Abstract
In the lamprey brain, there are 18 pairs of identified spinal-projecting neurons whose regenerative abilities have been characterized. The “bad-regenerating” neurons show a very delayed form of apoptosis after axotomy (Shifman et al., 2008). Theoretically, this should provide a long window of opportunity to intervene therapeutically, so it would be helpful if we could identify the early stages of this process in vivo. Until now, there has been no method to link mRNA or protein expression directly to early-stages neuronal apoptosis in vivo. Here we describe a double-labeling protocol in whole-mounted lamprey brain for simultaneous detection of early stage apoptosis, using Fluorochrome-Labeled Inhibitors of Caspases (FLICA), and either mRNA, using in situ hybridization, or protein expression, using immunohistochemistry. To improve brain preservation, the working temperature during the FLICA stage was lowered from 37°C to 4°C (Barreiro-Iglesias and Shifman, 2012). Using this method, neurofilament protein was demonstrated by immunohistochemistry in neurons previously reacted by FLICA. The method also revealed that mRNA for the receptor protein tyrosine phosphatase PTPσ is expressed selectively in FLICA-positive neurons. In addition, our study showed that a retrograde labeling technique can be used in the context of FLICA labeling. FLICA label colocalized with TUNEL staining, confirming that FLICA labeling is a reliable marker of apoptosis in lamprey brain. Our results suggested that we can combine caspase detection with other techniques in vivo to investigate the roles and mechanisms of activated caspases and other molecules in retrograde cell deaths and regenerative abilities of neurons.
Keywords: Caspase, Apoptosis, Wholemount in situ hybridization, Wholemount immunohistochemistry, Lamprey, Brain
1. Introduction
Apoptosis is regulated by genetically encoded mechanisms and can be recognized by many biologically and morphologically distinct characteristics (Arama and Steller, 2006). Cells undergoing apoptosis may have changes in mitochondrial membrane potential, caspase activation, nuclear DNA fragmentation, and membrane blebbing. Many different assays have been designed to detect apoptotic cells, including Annexin-V binding, caspase enzyme activity, and terminal deoxynucleotidyl transferase-mediated dUTP nick-end-labeling (TUNEL). For a long time, however, only TUNEL has been recommended and used on tissue specimens to detect late-stage apoptosis. Excitingly, Fluorochrome-Labeled Inhibitors of Caspases (FLICA) have been shown to detect activated caspases in live lamprey brain (Barreiro-Iglesias and Shifman, 2012), which may provide new insights into apoptosis in vivo. The lamprey has several advantages for neurobiological investigation, including an absence of myelin (Bullock et al., 1984). This makes the whole-mounted brain less opaque and facilitates immunohistochemistry, in situ hybridization and other imaging procedures. A schematic of large larval lamprey brain is shown in Figure 1. There are 18 pairs of large reticulospinal neurons in the lamprey brain. Some are bad regenerators and others good regenerators (Jacobs et al., 1997); the probability that each identified neuron will regenerate after spinal cord transection has been defined previously (Davis and McClellan, 1994; Jacobs et al., 1997). Recent evidence shows that spinal cord transection induces delayed cell death in lamprey spinal-projecting neurons (Shifman et al., 2008). The neuronal death, shown by TUNEL staining, began 4 weeks after spinal cord transection and reached its peak at 12–16 weeks (Shifman et al., 2008). Interestingly, at earlier times, the neurons were swollen and lacked Nissl-staining, which indicated that changes in these neurons began long before death. However, the mechanisms by which neurons in the brain detect and respond to spinal cord transection are unknown. It would be very useful to develop techniques to detect the early stages of apoptosis and to define its relationship to the expression of other important peptides or mRNAs.
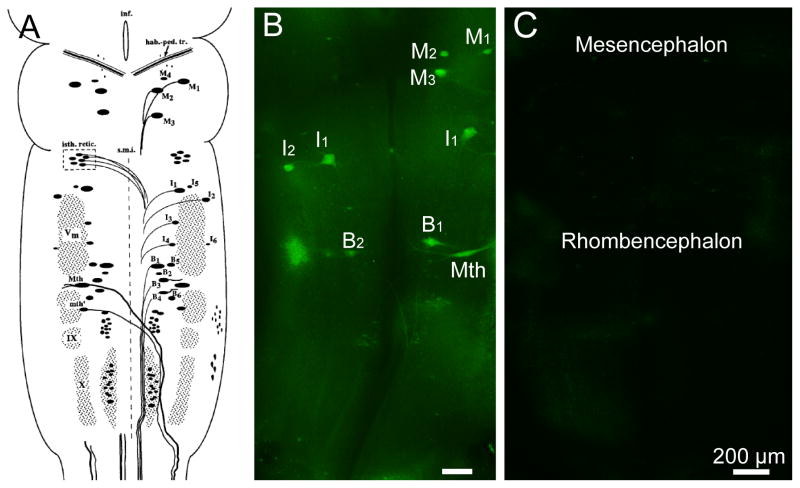
Figure 1. Cytoarchitecture of the identified reticulospinal neurons in lamprey brain.
