Abstract
Aim
To determine if physiological, rhythmic fluctuations of vagal baroreflex gain persist during exercise, post-exercise ischaemia, and recovery.
Methods
We studied responses of six supine healthy men and one woman to a stereotyped protocol comprising rest, handgrip exercise at 40 % maximum capacity to exhaustion, post-exercise forearm ischaemia, and recovery. We measured electrocardiographic R-R intervals, photoplethysmographic finger arterial pressures, and peroneal nerve muscle sympathetic activity. We derived vagal baroreflex gains from a sliding (25 s window moved by 2 s steps) systolic pressure – R-R interval transfer function at 0.04 – 0.15 Hz.
Results
Vagal baroreflex gain oscillated at low, nearly constant frequencies throughout the protocol (at ~ 0.06 Hz – a period of about 18 s); however, during exercise, most oscillations were at low gain levels, and during ischaemia and recovery, most oscillations were at high gain levels.
Conclusions
Vagal baroreflex rhythms are not abolished by exercise, and they are not overwhelmed after exercise during ischaemia and recovery.
Keywords: sympathetic, vagal, exercise, resetting
Vagal baroreflex gain diminishes during exercise (Bristow et al., 1971) in proportion to the intensity of the exercise (McRitchie et al., 1976, Mancia et al., 1978). A recent study (Fisher et al., 2007) indicates that this reduction, as reflected by the magnitude of heart rate slowing caused by neck suction (Eckberg et al., 1975), is not constant throughout exercise, but rather, after an early reduction, tends to recover as exercise continues. This interesting finding is not unexpected: early (Smyth et al., 1969) and late (Eckberg & Kuusela 2005, Westerhof et al., 2006) studies of human vagal baroreflexes document major ongoing variability of human vagal baroreflex gain. One of the later studies (Eckberg & Kuusela 2005) adds a provocative new twist: fluctuations of human baroreflex gain are organized, as a very low-frequency baroreflex rhythm.
We studied vagal baroreflex oscillations in healthy supine subjects during handgrip followed by ischaemia, and tested the null hypothesis that during exercise, when vagal baroreflex gain is diminished, and during post-exercise ischaemia, when vagal baroreflex gain is large (Carrington & White 2001), vagal baroreflex rhythmicity is absent.
Methods
Subjects
We studied five male astronauts and one male and one female backup astronaut who participated in the Neurolab Space Shuttle mission. The handgrip – ischaemia experiment was part of a larger series of autonomic studies (Cox et al., 2002). An article on the influence of microgravity on broad haemodynamic and autonomic responses to handgrip and ischaemia was published earlier for the five astronauts (Fu et al., 2002). We now report a detailed analysis of baroreflex transients in all seven subjects, as recorded during one pre-spaceflight session. Astronaut subjects are designated by the same numbers used in three earlier publications (Ertl et al., 2002, Fu et al., 2002, Levine et al., 2002). At the time of study, subjects’ average age (range) was 41 (38 – 46) years, and their average heights and weights were 179 (159 – 187) cm and 79 (57 – 99) kg. All were healthy and none were taking medications. This study conformed with principles framed as Good Publishing Practices in Physiology (Persson & Henriksson 2011).
Measurements
We recorded the electrocardiogram with surface electrodes; finger arterial pressure with a Finapres photoplethysmograph (Model 2300, Ohmeda, Englewood, CO, USA); and muscle sympathetic nerve activity with a tungsten microelectrode inserted into the right peroneal nerve. The nerve signal was amplified 70,000 – 160,000 times, band-pass filtered (700 – 2,000 Hz), rectified, and integrated with a time constant of 0.1 s. Muscle nerve sympathetic bursts were identified by their pulse-rhythmicity, their increases with held expiration and Valsalva straining, and their indifference to unexpected auditory and tactile stimuli, as described previously (Badra et al., 2001). Respiration was neither measured nor controlled
Protocol
Subjects were studied supine in a quiet darkened room. Details of the fixed-order handgrip protocol were published earlier (Fu et al., 2002). Briefly, each subject performed three, 3 s handgrips with his or her dominant hand, to the maximum intensity possible. Subsequent handgrips were performed at 40 % of the highest intensity of the three trials, to exhaustion, defined as a 2 s period in which handgrip intensity fell and remained below 80 % of the targeted intensity. At exhaustion, a pneumatic cuff was inflated high on the exercise arm, to 250 mmHg, for 2 min. Ten s after the cuff was inflated, subjects ended their handgrips. After release of the cuff, subjects rested quietly for an additional 2 min. Subjects were trained to breathe normally during the protocol, and specifically, to avoid breath-holding and Valsalva straining.
Analyses
Each signal was digitized on-line at 500 Hz with Windaq hardware and software (DATAQ, Akron, OH, USA). We analysed most results with WinCPRS 1.60 (Absolute Aliens Oy, Turku, Finland). This program automatically detects R-wave peaks, systolic and diastolic pressures, and muscle sympathetic bursts. Automatic selections were over read and corrected by one experienced author.
Vagal-cardiac nerve activity was estimated as the square root of mean squared differences of successive normal R-R intervals, RMSSD, and as the proportion of successive normal R-R intervals greater than 50 ms, divided by the total number of normal R-R intervals, pNN50 (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996). The rationale for these measurements comes from the observations that abrupt R-R interval fluctuations are mediated by changes of vagal-cardiac nerve activity (Eckberg, 1980, Diedrich et al., 2013); sympathetically-mediated changes do not occur abruptly. Our study focuses on low- (0.04 – 0.15 Hz) and very low- (≤ 0.04 Hz) oscillations.
We estimated vagal baroreflex gain with a frequency-domain method, the transfer function (Robbe et al., 1987), calculated as the average value of the systolic pressure – R-R interval cross spectrum derived by Fourier transform, divided by the systolic pressure spectrum in the low-frequency range, 0.04 – 0.15 Hz. We obtained moving baroreflex gain estimates, by iteratively calculating the transfer function in 25 s windows, moved through the measurements by 2 s steps. We justify our measurements on the basis of published research: our calculated values are vagal, because they are nearly abolished by large-dose atropine (Eckberg & Kuusela 2005), and they accurately estimate baroreflex gain (see Discussion). We focused on low-frequency oscillations because scientists are not agreed that high-(respiratory) frequency oscillations reflect baroreflex physiology. [For a detailed discussion of this issue, see a recent Point:Counterpoint debate (Eckberg , 2009, Karemaker 2009).] We determined centre frequencies of baroreflex oscillations mathematically, with autoregression (model order: 20), as in our earlier study (Eckberg & Kuusela 2005).
