Abstract
An enzyme-linked immunosorbent assay (ELISA) based on a recombinant rhoptry-associated protein-1 (RAP-1) of Babesia bovis has been previously developed, but it was imperfect because some cross-reactions were still present in Babesia bigemina-infected bovine sera. To improve its accuracy for the specific detection of the antibodies to B. bovis, we constructed three C-terminal truncated recombinant antigens of the RAP-1—rCT1 (amino acids [aa] 301 to 408), rCT2 (aa 388 to 490), and rCT3 (aa 466 to 565)—by using a baculovirus expression system and evaluated their diagnostic potentials using ELISA. rCT1 and rCT2 were better diagnostic antigens in their sensitivities and diagnostic efficiencies than rCT3, although none of the recombinant antigens showed any cross-reactivity to B. bigemina-infected bovine sera. These results confirmed that the N-terminal 300-aa region caused cross-reactivity of the entire RAP-1 antigen, and the C-terminal truncated recombinant antigens were shown to be useful reagents for species-specific serodiagnosis.
Bovine babesiosis is an economically important tick-borne disease of cattle in tropical and subtropical regions of the world (8). The disease is caused by the intraerythrocytic protozoan parasites Babesia bovis and Babesia bigemina. The clinical signs developed as a result of B. bovis and B. bigemina infections are similar, and they are characterized by fever, anemia, and icterus in the infected cattle (3). Generally, the disease caused by B. bovis is more severe and more difficult to control than that caused by B. bigemina (9). Acute infections are usually diagnosed by microscopic examination of blood smears, whereas subclinical infections can be identified serologically (14). Differential diagnosis between B. bovis and B. bigemina infections will lead to a better understanding of the epidemiology with regard to species distribution and prevalence and also will provide useful information for disease control strategies (3). Therefore, accurate diagnosis of B. bovis infection is essential for the development of disease control measures and epidemiological surveys.
Previously, we demonstrated that the entire rhoptry-associated protein-1 (RAP-1) of B. bovis synthesized by a baculovirus expression system could be used as a diagnostic enzyme-linked immunosorbent assay (ELISA) antigen for the detection of antibodies to B. bovis, but there were some cross-reactions with another pathogen, B. bigemina (1). The cross-reactions may be due to the high degree of sequence identity in the first 300 amino acids (aa) located at the N-terminal region between the RAP-1 derivates of both parasites (11, 12). On the contrary, the C terminus is represented by conserved sequences that serve as B-cell epitopes that can be helpful in the development of species-specific diagnostic assays (3a). Therefore, in order to develop a more-specific serological test without cross-reactivity to B. bigemina, we constructed three truncated recombinant antigens derived from the C-terminal region of RAP-1 by the baculovirus system and then evaluated their sensitivities and specificities in ELISA.
MATERIALS AND METHODS
Parasites.
The Texas strain of B. bovis (3b) was maintained by a microaerophilous stationary-phase culturing system (7).
Cloning of truncated RAP-1 genes.
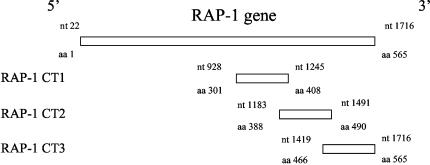
B. bovis genomic DNA was isolated from the culture as described previously (1). Oligonucleotide primers were synthesized with restriction enzyme-compatible ends for the subsequent cloning to find a specific immunogenic region in C-terminal RAP-1 (Table 1). Three kinds of DNA fragments encoding various regions of C-terminal RAP-1 were amplified from the genomic DNA by PCR with the primer pairs RCT11-RCT12, RCT21-RCT22, or RCT31-RCT32 (Fig. 1). Each of the amplified DNA samples was digested with the corresponding restriction enzymes, BamHI and XhoI, and then inserted into a pBluescript SK(+) cloning vector. The PCR amplification and confirmative nucleotide sequencing of the target DNA fragments were performed as described previously (1).
TABLE 1.