A, The spinal projecting neurons in the brain of a large larval sea lamprey were labeled and divided into cytoarchitectonic groups according to the nomenclature in (Swain et al., 1993). In addition to the nuclear groups of small neurons, several giant reticulospinal neurons are seen. These are the M ller and Mauthner neurons and also will be described in subsequent figures. M, Mesencephalic M ller cells; I, isthmic M ller cells; B, bulbar M ller cells; Mth, Mauthner cell; mth, auxiliary Mauthner cell; hab.-ped. tr., habenulopeduncular tract; inf., infundibulum; isth. retic., isthmic (anterior rhombencephalic) reticulospinal nucleus; s.m.i., sulcus medianus inferior; Vm, trigeminal motor nucleus; IX, glossopharyngeal motor nucleus; X, vagal motor nucleus. The unlabeled cell mass lateral to mth and lying between V and IX is the facial motor nucleus. Modified from (Jacobs et al., 1997). M2, M3, I1, B1, B2, B4, Mth are considered bad regenerators, while M1, M4, I2, I3, I4, I5, I6, B2, B5, B6 and mth as good regenerator (Davis and McClellan, 1994; Jacobs et al., 1997). B, FLICA labeling in the whole-mounted brain from a larval lamprey after 2 weeks post spinal cord transection. The identified neurons which are FLICA positive have been marked. C, FLICA labeling in the whole-mounted brain from a control larval lamprey without spinal cord transection. Images showed the mesencephalon and rhombencephalon as marked. Scale bar: 200 μm.
Caspases are a family of cysteine proteases that mediate apoptosis, which plays a critical role in development. Accumulating evidence suggests that in the mature nervous system, caspases are not only involved in apoptosis, but also have other important roles in physiological and pathological processes such as dendritic pruning (Kuo et al., 2006; Williams et al., 2006), synaptic plasticity (Li et al., 2010; Lu et al., 2006) and Alzheimer’s disease (D’Amelio et al., 2011; de Calignon et al., 2009; Jo et al., 2011). The multiple roles of caspases make it even more important to develop methods for combining in vivo caspase detection with other imaging techniques.
In the present study, FLICA labeling was subsequently combined with either immunohistochemistry for NF-180, the most prominent of the neurofilament (NF) subunits in lamprey, or in situ hybridization for protein tyrosine phosphatase sigma (PTPσ). NFs comprise most of the axonal cytoskeleton, providing mechanical support and regulating the diameter of axons. Thus far, four NF subunits have been identified in lamprey: NF-180, L-NFL, NF132 and NF95 (Zhang G, et al., 2004; Jin LQ, et al., 2010; Zhang G, et al., 2011;). NF-180 was the first discovered and is required for the formation of normal NF bundles (Zhang G, et al., 2011). It is of interest to our laboratory because after axotomy, NF-180 mRNA expression first disappeared, but then returned selectively in identified reticulospinal neurons that are “good regenerators”, whereas in bad-regenerating neurons, NF-180 expression was permanently downregulated (Jacobs AJ et al., 1997; Zhang G et al., 2011). PTPσ, a member of transmembrane receptor protein tyrosine phosphatase (RPTP) family, is of interest to our laboratory because it acts as a functional receptor for chondroitin sulfate proteoglycans (CSPGs) and appears to mediate a significant part of their inhibitory effects on axon regeneration (Shen et al., 2009).
In summary, using the lamprey nervous system as a model, we have modified the FLICA method to detect activated caspases in vivo and combined this with immunohistochemistry, in situ hybridization and retrograde labeling. These combinatorial labeling techniques permit exploration of caspase-involved activities in vivo (Hyman and Yuan, 2012).
2. Materials and Methods
2.1 Spinal Cord Transection and Retrograde Labeling of Reticulospinal Neurons
Wild-type larval and adult lampreys, Petromyzon marinus, 10–14 cm in length, obtained from the Connecticut River (Massachusetts) or from streams feeding Lake Champlain (Vermont), were maintained in fresh water tanks at room temperature (RT) until brains were removed for the experiments described below. Animals were anesthetized by immersion in 0.1% tricaine methanesulfonate, and the spinal cord was exposed from the dorsal midline at the level of the fifth gill. Transection of the lamprey spinal cord was performed with Castroviejo scissors. Completeness of full transections was confirmed by retraction and visual inspection of the cut ends. For retrograde labeling, a pledget of Gelfoam soaked in 5% dextran tetramethylrhodamine (DTMR, 10 kDa, Molecular Probes) was placed at the site of a complete spinal cord transection. Transected lampreys recovered on ice for 2 hours (hrs) and then returned to fresh water at RT for 2 weeks until brainstems were removed for the following experiments.
2.2 FLICA on Whole-Mounted Lamprey Brains
Two weeks after spinal cord transection, animals were reanesthetized by immersion in saturated benzocaine solution. Brains were dissected out in ice-cold lamprey Ringer’s buffer. The posterior and cerebrotectal commissures of the freshly dissected brains were cut open along the dorsal midline. Brains were incubated immediately in 150 μL 1 x FLICA labeling solution at 4°C for 1 hr, which was diluted from 150 x FLICA labeling solution with phosphate buffered saline (PBS). Afterwards, brains were washed 5 times with 1x wash buffer on a nutator at 4°C, 5 min per time. The alar plates of brains were deflected laterally and pinned flat to a small strip of Sylgard (Dow Corning Co., USA) and fixed in 4% paraformaldehyde (PFA) in PBS for 2 hrs at RT and then washed 3 times in PBS at RT. The fluorescence images of brains were captured immediately with a Nikon 80i microscope. The whole procedure should be conducted in the dark and all the samples should be protected carefully from light. The brains were placed in 70% EtOH and kept at −20°C for immunohistochemistry or in situ hybridization. Control experiments were performed using brains from lampreys without spinal cord transection. The specificity of FLICA labeling was demonstrated by pre-incubating the brains of spinal-transected animals with an excess (20 μM) of unlabeled pan-caspase inhibitor Z-VAD-FMK (Promega, Cat. No. G7231) in PBS for 1 hr at 4 °C and then demonstrating the absence of labeling when FLICA was applied as described above. All images were acquired under the same parameters.
