Abstract
Rationale
Amount and type of food can alter dopamine systems and sensitivity to drugs acting on those systems.
Objectives
This study examined whether changes in body weight, food type, or both body weight and food type contribute to these effects.
Methods
Rats had free or restricted access (increasing, decreasing, or maintaining body weight) to standard (5.7% fat) or high fat (34.3%) chow.
Results
In rats gaining weight with restricted or free access to high fat chow, both limbs of the quinpirole yawning dose-response curve (0.0032–0.32 mg/kg) shifted leftward compared with rats eating standard chow. Restricting access to standard or high fat chow (maintaining or decreasing body weight) decreased or eliminated quinpirole-induced yawning; within one week of resuming free feeding, sensitivity to quinpirole was restored, although the descending limb of the dose-response curve was shifted leftward in rats eating high fat chow. These are not likely pharmacokinetic differences because quinpirole-induced hypothermia was not different among groups. PG01037 and L-741,626 antagonized the ascending and descending limbs of the quinpirole dose-response curve in rats eating high fat chow, indicating D3 and D2 receptor mediation, respectively. Rats eating high fat chow also developed insulin resistance.
Conclusions
These results show that amount and type of chow alter sensitivity to a direct-acting dopamine receptor agonist with the impact of each factor depending on whether body weight increases, decreases, or is maintained. These data demonstrate that feeding conditions, perhaps related to insulin and insulin sensitivity, profoundly impact the actions of drugs acting on dopamine systems.
Keywords: Direct-acting dopamine receptor agonist, quinpirole, yawning, high fat chow, body weight, insulin
Factors that impact individual differences in response to therapeutic drugs and vulnerability to substance abuse are not fully understood. Some factors that might contribute to individual differences include age, gender, and genetics; an additional factor that has not been rigorously examined, but which impacts brain neurochemistry and drug effects, is diet. Nutritional factors impact neurochemical systems (e.g., dopamine) that are thought to be altered in certain psychopathologies; many of the same neurochemical systems mediate the effects of both psychotherapeutic drugs and drugs of abuse (France et al. 2009; Sevak et al. 2008). Thus, nutritional factors might contribute to the development of psychopathology (e.g., substance abuse) and also modify drug responses, thereby affecting therapeutic outcome or likelihood of abuse. The importance of this topic for substance abuse and other psychiatric disorders is underscored by the following: 1) there is a worldwide obesity epidemic and obesity is correlated with altered responsiveness to drugs (Khan et al. 2007); 2) diabetes is increasing worldwide and insulin is known to regulate neurochemical systems that mediate effects of therapeutic and abused drugs (Sevak et al. 2007); 3) efforts to reduce obesity include dieting (restricting food intake) which can decrease responsiveness to drugs (Li et al. 2009); 4) some (pathological) conditions of food restriction render patients insensitive to some therapeutic drugs (Kaye et al. 1998); and 5) diet-induced changes in some neurochemical systems (e.g., dopamine) can persist for a very long time and could predispose individuals to reduced responsiveness to drugs for an extended period (Bailer et al. 2005; Naef et al. 2008).
Five subtypes of dopamine receptors are classified as D1- or D2-like and many drugs act at one or more of these receptors. For example, some drugs of abuse inhibit reuptake or promote release of dopamine, acting as indirect dopamine receptor agonists. Other drugs act directly as agonists or antagonists at dopamine receptors. D3 and D2 receptors are the targets for drugs used to treat schizophrenia and Parkinson’s disease (Abi-Dargham and Laruelle 2005; Foley et al. 2004; Uitti and Ahlskog 1996) and these receptors are thought to mediate many effects of indirect-acting dopamine receptor agonists (Acri et al. 1995; Caine and Koob 1993; Sinnott et al. 1999; Spealman 1996).
Many factors influence the effects of drugs acting at dopamine receptors, including drug history (Collins and Woods 2007; Nader and Mach 1996), age (Kostrzewa et al. 1992), and feeding condition (Baladi and France 2009; Sevak et al. 2008). Dopamine systems are activated by drugs and by food (Di Chiara and Imperato 1988; Hernandez and Hoebel, 1988; Wise and Rompre 1989) and both the amount and type of food can alter the behavioral effects of drugs acting on dopamine systems. For example, food restriction or eating high fat chow alters sensitivity of rats to yawning produced by direct-acting dopamine receptor agonists (Baladi and France 2009; Collins et al. 2008; Sevak et al. 2008). In prior studies that examined food restriction and direct-acting dopamine receptor agonists, body weight was either maintained (e.g., 85% of free-feeding weight; Collins et al. 2008) or progressively decreased (e.g., 10 g/day; Baladi and France 2009). In prior studies examining high fat chow and dopamine receptor agonists, body weight of subjects eating high fat chow increased more than body weight of subjects eating standard chow (Baladi and France 2009). Thus, it is unclear whether changes in body weight, chow type, or both factors impact sensitivity to drugs acting on dopamine systems.
The role of different receptors in mediating drug effects might vary according to feeding conditions. For example, discriminative stimulus effects of quinpirole appear to be mediated by D3 receptors in free-feeding rats and by D2 receptors in food-restricted rats (Baladi et al. 2010). In rats eating standard chow, D3 and D2 receptors mediate the ascending and descending limbs, respectively, of the agonist-induced yawning dose-response curve (Baladi et al. 2010; Collins et al. 2005) and food restriction eliminates yawning by increasing sensitivity at D2 receptors (i.e., quinpirole-induced yawning is restored in the presence of a D2 receptor-selective antagonist [Collins et al. 2008]). It is not clear whether increased sensitivity to agonist-induced yawning in rats eating high fat chow reflects increased sensitivity at D3 and/or D2 receptors. Importantly, dopamine agonist-induced yawning and hypothermia are mediated by dopamine receptor subtypes (i.e., D3 and D2) that are known to be involved in the therapeutic and abuse-related effects of drugs acting on dopamine systems. This study examined the impact of body weight and chow type on quinpirole-induced yawning and hypothermia. Rats ate standard or high fat chow such that body weight increased (Experiment 1), decreased (Experiment 2), or was maintained (Experiment 3). Experiment 4 tested whether D3 and D2 receptors mediate the ascending and descending limbs of the quinpirole dose-response curve, respectively, in rats eating high fat chow. Experiment 5 compared insulin sensitivity in rats eating standard or high fat chow.
Materials and Methods
Subjects
Eighty-two male Sprague–Dawley rats (Harlan, Indianapolis, IN), weighing 250–300 g upon arrival, were housed individually in an environmentally controlled room (24 ± 1 °C, 50 ± 10% relative humidity) under a 12/12 h light/dark cycle (light period 0700–1900 hrs) with free access to water. Fifty-six rats (seven groups of 8 rats each; Experiments 1, 2, and 3) were used to examine the impact of body weight and chow type on sensitivity to quinpirole (Table 1). Eighteen rats (three groups of 6 rats each; Experiment 4) were used to study antagonism of quinpirole-induced yawning in rats eating high fat chow (Table 1). Eight rats (two groups of 4 rats each; Experiment 5) were used to examine the effect of eating high fat chow on insulin. Animals were maintained and experiments were conducted in accordance with the Institutional Animal Care and Use Committee, the University of Texas Health Science Center at San Antonio, and with the 1996 Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, the National Research Council, and the National Academy of Sciences).
