. Ebolavirus VP35 uses a bimodal strategy to bind dsRNA for innate immune suppression. Proc Natl Acad Sci USA. 2010;107:314–319. doi: 10.1073/pnas.0910547107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok KH, Ng MH, Ching YP, Jin DY. Human TRBP and PACT directly interact with each other and associate with dicer to facilitate the production of small interfering RNA. J Biol Chem. 2007;282:17649–17657. doi: 10.1074/jbc.M611768200. [DOI] [PubMed] [Google Scholar]
- Kok KH, Lui PY, Ng MH, Siu KL, Au SW, Jin DY. The double-stranded RNA-binding protein PACT functions as a cellular activator of RIG-I to facilitate innate antiviral response. Cell Host Microbe. 2011;9:299–309. doi: 10.1016/j.chom.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Lee Y, Hur I, Park SY, Kim YK, Suh MR, Kim VN. The role of PACT in the RNA silencing pathway. EMBO J. 2006;25:522–532. doi: 10.1038/sj.emboj.7600942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HY, Zhou K, Smith AM, Noland CL, Doudna JA. Differential roles of human Dicer-binding proteins TRBP and PACT in small RNA processing. Nucleic Acids Res. 2013 doi: 10.1093/nar/gkt361. http://dx.doi.org/10.1093/nar/gkt361. [DOI] [PMC free article] [PubMed]
- Leung DW, Ginder ND, Fulton DB, Nix J, Basler CF, Honzatko RB, Amarasinghe GK. Structure of the Ebola VP35 interferon inhibitory domain. Proc Natl Acad Sci USA. 2009;106:411–416. doi: 10.1073/pnas.0807854106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung DW, Prins KC, Borek DM, Farahbakhsh M, Tufariello JM, Ramanan P, Nix JC, Helgeson LA, Otwinowski Z, Honzatko RB, et al. Structural basis for dsRNA recognition and interferon antagonism by Ebola VP35. Nat Struct Mol Biol. 2010;17:165–172. doi: 10.1038/nsmb.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung LW, Park MS, Martinez O, Valmas C, López CB, Basler CF. Ebolavirus VP35 suppresses IFN production from conventional but not plasmacytoid dendritic cells. Immunol Cell Biol. 2011;89:792–802. doi: 10.1038/icb.2010.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung DW, Basler CF, Amarasinghe GK. Molecular mechanisms of viral inhibitors of RIG-I-like receptors. Trends Microbiol. 2012;20:139–146. doi: 10.1016/j.tim.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Peters GA, Ding K, Zhang X, Qin J, Sen GC. Molecular basis for PKR activation by PACT or dsRNA. Proc Natl Acad Sci USA. 2006;103:10005–10010. doi: 10.1073/pnas.0602317103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlberger E, Lötfering B, Klenk HD, Becker S. Three of the four nucleocapsid proteins of Marburg virus, NP, VP35, and L, are sufficient to mediate replication and transcription of Marburg virus-specific monocistronic minigenomes. J Virol. 1998;72:8756–8764. doi: 10.1128/jvi.72.11.8756-8764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlberger E, Weik M, Volchkov VE, Klenk HD, Becker S. Comparison of the transcription and replication strategies of marburg virus and Ebola virus by using artificial replication systems. J Virol. 1999;73:2333–2342. doi: 10.1128/jvi.73.3.2333-2342.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel RC, Sen GC. PACT, a protein activator of the interferon-induced protein kinase, PKR. EMBO J. 1998;17:4379–4390. doi: 10.1093/emboj/17.15.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel CV, Handy I, Goldsmith T, Patel RC. PACT, a stress-modulated cellular activator of interferon-induced double-stranded RNA-activated protein kinase, PKR. J Biol Chem. 2000;275:37993–37998. doi: 10.1074/jbc.M004762200. [DOI] [PubMed] [Google Scholar]