Muscle sympathetic bursts were detected automatically as follows: each entire mean voltage neurogram was scanned to identify upward voltage spikes occurring within a 0.5 s window, centred initially 1.3 s after the preceding (1 removed) R wave. The latency for the window was changed, according to the results of the scan, and the scan was repeated. When two scans of the entire record yielded identical average latencies, burst selection was considered final. Sympathetic neurograms were advanced 1.3 s, to compensate for conduction delays to the peroneal nerve recording site (Fagius & Wallin 1980). Muscle sympathetic nerve activity was expressed as bursts min−1, bursts 100 heart beats−1, and electronically integrated areas between the beginnings and endings of bursts and burst volleys (the latter measurement includes the areas beneath bursts, extending to the baseline). We normalized all muscle sympathetic nerve activity by dividing each burst by the average area of sympathetic bursts recorded during the initial pre-exercise control period.
Statistical analyses
Statistical analyses were performed with SigmaStat 3.11 (Systat Software, San Jose, CA, USA). Many data sets were not distributed normally; therefore, we report individual responses, medians, 25th and 75th percentile values, and ranges. Choices of individual statistical tests were dictated by the distribution of data sets and are indicated for each result. We considered P ≤ 0.05 as being statistically significant. Since responses to interventions varied greatly among subjects, data are normalized, as percentages of the maximum values registered during the entire experiment for each subject; actual numerical values are given in the Table.
Table.
Median (25th, 75th percentile) general results from seven subjects
| Control | Last 50 s handgrip | First 50 s ischaemia | Last 50 s ischaemia | First 50 s recovery | |
|---|---|---|---|---|---|
| †Systolic pressures (mmHg) | 135 (126, 145) A |
187 (170, 197) B |
167 (151, 193) C |
163 (157,190) C |
136 (134, 166) A |
| ‡R-R intervals (s) | 1.01 (0.92, 1.03) A |
0.75 (0.67, 0.83) B |
0.93 (0.9, 0.96) | 0.95 (0.85, 1.03) A |
0.97 (0.9, 1.05) A |
| ‡R-R interval RMSSD (ms) | 23 (20, 42) | 18 (16, 34) A |
57 (28, 119) B |
33 (23, 40) | 35 (25, 45) B |
| †R-R interval pNN50 (%) | 3.3 (0.5, 23.1) | 1.9 (0.4, 12.5) A |
13.7 (4.1, 31) B |
11.4 (2.6, 23.5) | 12 (3.6, 19.9) |
| ‡Low-frequency transfer function vagal baroreflex peak gain (ms/mmHg) | 16.6 (11.9, 19.1) | 8.4 (4.6, 11.9) A |
17.7 (12.8, 26.5) | 13, (11.1, 15.8) | 16.2 (1426.7) B |
| ‡Diastolic pressure (mmHg) | 67 (66, 74) A |
94 (93, 105) B |
81 (78, 99) | 89 (81, 101) B |
68 (65, 82) AC |
| †Muscle sympathetic nerve activity (normalized area/burst * bursts) | 17 (8, 20) A |
64 (49, 73) B |
61 (46, 78) B |
62 (60, 88) B |
28 (26, 38) A |
Values with the same letter are not significantly different; values with different letters are significantly different. RMSSD = square root of the mean squared differences of successive normal R-R intervals; pNN50 = number of successive normal R-R intervals ≥ 50 ms total number−1.
One way repeated measures analysis of variance, Holm-Sidak method
Friedman repeated measures analysis of variance on ranks, Tukey test.
Results
All subjects completed the protocol. Since the shortest pre-exercise control period for any subject was 50 s, all control values were calculated for the 50 s period immediately preceding the onset of handgrip. Haemodynamic data and statistical comparisons are given in the Table. Median control muscle sympathetic nerve values were 36 (25 – 39) bursts min−1 and 56 (44 – 59) bursts 100 heart beats−1. The median duration of handgrip was 172 (110 – 250) s, and the median duration of ischaemia was 121 (118 – 122) s. (The duration of handgrip was different for each subject because handgrip was continued to exhaustion; the duration of ischaemia, however, was fixed, and therefore, was close to the 120 s target in all subjects.) All median responses for all subjects and interventions are given in Table, with statistical comparisons.
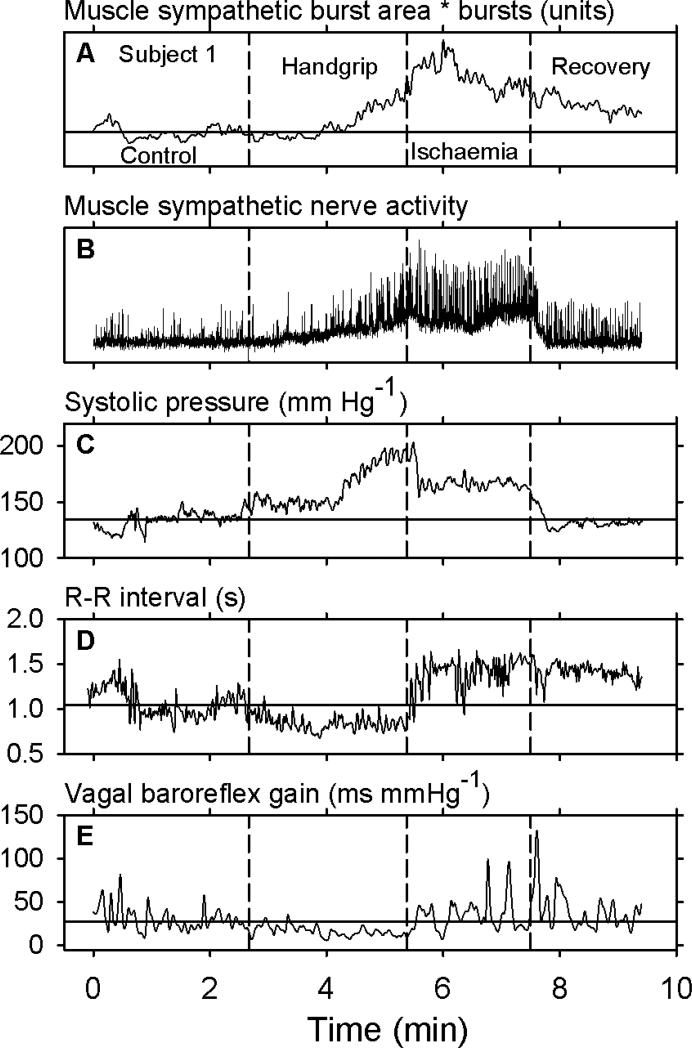
Figure 1 shows data obtained during an entire experiment from one subject who maintained handgrip for 162 s. (In this and subsequent figures, control values are indicated by horizontal dashed lines.) In this subject, control levels of muscle sympathetic nerve activity were 32 bursts min−1 and 56 bursts 100 heart beats−1. The numbers of bursts (not shown) declined during the first ~70 seconds of handgrip; minima and median values during this time were 16 and 27 bursts min−1 and 25 and 56 bursts 100 heart beats−1. After this initial period, sympathetic nerve activity increased until the end of handgrip, and then increased further during early ischaemia (Panel A). Sympathetic nerve activity then drifted downwards, but did not return to control levels (horizontal line) during the 120 s period after release of the occlusive cuff and end of ischaemia.