Table 1.
Experimental design: groups and feeding conditions
| Experiment | Group | Feeding Condition | ||
|---|---|---|---|---|
| Days 1–35 (Weeks 1–5) | Days 36–56 (Weeks 6–8) | |||
| 1 (Weight Increased) | 1 | free-feeding standard | → | free-feeding standard |
| 2 | free-feeding high fat | → | free-feeding high fat | |
| 3 | restricted high fat | → | free-feeding high fat | |
| 2 (Weight Decreased) | 4 | restricted standard | → | free-feeding standard |
| 5 | restricted high fat | → | free-feeding high fat | |
| 3 (Weight Maintained) | 6 | restricted standard | → | free-feeding standard |
| 7 | restricted high fat | → | free-feeding high fat | |
| Days 1–21 (Weeks 1–3) | ||||
| 4 (Weight Increased) | 8 | free-feeding high fat | ||
| 9 | free-feeding high fat | |||
| 10 | free-feeding high fat | |||
| Days 1–28 (Weeks 1–4) | ||||
| 5 (Weight Increased) | 11 | free-feeding standard | ||
| 12 | free-feeding high fat | |||
Feeding Conditions
Seven groups (n = 8/group) of rats had either free or restricted access (adjusted daily to increase, decrease, or maintain body weight, depending on the experiment; see Table 1) to standard or high fat chow (body weights were matched for separate groups of rats eating different chows). Animals maintained under restricted feeding conditions were fed daily at 1700 hrs. Feeding conditions were such that in Experiment 1 body weight increased in all three groups (Groups 1, 2, and 3), in Experiment 2 body weight decreased in both groups (Groups 4 and 5), and in Experiment 3 body weight was maintained in both groups (Groups 6 and 7). Behavioral tests occurred during an 8-week period when rats first were maintained for 5 weeks (35 days) on one of the feeding conditions shown in Table 1 followed by 3 weeks (21 days) of free access to the same chow (standard or high fat) they ate during the first 5 weeks of the study (Table 1). Three groups of rats (n = 6/group) had free access to high fat chow and were tested with quinpirole once per week for three weeks (Groups 8, 9, and 10; Experiment 4); different groups of rats received saline or a dopamine receptor antagonist prior to the last quinpirole test. Two additional groups of rats (n = 4/group) had free access to standard or high fat chow for 4 weeks (Groups 11 and 12; Experiment 5); insulin tolerance tests were conducted once per week for 4 weeks.
The nutritional content of the standard chow (Harlan Teklad 7912) was 5.7% fat and 19.9% protein (by weight), with a calculated gross energy content of 4.1 kcal/g. The high fat chow (Harlan Teklad 06414) contained 34.3% fat and 23.5% protein (by weight), with a calculated energy content of 5.1 kcal/g.
Yawning
Yawning was defined as an opening of the mouth such that the lower incisors were completely visible (Baladi and France 2009; Sevak et al. 2008). On the day of testing, rats were transferred from a clear plastic home cage to a test cage (the cages were identical; however, there was no food, water, or bedding present during testing) and allowed to habituate for 15 min. All experiments began with assessment of yawning after injection of vehicle. Initially in Experiments 1, 2 and 3, and while all rats had free access to standard laboratory chow, dose–response curves were generated for cumulative doses of quinpirole (0.0032, 0.01, 0.032, 0.1, 0.32 mg/kg i.p.) administered every 30 min. Beginning 20 min after each injection, the total number of yawns observed for 10 min was recorded. Rats were then randomly assigned to a feeding condition (n = 8/group; Groups 1–7) and cumulative dose-response curves for quinpirole-induced yawning were generated once per week for the next 8 weeks: 5 weeks (tests on days 7, 14, 21, 28, and 35) when the type and the amount of food varied across groups; and 3 weeks (tests on days 42, 49, and 56) when all rats had free access to one type of chow.
The ascending and descending limbs of the quinpirole yawning dose-response curve in rats eating standard chow are mediated by D3 and D2 receptors, respectively (e.g., Baladi et al. 2010); it is unclear whether the same receptors mediate the ascending and descending limbs of the quinpirole dose-response curve that is shifted leftward in rats eating high fat chow. Three separate group of rats (n = 6/group) had free access to high fat chow and were tested once per week for 3 weeks (Experiment 4). For the first two tests, all rats received only quinpirole in order to confirm a leftward shift in the quinpirole dose-response curve in rats eating high fat chow. For the third and final test, groups of rats were randomly assigned to receive quinpirole alone (Group 8) or quinpirole in combination with either the D2 dopamine receptor-selective antagonist L-741,626 (1.0 mg/kg s.c.; Group 9) or the D3 dopamine receptor-selective antagonist PG01037 (56.0 mg/kg s.c.; Group 10). In rats and after subcutaneous administration, these doses of L-741,626 and PG01037 have antagonist actions selectively at D2 and D3 dopamine receptors, respectively (Baladi et al. 2010; Collins et al. 2005). Antagonists were administered 30 min before administration of the first dose of quinpirole.
Body Temperature
Rectal body temperature was measured in a temperature controlled room (24 ± 1 °C and 50 ± 10% relative humidity) by inserting a lubricated thermal probe attached to a thermometer 3 cm into the rectum. Animals were adapted to the procedure by measuring body temperature on multiple occasions before assignment to feeding condition. During yawning experiments, body temperature was measured after completion of each 10 min observation period and prior to the next injection.
Insulin Tolerance
Insulin tolerance tests were performed once per week for 4 weeks (the same day and time [1600 hrs]) in 8 rats: 4 rats had free access to standard chow and 4 had free access to high fat chow throughout the 4-week period. A small sample of blood was collected from the tip of the tail then expressed on a blood glucose test trip. Glucose values were measured with a commercially available glucose meter (Accu-Chek Aviva; CVS). On each test occasion, glucose was measured prior to as well as 15, 30, 45, and 75 min after an i.p. injection of 0.75 U/kg insulin.
Data Analyses
Results are expressed as the average (± SEM) number of yawns during a 10-min observation period or average change in body temperature and plotted as a function of dose. Data are also presented as body weight in g and plotted as a function of day. For each group, differences between quinpirole dose–response curves for yawning (both the ascending and descending limbs) and hypothermia were analyzed by simultaneously fitting straight lines to the linear portion of the dose-response curves by means of GraphPad Prism (GraphPad Software Inc., San Diego, CA). The linear portion included doses that spanned the 50% level of effect, and included not more than one dose with greater than 75% effect and not more than one dose with less than 25% effect. Differences between the slopes and intercepts of the curves were analyzed with the F-ratio test (GraphPad Prism), as detailed elsewhere (Koek et al. 2006). The common slope values calculated by GraphPad Prism were used to constrain the fit of the parallel line assay. When slopes and intercepts of the yawning dose-response curves could not be analyzed, two-way, repeated-measures ANOVA with dose and feeding condition as factors was used to determine whether yawning in rats with restricted access to standard or high fat chow was significantly different from yawning in rats with free access to standard chow; post hoc multiple comparisons were made with the Bonferroni test (GraphPad Prism). ED50 values were calculated for individual rats using linear regression. To calculate ED50 values for quinpirole-induced hypothermia, a maximum effect was selected for individual rats. The 95% confidence limits (CL) were calculated from ED50 values averaged among rats within a group. For the antagonism study, differences between quinpirole dose-response curves in the presence and absence of antagonist were analyzed by line analysis methods described above.