- Peters GA, Hartmann R, Qin J, Sen GC. Modular structure of PACT: distinct domains for binding and activating PKR. Mol Cell Biol. 2001;21:1908–1920. doi: 10.1128/MCB.21.6.1908-1920.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters GA, Li S, Sen GC. Phosphorylation of specific serine residues in the PKR activation domain of PACT is essential for its ability to mediate apoptosis. J Biol Chem. 2006;281:35129–35136. doi: 10.1074/jbc.M607714200. [DOI] [PubMed] [Google Scholar]
- Prins KC, Cárdenas WB, Basler CF. Ebola virus protein VP35 impairs the function of interferon regulatory factor-activating kinases IKKepsilon and TBK-1. J Virol. 2009;83:3069–3077. doi: 10.1128/JVI.01875-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins KC, Binning JM, Shabman RS, Leung DW, Amarasinghe GK, Basler CF. Basic residues within the ebolavirus VP35 protein are required for its viral polymerase cofactor function. J Virol. 2010a;84:10581–10591. doi: 10.1128/JVI.00925-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins KC, Delpeut S, Leung DW, Reynard O, Volchkova VA, Reid SP, Ramanan P, Cárdenas WB, Amarasinghe GK, Volchkov VE, Basler CF. Mutations abrogating VP35 interaction with double-stranded RNA render Ebola virus avirulent in guinea pigs. J Virol. 2010b;84:3004–3015. doi: 10.1128/JVI.02459-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanan P, Edwards MR, Shabman RS, Leung DW, Endlich-Frazier AC, Borek DM, Otwinowski Z, Liu G, Huh J, Basler CF, Amarasinghe GK. Structural basis for Marburg virus VP35-mediated immune evasion mechanisms. Proc Natl Acad Sci USA. 2012;109:20661–20666. doi: 10.1073/pnas.1213559109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoggins JW, Rice CM. Interferon-stimulated genes and their antiviral effector functions. Curr Opin Virol. 2011;1:519–525. doi: 10.1016/j.coviro.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schümann M, Gantke T, Mühlberger E. Ebola virus VP35 antagonizes PKR activity through its C-terminal interferon inhibitory domain. J Virol. 2009;83:8993–8997. doi: 10.1128/JVI.00523-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabman RS, Leung DW, Johnson J, Glennon N, Gulcicek EE, Stone KL, Leung L, Hensley L, Amarasinghe GK, Basler CF. DRBP76 associates with Ebola virus VP35 and suppresses viral polymerase function. J Infect Dis. 2011;204(Suppl 3 ):S911–S918. doi: 10.1093/infdis/jir343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Castillo D, Patel CV, Patel RC. Stress-induced phosphorylation of PACT reduces its interaction with TRBP and leads to PKR activation. Biochemistry. 2011;50:4550–4560. doi: 10.1021/bi200104h. [DOI] [PubMed] [Google Scholar]
- Spiropoulou CF, Ranjan P, Pearce MB, Sealy TK, Albariño CG, Gangappa S, Fujita T, Rollin PE, Nichol ST, Ksiazek TG, Sambhara S. RIG-I activation inhibits ebolavirus replication. Virology. 2009;392:11–15. doi: 10.1016/j.virol.2009.06.032. [DOI] [PubMed] [Google Scholar]
- Theriault S, Groseth A, Neumann G, Kawaoka Y, Feldmann H. Rescue of Ebola virus from cDNA using heterologous support proteins. Virus Res. 2004;106:43–50. doi: 10.1016/j.virusres.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Trunschke M, Conrad D, Enterlein S, Olejnik J, Brauburger K, Mühlberger E. The L-VP35 and L-L interaction domains reside in the amino terminus of the Ebola virus L protein and are potential targets for antivirals. Virology. 2013;441:135–145. doi: 10.1016/j.virol.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Cherukuri NC, Jackel JN, Wu Z, Crary M, Buckley KJ, Bisaro DM, Parris DS. Characterization of the RNA silencing suppression activity of the Ebola virus VP35 protein in plants and mammalian cells. J Virol. 2012;86:3038–3049. doi: 10.1128/JVI.05741-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.