Figure 1.
Entire recording from one subject. The vertical dashed lines indicate the beginning of handgrip, the end of handgrip and beginning of ischaemia, and the end of ischaemia. The horizontal lines indicate average control values. As expected, muscle sympathetic nerve activity and arterial pressure increased during handgrip, and R-R intervals decreased. The major observation in this recording is that after handgrip, vagal baroreflex gain oscillated in major ways (Panel E, right).
The baseline level of the sympathetic neurogram began to increase about 22 s after the onset of handgrip (Fig. 1, Panel B), and did not return to control levels until about 16 s after the end of ischaemia. Five of the seven subjects had similar upward baseline shifts. The consistent return of the baseline of the sympathetic neurogram to control levels early during recovery from ischaemia suggests that increases of baseline voltage during the experiment reflected increased levels of sympathetic nerve activity caused by handgrip and ischaemia, and not shifts of the microneurography electrode position.
Systolic pressure (Fig. 1, Panel C) rose during handgrip, fell, but not to control levels after handgrip during ischaemia, and returned to control levels after ischaemia. R-R intervals (Panel D) declined – the heart rate sped – during handgrip, rose abruptly at the end of handgrip and onset of ischaemia, and did not return to control levels during the 120 s recovery period. Transfer function vagal baroreflex gain (Panel E) declined modestly during handgrip, in parallel with the decline of R-R intervals, and returned to and exceeded control levels at the end of handgrip during ischaemia and recovery. Fluctuations of vagal baroreflex gain were present as spikes throughout the recording; their magnitudes decreased during handgrip, and increased greatly after handgrip during and following ischaemia. [Note that vagal baroreflex gain reductions during handgrip (Panel E, centre) are sharply constrained – baroreflex gain cannot fall below zero.]
Vagal mechanisms
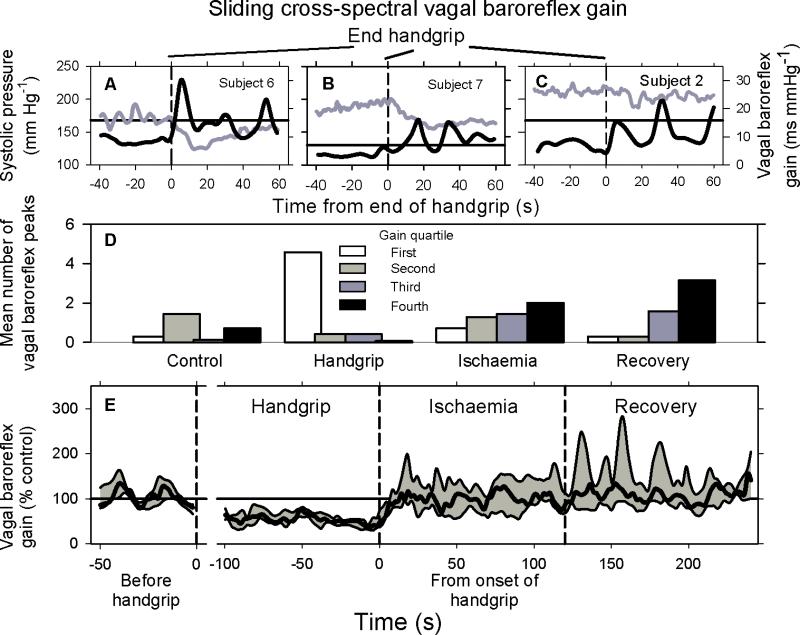
Figure 2 shows individual sliding transfer function vagal baroreflex gains for three subjects (top panels), and other data for all subjects. Rhythmic baroreflex surges are seen clearly after the end of handgrip (Panels A – C, vertical dashed lines) in responses of the three subjects. In these subjects, vagal baroreflex oscillations were small (or unapparent) during, and large after handgrip. The frequency of baroreflex gain oscillations in these three subjects averaged 0.054 Hz (period: 18.5 s), and the post-handgrip baroreflex gain peaks averaged 185, 228, and 141 % of control baroreflex peak values.
Figure 2.
Vagal baroreflex transfer function gain rhythms. Data shown in Panels A – C were obtained from three subjects different from the one whose responses are shown in Fig. 1. Panel D shows the distribution of median vagal baroreflex gains during 50 s of control measurements, the last 100 s of handgrip (exhaustion), the first 100 s of ischaemia, and the first 100 s of recovery. The magnitudes of all baroreflex gains were divided into quartiles, with the first quartile being the smallest gains, and the fourth quartile being the largest gains. The number of baroreflex peaks falling within each period was determined for each subject, and the percentage of this number falling within each quartile for each measurement period was calculated. Panel E shows median (heavy black line), and 25th and 75th percentile (gray shading) measurements during the periods indicated.