Oligonucleotides used for DNA amplification
| Oligonucleotide | Sequence (5′ to 3′) |
|---|---|
| RCT11 | ACGGATCCAGATGGATAAAGAAA |
| RCT12 | ACCTCGAGTTGACCGATGTTTTC |
| RCT21 | ACGGATCCGAGTTTTTCAGGGAA |
| RCT22 | ACCTCGAGAAACTCATGTATGAT |
| RCT31 | ACGGATCCGCTACCATGAAAGTCTTG |
| RCT32 | ACCTCGAGAAACGCATCTCATCAG |
FIG. 1.
Schematic representation of C-terminal fragments of the RAP-1 gene. Nucleotide (nt) and amino acid positions are indicated by numbers based on the sequence.
Construction of recombinant baculoviruses containing a deletion series of the RAP-1 gene.
Each of the 3′-terminal RAP-1 gene fragments was subcloned into the BamHI and XhoI sites of a baculovirus transfer vector, pFastBac Ht (Life Technologies, Grand Island, N.Y.), and the vectors were designated pFB/RAP-1 CT1, pFB/RAP-1 CT2, and pFB/RAP-1 CT3. By using these transfer vectors, three kinds of recombinant viruses, AcRAP-1 CT1, AcRAP-1 CT2, and AcRAP-1 CT3, were produced, respectively, according to the previously described method (1).
Immunoblot analysis.
Immunoblot analysis was carried out by using the RAP-1-specific mouse immune serum (1).
ELISA.
According to the same method described previously (1), soluble lysates of the recombinant baculovirus-infected insect cells were prepared as ELISA antigens. All sera were examined by using the recombinant RAP-1 (rRAP-1) antigen (1) and three C-terminal truncated recombinant antigens, rCT1, rCT2, and rCT3. Ninety-six-well microtiter plates (Nunc, Roskilde, Denmark) were coated with 50 μl of each recombinant antigen (10 μg/ml) at 4°C overnight. The details of the ELISA procedure were described previously (1). The cutoff line was defined as the mean value plus threefold standard deviations of the optical density (OD) obtained from 30 noninfected serum samples. The following definitions were used to calculate the corresponding diagnostic parameters—true-positive number (tp) of B. bovis-infected bovine sera showing a positive reading, false-negative number (fn) of B. bovis-infected bovine sera showing a negative reading, false-positive number (fp) of sera from healthy or B. bigemina-infected cattle showing a positive reading, true-negative number (tn) of sera from healthy or B. bigemina-infected cattle showing a negative reading: sensitivity = tp × 100/(tp + fn); specificity = tn × 100/(tn + fp); diagnostic efficiency = (tn + tp) × 100/(tp + fp + tn + fn) (4).
Sera.
Serum samples from cattle experimentally infected with B. bovis (n = 14) or B. bigemina (n = 12) and noninfected control serum samples (n = 30) from healthy cattle were kindly provided by Washington State University (Pullman) and Texas A&M University (College Station).
RESULTS
Cloning and expression of RAP-1 deletion clones.
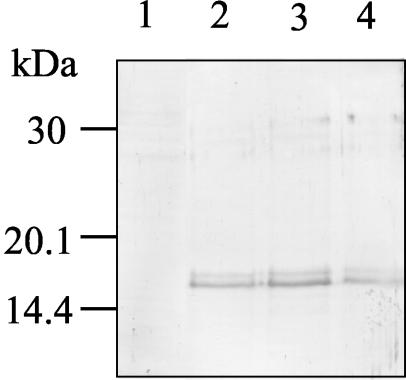
Three 3′-terminal RAP-1 gene fragments, RAP-1 CT1 (encoding aa 301 to 408), RAP-1 CT2 (encoding aa 388 to 490), and RAP-1 CT3 (encoding aa 466 to 565), were cloned by PCR (Fig. 1) and inserted into the recombinant baculovirus vectors. The extracts of recombinant baculovirus-infected insect cells with the corresponding AcRAP-1 CT1, AcRAP-1 CT2, or AcRAP-1 CT3 were subjected to immunoblot analysis with the anti-RAP-1-specific mouse serum. The serum recognized the expected recombinant proteins with a molecular mass of approximately 16 kDa in the recombinant baculovirus-infected cell extracts, but it did not react with the noninfected cell extract (Fig. 2). All recombinant proteins reacted with B. bovis-infected bovine serum but not with B. bigemina-infected bovine serum (data not shown). The results indicated the successful expression of three of the recombinant proteins derived from the C-terminal B. bovis RAP-1 in the insect cells.