Pilot studies (data not shown) indicated that maximal changes in blood glucose occurred approximately 45 min after i.p. administration of 0.75 U/kg insulin. Changes in glucose values after administration of insulin were used to quantify insulin sensitivity since the rate of recovery from insulin-induced hypoglycemia is thought to reflect hepatic glycogenolysis and not insulin sensitivity per se (Durham and Truett 2005). Data are expressed as the average (± SEM) change in glucose (mg/dL) from baseline (pre-insulin injection) levels as a function of week. A two-way ANOVA was used to assess whether glucose values before insulin injection were different from glucose values 45 min after insulin injection for rats with free access to standard chow (Group 11) and for rats with free access to high fat chow (Group 12).
Drugs
Quinpirole dihydrochloride and L-741,626 were purchased from Sigma-Aldrich (St. Louis, MO). PG01037 (N-{4-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-trans-but-2-enyl}-4-pyridine-2-yl benzamide HCl) was synthesized by Jianjing Cao (Medicinal Chemistry Section, National Institute on Drug Abuse, Baltimore, MD) using methods reported previously (Grundt et al., 2005). The vehicle for quinpirole dihydrochloride was sterile 0.9% saline. L-741,626 was dissolved in sterile 0.9% saline and 5% ethanol with 1 M HCl. PG01037 was dissolved in sterile 0.9% saline and 10% β-cyclodextrin. L-741,626 and PG01037 were administered s.c., typically in a volume of 1 ml/kg; quinpirole was administered i.p. in a volume of 1 ml/kg. Insulin (protamine zinc recombinant human insulin; Boehringer Ingelheim Vetmedica, Inc., St. Joseph, MO) was dissolved in sterile 0.9% saline and injected i.p.
Results
Experiment 1 (Groups 1, 2, and 3): Yawning; Weight Gain
After 35 days of free access to standard (Group 1) or high fat (Group 2) chow, body weight increased an average of 80 ± 3 (mean ± SEM) and 106 ± 7 g, respectively (Fig 1A, closed and open circles). Rats with restricted access to high fat chow for 35 days (Group 3; body weight matched to rats with free access to standard chow) gained an average of 79 ± 5 g (Fig 1A, open triangles). During the last 21 days of the study when all three groups of rats had free access to food, rats that continued eating standard chow (Group 1) gained an additional of 28 ± 2 g, rats that continued eating high fat chow (Group 2) gained 35 ± 3 g, and rats that previously had restricted access but then free access to high fat chow (Group 3) gained 40 ± 4 g (Fig 1A, closed circles, open circles, and closed triangles, respectively).
Fig. 1.
Body weight (panel A) and quinpirole-induced yawning (panels B–E) in three groups of rats over the 56-day (8-week) study: Group 1, free-feeding standard chow throughout the study; Group 2, free-feeding high fat chow throughout the study; and Group 3, restricted high fat chow (body weight matched to group 1) followed by free-feeding high fat chow (the vertical dashed line separates the two phases [feed conditions] of the study). Each symbol represents the mean (± SEM) of 8 rats. Abscissa: day of study (panel A) or dose in milligrams per kilogram of body weight; “V” = vehicle (panels B–E). Ordinates: mean (± SEM) body weight in grams (panel A) and mean (± SEM) number of yawns in 10 minutes (panels B–E).
Small doses of quinpirole increased and larger doses decreased yawning in all 3 groups of rats, resulting in an inverted U-shaped dose-response curve (Fig 1B). Repeated testing in rats with free access to standard chow yielded similar results over 8 once weekly quinpirole dose-response determinations (compare closed circles, Fig 1B–E). There was no significant change in sensitivity to quinpirole after just 7 days under these eating conditions (data not shown). After 14 days of free (Group 2) or restricted (Group 3) access to high fat chow, the ascending limb of the quinpirole dose-response curve was shifted up and to the left, compared with rats with free access to standard chow (compare open and closed symbols, Fig 1B). The linear portion corresponding to the ascending limb of the dose-response curves in rats with access to a high fat or standard chow could be fitted with a common slope but different x-intercepts, indicating that eating high fat chow shifted the curve significantly to the left. After 21 days of free access (data not shown) or after 28 days of restricted access to high fat chow (Fig 1C), the descending limb of the quinpirole dose-response curve was also shifted leftward, compared to rats with free access to standard chow. In addition, the linear portion corresponding to the descending limb of the dose-response curves in rats with access to a high fat or standard chow could be fitted with a common slope but different x-intercepts, indicating that eating high fat chow shifted the curve significantly to the left. The quinpirole dose-response curve (both limbs) remained shifted leftward, both in rats that continued eating high fat chow (Group 2) and in rats (Group 3) that previously had 35 days of restricted access to high fat chow followed by 21 days of free access to high fat chow (i.e., Days 42 and 56, Fig 1D and E, respectively).
Experiment 2 (Groups 4 and 5): Yawning; Weight Loss
After 14 days of restricted access to either standard (Group 4; 10 g/day) or high fat (Group 5; food adjusted daily to match body weight of Group 4) chow, the body weight of rats decreased an average of 62 ± 3 and 58 ± 3 g, respectively (open symbols, Fig 2A). For the next 21 days (Days 15–35), feeding was adjusted daily so that body weights were maintained (264–266 g in rats eating standard chow and 260–265 in rats eating high fat chow). Subsequently rats were given free access to their respective chows (i.e., the last 21 days of the study) and rats that previously had restricted access to standard chow (Group 4) gained an average of 109 ± 5 g while rats that previously had restricted access to high fat chow (Group 5) gained 135 ± 7 g (closed symbols, Fig 2A).
Fig. 2.
Body weight (panel A) and quinpirole-induced yawning (panels B–E) in two groups of rats over the 56-day (8-week) study: Group 4, restricted access to standard chow (10 g/day) followed by free-feeding standard chow; and Group 5, restricted access to high fat chow (weight matched to group 4) followed by free-feeding high fat chow. Data shown by shaded circles and dashed lines in panels B–E are the effects of quinpirole in rats with free access to standard chow throughout the study (Group 1, Fig 1). See Fig 1 for other details.
