Abstract
Objectives
To investigate the association of chronic obstructive pulmonary disease (COPD) with mild cognitive impairment (MCI) and MCI sub-types, amnestic MCI (a-MCI) and non-amnestic MCI (na-MCI), in a population-based study of elderly.
Patients and Methods
Participants included 1,927 individuals, aged 70 to 89 years, enrolled in the population-based, Mayo Clinic Study of Aging. Participants were evaluated with a nurse assessment, neurological evaluation, and neuropsychological testing and the diagnosis of MCI was made according to the standardized criteria by a consensus panel. COPD was identified by the review of medical records. The study was conducted from October 1, 2004, through July 31, 2007. The associations of COPD, and disease duration with MCI, and its subtypes were evaluated using logistic regression models adjusted for potential covariates.
Results
Of 1,927 subjects, 288 had COPD (men vs women 17.9% vs 11.8%, p<0.001). As compared to subjects without COPD, the subjects with COPD had higher prevalence of MCI (27.1% vs 14.6%, p<0.001). The odds ratio (OR) of MCI was almost two times higher in subjects with COPD (OR =1.90, 95 %CI =1.35 – 2.65), with a similar effect in men and women. The OR for MCI increased from 1.67 (97% CI, 1.00 – 2.69) in subjects with COPD duration of ≤ 5 years to 2.08 (95% CI, 1.36 – 3.14) in subjects > 5 years.
Conclusion
This population-based study suggests that COPD is associated with increased odds of having MCI and its sub-types. There was a dose-response association with duration of COPD, after controlling for the potential covariates.
INTRODUCTION
According to recent estimates, the cost of health care in 2012, including long-term care and hospice services, for individuals’ age 65 years and older with dementia was expected to be around $ 200 billion.1 With the aging population, costs associated with cognitive impairment will continue to soar and pose a critical burden on our health care system.2 Mild cognitive impairment (MCI) is an intermediate stage between normal cognitive aging and dementia3,4 with two major subtypes, amnestic (a-MCI) and non-amnestic (na-MCI), based on the affected cognitive domains.5 Individuals with MCI have a higher risk of dementia (10 - 15% per year) compared with general population (1- 2% per year).5 In the absence of any effective therapy for dementia, identification of risk factors for the development of MCI may hold the best promise for preventing or delaying the progression of early cognitive changes to clinical dementia.6,7
Chronic obstructive pulmonary disease (COPD) is defined as “chronic airflow limitation which is usually both progressive and associated with an abnormal inflammatory response of the lungs to noxious particles or gases”.8 According to a recent systematic review, the prevalence of COPD in adults aged 40 years and older is estimated to be 9–10%.9 The risk of developing COPD increases with age such that approximately 28% of individuals aged 80 years or older have a COPD diagnosis.10 Patients with COPD have increased risk of neuronal injury, either due to hypoxia or associated comorbidities, especially cardiovascular diseases.11 Recent studies suggest that up to 77% of patients with both COPD and hypoxemia11,12 have some form of cognitive impairment. However, few well-designed population-based studies have examined the relationship between COPD and MCI. Therefore, we examined the cross-sectional association between COPD and MCI among individuals aged 70 to 89 years in the population-based Mayo Clinic Study of Aging.
METHODS
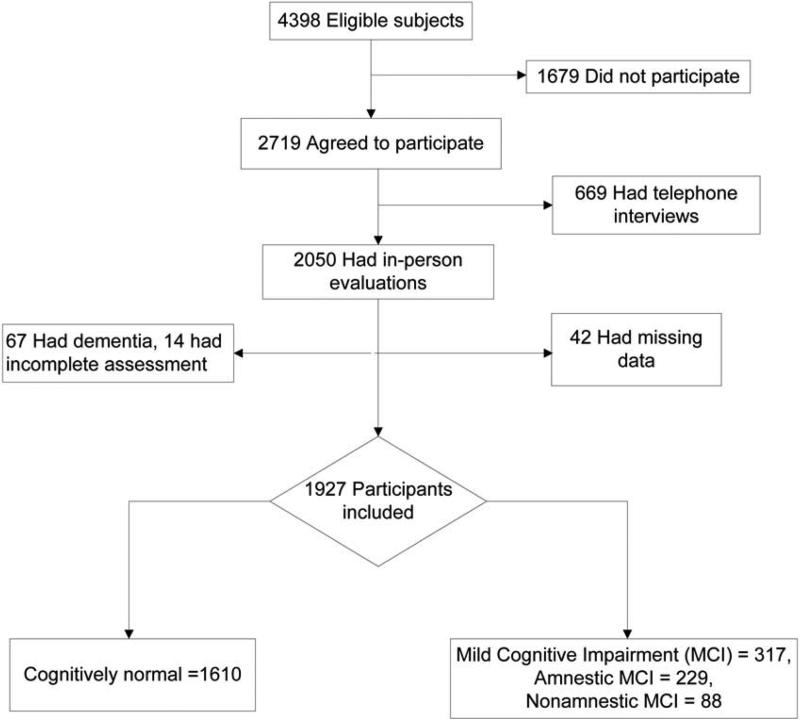
The Mayo Clinic Study of Aging (MCSA) is a population-based study of cognitive aging, initially started in 2004, that enrolled non-demented Olmsted County, MN residents aged 70 to 89 years on October 1, 2004. The design of the study design has been previously published.6 The study cohort was randomly selected from the population by age- and sex-stratification using the Rochester Epidemiology Project (REP) medical records linkage system. From a total of 9,953 individuals identified, a sample of 5,233 was randomly selected and evaluated for eligibility. Of the 4,398 eligible individuals, 2,719 agreed to participate, of which 2,050 were evaluated in–person and 669 were evaluated via telephone interview. The study was conducted from October 1, 2004, through July 31, 2007. Figure 1 provides the details of the subject selection. The present analysis includes 1,927 subjects who received a full evaluation at baseline and had complete data. The study protocol was approved by the institutional review boards of the Mayo Clinic and Olmsted Medical Center (OMC). All individuals provided written informed consent prior to participating.