2.3 Immunohistochemistry on Whole-Mounted Lamprey Brains after FLICA
Immunohistochemistry for neurofilaments (NFs) was performed on whole-mounted lamprey brains after FLICA. After 3 washes with PBS at RT on a nutator, 20 min per time, brains were treated with 5% H2O2/methanol for 30 min at RT on a nutator to remove the endogenous peroxidase. After 2 washes with PBS/0.5%Tween-20 (PBST) at RT on a nutator, 30 min per time, 0.1% collagenase in Ca2+ free Ringer’s was applied onto brains for 1 min to loosen up the structure and enhance penetration. After 2 washes with PBST, 30 min per time, at RT on a nutator, brains were placed in blocking buffer 10% fetal calf serum (FBS)/0.5% Tween-20/PBS for 1 hr at RT on a nutator. The primary antibody LCM16 was diluted at 1:100 in the blocking buffer and applied to whole-mounted brains for 48 hrs at 4°C on a nutator. Brains were washed with PBST 3 times, 1 hr each time, and incubated with blocking buffer again for 1 hr at RT on a nutator. Goat anti-mouse conjugated with HRP (Santa Cruz, Cat. No. SC-2005) in blocking buffer was applied onto whole-mounted brains overnight at 4°C on a nutator. After vigorous washing with PBST 3 times and PBS 3 times, 30 min per time, brains were incubated in metal-enhanced diaminobenzidine (DAB) substrate (Pierce, Cat. No. 34002) on ice for 5–10 min. The reaction was stopped by rinsing with dH2O x 3, and washing with PBS x 3, 30 min/time.
2.4 PTPσ Riboprobe Synthesis
The lamprey genome was searched via the Pre-Ensemble database. The assembly consisted of a series of unlinked, overlapping contigs of varying lengths, encompassing the entire genome. Using sequences from several species including human, contigs were found homologous to PTPσ and almost its entire sequence was assembled. Mammalian PTPσ consist of three extracellular Ig-like domains, eight extracellular type-III fibronectin (FNIII) repeats and two intracellular phosphatase domains (Pulido et al., 1995). In order to generate probes specific for the tested gene, we aligned the cloned PTPσ fragments. The low homology (31 %) region of the FNIII domain was selected for synthesis of probes for PTPσ. The cloned PTPσ cDNA fragment was used as template for the generation of digoxigenin (Dig)-labeled antisense riboprobes for in situ hybridization. The PCR primers were designed to amplify the targeted region in the FNIII domain, and the T7 promoter sequence was added to the 5′ end of the antisense primer. The Dig-labeled antisense RNA probes were constructed from amplified cDNA template, which contained the T7 promoter sequence upstream of the antisense-strand, using an RNA transcription kit (Stratagene) with Dig RNA-labeling Mix, as recommended by the manufacturer (Roche Applied Science). The probe sequence is on contigs AEFG01036029 (a part of the scaffold of GL 481841), which is as follows: gcctctcttggactacagactcacttacaaacgcatggatgcagccactcaagcgtccaccctgtacctcgcggtgtccgagtctagtgtcactgtgcgcaacctgcatcgcggggcctcctactcgtttgtcatggtggcacggagtgtgggcggggtgagcggaggcagtctcttgggcgaggaggcggtgattacagtggtaacgcccgaggctccgccgtcagctccgccgttgaacgtgagtgtggggcgcatgggccgctcggcggccgcgctccattgggagcctccacccctggactctcgcaatggagtgatcgtaaggtatacggtgctttaccgagccctgcccattggaggagtaggacatcgtgagggtacaggagcagagagagcagaaggaagtattgctggagatggaagaggaaaaggagattacaaagcaggtggagtttctcaacgtgg tggaagaggtactagaggtacaatttctgaaggtggaagaggtcctgcaagtggaggtgaacgagg.
2.5 In Situ Hybridization on Whole-Mounted Lamprey Brains after FLICA
In situ hybridization was carried out according to modifications of the chromogenic method previously described (Swain et al., 1994). Briefly, whole-mounted brainstem preparations were washed in PTW (0.1% Tween-20 in PBS) and pre-hybridized at 50 – 55 °C in hybridization solution (50% deionized formamide, 5X SSC, 100 mg/ml Torula yeast RNA, 100 mg/ml wheat germ tRNA, 50 mg/ml heparin, 0.1% Tween-20) for 60 min, followed by hybridization overnight on a nutator at 50–55 °C in the same solution plus 1 μg/ml Dig-labeled antisense RNA probes of PTPσ. Specimens were washed in hybridization solution at 55 °C followed by washes in PTW and PBT (0.1% bovine serum albumin, 0.2% Triton X-100 in PBS) at RT. Alkaline phosphatase (AP)-conjugated anti-Dig antibody (1:1,000; Roche Applied Science) was applied to the tissue overnight at 4 °C. The tissue was washed sequentially in PBT and SMT (100 mM NaCl, 50 mM MgCl2, 100 mM Tris, pH 9.5, 0.1% Tween-20) and the chromogenic reaction was carried out in a solution containing 20 μl of NBT/BCIP stock solution (Roche Applied Science) in every 1 ml of SMT on ice in the dark for 1 hr or until reaction was completed as determined by monitoring staining under a dissecting microscope. Finally, specimens were washed in PBS, dehydrated in serial ethanols, cleared with toluene, and mounted in Permount.