Restricted access to standard chow (Group 4) or high fat chow (Group 5) decreased quinpirole-induced yawning, compared to rats that had free access to standard chow throughout the study (Figs 2B and C). After 7 days of restricted access to food, quinpirole-induced yawning was decreased in both groups, but was decreased further in rats eating standard chow as compared to rats eating high fat chow (Fig 2B), despite similar body weight reductions in these two groups of rats (Fig 2A). After 14 days of food restriction (and continued body weight loss), quinpirole failed to produce any yawning in any rat in Groups 4 and 5 (Fig 2C); with continued food restriction and maintenance of the reduced body weight there was no yawning observed after administration of quinpirole (up to a dose of 0.32 mg/kg) on days 21, 28, and 35 (data not shown). Within 7 days of rats in Groups 4 and 5 having free access to the same chow they had eaten (i.e., under restricted conditions) earlier in the study (i.e., Day 42), quinpirole-induced yawning re-emerged and in Group 5 was similar, but not identical, to what was observed in rats free-feeding on a standard chow (Fig 2D). Whereas a dose of 0.1 mg/kg of quinpirole produced yawning in rats that had free access to standard chow throughout the study (Group 1), and in rats that had restricted followed by free access to standard chow (Group 4), this dose produced very little yawning in rats that had restricted followed by free access to high fat chow (Group 5), similar to what was observed in rats eating high fat chow in Experiment 1 (i.e., Groups 2 and 3, Fig 1E). The linear portion corresponding to the descending limb of the dose-response curves in rats with access to a high fat (Group 5) or standard chow (Group 1) could be fitted with a common slope but different x-intercepts and decreased effectiveness of this dose of quinpirole in rats eating high fat chow was still evident at the end of the study (Day 56; Fig 2E).
Experiment 3 (Groups 6 and 7): Yawning; Weight Maintained
For 35 days access to standard (Group 6) or high fat (Group 7) chow was adjusted daily so that body weight was maintained (on average, not more than 2 g change in body weight; Fig 3A). When given free access to their respective chows (i.e. the last 21 days of the study), rats that previously had restricted access to standard chow gained an average of 68 ± 4 g and rats that previously had restricted access to high fat chow gained an average of 97 ± 3 g (closed symbols, Fig 3A).
Fig. 3.
Body weight (panel A) and quinpirole-induced yawning (panels B–E) in two groups of rats over the 56-day (8-week) study: Group 6, restricted access to standard chow (weight maintained) followed by free-feeding standard chow; and Group 7, restricted access to high fat chow (weight maintained) followed by free-feeding high fat chow. Data shown by shaded circles and dashed lines in panels B–E are the effects of quinpirole in rats with free access to standard chow throughout the study (Group 1, Fig 1). See Fig 1 for other details.
After 14 days of restricted access (with body weight maintained) to standard chow (Group 6), the descending limb of the quinpirole dose-response curve shifted leftward, as compared to rats with free access to standard chow throughout the study (compare closed circles to open diamonds, Fig 3B). That is, line analysis of the descending limb of the dose-response curves (Groups 1 and 6) could be fitted with a common slope but different x-intercepts. After 14 days of restricted access (with body weight maintained) to high fat chow (Group 7), the ascending and descending limbs of the quinpirole dose-response curve shifted left (compare closed circles to open inverted triangles, Fig 3B). Line analysis of the ascending and descending limbs of the dose-response curves (Groups 1 and 7) could be fitted with a common slope but different x-intercepts. Continued food restriction while maintaining body weight (standard or high fat chow) further decreased quinpirole-induced yawning in both groups of rats (i.e., Fig 3C). When rats that previously had restricted access to standard (Group 6) or high fat (Group 7) chow were given free access to the same chow type, sensitivity to quinpirole-induced yawning recovered rapidly (i.e., within 7 days; Day 42, Fig 3D) and was not different from rats that were free-feeding on standard chow throughout the study (Fig 3D). Continued free access to standard chow (Group 6) did not further change sensitivity to quinpirole-induced yawning; however, continued free access to high fat chow (Group 7) shifted the ascending and descending limbs of the quinpirole dose-response curve to the left (compare circles and inverted triangles, Fig 3E) as indicated by common slopes but different x-intercepts.
Experiments 1, 2, and 3: Quinpirole-Induced Hypothermia
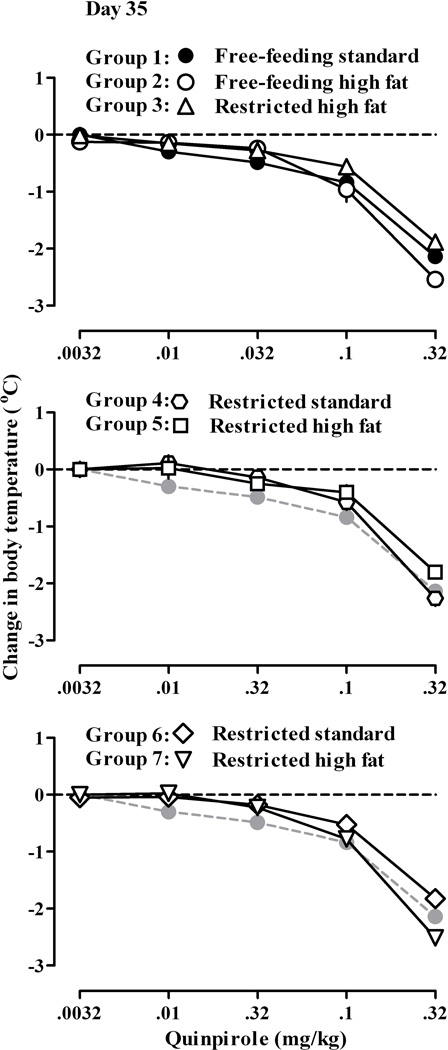
Quinpirole dose-dependently decreased body temperature in all tests in all rats in Groups 1–7 (Fig 4). For all tests, there was no significant difference in the potency of quinpirole to decrease body temperature between rats with free access to standard chow throughout the study (Group 1) and all other (2–7) groups (i.e. ED50 values and 95% CLs were not significantly different).
Fig. 4.