FIG. 2.
Immunoblot analysis of C-terminal recombinant antigens of B. bovis RAP-1 expressed in insect cells. Noninfected (lane 1) and infected insect cells with AcRAP-1 CT1 (lane 2), AcRAP-1 CT2 (lane 3), and AcRAP-1 CT3 (lane 4) were reacted with mouse anti-B. bovis RAP-1 polyclonal antibody.
Evaluation of ELISAs with rRAP-1 deletion antigens.
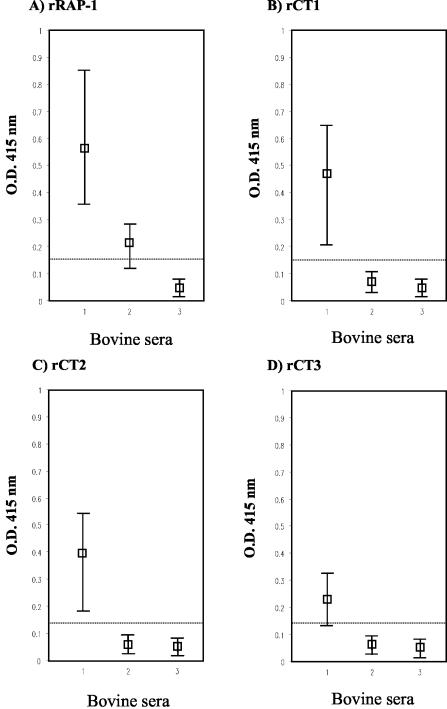
In order to improve the specificity of the rRAP-1 antigen, three C-terminal truncated antigens were constructed and subjected to ELISA, and their sensitivity and specificity were evaluated. From the control results using 30 noninfected sera, cutoffs for the OD at 415 nm were determined as 0.141, 0.140, 0.136, and 0.133 for rRAP-1, rCT1, rCT2, and rCT3, respectively (Table 2). The actual reactivities of the recombinant antigens against each group of the serum samples are shown in Fig. 3. Maximum OD values were observed in the rRAP-1 ELISA (Fig. 3A), whereas the absorbance decreased in the rCT1, rCT2, and rCT3 ELISAs (Fig. 3B to D). All of the B. bovis-infected serum samples showed a certain positive value in the rRAP-1, rCT1, and rCT2 ELISAs (Fig. 3A to C), but one of these samples had a lower OD value than the defined cutoff line in the rCT3 ELISA (Fig. 3D), resulting in sensitivities of 100% for the rRAP-1, rCT1, and rCT2 ELISAs and 92.8% for the rCT3 ELISA (Table 2). No OD value from the B. bigemina-infected bovine serum samples was over the cutoff in the rCT1, rCT2, and rCT3 ELISAs (Fig. 3B to D), whereas 10 B. bigemina-infected bovine serum samples had OD values above the cutoff in the rRAP-1 ELISA (Fig. 3A), resulting in specificities of 100% for the rCT1, rCT2, and rCT3 ELISAs and 76.2% for the rRAP-1 ELISA (Table 2). The diagnostic efficiency of the rCT1 and rCT2 antigens showed a perfect percentage, 100% (Table 2). The results indicated that the ELISA with the rCT1 or rCT2 antigen provided a highly specific and sensitive system for the serological diagnosis of B. bovis infection.
TABLE 2.
Diagnostic performance of recombinant RAP-1 and C-terminal truncated antigens by ELISA
| Antigen | OD415 cutoff valuea | Sensitivity (%) | Specificity (%) | Diagnostic efficiency (%) |
|---|---|---|---|---|
| rRAP-1 | 0.141 | 100 | 76.2 | 82.1 |
| rCT1 | 0.140 | 100 | 100 | 100 |
| rCT2 | 0.136 | 100 | 100 | 100 |
| rCT3 | 0.133 | 92.8 | 100 | 98.2 |
OD415, optical density at 415 nm.
FIG. 3.
Reactivities of several panels of bovine sera in ELISA with the C-terminal recombinant antigens of RAP-1. Maximum and minimum values (bars) and median values (□) from the ELISA are shown. The bar represents the OD at 415 nm of B. bovis-infected bovine sera (n = 14) (column 1), B. bigemina-infected bovine sera (n = 12) (column 2), and noninfected bovine sera (n = 30) (column 3).