2.6 TUNEL Staining in Whole-mounted Lamprey Brain
TUNEL staining was performed on whole-mounted lamprey brains after the FLICA labeling. Brains were washed with 0.5% Triton X-100/PBS 30 min x 4 times and washed twice for 30 minutes with PBS. Brains were equilibrated with equilibration buffer (100 μl per brain) at RT for 30 min. They were labeled with 100 μl TdT reaction mix (1 μl of biotinylated nucleotide mix and 1 μl of rTdT enzyme in 98 μl of equilibrate buffer) (Promega, Cat. No. G7131) overnight at RT. The reactions were terminated by 2 x SSC 30 min at RT. Wash with PBS at RT 30 min x 2 times. Wash with 0.1% Tween-20/PBS 30 min x 2 times. Brains were blocked with 0.1%BSA/0.2%Triton X-100/PBS 30 min x 2 times, and then incubated brains with streptavidin-AP in 0.1%BSA/0.2%Triton X-100/PBS (1:1000) overnight at 4 °C. Brains were washed with 0.5% Triton X-100/PBS 3x 30 min and SMT (100 mM NaCl, 50 mM MgCl2, 100 mM Tris, pH 9.5, 0.1% Tween-20) 3x 30 min, after which they were incubated in AP substrate for 30 min or until signal showed. Brains were washed several times in dH2O. Finally, brains were washed in PBS, dehydrated in serial ethanols, cleared with toluene, and mounted in Permount. As positive controls, brains were pre-treated with DNase I (340 units/ml in 50mM Tris-HCl, pH 7.5, 10 mM MgCl2, 1 mg/ml BSA) (Promega, Cat. No. G7131) for 30 min at RT prior to labeling procedures (data not shown).
3. Results
3.1 Distribution of caspase positive neurons in lamprey brain after spinal cord transection
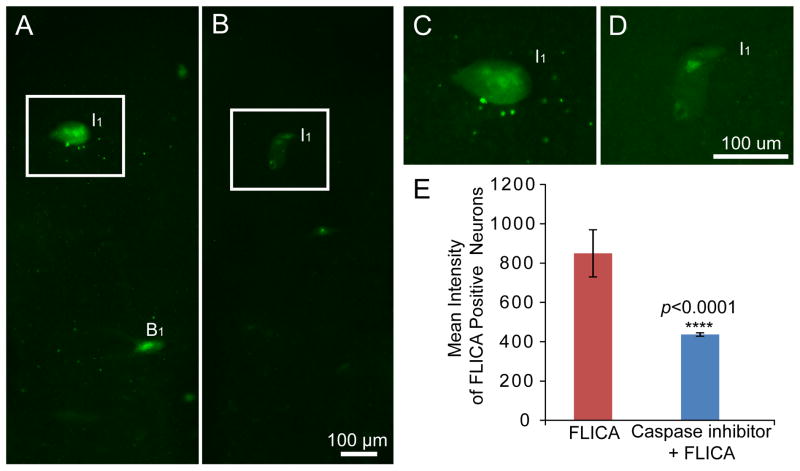
Restricted and localized activation of caspases is involved in the normal physiology of living neurons (Hyman and Yuan, 2012), but caspases are nevertheless the central components of the apoptotic response (Riedl and Shi, 2004) and can be considered as an important hallmark of apoptosis. Our present study showed that incubation of the brains with the polycaspase FLICA reagent (FAM-VAD-FMK) at 4°C can detect activated caspases in identified reticulospinal neurons of larval and adult lamprey brains 2 weeks after spinal cord transection (Figure 2A, 2C, 2E). FAM-VAD-FMK labeling, which targets a peptide region common to all activated caspases and thus detects any activated caspase non-selectively, was strongly concentrated in the neurons that previously have been documented to be bad regenerators (Shifman et al., 2008), which is consistent with the findings of a previous publication from our laboratory using a different incubation temperature (37°C) (Barreiro-Iglesias and Shifman, 2012). Moreover, identified neurons in brains of animals without spinal cord transection show no FLICA labeling (Figure 1C). The specificity of FLICA labeling was evaluated by pre-incubating the brains of spinal-transected animals with an excess (20 μM) of unlabeled pan-caspase inhibitor Z-VAD-FMK in PBS for 1 hr at 4 °C, after which FLICA was applied as described above. The unlabeled pancaspase inhibitor Z-VAD-FMK reduced FLICA-labeling in identified neurons, such as I1 and B1 (Figure 6A–D). The mean intensity of all identified neurons was determined and compared between these two groups. Pan-caspase inhibition with Z-VAD-FMK significantly reduced FLICA labeling by approximately 50% (p<0.0001; Figure 6E), which strongly support the specificity of the FLICA labeling.