Quinpirole-induced hypothermia in seven groups (1–7) of rats at day 35 of the study. Data shown by closed circles in the upper panel are the same data shown by the gray circles in the middle and lower panels and show the effects of quinpirole in rats with free access to standard chow throughout the study (Group 1, Fig 1). Ordinate: mean (± SEM) change in body temperature in °C. See Fig 1 for other details
Experiment 4 (Groups 8, 9, and 10): Weight Gain (Antagonism of Quinpirole-Induced Yawning)
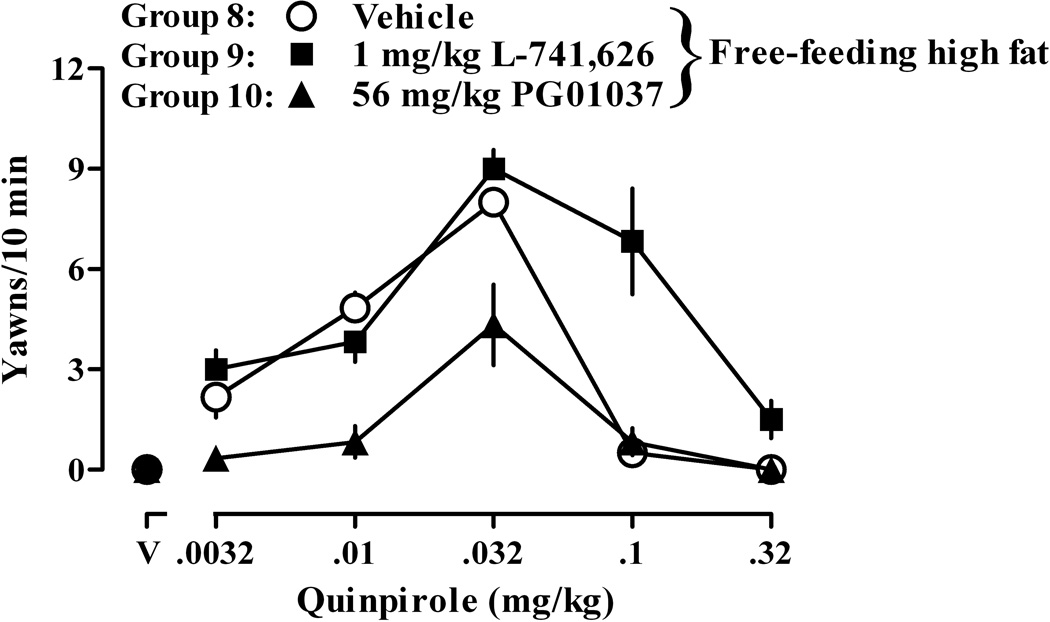
The average body weight of rats in Groups 8, 9 and 10 was not different at the beginning of this experiment: 330 ± 3, 328 ± 4, and 332 ± 3 g, respectively. After 21 days of free access to high fat chow, body weight increased an average of 62 ± 6, 62 ± 4, and 63 ± 7 g in Groups 8, 9, and 10, respectively (data not shown). In rats with free access to high fat chow, small doses of quinpirole increased, while larger doses decreased, yawning, resulting in an inverted U-shaped dose-response curve. In these 18 rats eating high fat chow, the quinpirole dose-response curve shifted progressively leftward over repeated weekly tests (data not shown), compared with rats eating standard chow, in a manner similar to what was observed in other rats eating high fat chow (i.e., Group 2). For example, the quinpirole dose-response curve determined after rats had eaten high fat chow for three weeks (Group 8) was similar to the dose-response curve determined for other rats eating high fat chow (Group 2) and was shifted leftward of the quinpirole dose-response curve determined in rats eating standard chow (Group 1; compare open circles in Fig 5 to open and closed circles in Fig 1, respectively). A dose of 1.0 mg/kg of L-741,626 shifted the descending, but not the ascending, limb of the yawning dose-response curve to the right (Fig 5; Group 9, closed squares). Conversely, a dose of 56.0 mg/kg of PG01037 shifted the ascending, but not the descending, limb of the yawning dose-response curve to the right (Fig 5; Group 10, closed triangles).
Fig. 5.
Quinpirole-induced yawning when quinpirole was administered alone (Group 8, open circles) and in combination with 1.0 mg/kg L-741,626 (Group 9, closed squares) or 56 mg/kg PG01037 (Group 10, closed triangles) in rats with free access to high fat chow. Each symbol represents the mean (± SEM) of 6 rats. See Fig 1 for other details.
Experiment 5 (Groups 11 and 12): Insulin Tolerance; Weight Gain
The average body weight of rats in Groups 11 and 12 was not different at the beginning of this experiment: 315 ± 4 and 314 ± 3 g, respectively. After 4 weeks of free access to either standard (Group 11) or high fat (Group 12) chow, body weight increased an average of 68 ± 8 and 91 ± 7 g, respectively. Basal glucose values were not significantly different across weeks for groups of rats eating the same chow or between groups of rats eating different chows (data not shown). For example, average (± SEM) basal glucose values after 1 week of free access to either standard or high fat chow were 99 ± 7 and 105 ± 3 mg/dL, respectively, and after 4 weeks were 103 ± 4 and 103 ± 3 mg/dL, respectively.
Blood glucose concentration was significantly decreased 45 min after the administration of insulin in rats eating standard chow (Group 11, open bars; Fig 6). In once weekly tests, insulin continued to significantly decrease glucose in rats eating standard chow. Similarly, blood glucose was significantly decreased in rats that had free access to high fat chow for one week (Group 12, closed bars; Fig 6); however, when rats had eaten high fat chow for 2 or more weeks, insulin failed to significantly alter blood glucose. Thus, rats were resistant to insulin-induced hypoglycemia after two weeks of eating high fat chow.
Fig. 6.
Change in blood glucose (mean ± SEM, mg/dL; ordinate) determined once per week for 4 weeks (abscissa) in rats with free access to standard (Group 11, open bars; n = 4) or high fat (Group 12, closed bars; n = 4) chow. * = significant difference (P<0.05) pre- versus post-insulin.
Discussion
Dopamine systems are altered in various diseases and they are the target of many drugs that are used clinically as well as drugs of abuse. Dopamine systems also mediate, at least in part, the reinforcing effects of many drugs and of food (Di Chiara and Imperato 1988; Wise and Rompre 1989). Moreover, it is clear that feeding conditions impact the activity of dopamine systems in brain as well as the effects of drugs acting on those systems. Because feeding conditions (i.e., amount and type of food) can vary markedly within and among individuals, it is possible that variations in food intake and nutritional status contribute to the development of psychopathologies (e.g., vulnerability to drug abuse) and impact the response of individuals to drugs. This study examined the impact of how much rats eat (increasing, decreasing, or maintaining body weight) and the type of chow they eat (standard or high fat) on the behavioral effects of the direct-acting dopamine receptor agonist quinpirole. While type of chow appeared to be the predominant factor in determining quinpirole effects under some conditions, under other conditions body weight appeared to be the predominant factor. For example, because different effects were obtained in rats of similar body weight and eating different types of chow (Groups 1 and 3), the type of chow appeared to be the important factor in determining response to quinpirole in Experiment 1 (weight gain). That is, rats with either free or restricted access to high fat chow were more sensitive to quinpirole (i.e., both the ascending [D3] and the descending [D2] limbs of the quinpirole dose-response curve shifted leftward) compared to rats with free access to standard chow. Thus, eating fat and not being fat (as compared with free-feeding standard chow controls) appeared to confer increased sensitivity to quinpirole in these rats. Furthermore, the ascending and descending limbs of the dose-response curve were shifted (back) to the right by antagonists with selectively for D3 or D2 receptors, respectively, demonstrating that these effects of quinpirole in rats eating high fat chow are mediated by the same receptors that mediate these effects in rats eating standard chow. On the other hand, the same effect (reduction or elimination of quinpirole-induced yawning) was eventually observed in rats of similar body weight and eating different types of chow (Groups 4 and 5 and Groups 6 and 7), suggesting that body weight was the predominant factor determining response to quinpirole in Experiments 2 (weight decrease) and 3 (weight maintenance).