DISCUSSION
The effective serodiagnosis of bovine babesiosis largely depends on the availability and quality of prepared antigens. Recombinant protein-based serological tests may achieve high sensitivity and specificity because of the high concentration of the immunoreactive antigen and the lack of host protein components from the crude antigen preparations (2). We previously introduced a serodiagnostic ELISA using an entire RAP-1 gene product of B. bovis for the detection of antibodies to B. bovis in cattle (1). However, some cross-reactions remained in the B. bigemina-infected bovine sera, and further improvement was required. The B. bovis RAP-1 shows a high identity to B. bigemina RAP-1 (p58) in the N-terminal 300-aa region (11). It has been demonstrated that the C terminus contains conserved, repeated amino acid sequences (13). Furthermore, a competitive ELISA using a monoclonal antibody specific for the repeat region of the C terminus has recently been shown to possess diagnostic potential with exquisite specificity (3a). In the present study, we synthesized three C-terminal recombinant antigens of B. bovis RAP-1 and then evaluated whether the recombinants were available for species-specific serodiagnosis without any cross-reactions to B. bigemina infection.
The present results showed that the entire recombinant antigen derived from the C-terminal region of RAP-1 had no cross-reactivity to B. bigemina-infected bovine sera, confirming that the N-terminal region of RAP-1 caused the cross-reactivity in ELISA (12). The rCT1 and rCT2 ELISAs gave satisfactory results, and their sensitivities, specificities, and diagnostic efficiencies were 100%. However, the number of bovine sera was small, and further evaluation with a large number of bovine serum samples will be necessary. The CT1 and CT2 fragments contain a large region of repeated 23-aa sequences (10). The periodicity of a tandem repeated sequence is 7 from aa 317 to 477 of RAP-1, and making a secondary structure with a predicted high antigenicity is considered necessary (10). When the same B. bovis-infected bovine sera were used, the OD values of B. bovis-infected bovine sera in the rCT1 ELISA were higher than those in the rCT2 and rCT3 ELISAs but lower than those in the rRAP-1 ELISA, indicating that rCT1 contains a larger immunodominant region capable of inducing a stronger humoral immune response to B. bovis infection than rCT2 and rCT3 but which induces a weaker response than rRAP-1. Various genes have been identified in Babesia parasites. They have been used to produce their corresponding recombinant antigens and evaluated for their diagnostic potentials in ELISA for Babesia infections (5, 6, 13, 15). The selection of target recombinants that are highly specific to the parasites is important for the development of novel serodiagnosis (2).
In conclusion, we described the construction of three truncated C-terminal recombinant antigens of B. bovis RAP-1, rCT1, rCT2, and rCT3 and evaluated their diagnostic potential in ELISA. The results of ELISA indicated that rCT1 and rCT2 are novel diagnostic antigens. These antigens are available for the serodiagnostic ELISA of B. bovis infection. This serodiagnosis will allow accurate epidemiological surveys as well.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science and by a grant from The 21st Century COE Program (A-1), Ministry of Education, Culture, Sport, Science, and Technology, Japan.
REFERENCES
- 1.Boonchit, S., X. Xuan, N. Yokoyama, W. L. Goff, G. Wagner, and I. Igarashi. 2002. Evaluation of an enzyme-linked immunosorbent assay with recombinant rhoptry-associated protein-1 antigen of Babesia bovis for the detection of specific antibodies in cattle. J. Clin. Microbiol. 40:3771-3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bose, R., W. K. Jorgensen, R. J. Dalgliesh, K. T. Friedhoff, and A. J. de Vos. 1995. Current state and future trends in the diagnosis of babesiosis. Vet. Parasitol. 57:61-74. [DOI] [PubMed] [Google Scholar]
- 3.de Vos, A. J., and F. T. Potgieter. 1994. Bovine babesiosis, p. 278-294. In J. A. W. Coetzer, G. R. Thomson, and R. C. Tustin (ed.), Infectious diseases of livestock. Oxford University Press, Cape Town, South Africa.