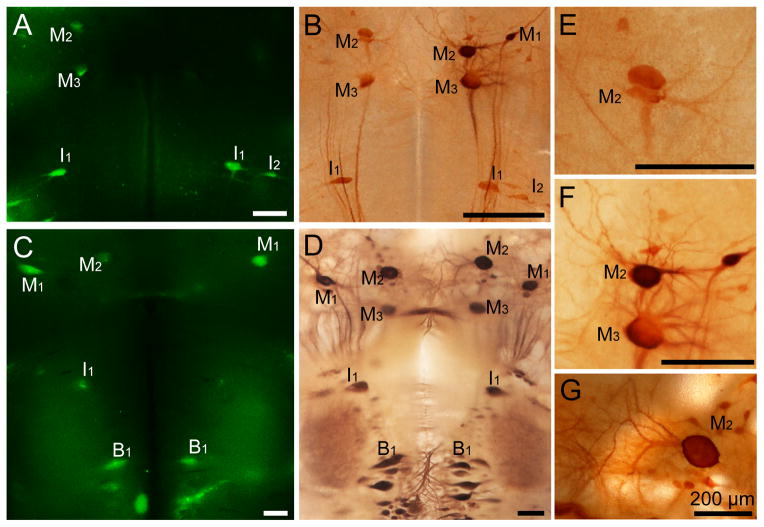
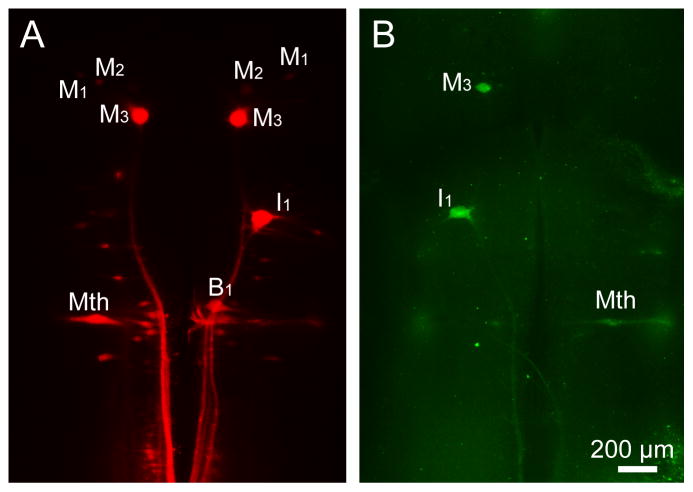
Figure 2. Double-labeling by FLICA and neurofilament immunohistochemistry in whole-mounted lamprey brains at 2 weeks post spinal cord transection.
A, FLICA labeling in the whole-mounted brain from a larval lamprey (12 cm, approximately 4 year old). FLICA-labeled identified neurons have been marked. B, The immunohistochemical staining of neurofilaments performed after FLICA staining (the same animal in A). The morphology of identified neurons has been kept intact and the distribution of neurofilaments is normal. C, FLICA staining in the whole-mounted brain from a post-metamorphic adult lamprey. D, The immunohistochemical staining of neurofilaments conducted after FLICA labeling (the same animal used in C). The distribution pattern of neurofilaments is normal. E, F, Higher magnification images of identified neurons from B. G, Higher magnification images of identified neurons M2 from D. The identified neurons have been kept intact in both lamprey brains. Scale bar: 200 μm.
Figure 6. Caspase inhibitor Z-VAD-FMK can inhibit FLICA labeling in lamprey brains.
A, FLICA labeling in the whole-mounted brain of a larval lamprey at 2 weeks post-transection. B, The fluorescent intensity of FLICA labeling was greatly reduced by pre-incubation with the unlabeled caspase inhibitor Z-VAD-FMK in the larval lamprey at 2 weeks post-transection. C and D, Higher maginification images from A and B. E, Effect of pre-incubation with unlabeled caspase inhibitor Z-VAD-FMK on intensity of FLICA labeling (means + SD). Pre-incubation reduced the intensity of FLICA labeling approximately 50% (p<0.0001, n=11 for FLICA labeling, n=20 for Z-VAD-FMK with FLICA labeling). Scale bar: 100 μm.
3.2 Pattern of neurofilament immunohistochemistry and PTPσ in situ hybridization after FLICA
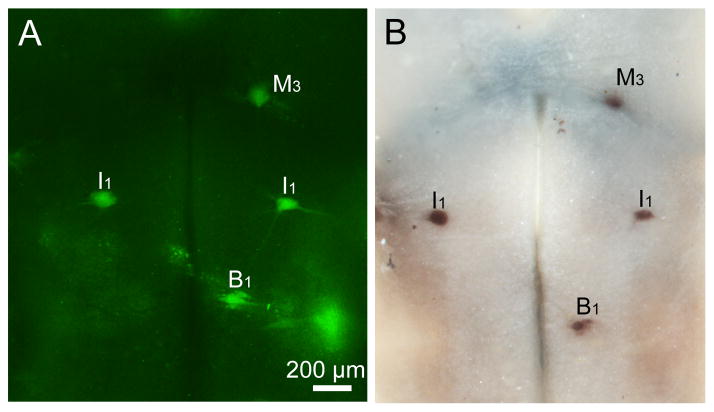
In order to test whether the identified neurons studied with the FLICA procedure remain sufficiently intact to allow further investigation of additional antigens or mRNA, we have performed immunohistochemistry and in situ hybridization on whole-mount preparations after FLICA labeling. As described in Materials and Methods (2.2), brains were fixed in 4% PFA in PBS for 2 hrs at RT. 4% PFA was used as a conventional fixative and preserved lamprey wholemounted brains well. Neuronal morphology was preserved after FLICA staining (Figure 2A, 2C) and in NF immunohistochemical preparations (Figure 2B, 2D). The in situ hybridization of PTPσ mRNA after FLICA labeling also showed excellent staining (Figure 3A and 3B). This result demonstrates that mRNAs do not undergo obvious degradation during the FLICA incubations and are well enough preserved to perform successful in situ hybridizations.