Figure 1.
The study flow diagram
Measurements of Cognitive Function
All study participants were interviewed by a nurse or study coordinator, had a neurologic evaluation by a physician, and completed neuropsychological testing administered by a psychometrist.6 The nurse interview included a semi-structured questionnaire that included questions about memory administered to the participant, and the Clinical Dementia Rating scale (CDR)13 that was administered to an informant. The physician examination included a medical history review, a complete neurological examination, and administration of the Short Test of Mental Status 14 and the Unified Parkinson's Disease Rating Scale.15 The neuropsychological battery consisted of 9 tests to assess function in four cognitive domains (memory, language, executive function and visuospatial skills). For each test, the scores were age-adjusted and scaled using normative data from the Mayo's Older American Normative Studies.16 Within each domain, the scaled test scores were summed and scaled to compute domain-specific z-scores.6 The raw scores in each domain were also summed and scaled, and the domain scores were scaled to obtain the final global cognitive z score. In each domain, a score less than 1.0 SD below the age-specific mean among the general population was considered as possible cognitive impairment. However, a final decision to assign a diagnosis of MCI was based on a review of all available information and was made by a consensus agreement between the interviewing nurse, examining physician, and the neuropsychologist.6,17 MCI cases were further classified into amnestic or non-amnestic MCI depending on whether or not the memory domain was impaired.
Ascertainment of COPD diagnosis
Potential cases of COPD were identified using two sources of information: a) automated digital algorithms18 and b) ascertainment of diagnoses through the REP medical records linkage system,19,20
a. Automated digital algorithm
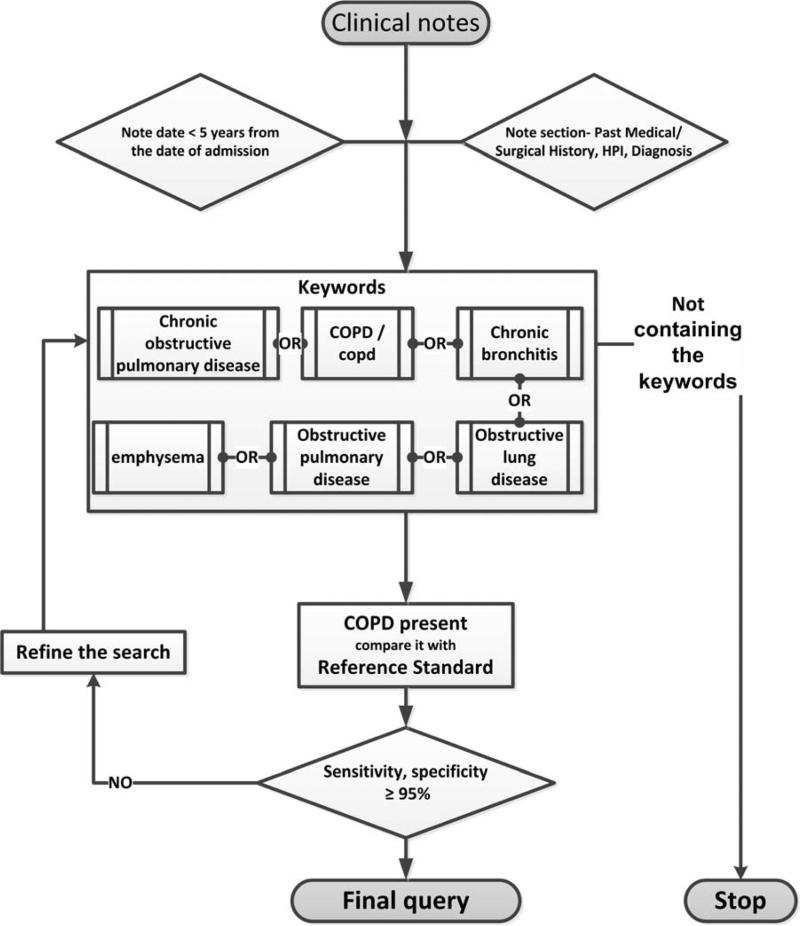
The automated digital algorithm is a highly accurate automatic method of extracting comorbidities, including COPD, from the electronic medical records (EMR) using Boolean combinations of clinical variables and natural language processing data feeds (Figure 2). The implementation of an automatic note search strategy to extract COPD from the EMR is advantageous in that it facilitates fast recognition of COPD cases and has high sensitivity (>98%) and specificity (>99%).18,21,22 A total of 369 potential COPD cases were identified using automated digital algorithms. Out of the remaining, 50 random non-COPD cases were manually reviewed to confirm the non-COPD cases, which were all negative. However, OMC medical records cannot be accessed by the digital algorithms; therefore, we used the REP codes to identify all the cases.