It is well established that restricting access to food significantly alters brain neurochemistry and the behavioral effects of drugs (e.g., Sevak et al. 2008). It is also clear that allowing animals to eat certain foods (e.g., high fat) can affect brain neurochemistry and the behavioral effects of drugs (Baladi and France 2009, 2010; Rada et al. 2010). In this study, quinpirole-induced yawning varied significantly across eating conditions and across different periods of time. For example, after 21 (free-feeding) or 28 (restricted) days of eating high fat chow while gaining body weight (Experiment 1), the descending limb of the quinpirole dose-response curve was shifted leftward, reflecting increased sensitivity at dopamine D2 receptors. In contrast, the descending limb of the quinpirole dose-response curve was shifted leftward after just 7 or 14 days in rats eating high fat chow and losing (Experiment 2) or maintaining (Experiment 3) body weight, respectively. Thus, although qualitatively similar effects were observed across conditions (shift left in the descending limb of the dose-response curve), those effects occurred at different times among groups. Moreover, increased sensitivity to quinpirole-induced yawning was correlated with the emergence of insensitivity to insulin-induced hypoglycemia (Experiment 5). Upon resumption or continuation of free-feeding, the descending limb of the quinpirole dose-response curve remained shifted leftward in all rats eating high fat chow. In contrast, when rats that previously had restricted access to standard chow were allowed free access to standard chow, their sensitivity to quinpirole returned and was not different from rats that had only free access to standard chow throughout the study (compare Group 1 to Groups 4 and 6, Panel E, Figs 2 and 3), indicating the reversibility of food restriction (standard chow)-induced changes in sensitivity to quinpirole.
In rats eating high fat chow and gaining weight, the shift leftward in the ascending limb of the dose-response curve was evident after 14 days and persisted until the end of the study. A similar shift leftward in the ascending limb (D3) of the dose-response curve was evident after 14 days in rats eating high fat chow and maintaining body weight, although that shift was no longer evident 7 days later, presumably because of an even greater increase in sensitivity at D2 receptors that masked any expression of yawning. In the same rats (i.e., eating high fat chow with body weight maintained, Group 7), normal sensitivity to quinpirole was apparent after just 7 days of free access to high fat chow (Day 42, panel D, Fig 3). With continued free access to high fat chow and with further body weight gain, the ascending limb of the quinpirole dose-response curve shifted leftward and was not different from what was observed in rats that had free access to high fat chow throughout the study (compare Groups 2 and 7). In rats losing weight, eating high fat chow also appeared to delay, but not prevent, the elimination of quinpirole-induced yawning, perhaps reflecting increased sensitivity at D3 receptors that eventually was overcome by a greater increase at D2 receptors. Collectively, two major trends are apparent from these data: 1) eating high fat food increases sensitivity to D3 (leftward shift in the ascending limb) and D2 (leftward shift in the descending limb) receptor-mediated effects of quinpirole; and 2) regardless of chow type, food restriction (so that body weight does not increase) increases sensitivity to D2 receptor-mediated effects of quinpirole and, eventually, suppresses quinpirole-induced yawning.
Sensitivity to the behavioral effects of drugs acting on dopamine systems sometimes increases after repeated intermittent drug administration (i.e., sensitization) and feeding conditions can impact those increases in sensitivity. For example, eating high fat chow accelerates the rate at which sensitivity increases to the locomotor-stimulating effects of the indirect-acting dopamine receptor agonist methamphetamine in rats (e.g., McGuire et al. 2011). Although sensitization can also develop to the locomotor-stimulating effects of quinpirole (Lomanowska et al. 2004; Szechtman et al. 1994), there was no evidence in the current study for sensitization to quinpirole-induced yawning. The quinpirole yawning dose-response curve in rats with free access to standard chow was very consistent across many weeks of once per week quinpirole testing. It also appears unlikely that feeding conditions (e.g., eating high fat chow) selectively enhance the development of sensitization to quinpirole since rats that eat high fat chow and are tested just once with quinpirole (Baladi, unpublished data) show the same changes in sensitivity that were observed in the current study in rats that were tested weekly with quinpirole.
Feeding conditions (e.g., food restriction) that significantly altered the descending limb of the quinpirole yawning dose-response curve (mediated by D2 receptors) had no effect on quinpirole-induced hypothermia, which also is thought to be mediated by D2 receptors (Baladi et al. 2010; Chaperon et al. 2003; Collins et al. 2007; Nunes et al. 1991). That quinpirole-induced hypothermia did not vary across feeding conditions suggests that changes in quinpirole-induced yawning were not due to pharmacokinetic factors and, possibly, that the receptors mediating the hypothermic effects of quinpirole are not the same receptors mediating yawning (i.e., D2 and D1, Chaperon et al. 2003). Both yawning and hypothermia are thought to be centrally mediated (Dourish and Hutson, 1985; Nunes et al. 1991), although different populations of dopamine receptors might mediate these effects (the anterior hypothalamus/preoptic area for quinpirole-induced hypothermia [Lin et al. 1982] and the paraventricular nucleus of the hypothalamus for quinpirole-induced yawning [Argiolas and Melis, 1998]). Moreover, other (non-dopamine) mechanisms might also contribute to the regulation of body temperature in a manner that attenuates nutrition-related changes in dopamine receptor sensitivity that might otherwise be expected to impact body temperature (Jinka et al. 2010). While the current study examined changes in a behavioral effect (i.e., yawning) that is thought to be mediated by dopamine receptors in the paraventricular nucleus of the hypothalamus (Argiolas and Melis, 1998), it is likely that dopamine receptors in some other brain regions are also impacted by feeding conditions. For example, food restriction increases sensitivity to the locomotor stimulating effects of direct-acting dopamine receptor agonists, including quinpirole (Carr et al. 2001; Collins et al. 2008), possibly indicating changes in receptor sensitivity in the mesolimbic dopamine pathway (Ouagazzal and Creese, 2000).
The mechanism(s) mediating changes in the behavioral effects of quinpirole across eating conditions are not known and might include changes in dopamine content or turnover, receptor number or function. It is well established that different feeding conditions (amount and type of food) can significantly modify circulating concentrations of hormones such as insulin, leptin, and ghrelin that are known to have effects on dopamine systems (Figlewicz et al. 1998; Palmiter, 2007; Patterson et al. 1998). These hormones can activate specific receptors on dopamine neurons and either inhibit (insulin and leptin) or stimulate (ghrelin) dopamine signaling. Food restriction decreases (Kinzig et al. 2009; Carr, 1996) while eating high fat chow increases (Shiraev et al. 2009) circulating insulin. Moreover, plasma concentrations of insulin and leptin are increased similarly in rats with free or restricted (body weight matched to rats with free access to high fat chow) access to high fat chow (Shiraev et al. 2009), consistent with the similar behavioral effects observed in the current study between rats with free or restricted access to high fat chow (Experiment 1, Fig 1). Whether hormonal changes contribute to altered behavioral effects of drugs acting on dopamine systems is yet to be determined, although a growing body of literature obtained in diabetic animals and in animals eating different amounts and types of chow strongly implicates insulin and leptin as playing major roles in drug effects that are mediated by dopamine systems (e.g., reinforcing effects; Davis et al. 2010). For example, in the current study eating high fat chow changed the behavioral effects of quinpirole and, in parallel, induced insulin resistance, supporting the view that insulin-signaling pathways play a role in diet-induced changes in drug response.