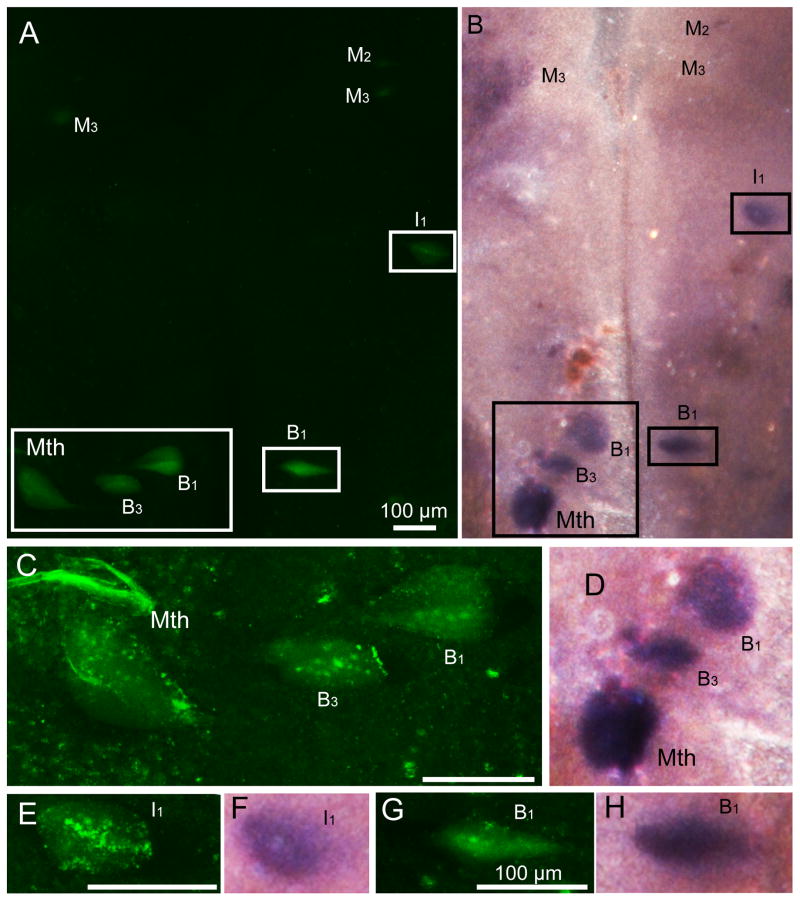
Figure 3. Double-labeling by FLICA and PTPσ in situ hybridization in whole-mounted lamprey brains.
Lampreys underwent spinal cord transection and were allowed to recover for 2 weeks. The brains were then removed and reacted for FLICA and PTPσ. A, Activated caspases in identified reticulospinal neurons such as M2, I1, Mth, B1, B3, and B4 neurons, as revealed by FAM-VAD-FMK labeling (green, FLICA labeling). B, In situ hybridization shows PTPσ mRNA distribution after FLICA labeling (in the same larval lamprey brain used in A). C and D, Enlarged images for neuron I1 from A and B. Scale bar: 200 μm.
3.3 Retrograde labeling techniques combined with FLICA
In addition to the methods that we tested after FLICA labeling, we also tested whether retrograde labeling techniques could be used successfully before performing FLICA. As described in Materials and Methods (2.1), we performed spinal cord transection and at the same time applied DTMR (red) at the transection site. FLICA labeling was performed 2 weeks later on whole-mount preparations of lamprey brain (Figure 4). Retrogradely labeled reticulospinal neurons were present (Figure 4A), and the FLICA labeling pattern resembled the pattern that we observed without the retrograde-labeling procedures (Figures 4B, 2A, 2C, 3A). These results suggest that the retrograde labeling procedures did not interfere with FLICA labeling and therefore that these techniques can be used in combination with FLICA labeling to further characterize the neurons injured by spinal cord transection.
Figure 4. Double-labeling by FLICA and retrograde labeling tracer DTMR in whole-mounted larval lamprey brain at 2 weeks after spinal cord transection.
A, Retrograde labeling tracer DTMR (red) shows neurons in larval lamprey brain 2 weeks after application at the time of transection. B, Activated caspases in identified reticulospinal neurons, such as M1 and I1 neurons, revealed by FAM-VAD-FMK labeling (green, FLICA labeling) in the same brain used in A. Scale bar: 200 μm.
3.4 TUNEL staining colocalized with FLICA in identified neurons
Spinal-projecting neurons in lamprey brain undergo delayed cell death after spinal cord transection, and this can be detected with TUNEL staining beginning at 4 weeks post-transection (Shifman et al., 2008). FLICA reagents have been shown to be reliable reporters of caspase activation (Darzynkiewicz and Pozarowski, 2007; Grabarek et al., 2002; Kuzelova et al., 2007), but these studies were conducted in vitro. To determine whether FLICA positive neurons were undergoing apoptosis in our in vivo system, we performed TUNEL staining after FLICA labeling in lamprey brain at 24 weeks post-transection. We found 100% colocalization between TUNEL staining (Figure 5B, 5D, 5F, 5H.) and FLICA positivity in neurons (Figure 5A, 5C, 5E, 5G), which strongly suggested that FLICA labeling is a reliable marker of apoptosis in the lamprey CNS.
Figure 5. TUNEL staining after FLICA labeling in whole-mounted lamprey brains at 24 weeks post-transection.
A, Activated caspases in identified reticulospinal neurons by FAM-VAD-FMK labeling (green, FLICA labeling) in larval lamprey brain 24 weeks after spinal cord transection. B, TUNEL colocalized with FLICA labeling in identified reticulospinal neurons. C, E, G, Higher-maginification images showed FLICA positive neurons. D, F, H, Higher-maginification images showing TUNEL-labeled neurons. Scale bar: 100 μm.