Handgrip and ischaemia strongly influenced peak vagal baroreflex gains and the distribution of vagal baroreflex gains among oscillations. Figure 2, Panel D shows the distribution of baroreflex gains, ranked in quartiles (in this scheme, the first quartile comprises peaks with the smallest baroreflex gains, and the fourth quartile comprises peaks with the largest baroreflex gains). Baroreflex gains during the control period (Fig. 2, Panel D, left) were distributed over all quartiles; however, the largest number of measurements fell in the second (moderate gain) quartile. Baroreflex gain distribution during the last 100 s of handgrip was prominent in the first quartile (lowest baroreflex gain), and nearly absent in the fourth quartile (highest baroreflex gain). Baroreflex gain during the last 100 s of recovery aggregated in the third and fourth quartiles (moderate to high baroreflex gain). Distributions of baroreflex gains during handgrip and recovery tended to be mirror images of each other.
Figure 2, Panel E, shows median (heavy black line), and 25th and 75th (gray shading) percentile peak vagal baroreflex gains for the seven subjects. Vagal baroreflex peak gains declined steadily during handgrip and increased abruptly at the end of handgrip and beginning of ischaemia. Vagal baroreflex gain peaks were significantly less during handgrip than during recovery (Table).
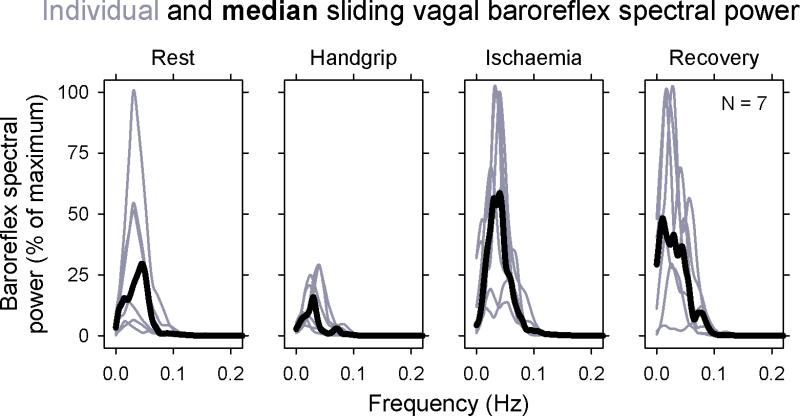
Figure 3 shows individual and median spectral powers of sliding transfer function vagal baroreflex gains during four periods (the last 50 s of control measurements; the last 100 s during handgrip at exhaustion; the first 100 s of ischaemia; and the last 100 s of recovery). During all epochs, baroreflex peaks aggregated between 0 and ~ 0.1 Hz. Although the magnitudes of baroreflex gain oscillations were much diminished by handgrip, their periodicities all fell below 0.1 Hz. Median centre frequencies, identified by autoregressive analyses of vagal baroreflex time series for each period were 0.053, 0.056, 0.053, and 0.059 Hz, (17 to 18.9 s periods), P = 0.55, repeated measures one way analysis of variance. Although vagal baroreflex gain oscillations were small during handgrip, this spectral analysis makes a strong case that they were present.
Figure 3.
Sliding transfer function vagal baroreflex spectral power for all subjects. Individual measurements are shown in gray and median values are shown in black. The period of control measurements was 50 s; all other periods were 100 s. Despite very low values of baroreflex gains during handgrip, their frequencies were in the same range as during other parts of the protocol.
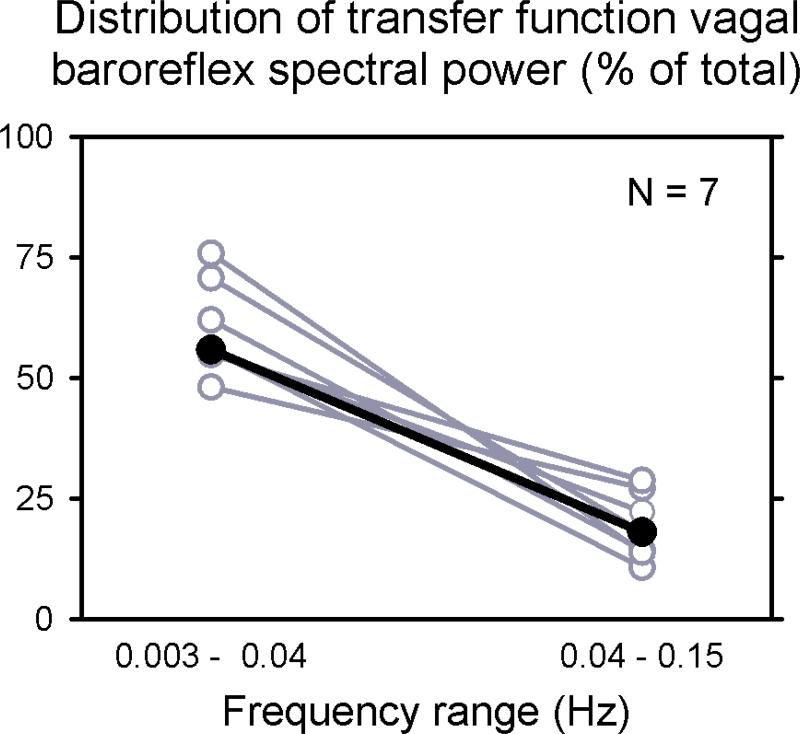
The goal of our study was to determine if vagal baroreflex oscillations persist during exercise, ischaemia, and recovery, and not necessarily to define their frequencies. Our results (Fig.3 and statistical analyses) indicate that baroreflex rhythms persist, and more importantly, that they persist within the same frequency range. On the basis of this latter observation, we analysed entire recordings (median duration: 405.7; range: 329 – 471.9) s to define baroreflex frequencies more precisely. Figure 4 depicts the frequency distribution of baroreflex oscillations (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996), and indicates that our study deals primarily with low-frequency oscillations (median values were 56 % at very low-frequencies, and 18 % at low-frequencies, P < 0.001).
Figure 4.
Distribution of baroreflex oscillatory frequencies during entire recordings. Eighty percent of all baroreflex spectral power fell in the very low- and low-frequency ranges.