Figure 2.
The automated digital algorithm for chronic obstructive pulmonary diseases.
b. Medical records ascertainment
The medical records of all Olmsted County residents, from all sources, are linked and accessible through the REP.19,20 The REP compiles the residency status of each person who visited any health care provider in Olmsted County from 1/1/1966; this primarily includes the Mayo Clinic, Olmsted Medical Center, and their satellites.19 Using the REP records linkage system, we identified all MCSA subjects with any of the following primary International Classification of Diseases [ICD], Ninth Revision or relevant Adapted Codes for Hospitals [HICDA codes) indicative of possible COPD: 491.xx - chronic bronchitis, 492.xx - emphysema, 496.xx - chronic airway obstruction, not elsewhere classified.23 There were 333 MCSA participants with at least one of these codes.
After identification of possible cases of COPD by both the methods (automated digital algorithm and ICD-9-code), records of the subjects were reviewed to make a confirmatory diagnosis. Clinical notes and laboratory results were reviewed to confirm the diagnoses. Patients were determined to have COPD if the following criteria were met: physician diagnosis of COPD (documented diagnosis of chronic bronchitis, emphysema, chronic obstructive pulmonary disease, or COPD in the admission note, progress note, or discharge summary from the index hospitalization) and / or use of COPD medication therapy for treatments, or COPD exacerbations. After a comprehensive review of the EMR, 288 unique COPD cases were identified. The kappa for both the automated digital algorithms and REP for identification of COPD cases was excellent ~ 0.8, with 94% agreement. Of the total 288 unique COPD cases, both automated digital algorithms and ICD-9CM codes identified 235 common cases, the automated digital algorithm identified 32 additional cases missed by ICD-9 and HICDA codes, and 21 cases were identified by ICD-9 codes which were missed by automated digital algorithm (due to the non-availability of an EMR for cases receiving care at OMC).
Quality assessment for COPD diagnoses
Prior to beginning the study, the EMR of 50 randomly selected subjects (10 COPD and 40 non-COPD cases) were reviewed independently by two reviewers (AKP and BS) to determine the reliability of the diagnosis of COPD. Physician notes before the date of the visit, and outpatient and inpatient data from the EMR were reviewed. Interrater reliability for COPD diagnoses was calculated using Cohen's kappa statistics (see results).24 Investigators making a diagnosis of COPD were blinded to the diagnosis of MCI, thus avoiding diagnostic suspicion bias.
Measure of potential confounders
The following variables were examined as potential confounders: Age, sex, body mass index (BMI), and education obtained at the study visit, and medical history of diabetes, depression, hypertension, stroke, and coronary artery disease (angina, myocardial infarction, coronary revascularization, or coronary artery bypass grafting) ascertained from the medical record chart review. Current symptoms of depression were assessed according to the participant interview using the Beck Depression Inventory scale.25 Apolipoprotein E (APOE) genotyping were assessed for each subject using standard methodology.
ANALYSIS
Continuous variables were reported as medians with the interquartile range (IQR), and categorical variables as counts with percentages. The differences in the baseline demographic and health-related characteristics between subjects with and without MCI were examined using chi-square tests for categorical variables and Wilcoxon Rank Sum tests for continuous variables.
The association between COPD and MCI were evaluated using logistic regression models, reported as odds ratio (OR) and 95 % confidence interval (CI). Outcomes included any MCI, a-MCI, and na-MCI. Three models were evaluated to investigate the association, each building upon the previous model. Model 1 adjusted for age, education, and sex (as these 3 variables have been shown to be strongly associated with cognitive function). Model 2 controlled for the variables in Model 1 plus BDI-II depression scores (as a categorical variable, < 13 and ≥ 13) and history of stroke. Model 3 controlled for the variables in Model 2 and also included APOE genotype (any ε4 vs. no ε4), history of diabetes, hypertension, coronary artery disease, and BMI. Smoking status (ever/never) was examined as both a confounder and an effect modifier, but as it did not fit either of these definitions and had little impact on the models, it was not included. In subsequent analyses, the models were stratified by sex and duration of COPD (≤5 years versus >5 years).
All the statistical tests were performed at the conventional 2-tailed alpha level of 0.05. JMP 9.0.1 computer software (SAS Institute, Cary, NC) was used for all the analyses.
RESULTS
Out of 1,927 subjects with available data, 317 (16.5%) were diagnosed with MCI, of which 229 had a-MCI (72%) and 88 (18%) had na-MCI (Figure 1). The baseline characteristics of the study participants are shown in table 1. As compared to cognitively normal subjects, a higher percent of MCI subjects were men (58.7% vs 49.9%, p = 0.004). MCI subjects were also older (82.7 yrs vs. 79.7 yrs, p<0.0001), less educated (median 12 yrs vs. 13 yrs, p <0.0001), and had a higher frequency of APOE ε4 genotype as compared to the cognitive normal individuals (30.7% vs. 23.9%, p=0.01). Subjects with MCI were also significantly more likely to have a history of stroke, CAD and depression as compared to cognitively normal subjects (all p<0.05). There was no difference in the smoking status between the two groups.
Table 1.