Finally, these data indicate that response to drugs (therapeutic and recreational) might be profoundly impacted by eating conditions, including body weight and type of food, and that changes in drug sensitivity might be long lasting. Thus, modest weight loss or weight gain might contribute to individual differences in response to drugs. Moreover, the type of food eaten might impact brain neurochemistry resulting in an altered response to drugs. While the current obesity epidemic (more than one-third of adults in the US were obese in 2007–2008; Flegal et al., 2010) is a pressing public health problem that is relevant to the current study, data in rats indicate that being overweight per se might not be the most important factor affecting response to drugs. Rather, it appears to be the consumption of fat and, perhaps, resulting hormonal changes, that markedly alter dopamine systems. Understanding the relationship between feeding conditions and drug response could facilitate our understanding of individual differences in response to therapeutic drugs and in vulnerability to drug abuse.
Acknowledgements
CPF is supported by a Senior Scientist Award (KO5 DA17918).
Footnotes
The authors have no conflict of interest.
Contributor Information
Michelle G Baladi, Department of Pharmacology, University of Texas Health Science Center at San Antonio, San Antonio, Texas, USA.
Amy H Newman, Medicinal Chemistry Section, National Institute on Drug Abuse, Intramural Research Program, National Institutes of Health, Baltimore, Maryland, USA.
Charles P France, Department of Pharmacology, University of Texas Health Science Center at San Antonio, San Antonio, Texas, USA; Department of Psychiatry, University of Texas Health Science Center at San Antonio, San Antonio, Texas, USA.
References
- Abi-Dargham A, Laruelle M. Mechanisms of action of second generation antipsychotic drugs in schizophrenia: insights from brain imaging studies. Eur Psychiatry. 2005;20:15–27. doi: 10.1016/j.eurpsy.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Acri JB, Carter SR, Alling K, Geter-Douglass B, Dijkstra D, Wikström H, Katz JL, Witkin JM. Assessment of cocaine-like discriminative stimulus effects of dopamine D3 receptor ligands. Eur J Pharmacol. 1995;281:R7–R9. doi: 10.1016/0014-2999(95)00411-d. [DOI] [PubMed] [Google Scholar]
- Argiolas A, Melis MR. The neuropharmacology of yawning. Eur J Pharmacol. 1998;343:1–16. doi: 10.1016/s0014-2999(97)01538-0. [DOI] [PubMed] [Google Scholar]
- Bailer UF, Frank GK, Henry SE, Price JC, Meltzer CC, Weissfeld L, Mathis CA, Drevets WC, Wagner A, Hoge J, Ziolko SK, McConaha CW, Kaye WH. Altered brain serotonin 5-HT1A receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [carbonyl11C]WAY-100635. Arch Gen Psychiatry. 2005;62:1032–1041. doi: 10.1001/archpsyc.62.9.1032. [DOI] [PubMed] [Google Scholar]
- Baladi MG, Newman AH, France CP. Dopamine D3 receptors mediate the discriminative stimulus effects of quinpirole in free-feeding rats. J Pharmacol Exp Ther. 2010;332:308–315. doi: 10.1124/jpet.109.158394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baladi MG, France CP. Eating high-fat chow increases the sensitivity of rats to quinpirole-induced discriminative stimulus effects and yawning. Behav Pharmacol. 2010;21:615–620. doi: 10.1097/FBP.0b013e32833e7e5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baladi MG, France CP. High fat diet and food restriction differentially modify the behavioral effects of quinpirole and raclopride in rats. Eur J Pharmacol. 2009;610:55–60. doi: 10.1016/j.ejphar.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Koob GF. Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Science. 1993;260:1814–1816. doi: 10.1126/science.8099761. [DOI] [PubMed] [Google Scholar]
- Carr KD. Feeding, drug abuse, and the sensitization of reward by metabolic need. Neurochem Res. 1996;21:1455–1467. doi: 10.1007/BF02532386. [DOI] [PubMed] [Google Scholar]
- Carr KD, Kim GY, Cabeza de Vaca S. Rewarding and locomotor-activating effects of direct dopamine receptor agonists are augmented by chronic food restriction in rats. Psychopharmacology. 2001;154:420–428. doi: 10.1007/s002130000674. [DOI] [PubMed] [Google Scholar]
- Chaperon F, Tricklebank MD, Unger L, Neijt HC. Evidence for regulation of body temperature in rats by dopamine D2 receptor and possible influence of D1 but not D3 and D4 receptors. Neuropharmacology. 2003;44:1047–1053. doi: 10.1016/s0028-3908(03)00113-8. [DOI] [PubMed] [Google Scholar]
- Collins GT, Calinski DM, Newman AH, Grundt P, Woods JH. Food restriction alters N’-propyl-4,5,6,7-tetrahydrobenzothiazole-2,6-diamine dihydrochloride (pramipexole)-induced yawning, hypothermia, and locomotor activity in rats: evidence for sensitization of dopamine D2 receptor-mediated effects. J Pharmacol Exp Ther. 2008;325:691–697. doi: 10.1124/jpet.107.133181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Woods JH. Drug and reinforcement history as determinants of the response-maintaining effects of quinpirole in the rat. J Pharmacol Exp Ther. 2007;323:599–605. doi: 10.1124/jpet.107.123042. [DOI] [PubMed] [Google Scholar]
- Collins GT, Newman AH, Grundt P, Rice KC, Husbands SM, Chauvignac C, Chen J, Wang S, Woods JH. Yawning and hypothermia in rats: effects of dopamine D3 and D2 agonists and antagonists. Psychopharmacology (Berl) 2007;193:159–170. doi: 10.1007/s00213-007-0766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Witkin JM, Newman AH, Svensson KA, Grundt P, Cao J, Woods JH. Dopamine agonist-induced yawning in rats: a dopamine D3 receptor-mediated behavior. J Pharmacol Exp Ther. 2005;314:310–319. doi: 10.1124/jpet.105.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JF, Choi DL, Benoit SC. Insulin, leptin and reward. Trends Endocrinol Metab. 2010;21:68–74. doi: 10.1016/j.tem.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Nal Acad Sci USA. 1988;85:5274–5280. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dourish CT, Hutson PH. Bilateral lesions of the striatum induced with 6-hydroxydopamine abolish apomorphine-induced yawning in rats. Neuropharmacology. 1985;24:1051–1055. doi: 10.1016/0028-3908(85)90190-x. [DOI] [PubMed] [Google Scholar]
- Durham HA, Truett GE. Development of insulin resistance and hyperphagia in Zucker fatty rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R652–R658. doi: 10.1152/ajpregu.00428.2004. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Patterson TA, Johnson LB, Zavosh A, Israel PA, Szot P. Dopamine transporter mRNA is increased in the CNS of Zucker fatty (fa/fa) rats. Brain Res Bull. 1998;46:199–202. doi: 10.1016/s0361-9230(98)00009-4. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;20:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Foley P, Gerlach M, Double KL, Riederer P. Dopamine receptor agonists in the therapy of Parkinson’s diseases. J Neural Transm. 2004;111:1375–1446. doi: 10.1007/s00702-003-0059-x. [DOI] [PubMed] [Google Scholar]