- 3a.Goff, W. L., T. F. McElwain, C. E. Suarez, W. C. Johnson, W. C. Brown, J. Norimine, and D. P. Knowles. 2002. Competitive enzyme-linked immunosorbent assay based on a rhoptry-associated protein 1 epitope specifically identifies Babesia bovis-infected cattle. Clin. Diagn. Lab. Immunol. 10:38-42. [DOI] [PMC free article] [PubMed]
- 3b.Goff, W. L., W. C. Johnson, and C. W. Cluff. 1998. Babesia bovis immunity: in vitro and in vivo evidence for IL-10 regulation of IFN-γ and iNOS. Ann N. Y. Acad. Sci. 849:161-180. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez-Sapienza, G., C. Lorenzo, and A. Nieto. 2000. Improved immunodiagnosis of cystic hydratid disease by using a synthetic peptide with higher diagnostic value than that of the parent protein, Echinococcus granulosus antigen B. J. Clin. Microbiol. 38:3979-3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikadai, H., X. Xuan, I. Igarashi, S. Tanaka, T. Kanemaru, H. Nagasawa, K. Fujisaki, N. Suzuki, and T. Minami. 1999. Cloning and expression of a 48-kDa Babesia caballi merozoite rhoptry protein and potential use of the recombinant antigen in an enzyme-linked immunosorbent assay. J. Clin. Microbiol. 37:3475-3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kappmeyer, L. S., L. E. Perryman, S. A. Hines, T. V. Baszler, J. B. Katz, S. G. Hennager, and D. P. Knowles. 1999. Detection of equine antibodies to Babesia caballi by recombinant B. caballi rhoptry-associated protein 1 in a competitive inhibition enzyme-linked immunosorbent assay. J. Clin. Microbiol. 37:2285-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy, M. G., and M. Rustic. 1980. Babesia bovis: continuous cultivation in a microaerophilus stationary phase culture. Science 204:1218-1220. [DOI] [PubMed] [Google Scholar]
- 8.McCosker, P. J. 1981. The global importance of babesiosis, p. 1-24. In M. Ristic and J. P. Kreier (ed.), Babesiosis. Academic Press, New York, N.Y.
- 9.Ristic, M. 1981. Babesiosis, p. 443-468. In M. Ristic and I. MacIntyre (ed.), Diseases of cattle in the tropics. Martinus Nijhof Publishers, The Hague, The Netherlands.
- 10.Suarez, C. E., G. H. Palmer, D. P. Jasmer, S. A. Hines, L. E. Perryman, and T. F. McElwain. 1991. Characterization of the gene encoding a 60-kilodalton Babesia bovis merozoite protein with conserved and surface-exposed epitopes. Mol. Biochem. Parasitol. 46:45-52. [DOI] [PubMed] [Google Scholar]
- 11.Suarez, C. E., T. F. McElwain, E. B. Stephens, V. S. Mishra, and G. H. Palmer. 1991. Sequence conservation among merozoite apical complex proteins of Babesia bovis, Babesia bigemina, and other apicomplexa. Mol. Biochem. Parasitol. 49:329-332. [DOI] [PubMed] [Google Scholar]
- 12.Suarez, C. E., G. H. Palmer, A. Hines, and T. F. McElwain. 1993. Immunogenic B-cell epitopes of Babesia bovis rhoptry-associated protein 1 are distinct from sequences conserved between species. Infect. Immun. 61:3511-3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tebele, N., R. A. Skilton, J. Katende, C. W. Wells, V. Nene, T. McElwain, S. P. Morzaria, and A. J. Musoke. 2000. Cloning, characterization, and expression of a 200-kilodalton diagnostic antigen of Babesia bigemina. J. Clin. Microbiol. 38:2240-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiland, G., and I. Reiter. 1988. Methods for serological response to Babesia, p. 143-158. In M. Ristic (ed.), Babesiosis of domestic animal and man. CRC Press, Boca Raton, Fla.
- 15.Xuan, X., A. Larsen, H. Ikadai, T. Tanaka, I. Igarashi, H. Nagasawa, K. Fujisaki, Y. Toyoda, N. Suzuki, and T. Minami. 2001. Expression of Babesia equi merozoite antigen 1 in insect cells by a recombinant baculovirus and evaluation of its diagnostic potential in an enzyme-linked immunosorbent assay. J. Clin. Microbiol. 39:705-709. [DOI] [PMC free article] [PubMed] [Google Scholar]