Baseline characteristics of the study participants
| Baseline Variables | MCI (n=317) | Normal cognition (n=1610) | p-value |
|---|---|---|---|
| Male sex, N (%) | 186 (58.7) | 803 (49.9) | 0.004 |
| Age (years) -- median (IQR) | 82.7 (79.2, 85.8) | 79.7 (75.2, 83.6) | <.001 |
| Education (years) -- median (IQR) | 12 (12, 15) | 13 (12, 16) | <.001 |
| APOE E4 allele (E24/34/44 vs. 22/23/33), N (%)* | 96 (30.7) | 379 (23.9) | 0.01 |
| Hypertension, N (%) | 254 (80.1) | 1223 (76.0) | 0.11 |
| Stroke, N (%) | 62 (19.6) | 157 (9.8) | <.001 |
| BDI-II Depression (>13), N (%) ** | 45 (15.0) | 124 (8.0) | 0.001 |
| BMI, median (IQR)*** | 26.9 (24.0, 29.8) | 27.20 (24.4, 30.3) | 0.04 |
| Smokers, N (%) | 0.90 | ||
| Never | 156 (49.2) | 811 (50.4) | |
| Current | 15 (4.7) | 69 (4.3) | |
| Former | 146 (46.1) | 730 (45.3) | |
| Coronary artery disease, N (%) | 156 (49.2) | 654 (40.6) | 0.005 |
| Diabetes Mellitus, N (%) | 69 (21.8) | 277 (17.2) | 0.05 |
| COPD, N (%) | 78 (24.6) | 210 (13.0) | <.001 |
Notes: Data are n (%) or median (IQR). IQR = interquartile ratio; BMI = body mass index; BDI = Beck Depression Inventory; COPD = chronic obstructive pulmonary disease; MI = Myocardial infarction
25 patients missing APOE e4 status (21 Normal, 4 MCI).
75 patients missing BDI-II Depression (59 Normal, 16 MCI).
43 patients missing BMI (33 Normal, 10 MCI)
Of 1,927 subjects, 288 (15%) had a diagnosis of COPD. The interobserver agreement between the two investigators for the diagnosis of COPD was excellent, κ = 0.88 (95 CI, 0.73 - 1.00) with 96% agreement. Men had a higher frequency of COPD as compared to women (17.9% vs 11.8%, p <0.001). Only 84 (29.4%) subjects with diagnosed COPD were on regular treatment for COPD. As compared to subjects without COPD, the subjects with COPD had a higher frequency of MCI (27.1% vs 14.6%, p<0.001), a-MCI (19.1 vs 10.6%, p<0.001) and na-MCI (8% vs 4% p=0.003).
Table 2 shows the association between COPD and MCI and its subtypes among all subjects and also stratified by sex. In this elderly cohort, the odds of having MCI were almost two times higher in subjects with a diagnosis of COPD compared to those without. In Model 1, COPD was associated with increased odds of MCI (OR = 1.98, 95 %CI = 1.46 – 2.67) after adjusting for age, sex, and education. The effect remained even after adjusting for other covariates in Model 2 (OR =1.90, 95 %CI =1.39 – 2.59) and Model 3 (OR =1.87, 95 %CI =1.34 – 2.61) and was similar in both men and women. Examining MCI subtypes, COPD was associated with significantly elevated odds of a-MCI in men and women separately, and in both sexes combined even after adjustment for all the covariates in Model 3 (Table 2). In contrast, COPD was only associated with na-MCI in men and women combined, and in men separately, after adjustment for age, sex, education, depression and history of stroke. The relationship was attenuated, and no longer significant in Model 3, after further adjustment for APOEe4 genotype, diabetes, hypertension, coronary artery disease, and BMI (all participants: OR = 1.39, 95% CI: 0.77-2.39; men only: OR = 1.99, 95% CI: 0.94 - 4.06).
Table 2.
Cross-sectional association between COPD and MCI
| Model 1a | Model 2b | Model 3c | ||||
|---|---|---|---|---|---|---|
| MCI Type | Cases/total N | OR (95% CI) | Cases/total N | OR (95% CI) | Cases/total N | OR (95% CI) |
| Any MCI (n=317) | ||||||
| Men (n=186) | 186/989 | 2.08 (1.41, 3.05) | 172/943 | 2.00 (1.34, 2.97) | 166/916 | 2.12 (1.38, 3.22) |
| Women (n=131) | 131/938 | 1.83 (1.09, 2.99) | 129/909 | 1.73 (1.02, 2.85) | 121/871 | 1.57 (0.88, 2.71) |
| Both sexes | 317/1,927 | 1.98 (1.46, 2.67) | 301/1,852 | 1.90 (1.39, 2.59) | 287/1,787 | 1.87 (1.34, 2.61) |
| Amnestic MCI (n = 229) | ||||||
| Men (n=143) | 143/989 | 1.72 (1.11, 2.61) | 131/943 | 1.72 (1.10, 2.66) | 127/916 | 1.93 (1.20, 3.06) |
| Women (n=86) | 86/938 | 1.96 (1.07, 3.43) | 84/909 | 1.84 (0.99, 3.26) | 78/871 | 1.98 (1.01, 3.68) |
| Both sexes | 229/1,927 | 1.78 (1.26, 2.49) | 215/1,852 | 1.77 (1.23, 2.50) | 205/1,787 | 1.94 (1.32, 2.80) |
| Non-amnestic MCI (n = 88) | ||||||
| Men (n=43) | 43/989 | 2.48 (1.27, 4.71) | 41/943 | 2.23 (1.11, 4.34) | 39/916 | 1.99 (0.94, 4.06) |
| Women (n=45) | 45/938 | 1.37 (0.55, 2.97) | 45/909 | 1.32 (0.52, 2.87) | 43/871 | 0.86 (0.28, 2.10) |
| Both sexes | 88/1,927 | 1.99 (1.18, 3.23) | 86/1,852 | 1.79 (1.05, 2.95) | 82/1,787 | 1.39 (0.77, 2.39) |
COPD = chronic obstructive pulmonary disease; MCI = mild cognitive impairment; OR= Odds ratio
Model 1 adjusted for age as a continuous variable, sex where applicable, and education at the baseline.