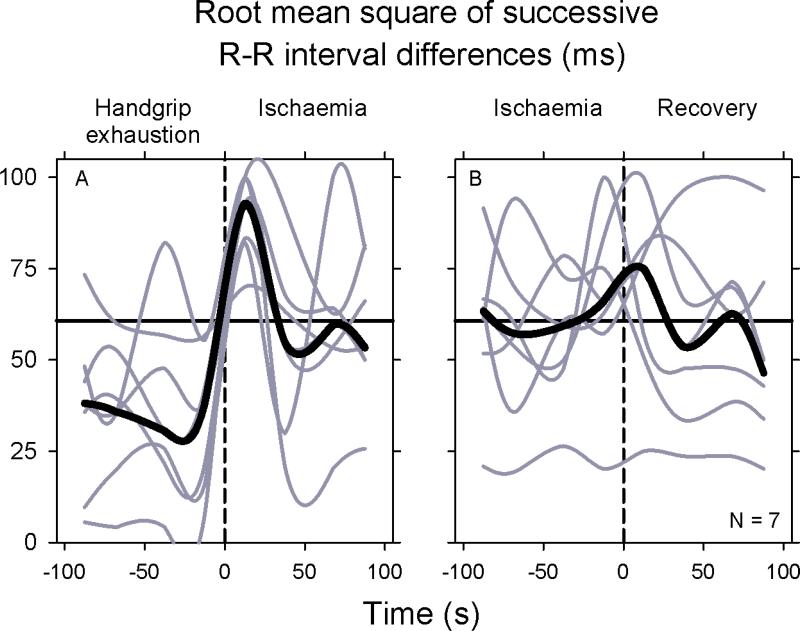
R-R interval fluctuations shown in the preceding figures likely reflect both sympathetic and vagal influences. Therefore, we estimated levels of vagal-cardiac outflow with different metrics. Figure 5 shows one R-R interval index, the square root of the mean squared differences of successive normal R-R intervals, RMSSD, which is thought to be mediated exclusively by fluctuations of vagal-cardiac nerve activity [but likely dampened by ongoing sympathetic stimulation (Taylor et al., 2001)]. This fig. provides new information regarding fluctuations of vagal outflow. First, as cross-spectral vagal baroreflex gain, vagal outflow also waxed and waned during handgrip and ischaemia throughout the protocol in all subjects (gray). Second, fluctuations of vagal outflow, which, as expected, occurred in no particular temporal relation among individuals, were synchronized as prominent peaks and rose together, at the end of handgrip and beginning of ischaemia, and at the end of ischaemia and beginning of recovery. The surges of vagal outflow after the two transitions were evanescent, however, and in all subjects, vagal outflows returned to or below control levels within ~ 30 s after the transitions. Similar oscillations were observed in another vagal index, the proportion of successive R-R intervals greater than 50 ms divided by the total number of normal R-R intervals, pNN50 (not shown).
Figure 5.
Individual and median square root of mean squared differences of successive sinus R-R intervals (RMSSD). Individual (gray) and median (black) values were determined at 25 s intervals. These data document major fluctuations of vagal-cardiac nerve activity, which are synchronized as short-lived surges during transitions between exercise and ischaemia, and ischaemia and recovery.
Discussion
Human vagal baroreflex gain oscillates at very low-frequencies (Eckberg & Kuusela 2005). We studied the effects of handgrip exercise and post-exercise forearm ischaemia on vagal baroreflex rhythms and vagal-cardiac nerve activity and report two new findings. First, we rejected the null hypothesis that exercise followed by muscle ischaemia abolish low-frequency human baroreflex rhythms: vagal baroreflex rhythmicity persists during exercise and ischaemia, notwithstanding major fluctuations of baroreflex gain. Second, vagal-cardiac nerve activity, as reflected by the square root of mean squared differences of successive normal R-R intervals, RMSSD, is synchronized by transitions from handgrip to ischaemia, and from ischaemia to recovery, and appears as major, short-lived surges.
The rationale for this research comes from studies of another vagal baroreflex oscillation, that associated with breathing [styled as the human respiratory ‘gate’ (Eckberg, 2003)]. Respiration imposes its rhythm on vagal-cardiac motoneurones centrally, by phasically altering motoneurone membrane susceptibility to stimulatory inputs (McAllen & Spyer 1978, Eckberg et al., 1980, Gilbey et al., 1984). The level of respiratory sinus arrhythmia, which reflects the ebb and flow of vagal-cardiac motoneurone firing (Katona & Jih 1975), is proportional to arterial pressure, from low to usual arterial pressure levels (Eckberg et al., 1988). However, when arterial pressure is very low, and when arterial pressure or the level of baroreceptor stimulation is very high, respiratory sinus arrhythmia is absent (Anrep et al., 1936, Eckberg & Orshan 1977). Based on the precedent of respiration, we considered that non-respiratory vagal baroreflex rhythms might also be absent when baroreflex gain is diminished by exercise or when baroreflex gain is greatly augmented by the cessation of exercise and onset of ischaemia.
Vagal baroreflex rhythms
Numerous articles attest to the great variability of vagal baroreflex gain in healthy individuals. Large increases of baroreflex gain occur when supine subjects sleep (Smyth et al., 1969, Pagani et al., 1988), and when upright subjects abruptly return to the supine position (Westerhof et al., 2006). Moreover, simple repeated estimates of vagal baroreflex gain in subjects who are, as well as can be determined, in a ‘steady-state’, document surprisingly large ongoing fluctuations of vagal baroreflex gain (Yamamoto et al., 1989, Badra et al., 2001, Ichinose et al., 2004, Eckberg & Kuusela 2005).
Our earlier article (Eckberg & Kuusela 2005), based on the same sliding transfer function baroreflex estimates that we used in the present study, showed that vagal baroreflex gain fluctuations are organized at very low-frequencies. This new observation led us to search for baroreflex rhythms in time series altered by exercise and ischaemia. We identified unmistakable low-frequency vagal baroreflex rhythms throughout the protocol (Figs. 1 – 3), but with gains that varied widely. During exercise, baroreflex gain oscillations are skewed toward low levels, and during ischaemia and recovery, baroreflex gain oscillations are skewed toward high levels (Fig. 2, Panel D, Fig. 3). Our short measurement periods precluded meaningful evaluation of very low-frequency baroreflex rhythms. Analysis of entire recordings (Fig. 4), however, indicated that baroreflex rhythms fell primarily in the very low-frequency range. This is consistent with our earlier study (Eckberg & Kuusela 2005), which documented average centre frequencies of baroreflex oscillations of 0.011 Hz during recordings lasting 20 min.