4. Discussion
Caspases are a family of cysteine proteases involved in apoptosis that are found in a wide range of animals, from worms to human. They are generally synthesized as inactive zymogens; activation requires specific cleavage and allosteric conformational changes (Hyman and Yuan, 2012; Riedl and Salvesen, 2007). Caspases can be classified as initiators (caspase 8, caspase 9) and executioners (caspase 3, caspase 7) according to their functions in apoptosis. They are activated by two main pathways: an extrinsic pathway and an intrinsic pathway. The extrinsic pathway is triggered by the binding of specific extracellular ligands to transmembrane death receptors, such as tumor necrosis factor receptor 1 (TNFR1), Fas and nerve growth factor receptor p75NTR (Benn and Woolf, 2004; Lavrik et al., 2005). The death receptors cluster, recruit and activate initiator caspase-8, which in turn activates executioner caspase-3 and leads to apoptosis. In the intrinsic pathway, apoptotic stimuli alter mitochondrial permeability and induce cytochrome c release. Apoptosomes containing APAF1 and cytochrome C mediate caspase-9 activation (Garrido et al., 2006; Ow et al., 2008), which cleaves executioner caspase-3 or -7 (Jiang and Wang, 2004). Mounting evidence suggests that caspases are not only involved in apoptosis but also play critical roles in multiple cellular processes in the healthy nervous system, including dendritic pruning (Kuo et al., 2006; Williams et al., 2006), synapse plasticity (Li et al., 2010; Lu et al., 2006), and in the processes involved in Alzheimer’s disease (D’Amelio et al., 2011; de Calignon et al., 2009; Jo et al., 2011).
FLICAs are cell permeable and noncytotoxic and can bind to activated caspases within cells that undergo apoptosis. Unbound FLICA molecules can be washed out of cells that do not contain active caspases, so that the remaining green fluorescent signal is a direct indicator of active caspases. The FLICA reagent that we used in this study (FAM-VAD-FMK) is non-specific and labels all active caspases, but the same protocol can also be applied to identify other FLICAs.
The present study demonstrates the feasibility of simultaneous detection of early stage apoptosis and mRNA or protein expression in whole-mounted lamprey brain, using FLICA in combination with double-labeling by in situ hybridization or immunohistochemistry. The method for FLICA labeling described in the current study is a modification of the FLICA labeling technique reported previously (Barreiro-Iglesias and Shifman, 2012). The revision is necessary because the lower temperature used in the present procedure (4°C) allows superior tissue preservation before fixation than the working temperature that was used for FLICA labeling in the earlier study (37°C) (Shifman et al., 2008). We found that the present method insures excellent staining for neurofilament protein and PTPσ mRNA after FLICA labeling. Our results also showed that retrograde labeling techniques can also be used in combination with FLICA labeling.
Conclusion
The methods that we describe for FLICA labeling of whole-mount preparations of lamprey brain in combination with immunohistochemistry or in situ hybridization can help identify peptides and species of mRNA related to apoptosis. FLICA staining combined with a retrograde labeling technique allows further characterization of the diverse neurons axotomized by spinal cord injury. The methods also provide tools to probe the multiple roles of caspases in the normal and injured nervous system in vivo.
Highlights.
During the course of an experiment, it often is desirable to know when neurons are undergoing early apoptosis before the tissue is fixed and processed for molecular correlates. This manuscript describes methods to allow imaging of caspase activation in living tissue and then double labeling the same tissue after fixation, so that protein or mRNA expression patterns can be assessed in neurons known to have undergone caspase activation.
Highlights.
Often there is a need to detect early apoptosis before tissue is fixed and processed for molecular correlates.
Activation of caspases was detected in living lamprey brains with fluorescently-labeled inhibitors of caspase (FLICA).
The same tissue was then fixed and processed for immunohistochemistry or in situ hybridization.
“Bad-regenerator” neurons undergo slow apoptosis post-axotomy and selectively express the mRNA for the CSPG receptor PTPσ.
Acknowledgments
NIH Grants R24 HD050838, R01 NS038537 and Shriners Grant # SHC-85220
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arama E, Steller H. Detection of apoptosis by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling and acridine orange in Drosophila embryos and adult male gonads. Nat Protoc. 2006;1:1725–31. doi: 10.1038/nprot.2006.235. [DOI] [PubMed] [Google Scholar]
- Barreiro-Iglesias A, Shifman MI. Use of Fluorochrome-Labeled Inhibitors of Caspases to Detect Neuronal Apoptosis in the Whole-Mounted Lamprey Brain after Spinal Cord Injury. Enzyme Res. 2012;2012:835731. doi: 10.1155/2012/835731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benn SC, Woolf CJ. Adult neuron survival strategies--slamming on the brakes. Nat Rev Neurosci. 2004;5:686–700. doi: 10.1038/nrn1477. [DOI] [PubMed] [Google Scholar]
- Bullock TH, Moore JK, Fields RD. Evolution of myelin sheaths: both lamprey and hagfish lack myelin. Neurosci Lett. 1984;48:145–8. doi: 10.1016/0304-3940(84)90010-7. [DOI] [PubMed] [Google Scholar]