Model 2 additionally adjusted for BDI-II Depression, and history of stroke.
Model 3 includes model 2 variables, with additional adjustment for APOEe4 genotype, diabetes, hypertension, coronary artery disease, and BMI.
COPD duration and odds of MCI
There was a dose-response relationship between duration of COPD and the odds of any MCI and a-MCI (Table 3). The overall OR for any MCI increased from 1.60 (97% CI, 0.97 – 2.57) in subjects with COPD duration of ≤ 5 years to 2.10 (95% CI, 1.38 – 3.14) in subjects > 5years in model 3. This dose-response effect was similar for both any MCI and a-MCI in men, but was not observed in women, or for the association with na-MCI.
Table 3.
Association of COPD with Mild Cognitive Impairment by duration of COPD
| Model 1a | Model 2b | Model 3c | |||||||
|---|---|---|---|---|---|---|---|---|---|
| COPD duration ≤ 5years (n=118) | COPD duration > 5years (n=170) | COPD duration ≤ 5years (n=112) | COPD duration > 5years (n=167) | COPD duration ≤ 5years (n=106) | COPD duration > 5years (n=146) | ||||
| MCI Type | Cases/total N | OR (95% CI)a | OR (95% CI) | Cases/total N | OR (95% CI)b | OR (95% CI) | Cases/total N | OR (95% CI) | OR (95% CI) |
|
Any MCI (n=317)
| |||||||||
| Men (n=186) | 186/989 | 1.63 (0.90, 2.84) | 2.45 (1.53, 3.88) | 172/943 | 1.52 (0.81, 2.73) | 2.38 (1.47, 3.81) | 166/916 | 1.55 (0.81, 2.84) | 2.54 (1.50, 4.25) |
| Women (n=131) | 131/938 | 1.97 (0.89, 4.01) | 1.75 (0.90, 3.21) | 129/909 | 1.87 (0.83, 3.86) | 1.65 (0.84, 3.06) | 121/871 | 1.75 (0.77, 3.71) | 1.43 (0.65, 2.90) |
| Both sexes | 317/1,927 | 1.73 (1.08, 2.69) | 2.17 (1.49, 3.12) | 301/1,852 | 1.65 (1.01, 2.61) | 2.09 (1.41, 3.03) | 287/1,787 | 1.60 (0.97, 2.57) | 2.10 (1.38,3.14) |
|
Amnestic MCI (n = 229) | |||||||||
| Men (n=143) | 143/989 | 1.20 (0.59, 2.27) | 2.13 (1.27, 3.50) | 131/943 | 1.21 (0.58, 2.35) | 2.12 (1.24, 3.51) | 127/916 | 1.21 (0.56, 2.41) | 2.59 (1.48, 4.45) |
| Women (n=86) | 86/938 | 2.07 (0.81, 4.61) | 1.89 (0.87, 3.76) | 84/909 | 1.94 (0.75, 4.42) | 1.78 (0.81, 3.58) | 78/871 | 1.97 (0.74, 4.63) | 1.99 (0.83, 4.31) |
| Both sexes | 229/1,927 | 1.43 (0.82, 2.37) | 2.04 (1.34, 3.05) | 215/1,852 | 1.44 (0.81, 2.42) | 2.00 (1.30, 3.02) | 205/1,787 | 1.43 (0.79,2.47) | 2.37 (1.50, 3.68) |
|
Non,amnestic MCI (n = 88) | |||||||||
| Men (n=43) | 43/989 | 2.64 (1.02, 6.07) | 2.37 (1.01, 5.07) | 41/943 | 2.20 (0.77, 5.40) | 2.25 (0.95, 4.88) | 39/916 | 2.34 (0.82, 5.83) | 1.74 (0.65, 4.14) |
| Women (n=45) | 45/938 | 1.49 (0.35, 4.36) | 1.29 (0.38, 3.36) | 45/909 | 1.44 (0.34, 4.24) | 1.24 (0.36, 3.23) | 43/871 | 1.19 (0.27, 3.58) | 0.60 (0.09, 2.10) |
| Both sexes | 88/1,927 | 2.14 (1.00, 4.15) | 1.88 (0.96, 3.41) | 86/1,852 | 1.85 (0.83, 3.70) | 1.75 (0.90, 3.20) | 82/1,787 | 1.66 (0.73, 2.36) | 1.19 (0.53, 2.38) |
OR = Odds Ratio; CI = confidence interval; COPD = chronic obstructive pulmonary disease; MCI = mild cognitive impairment;
Model 1 adjusted for age as a continuous variable, sex where applicable, and education at the baseline. No,COPD is the reference group for all the analyses.