The earlier work (Eckberg & Kuusela 2005) showed that the frequency of baroreflex gain oscillations is not altered by cholinergic, beta-adrenergic, or angiotensin converting enzyme blockade. We now report that the frequency of baroreflex gain oscillations is not altered by central command, increased mechano- and metaboreceptor activity, reduced or augmented vagal inhibition, and increased sympathetic stimulation. We show also that although vagal baroreflex gain does not increase to a sustained high level during and following ischaemia, baroreflex gain fluctuates hugely during these times, with peaks that may rise well above those observed before exercise (Figs. 1 and 2). These peaks occur despite persistence of major sympathetic opposition [Fig. 1, Panels A and B, Table (Taylor et al., 2001)].
Human haemodynamic rhythms
Autonomic and haemodynamic measurements in resting humans oscillate over a range of frequencies, from cardiac- to very, and ultra low-frequencies. It is not known if very low-frequency vagal baroreflex rhythms reflect the existence of an independent baroreflex oscillator, or a response to other factors that contribute to very low-frequency oscillations, including renin-angiotensin-aldosterone (Akselrod et al., 1981, Taylor et al., 1998), endothelial (Kvandal et al., 2003), and thermoregulatory (Shiogai et al., 2010, Sheppard et al., 2011, Bernjak et al., 2012) mechanisms.
Our study provides no firm indication of what secondary factors might cause very low-frequency vagal baroreflex rhythms. We can say that respiration probably played no role; a very recent study (Stankovski et al., unpublished) indicates that breathing frequency does not modulate vagal baroreflex rhythms. Baroreflex rhythms may be secondary to muscle sympathetic nerve activity or arterial pressure, which also oscillate at very low frequencies (Eckberg et al., 1985, Badra et al., 2001). The relation of arterial pressure to oscillations of vagal baroreflex gain in our study is unclear. In two subjects, but not a third one (Fig. 2, Panels A – C), the transition from handgrip to ischaemia provoked sharp arterial pressure reductions; nonetheless, strong baroreflex oscillations occurred in all three subjects. Moreover, the magnitude of baroreflex oscillations was much larger during the recovery than the control periods, but arterial pressures were similar (Table).
Vagal baroreflex rhythms as experimental artefacts
An alternative interpretation of our results is that what we consider to be vagal baroreflex rhythms are artefacts. Several studies document significant coherence between gains estimated with the low-frequency transfer function we used and those calculated other spontaneous baroreflex indices (di Rienzo et al., 1997, Tank et al., 2000, Laude et al., 2004), or after vasoactive drug injections (Pagani et al., 1988, Lipman et al., 2003). A recent study (Diedrich et al., 2013) compared baroreflex sequences after isolated muscle sympathetic nerve bursts with the low-frequency transfer function estimates we used. Both linear regression and Bland-Altman analyses documented very strong correlations between these two estimates of spontaneous baroreflex gain (the linear regression correlation coefficient was 0.96).
Provoked oscillations of vagal-cardiac nerve activity
We confirm an earlier observation (Nishiyasu et al., 1994), that vagal-cardiac neural outflow (as reflected by the R-R interval standard deviations) increases during ischaemia after exercise. We refine this observation by showing first, that, as with vagal baroreflex gain, levels of vagal-cardiac neural outflow fluctuate continuously (Fig. 5, gray lines), and second, that these fluctuations are synchronized by the transitions between exercise and ischaemia, and ischaemia and recovery. We show further that vagal outflow after these transitions does not simply increase to new steady-state levels, but increases as short-lived impulses.
Limitations
We studied only seven subjects. Nonetheless, we report highly significant differences in responses during the protocol. Obviously, statistically-insignificant results must be interpreted with caution; we cannot rule out the possibility that some negative results reflect beta-statistical errors. In our study, we used only one intensity of forearm exercise; therefore, we cannot exclude the possibility that more intense exercise, or exercise involving larger muscle groups might abolish vagal baroreflex rhythmicity. Although we did not measure or control breathing frequency or tidal volume, this failure is unlikely to have influenced our results, since a very recent study (Stankowski et al., unpublished) indicates that breathing frequency exerts no influence on transfer function vagal baroreflex gain.
In conclusion, we measured fluctuations of vagal baroreflex gains and vagal-cardiac nerve activity in healthy supine subjects during handgrip exercise, followed by forearm ischaemia and recovery, and documented great fluidity of baroreflex mechanisms and vagal outflow. We show that vagal baroreflex rhythms persist throughout rest, exercise, ischaemia, and recovery, at nearly constant, low-frequencies. However, gains within rhythmic baroreflex surges are shifted to low levels during exercise, and to high levels during ischaemia and recovery. We show also that transitions from exercise to ischaemia, and from ischaemia to recovery, are marked by major, synchronized, short-lived surges of vagal-cardiac outflow. These moment-by-moment fluctuations of autonomic outflows underscore the highly dynamic, rhythmic nature of human responses to routine activities of daily life.
Acknowledgments
We thank the astronauts and backup astronauts who gave of themselves unstintingly to make this research successful. We also thank the large number of scientists and engineers at Lyndon B. Johnson Space Center. This work was supported by National Aeronautics and Space Administration contracts NAS0-19541 and NAG2-408, National Heart, Lung, and Blood Institute, UO1HL-56417. It was also supported by a grant from the Centennial Foundation of Helsingin Sanomat, Finland.
Footnotes
Conflict of Interest
None of the authors have a conflict of interest.