- France CP, Li JX, Owens WA, Koek W, Toney GM, Daws LC. Reduced effectiveness of escitalopram in the forced swimming test is associated with increased serotonin clearance rate in food-restricted rats. Int. J. Neuropsychopharmacol. 2009;12:731–736. doi: 10.1017/S1461145709000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundt P, Carlson EE, Cao J, Bennett CJ, McElveen E, Taylor M, Luedtke RR, Newman AH. Novel heterocyclic trans olefin analogues of N-{4-[4-(2,3 dichlorophenyl)piperazin-1-yl]butyl}arylcarboxamides as selective probes with high affinity for the dopamine D3 receptor. J Med Chem. 2005;48:839–848. doi: 10.1021/jm049465g. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG. Feeding and hypothalamic stimulation increase dopamine turnover in the accumbens. Physiol Behav. 1988;44:599–606. doi: 10.1016/0031-9384(88)90324-1. [DOI] [PubMed] [Google Scholar]
- Jinka TR, Carlson ZA, Moore JT, Drew KL. Altered thermoregulation via sensitization of A1 adenosine receptors in dietary-restricted rats. Psychopharmacology. 2010;209:217–224. doi: 10.1007/s00213-010-1778-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye W, Gendall K, Strober M. Serotonin neuronal function and selective serotonin reuptake inhibitor treatment in anorexia and bulimia nervosa. Biol Psychiatry. 1998;44:825–838. doi: 10.1016/s0006-3223(98)00195-4. [DOI] [PubMed] [Google Scholar]
- Khan A, Schwartz KA, Kolts RL, Brown WA. BMI, sex, and antidepressant response. J Affect Disord. 2007;99:101–106. doi: 10.1016/j.jad.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Kinzig KP, Hargrave SL, Tao EE. Central and peripheral effects of chronic food restriction and weight restoration in the rat. Am J Physiol Endocrinol Metab. 2009;296:E282–E290. doi: 10.1152/ajpendo.90523.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koek W, Carter LP, Wu H, Coop A, France CP. Discriminative stimulus effects of flumazenil: perceptual masking by baclofen, and lack of substitution with gamma-hydroxybutyrate and its precursors 1,4-butanediol and gamma-butyrolactone. Behav Pharmacol. 2006;17:239–247. doi: 10.1097/00008877-200605000-00005. [DOI] [PubMed] [Google Scholar]
- Kostrzewa RM, Brus R, Rykaczewska M, Plech A. Low-dose quinpirole ontogenically sensitizes to quinpirole-induced yawning in rats. Pharmacol Biochem Behav. 1992;44:487–489. doi: 10.1016/0091-3057(93)90496-g. [DOI] [PubMed] [Google Scholar]
- Li J-X, Koek W, France CP. Food restriction and streptozotocin differentially modify sensitivity to the hypothermic effects of direct- and indirect-acting serotonin receptor agonists in rats. Eur J Pharmacol. 2009;613:60–63. doi: 10.1016/j.ejphar.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Chandra A, Tsay BL, Chern YF. Hypothalamic and striatal dopamine receptor activation inhibits heat production in the rat. Am J Physiol. 1982;242:471–481. doi: 10.1152/ajpregu.1982.242.5.R471. [DOI] [PubMed] [Google Scholar]
- Lomanowska A, Gormley S, Szechtman H. Presynaptic stimulation and development of locomotor sensitization to the dopamine agonist quinpirole. Pharmacol Biochem Behav. 2004;77:617–622. doi: 10.1016/j.pbb.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Nader MA, Mach RH. Self-administration of the dopamine D3 agonist 7-OH-DPAT in rhesus monkeys is modified by prior cocaine exposure. Psychopharmacology (Berl) 1996;125:13–22. doi: 10.1007/BF02247388. [DOI] [PubMed] [Google Scholar]
- Naef L, Srivastava L, Gratton A, Hendrickson H, Owens SM, Walker CD. Maternal high fat diet during the perinatal period alters mesocorticolimbic dopamine in the adult rat offspring: reduction in the behavioral responses to repeated amphetamine administration. Psychopharmacology. 2008;197:83–94. doi: 10.1007/s00213-007-1008-4. [DOI] [PubMed] [Google Scholar]
- Nunes JL, Sharif NA, Michel AD, Whiting RL. Dopamine D2-receptors mediate hypothermia in mice: ICV and IP effects of agonists and antagonists. Neurochem Res. 1991;16:1167–1174. doi: 10.1007/BF00966597. [DOI] [PubMed] [Google Scholar]
- Ouagazzal AM, Creese I. Intra-accumbens infusion of D(3) receptor agonists reduces spontaneous and dopamine-induced locomotion. Pharmacol Biochem Behav. 2000;67:637–645. doi: 10.1016/s0091-3057(00)00406-8. [DOI] [PubMed] [Google Scholar]
- Palmiter RD. Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. 2007;30:375–381. doi: 10.1016/j.tins.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Patterson TA, Brot MD, Zavosh A, Schenk JO, Szot P, Figlewicz DP. Food deprivation decreases mRNA and activity of the rat dopamine transporter. Neuroendocrinology. 1998;68:11–20. doi: 10.1159/000054345. [DOI] [PubMed] [Google Scholar]
- Rada P, Bocarsly ME, Barson JR, Hoebel BG, Leibowitz SF. Reduced accumbens dopamine in Sprague-Dawley rats prone to overeating a far-rich diet. Physiol Behav. 2010;101:394–400. doi: 10.1016/j.physbeh.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szechtman H, Dai H, Mustafa S, Einat H, Sullivan RM. Effects of dose and interdose interval on locomotor sensitization to the dopamine agonist quinpirole. Pharmacol Biochem Behav. 1994;48:921–928. doi: 10.1016/0091-3057(94)90201-1. [DOI] [PubMed] [Google Scholar]
- Sevak RJ, Koek W, Galli A, France CP. Insulin replacement restores the behavioral effects of quinpirole and raclopride in streptozotocin-treated rats. J Pharmacol Exp Ther. 2007;320:1216–1223. doi: 10.1124/jpet.106.115600. [DOI] [PubMed] [Google Scholar]
- Sevak RJ, Koek W, Owens WA, Galli A, Daws LC, France CP. Feeding conditions differentially affect the neurochemical and behavioral effects of dopaminergic drugs in male rats. Eur J Pharmacol. 2008;592:109–115. doi: 10.1016/j.ejphar.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraev T, Chen H, Morris MJ. Differential effects of restricted versus unlimited high-fat feeding in rats on fat mass, plasma hormones and brain appetite regulators. J Neuroendocrinol. 2009;21:602–609. doi: 10.1111/j.1365-2826.2009.01877.x. [DOI] [PubMed] [Google Scholar]
- Sinnott RS, Mach RH, Nader MA. Dopamine D2/D3 receptors modulate cocaine’s reinforcing and discriminative stimulus effects in rhesus monkeys. Drug Alcohol Depend. 1999;54:97–110. doi: 10.1016/s0376-8716(98)00162-8. [DOI] [PubMed] [Google Scholar]
- Spealman RD. Dopamine D3 receptor agonists partially reproduce the discriminative stimulus effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther. 1996;278:1128–1137. [PubMed] [Google Scholar]
- Uitti RJ, Ahlskog KE. Comparative review of dopamine receptor agonists in Parkinson’s disease. CNS Drugs. 1996;5:369–388. doi: 10.2165/00023210-199605050-00006. [DOI] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]