Model 2 additionally adjusted for BDI-II Depression, and history of stroke.
Model 3 includes model 2 variables, with additional adjustment for APOEe4 genotype, diabetes, hypertension, coronary artery disease, and BMI.
DISCUSSION
In this cross-sectional, population-based study of elderly individuals aged 70-89, COPD was associated with almost two-fold higher odds of any MCI and a-MCI, but not na-MCI. The non-significant association of COPD with na-MCI could be due to reduced power on stratification of data. This relationship was independent of age, sex, education, APOE genotype, BMI, depression, history of diabetes, hypertension, CAD, and stroke. Further, there was a dose-response effect such that the odds of MCI increased with the duration of COPD.
Previous studies have suggested that patients with COPD may have a higher risk of cognitive dysfunction as compared to non-COPD patients.11,12,26-29 However, few studies have investigated the association of COPD with MCI using standardized criteria. A recent study from Finland, found that a self-reported diagnosis of COPD in mid-life was associated with increased odds of developing MCI in the later life.30 However, in late-life, COPD was inversely associated with MCI and the authors suggest this could be due to survival bias. In contrast, in the present study we found that COPD was associated with higher odds of MCI. Further, a longer duration of COPD (> 5years) was associated with higher odds of MCI and this relationship was strongest in men. The reason for this difference could be due to the study design (prospective vs cross-sectional) and the methods of assessing COPD. Rusanen et al obtained self-reported diagnoses of COPD, which may not be accurately reported; while we extensively reviewed the medical records of potential cases identified using the REP and automated digital algorithms
In another recently published preliminary-study31, authors compared 45 patients with moderate to severe COPD with 50 healthy controls who were referred from an outpatient pulmonary clinic and estimated the frequency of MCI using standardized criteria.3,32 The frequency of MCI in moderate to severe patients with COPD was shown to be 36%, which is slightly higher than the 27.1% observed among the patients with COPD in our study. The reason for this difference is probably due to the different inclusion criteria and small sample size in the published preliminary study.31 In our cohort, less than one-third of the COPD patients were taking medications or treatment at the baseline. This finding is similar to the recent Medicare data wherein 70.9% patients with COPD were identified to be not on any maintenance therapy33, consistent with the facts that COPD is often mild, but is also commonly undertreated.
Longer duration of COPD could make the brain more vulnerable to hypoxic insults, which are associated with the generation of free radicals, inflammation, neuronal damage, and glial activation.34 Patients with COPD may have increased risk of neuronal injury, either due to hypoxia or associated comorbidities, especially cardiovascular diseases.11 Cardiac diseases may increase the risk of cerebrovascular diseases through hypoperfusion of brain due to impaired cardiovascular function and microemboli to the brain from atrial fibrillation.35 The association between COPD and MCI was significant even after adjusting for cardiovascular comorbidities and other co-variates; thus, strengthening the evidence that an association exist between COPD and MCI, independent of the comorbidities (i.e. the association is not due to confounding by vascular risk factors and stroke). In addition, COPD patients have associated chronic inflammatory process36, which may have a role in the cognitive impairment.37
This study has several strengths. First, it is a population-based cross-sectional study. Subjects were randomly selected from a cohort of elderly subjects, and thus it is less prone to selection bias. Second, the interobserver agreement for the diagnosis of COPD was excellent. Third, the investigators making a diagnosis of COPD were blinded to the diagnosis of MCI, thus avoiding diagnostic suspicion bias. Last, the diagnosis of the cognitive status of subjects was made by the consensus among examining physician/neurologist, nurse and a neuropsychologist. However, limitations of the study also warrant consideration. First, the study design was cross-sectional and precludes us from concluding there is a causal association between COPD and MCI. Second, we used physician diagnosed COPD cases identified by using REP data source and automated digital algorithms, followed by the EMR review and not spirometry, which is the recommended diagnostic test for COPD. As previous epidemiological studies have shown that COPD is underdiagnosed in the population,38-41 our estimates might be conservative. At the same time, some studies have shown that physician-diagnosed COPD may also over diagnose COPD if not confirmed by spirometry. 42,43 On the other hand, the misuse of spirometry has been shown to falsely enhance the prevalence of COPD, especially in an older age-group,44 and may actually contribute to misclassification bias. We therefore, examined in details the medical records of all the potential cases to confirm the date of onset and diagnosis of COPD. Third, the elderly population of the Olmsted County is primarily Caucasian; therefore generalizing the findings of our study to other races and ethnicity has to be done with caution. However, previous studies have shown that the findings of Olmsted County are generalizable to the Upper Midwest population,45 and do provide invaluable information regarding many diseases which are consistent with the national data.45
In conclusion, this study provides evidence that COPD is associated with increased odds of MCI and amnestic MCI. It also demonstrates a dose-response association with duration of COPD. Additional longitudinal studies in population-based cohorts are needed to determine whether COPD is indeed associated with risk of incident MCI and dementia.
Acknowledgement
We thank Kenneth O. Parker (Program analyst), Carl D. Mottram, RRT (Associate Professor of Medicine, Technical Director of the Pulmonary Function Laboratories at Mayo Clinic, Rochester, MN) and Margary Kurland, RN (Research Department Supervisor at OMC) for helping in the data collection. We wish to thank all members of the Alzheimer's disease Research Center group for constant and constructive feedback.