- D’Amelio M, Cavallucci V, Middei S, Marchetti C, Pacioni S, Ferri A, Diamantini A, De Zio D, Carrara P, Battistini L, Moreno S, Bacci A, Ammassari-Teule M, Marie H, Cecconi F. Caspase-3 triggers early synaptic dysfunction in a mouse model of Alzheimer’s disease. Nat Neurosci. 2011;14:69–76. doi: 10.1038/nn.2709. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z, Pozarowski P. All that glitters is not gold: all that FLICA binds is not caspase. A caution in data interpretation--and new opportunities. Cytometry A. 2007;71:536–7. doi: 10.1002/cyto.a.20425. [DOI] [PubMed] [Google Scholar]
- Davis GR, Jr, McClellan AD. Long distance axonal regeneration of identified lamprey reticulospinal neurons. Exp Neurol. 1994;127:94–105. doi: 10.1006/exnr.1994.1083. [DOI] [PubMed] [Google Scholar]
- de Calignon A, Spires-Jones TL, Pitstick R, Carlson GA, Hyman BT. Tangle-bearing neurons survive despite disruption of membrane integrity in a mouse model of tauopathy. J Neuropathol Exp Neurol. 2009;68:757–61. doi: 10.1097/NEN.0b013e3181a9fc66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006;13:1423–33. doi: 10.1038/sj.cdd.4401950. [DOI] [PubMed] [Google Scholar]
- Grabarek J, Du L, Johnson GL, Lee BW, Phelps DJ, Darzynkiewicz Z. Sequential activation of caspases and serine proteases (serpases) during apoptosis. Cell Cycle. 2002;1:124–31. [PubMed] [Google Scholar]
- Hyman BT, Yuan J. Apoptotic and non-apoptotic roles of caspases in neuronal physiology and pathophysiology. Nat Rev Neurosci. 2012;13:395–406. doi: 10.1038/nrn3228. [DOI] [PubMed] [Google Scholar]
- Jacobs AJ, Swain GP, Snedeker JA, Pijak DS, Gladstone LJ, Selzer ME. Recovery of neurofilament expression selectively in regenerating reticulospinal neurons. J Neurosci. 1997;17:5206–20. doi: 10.1523/JNEUROSCI.17-13-05206.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Wang X. Cytochrome C-mediated apoptosis. Annu Rev Biochem. 2004;73:87–106. doi: 10.1146/annurev.biochem.73.011303.073706. [DOI] [PubMed] [Google Scholar]
- Jo J, Whitcomb DJ, Olsen KM, Kerrigan TL, Lo SC, Bru-Mercier G, Dickinson B, Scullion S, Sheng M, Collingridge G, Cho K. Abeta(1-42) inhibition of LTP is mediated by a signaling pathway involving caspase-3, Akt1 and GSK-3beta. Nat Neurosci. 2011;14:545–7. doi: 10.1038/nn.2785. [DOI] [PubMed] [Google Scholar]
- Kuo CT, Zhu S, Younger S, Jan LY, Jan YN. Identification of E2/E3 ubiquitinating enzymes and caspase activity regulating Drosophila sensory neuron dendrite pruning. Neuron. 2006;51:283–90. doi: 10.1016/j.neuron.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Kuzelova K, Grebenova D, Hrkal Z. Labeling of apoptotic JURL-MK1 cells by fluorescent caspase-3 inhibitor FAM-DEVD-fmk occurs mainly at site(s) different from caspase-3 active site. Cytometry A. 2007;71:605–11. doi: 10.1002/cyto.a.20415. [DOI] [PubMed] [Google Scholar]
- Lavrik IN, Golks A, Krammer PH. Caspases: pharmacological manipulation of cell death. J Clin Invest. 2005;115:2665–72. doi: 10.1172/JCI26252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Jo J, Jia JM, Lo SC, Whitcomb DJ, Jiao S, Cho K, Sheng M. Caspase-3 activation via mitochondria is required for long-term depression and AMPA receptor internalization. Cell. 2010;141:859–71. doi: 10.1016/j.cell.2010.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Wang Y, Furukawa K, Fu W, Ouyang X, Mattson MP. Evidence that caspase-1 is a negative regulator of AMPA receptor-mediated long-term potentiation at hippocampal synapses. J Neurochem. 2006;97:1104–10. doi: 10.1111/j.1471-4159.2006.03800.x. [DOI] [PubMed] [Google Scholar]
- Ow YP, Green DR, Hao Z, Mak TW. Cytochrome c: functions beyond respiration. Nat Rev Mol Cell Biol. 2008;9:532–42. doi: 10.1038/nrm2434. [DOI] [PubMed] [Google Scholar]
- Pulido R, Serra-Pages C, Tang M, Streuli M. The LAR/PTP delta/PTP sigma subfamily of transmembrane protein-tyrosine-phosphatases: multiple human LAR, PTP delta, and PTP sigma isoforms are expressed in a tissue-specific manner and associate with the LAR-interacting protein LIP. 1. Proc Natl Acad Sci U S A. 1995;92:11686–90. doi: 10.1073/pnas.92.25.11686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl SJ, Salvesen GS. The apoptosome: signalling platform of cell death. Nat Rev Mol Cell Biol. 2007;8:405–13. doi: 10.1038/nrm2153. [DOI] [PubMed] [Google Scholar]
- Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol. 2004;5:897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- Shifman MI, Zhang G, Selzer ME. Delayed death of identified reticulospinal neurons after spinal cord injury in lampreys. J Comp Neurol. 2008;510:269–82. doi: 10.1002/cne.21789. [DOI] [PubMed] [Google Scholar]
- Swain GP, Jacobs AJ, Frei E, Selzer ME. A method for in situ hybridization in wholemounted lamprey brain: neurofilament expression in larvae and adults. Exp Neurol. 1994;126:256–69. doi: 10.1006/exnr.1994.1063. [DOI] [PubMed] [Google Scholar]
- Swain GP, Snedeker JA, Ayers J, Selzer ME. Cytoarchitecture of spinal-projecting neurons in the brain of the larval sea lamprey. J Comp Neurol. 1993;336:194–210. doi: 10.1002/cne.903360204. [DOI] [PubMed] [Google Scholar]
- Williams DW, Kondo S, Krzyzanowska A, Hiromi Y, Truman JW. Local caspase activity directs engulfment of dendrites during pruning. Nat Neurosci. 2006;9:1234–6. doi: 10.1038/nn1774. [DOI] [PubMed] [Google Scholar]