References
- Akselrod S, Gordon D, Ubel FA, Shannon DC, Barger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213:220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- Anrep GV, Pascual W, Rössler R. Respiratory variations of the heart rate. I - The reflex mechanism of the respiratory arrhythmia. Proc Roy Soc Lond B. 1936;119 B:191–217. [Google Scholar]
- Badra LJ, Cooke WH, Hoag JB, Crossman AA, Kuusela TA, Tahvanainen KUO, Eckberg DL. Respiratory modulation of human autonomic rhythms. Am J Physiol. 2001;280:H2674–H2688. doi: 10.1152/ajpheart.2001.280.6.H2674. [DOI] [PubMed] [Google Scholar]
- Bernjak A, Cui J, Iwase S, Mano T, Stefanovska A, Eckberg DL. Human sympathetic outflows to skin and muscle target organs fluctuate concordantly over a wide range of time-varying frequencies. J Physiol. 2012;590:363–375. doi: 10.1113/jphysiol.2011.214528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow JD, Brown EB, Jr., Cunningham DJC, Howson MG, Petersen ES, Pickering TG, Sleight P. Effect of bicycling on the baroreflex regulation of pulse interval. Circ Res. 1971;28:582–592. [Google Scholar]
- Carrington CA, White MJ. Exercise-induced muscle chemoreflex modulation of spontaneous baroreflex sensitivity in man. J Physiol. 2001;536:957–962. doi: 10.1111/j.1469-7793.2001.00957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JF, Tahvanainen KUO, Kuusela TA, Levine BD, Cooke WH, Iwase S, Saito M, Sugiyama Y, Ertl AC, Biaggioni I, Diedrich A, Robertson RM, Zuckerman JH, Lane LD, Ray CA, White RJ, Pawelczyk JA, Buckey JC, Jr., Baisch FJ, Blomqvist CG, Robertson D, Eckberg DL. Influence of microgravity on astronauts' sympathetic and vagal responses to Valsalva's manoeuvre. J Physiol. 2002;538:309–320. doi: 10.1113/jphysiol.2001.012574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Rienzo M, Parati G, Mancia G, Pedotti A, Castiglioni P. Investigating baroreflex control of circulation using signal processing techniques. IEEE Eng Med Biol Mag. 1997;16:86–97. doi: 10.1109/51.620499. [DOI] [PubMed] [Google Scholar]
- Diedrich A, Crossman AA, Beightol LA, Tahvanainen KUO, Kuusela TA, Ertl AC, Eckberg DL. Baroreflex physiology studied in healthy subjects with very infrequent muscle sympathetic bursts. J Appl Physiol. 2013;114:203–210. doi: 10.1152/japplphysiol.00509.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg DL. Nonlinearities of the human carotid baroreceptor-cardiac reflex. Circ Res. 1980;47:208–216. doi: 10.1161/01.res.47.2.208. [DOI] [PubMed] [Google Scholar]
- Eckberg DL. The human respiratory gate. J Physiol. 2003;548:339–352. doi: 10.1113/jphysiol.2003.037192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg DL. Point:Counterpoint: Respiratory sinus arrhythmia is due to a central mechanism vs. respiratory sinus arrhythmia is due to the baroreflex mechanism. J Appl Physiol. 2009;106:1740–1742. doi: 10.1152/japplphysiol.91107.2008. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, Cavanaugh MS, Mark AL, Abboud FM. A simplified neck suction device for activation of carotid baroreceptors. J Lab Clin Med. 1975;85:167–173. [PubMed] [Google Scholar]
- Eckberg DL, Kifle YT, Roberts VL. Phase relationship between normal human respiration and baroreflex responsiveness. J Physiol. 1980;304:489–502. doi: 10.1113/jphysiol.1980.sp013338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg DL, Kuusela TA. Human vagal baroreflex sensitivity fluctuates widely and rhythmically at very low frequencies. J Physiol. 2005;567:1011–1019. doi: 10.1113/jphysiol.2005.091090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg DL, Nerhed C, Wallin BG. Respiratory modulation of muscle sympathetic and vagal cardiac outflow in man. J Physiol. 1985;365:181–196. doi: 10.1113/jphysiol.1985.sp015766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg DL, Orshan CR. Respiratory and baroreceptor reflex interactions in man. J Clin Invest. 1977;59:780–785. doi: 10.1172/JCI108699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg DL, Rea RF, Andersson OK, Hedner T, Pernow J, Lundberg JM, Wallin BG. Baroreflex modulation of sympathetic activity and sympathetic neurotransmitters in humans. Acta Physiol Scand. 1988;133:221–231. doi: 10.1111/j.1748-1716.1988.tb08401.x. [DOI] [PubMed] [Google Scholar]
- Ertl AC, Diedrich A, Biaggioni I, Levine BD, Robertson RM, Cox JF, Zuckerman JH, Pawelczyk JA, Ray CA, Buckey JC, Jr., Lane LD, Shiavi R, Gaffney FA, Costa F, Holt C, Blomqvist CG, Eckberg DL, Baisch FJ, Robertson D. Human muscle sympathetic nerve activity and plasma noradrenaline kinetics in space. J Physiol. 2002;538:321–329. doi: 10.1113/jphysiol.2001.012576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagius J, Wallin BG. Sympathetic reflex latencies and conduction velocities in normal man. J Neurol Sci. 1980;47:433–448. doi: 10.1016/0022-510x(80)90098-2. [DOI] [PubMed] [Google Scholar]
- Fisher LD, Ogoh S, Young CN, Keller DM, Fadel PJ. Exercise intensity influences cardiac baroreflex function at the onset of isometric exercise in humans. J Appl Physiol. 2007;103:941–947. doi: 10.1152/japplphysiol.00412.2007. [DOI] [PubMed] [Google Scholar]
- Fu Q, Levine BD, Pawelczyk JA, Ertl A, Diedrich A, Cox JF, Zuckerman JH, Ray CA, Smith ML, Iwase S, Saito M, Sugiyama Y, Mano T, Zhang R, Iwasaki K, Lane LD, Buckey JC, Jr., Cooke WH, Robertson RM, Baisch FJ, Blomqvist CG, Eckberg DL, Robertson D, Biaggioni I. Cardiovascular and sympathetic neural responses to handgrip and cold pressor stimuli in humans before, during and after spaceflight. J Physiol. 2002;544:653–664. doi: 10.1113/jphysiol.2002.025098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbey MP, Jordan D, Richter DW, Spyer KM. Synaptic mechanisms involved in the inspiratory modulation of vagal cardio-inhibitory neurones in the cat. J Physiol. 1984;356:65–78. doi: 10.1113/jphysiol.1984.sp015453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose M, Saito M, Ogawa T, Hayashi K, Kondo N, Nishiyasu T. Modulation of control of muscle sympathetic nerve activity during orthostatic stress in humans. Am J Physiol. 2004;287:H2147–H2153. doi: 10.1152/ajpheart.00215.2004. [DOI] [PubMed] [Google Scholar]