Grant support: Supported by NIH grants P50 AG016574, U01 AG006786, K01 MH068351, and K01 AG028573, by the Robert Wood Johnson Foundation, and by the Robert H. and Clarice Smith and Abigail van Buren Alzheimer's disease Research Program. It was made possible by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH) and by the Rochester Epidemiology Project (R01 AG034676). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Dr. Mielke receives funding from the National Institute on Aging and the Alzheimer Drug Discovery Foundation. Dr. Roberts receives research support from the NIH and the Driskill Foundation. Dr. Yawn has received funding from BI, Merck, and Forrest related to COPD, but no funding related to COPD and cognitive issues. Dr. Petersen serves on scientific advisory boards for the Alzheimer's Association, the National Advisory Council on Aging (NIA), Elan/Janssen AI, Pfizer Inc (Wyeth), and GE Healthcare; receives publishing royalties from Mild Cognitive Impairment (Oxford University Press, 2003); serves as a consultant for Elan/Janssen AI and GE Healthcare; and receives research support from the NIH/NIA.
Glossary
- a-MCI
amnestic mild cognitive impairment
- APOE
Apolipoprotein E
- BDI
Beck Depression Inventory
- BMI
body mass index
- CAD
coronary artery disease
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- EMR
electronic medical records
- HICDA
Hospital International Classification of Disease Adaptation
- ICD
International Classification of Diseases
- IQR
interquartile range
- MCSA
Mayo Clinic Study of Aging
- MCI
mild cognitive impairment
- na-MCI
non-amnestic mild cognitive impairment
- OR
odds ratio
- OMC
Olmsted medical Center
- REP
Rochester Epidemiology Project
- SD
standard deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest disclosure: Drs. Singh and Parsaik report no disclosures.
REFERENCES
- 1.2012 Alzheimer's disease facts and figures. Alzheimers Dement. 2012;8(2):131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88(9):1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 4.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 5.Petersen RC, Roberts RO, Knopman DS, et al. Mild cognitive impairment: ten years later. Arch Neurol. 2009;66(12):1447–1455. doi: 10.1001/archneurol.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30(1):58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts RO, Geda YE, Knopman DS, et al. Association of duration and severity of diabetes mellitus with mild cognitive impairment. Arch Neurol. 2008;65(8):1066–1073. doi: 10.1001/archneur.65.8.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet. 2004;364(9434):613–620. doi: 10.1016/S0140-6736(04)16855-4. [DOI] [PubMed] [Google Scholar]
- 9.Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM. Global burden of COPD: systematic review and meta-analysis. Eur Respir J. 2006;28(3):523–532. doi: 10.1183/09031936.06.00124605. [DOI] [PubMed] [Google Scholar]
- 10.Gershon AS, Wang C, Guan J, Vasilevska-Ristovska J, Cicutto L, To T. Identifying individuals with physcian diagnosed COPD in health administrative databases. Copd. 2009;6(5):388–394. doi: 10.1080/15412550903140865. [DOI] [PubMed] [Google Scholar]
- 11.Dodd JW, Getov SV, Jones PW. Cognitive function in COPD. Eur Respir J. 2010;35(4):913–922. doi: 10.1183/09031936.00125109. [DOI] [PubMed] [Google Scholar]
- 12.Grant I, Heaton RK, McSweeny AJ, Adams KM, Timms RM. Neuropsychologic findings in hypoxemic chronic obstructive pulmonary disease. Arch Intern Med. 1982;142(8):1470–1476. [PubMed] [Google Scholar]
- 13.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 14.Kokmen E, Smith GE, Petersen RC, Tangalos E, Ivnik RC. The short test of mental status. Correlations with standardized psychometric testing. Arch Neurol. 1991;48(7):725–728. doi: 10.1001/archneur.1991.00530190071018. [DOI] [PubMed] [Google Scholar]
- 15.Fahn S, Elton R. Committee MotUD. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden C, Caine D, Lieberman A, editors. Recent Developments in Parkinson's Disease. Macmillan Health Care Information; Florham Park, NJ: 1987. pp. 153–163. [Google Scholar]
- 16.Ivnik RJ, Malec JF, Smith GE, et al. Mayo's Older Americans Normative Studies: WAIS-R norms for ages 56 to 97. The Clinical Neuropsychologist. 1992;6(Suppl.):1–30. [Google Scholar]
- 17.Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology. 2010;75(10):889–897. doi: 10.1212/WNL.0b013e3181f11d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh B, Singh A, Ahmed A, et al. Derivation and validation of automated electronic search strategies to extract Charlson comorbidities from electronic medical records. Mayo Clin Proc. 2012;87(9):817–824. doi: 10.1016/j.mayocp.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melton LJ., 3rd. History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 20.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173(9):1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alsara A, Warner DO, Li G, Herasevich V, Gajic O, Kor DJ. Derivation and validation of automated electronic search strategies to identify pertinent risk factors for postoperative acute lung injury. Mayo Clin Proc. 2011;86(5):382–388. doi: 10.4065/mcp.2010.0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh B, Singh A, Giri J, et al. Evaluation of digital signature of Charlson comorbidities using automatic electronic note search strategies in critically ill patients. Crit Care Med. 2011;39(12):160. [Google Scholar]
- 23.Cooke CR, Joo MJ, Anderson SM, et al. The validity of using ICD-9 codes and pharmacy records to identify patients with chronic obstructive pulmonary disease. BMC Health Serv Res. 2011;11:37. doi: 10.1186/1472-6963-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen JA. Coefficient of Agreement for Nominal Scales Educational and Psychological Measurement. Educ Psychol Meas. 1960;20:37–46. [Google Scholar]
- 25.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Huang Y, Fei GH. The evaluation of cognitive impairment and relevant factors in patients with chronic obstructive pulmonary disease. Respiration. 2013;85(2):98–105. doi: 10.1159/000342970. [DOI] [PubMed] [Google Scholar]
- 27.Fix AJ, Golden CJ, Daughton D, Kass I, Bell CW. Neuropsychological deficits among patients with chronic obstructive pulmonary disease. Int J Neurosci. 1982;16(2):99–105. doi: 10.3109/00207458209147610. [DOI] [PubMed] [Google Scholar]
- 28.Dodd JW, Charlton RA, van den Broek MD, Jones PW. Cognitive Dysfunction in Patients Hospitalized with Acute Exacerbation of Chronic Obstructive Pulmonary Disease (COPD). Chest. 2013 doi: 10.1378/chest.12-2099. [DOI] [PubMed] [Google Scholar]
- 29.Antonelli Incalzi R, Marra C, Giordano A, et al. Cognitive impairment in chronic obstructive pulmonary disease--a neuropsychological and spect study. J Neurol. 2003;250(3):325–332. doi: 10.1007/s00415-003-1005-4. [DOI] [PubMed] [Google Scholar]
- 30.Rusanen M, Ngandu T, Laatikainen T, Tuomilehto J, Soininen H, Kivipelto M. Chronic Obstructive Pulmonary Disease and Asthma and the Risk of Mild Cognitive Impairment and Dementia: a Population Based CAIDE Study. Curr Alzheimer Res. 2013;10(5):549–555. doi: 10.2174/1567205011310050011. [DOI] [PubMed] [Google Scholar]
- 31.Villeneuve S, Pepin V, Rahayel S, et al. Mild Cognitive Impairment in Moderate to Severe Chronic Obstructive Pulmonary Disease: A Preliminary Study. Chest. 2012 doi: 10.1378/chest.11-3035. [DOI] [PubMed] [Google Scholar]
- 32.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Make B, Dutro MP, Paulose-Ram R, Marton JP, Mapel DW. Undertreatment of COPD: a retrospective analysis of US managed care and Medicare patients. Int J Chron Obstruct Pulmon Dis. 2012;7:1–9. doi: 10.2147/COPD.S27032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De la Torre JC. Critical threshold cerebral hypoperfusion causes Alzheimer's disease? Acta Neuropathol. 1999;98(1):1–8. doi: 10.1007/s004010051044. [DOI] [PubMed] [Google Scholar]
- 35.Roberts RO, Geda YE, Knopman DS, et al. Cardiac Disease Associated With Increased Risk of Nonamnestic Cognitive Impairment: Stronger Effect on Women. JAMA Neurol. 2013:1–9. doi: 10.1001/jamaneurol.2013.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343(4):269–280. doi: 10.1056/NEJM200007273430407. [DOI] [PubMed] [Google Scholar]
- 37.Koyama A, O'Brien J, Weuve J, Blacker D, Metti AL, Yaffe K. The role of peripheral inflammatory markers in dementia and Alzheimer's disease: a meta-analysis. The journals of gerontology. Series A, Biological sciences and medical sciences. 2013;68(4):433–440. doi: 10.1093/gerona/gls187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bednarek M, Maciejewski J, Wozniak M, Kuca P, Zielinski J. Prevalence, severity and underdiagnosis of COPD in the primary care setting. Thorax. 2008;63(5):402–407. doi: 10.1136/thx.2007.085456. [DOI] [PubMed] [Google Scholar]
- 39.Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370(9589):741–750. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 40.De Marco R, Pesce G, Marcon A, et al. The Coexistence of Asthma and Chronic Obstructive Pulmonary Disease (COPD): Prevalence and Risk Factors in Young, Middle-aged and Elderly People from the General Population. PLoS One. 2013;8(5):e62985. doi: 10.1371/journal.pone.0062985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ford ES, Croft JB, Mannino DM, Wheaton AG, Zhang X, Giles WH. Chronic Obstructive Pulmonary Disease Surveillance-United States, 1999-2011. Chest. 2013 doi: 10.1378/chest.13-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prieto Centurion V, Huang F, Naureckas ET, et al. Confirmatory spirometry for adults hospitalized with a diagnosis of asthma or chronic obstructive pulmonary disease exacerbation. BMC Pulm Med. 2012;12:73. doi: 10.1186/1471-2466-12-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hnizdo E, Glindmeyer HW, Petsonk EL, Enright P, Buist AS. Case definitions for chronic obstructive pulmonary disease. Copd. 2006;3(2):95–100. doi: 10.1080/15412550600651552. [DOI] [PubMed] [Google Scholar]
- 44.Hardie JA, Buist AS, Vollmer WM, Ellingsen I, Bakke PS, Morkve O. Risk of over-diagnosis of COPD in asymptomatic elderly never-smokers. Eur Respir J. 2002;20(5):1117–1122. doi: 10.1183/09031936.02.00023202. [DOI] [PubMed] [Google Scholar]
- 45.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87(2):151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]