- Karemaker JM. Counterpoint: respiratory sinus arrhythmia is due to the baroreflex mechanism. J Appl Physiol. 2009;106:1742–1743. doi: 10.1152/japplphysiol.91107.2008a. [DOI] [PubMed] [Google Scholar]
- Katona PG, Jih F. Respiratory sinus arrhythmia: noninvasive measure of parasympathetic cardiac control. J Appl Physiol. 1975;39:801–805. doi: 10.1152/jappl.1975.39.5.801. [DOI] [PubMed] [Google Scholar]
- Kvandal P, Stefanovska A, Veber M, Kvenmmo HD, Kirkebøen KA. Regulation of human cutaneous circulation evaluated by laser Doppler flowmetry, iontophoresis, and spectral analysis: importance of nitric oxide and prostaglandines. Microvasc Res. 2003;65:160–171. doi: 10.1016/s0026-2862(03)00006-2. [DOI] [PubMed] [Google Scholar]
- Laude D, Elghozi J-L, Girard A, Bellard E, Bouhaddi M, Castiglioni P, Cerutti C, Cividjian A, Di Rienzo M, Fortrat J-O, Janssen B, Karemaker JM, Lefthériotis G, Parati G, Persson PB, Porta A, Quintin L, Regnard J, Rudiger H, Stauss HM. Comparison of various techniques used to estimate spontaneous baroreflex sensitivity (the EuroBaVar study). Am J Physiol. 2004;286:R226–R231. doi: 10.1152/ajpregu.00709.2002. [DOI] [PubMed] [Google Scholar]
- Persson PB, Henriksson J. Good publication practise in physiology. Acta Physiol. 2011;203:403–407. [Google Scholar]
- Levine BD, Pawelczyk JA, Ertl AC, Cox JF, Zuckerman JH, Diedrich A, Biaggioni I, Ray CA, Smith ML, Iwase S, Saito M, Sugiyama Y, Mano T, Zhang R, Iwasaki K, Lane LD, Buckey JC, Jr., Cooke WH, Baisch FJ, Robertson D, Eckberg DL, Blomqvist CG. Human muscle sympathetic neural and haemodynamnic responses to tilt following spaceflight. J Physiol. 2002;538:331–340. doi: 10.1113/jphysiol.2001.012575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman RD, Salisbury JK, Taylor JA. Spontaneous indices are inconsistent with arterial baroreflex gain. Hypertension. 2003;42:481–487. doi: 10.1161/01.HYP.0000091370.83602.E6. [DOI] [PubMed] [Google Scholar]
- Mancia G, Iannos J, Jamieson GG, Lawrence RH, Sharman PR, Ludbrook J. Effect of isometric hand-grip exercise on the carotid sinus baroreceptor reflex in man. Clin Sci Mol Med. 1978;54:33–37. doi: 10.1042/cs0540033. [DOI] [PubMed] [Google Scholar]
- McAllen RM, Spyer KM. The baroreceptor input to cardiac vagal motoneurones. J Physiol. 1978;282:365–374. doi: 10.1113/jphysiol.1978.sp012469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRitchie RJ, Vatner SF, Boettcher D, Heyndrickx GR, Patrick TA, Braunwald E. Role of arterial baroreceptors in mediating cardiovascular response to exercise. Am J Physiol. 1976;230:85–89. doi: 10.1152/ajplegacy.1976.230.1.85. [DOI] [PubMed] [Google Scholar]
- Nishiyasu T, Tan N, Morimoto K, Nishiyasu M, Yamaguchi Y, Murakami N. Enhancement of parasympathetic cardiac activity during activation of muscle metaboreflex in humans. J Appl Physiol. 1994;77:2778–2783. doi: 10.1152/jappl.1994.77.6.2778. [DOI] [PubMed] [Google Scholar]
- Pagani M, Somers V, Furlan R, Dell'Orto S, Conway J, Baselli G, Cerutti S, Sleight P, Malliani A. Changes in autonomic regulation induced by physical training in mild hypertension. Hypertension. 1988;12:600–610. doi: 10.1161/01.hyp.12.6.600. [DOI] [PubMed] [Google Scholar]
- Rein H, Schneider M. Einführung in die Physiologie des Menschen. Springer-Verlag; Berlin: 1956. pp. 178–179. [Google Scholar]
- Robbe HWJ, Mulder LJM, Rüddel H, Langewitz WA, Veldman JBP, Mulder G. Assessment of baroreceptor reflex sensitivity by means of spectral analysis. Hypertension. 1987;10:538–543. doi: 10.1161/01.hyp.10.5.538. [DOI] [PubMed] [Google Scholar]
- Sheppard LW, Vuksanović V, McClintock PVE, Stefanovska A. Oscillatory dynamics of vasoconstriction and vasodilation identified by time-localized phase coherence. Phys Med Biol. 2011;56:3583–3601. doi: 10.1088/0031-9155/56/12/009. [DOI] [PubMed] [Google Scholar]
- Shiogai Y, Stefanovska A, McClintock PVE. Nonlinear dynamics of cardiovascular ageing. Phys Rep. 2010;488:51–110. doi: 10.1016/j.physrep.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth HS, Sleight P, Pickering GW. Reflex regulation of arterial pressure during sleep in man. A quantitative method of assessing baroreflex sensitivity. Circ Res. 1969;24:109–121. doi: 10.1161/01.res.24.1.109. [DOI] [PubMed] [Google Scholar]
- Tank J, Baevski RM, Fender A, Baevski AR, Graves KF, Ploewka K, Weck M. Reference values of indices of spontaneous baroreceptor reflex sensitivity. Am J Hypertens. 2000;13:268–275. doi: 10.1016/s0895-7061(99)00172-7. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Taylor JA, Carr DL, Myers CW, Eckberg DL. Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation. 1998;98:547–555. doi: 10.1161/01.cir.98.6.547. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Myers CW, Halliwill JR, Seidel H, Eckberg DL. Sympathetic restraint of respiratory sinus arrhythmia: implications for vagal-cardiac tone assessment in humans. Am J Physiol. 2001;280:H2804–H2814. doi: 10.1152/ajpheart.2001.280.6.H2804. [DOI] [PubMed] [Google Scholar]
- Westerhof BE, Gisolf J, Karemaker JM, Wesseling KH, Secher NH, van Lieshout JJ. Time course analysis of baroreflex sensitivity during postural stress. Am J Physiol. 2006;291:H2864–H2874. doi: 10.1152/ajpheart.01024.2005. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Takabatake T, Nakamura S, Hashimoto N, Satoh S, Yamada Y, Ohta H, Hattori N. Sensitivity of arterial baroreflex changes during daily activity. Clin Exp Pharmacol Physiol (Suppl.) 1989;15:113–116. doi: 10.1111/j.1440-1681.1989.tb03005.x. [DOI] [PubMed] [Google Scholar]