Abstract
Objective
To evaluate symptom control and tolerability after abrupt conversion from oral extended-release methylphenidate (ER-MPH) to methylphenidate transdermal system (MTS) via a dose-transition schedule in children with attention-deficit/hyperactivity disorder (ADHD).
Methods
In a 4-week, prospective, multisite, open-label study, 171 children (164 intent-to-treat) with diagnosed ADHD aged 6–12 years abruptly switched from a stable dose of oral ER-MPH to MTS in nominal dosages of 10, 15, 20, and 30 mg using a predefined dose-transition schedule. After the first week on the scheduled dose, the dose was titrated to optimal effect. The primary effectiveness outcome was the change from baseline (while taking ER-MPH) to week 4 in ADHD-Rating Scale-IV (ADHD-RS-IV) total scores. Adverse events (AEs) were assessed throughout the study.
Results
Most subjects (58%) remained on the initial MTS dose defined by the dose-transition schedule; 38% increased and 4% decreased their MTS dose for optimization. MTS dose optimization resulted in significantly better ADHD-RS-IV total (mean ± SD) scores at week 4 than at baseline (9.9±7.47 vs 14.1±7.48; p<0.0001). The most commonly reported AEs included headache, decreased appetite, insomnia, and upper abdominal pain. Four subjects (2.3%) discontinued because of application site reactions and 3 discontinued because of other AEs.
Conclusions
Abrupt conversion from a stable dose of oral ER-MPH to MTS was accomplished using a predefined dose-transition schedule without loss of symptom control; however, careful titration to optimal dose is recommended. Most AEs were mild to moderate and, with the exception of application site reactions, were similar to AEs typically observed with oral MPH. Limitations of this study included its open-label sequential design without placebo, which could result in spurious attribution of improvement to the study treatment and precluded superiority determinations of MTS over baseline ER-MPH treatment. The apparent superiority of MTS was likely due to more careful titration and clinical monitoring rather than the product itself.
NCT
Key Terms: Methylphenidate Transdermal System, Extended-Release Methylphenidate, Attention-Deficit/Hyperactivity Disorder, Stimulants
INTRODUCTION
Attention-deficit/hyperactivity disorder (ADHD) is the most prevalent psychiatric disorder of childhood, affecting an estimated 5% to 8% of school-aged children1–3, and it may extend into adolescence and adulthood4. Individuals with ADHD are at greater risk for comorbid psychiatric problems including mood, anxiety, and substance abuse disorders1,4,5; social and academic impairments; underachievement; difficult interpersonal relationships; and poor quality of life6–9. These adverse effects of ADHD on patients and families make it a public health concern and affirm the need for effective treatment10.
Stimulants are considered the first-line agents for treatment of children with ADHD11,12. The positive effects of methylphenidate (MPH) on behavior and academic productivity are well established13,14. However, one of the most difficult aspects of managing ADHD in children is timing of the stimulant therapy15. Immediate-release formulations of MPH have a relatively short duration of action (3 to 6 hours) and multiple doses are required throughout the day to maintain symptom improvement12,16,17. Extended-release MPH (ER-MPH) formulations provide a longer duration of symptom improvement, require less frequent administration, and may lead to increased compliance, reduced social stigma from school dosing, and decreased misuse of controlled substances17.
In addition to oral ER-MPH formulations, a MPH transdermal system (MTS) patch dosage form (Daytrana*) is approved by the US Food and Drug Administration as part of a comprehensive treatment program for ADHD for children ages 6–12 years18. MTS uses DOT Matrix† technology to create a diffusion-based patch that delivers MPH through the skin for as long as the patch is worn. Efficacy and safety of MTS have been demonstrated in several clinical trials19–24. Within the clinical practice setting, the potential benefits of MTS, including convenience of once-daily administration, reduced fluctuation in blood concentration, visual compliance confirmation, elimination of need to swallow, and control over the duration of effect25 have motivated some patients to switch from ER-MPH to MTS. To the authors’ knowledge, there are no data regarding maintenance of symptom control and tolerability when switching from ER-MPH to MTS. Thus, data on such switching in ADHD therapy may enlighten clinical practice with respect to switching patients from oral MPH therapy to MTS. Furthermore, in clinical practice, the switch is likely to be made abruptly as the most practical procedure because of the short half-life of the drug.
In this study, ADHD symptom control and tolerability were evaluated in subjects after they were abruptly switched from stable doses of ER-MPH to MTS using a predefined dose-transition schedule. However, because of differences in bioavailability between products, MTS dose should be titrated based on individual response when patients are switched from oral ER-MPH formulations18.
METHODS
Subjects
Children aged 6–12 years, previously diagnosed with ADHD (any subtype) by Diagnostic and Statistical Manual of Mental Disorders criteria (DSM-IV-TR)1 were eligible to participate in the study. Subjects were required to be on a stable dose of an oral ER-MPH (Ritalin LA, Novartis AG, Basel, Switzerland; Concerta, Alza Corporation, Palo Alto, CA USA; or Metadate CD, UCB Inc., Atlanta, GA, USA) not to exceed 54 mg/day for at least 30 days prior to screening. At baseline, subjects were required to have a total score of ≤1.5 standard deviations (SDs) from age-appropriate norms on the ADHD Rating Scale-IV (ADHD-RS-IV)26 to provide a reasonable presumption that the patients had been benefiting from the oral MPH as generally, 1.5 SDs on this scale is considered a degree of severity warranting treatment. At baseline, subjects were required to have normal laboratory parameters, vital signs, electrocardiogram (ECG), and a body mass index (BMI) not exceeding the 90th percentile.
Children were excluded from study enrollment if they had any comorbid psychiatric diagnosis (with the exception of oppositional defiant disorder), a history of intellectual disability, or any concurrent illness or skin disorder that might compromise tolerability or study assessments. Subjects could not have taken clonidine, atomoxetine, antidepressants, antihypertensives, medications with central nervous system effects, sedatives, antipsychotics, anxiolytics, anticonvulsants, or other investigational medications within 30 days prior to screening.
Study Design
This was a prospective, open-label, multisite study of MTS designed to evaluate the tolerability and relative effectiveness of MTS in children previously treated for ADHD with oral ER-MPH. In addition, compliance, quality of life, and medication satisfaction were measured, but results have not been reported herein. Subjects were recruited from 18 sites located in the United States, and IRB approval was attained for each study site. The study was conducted between June 21, 2005 and February 6, 2006.
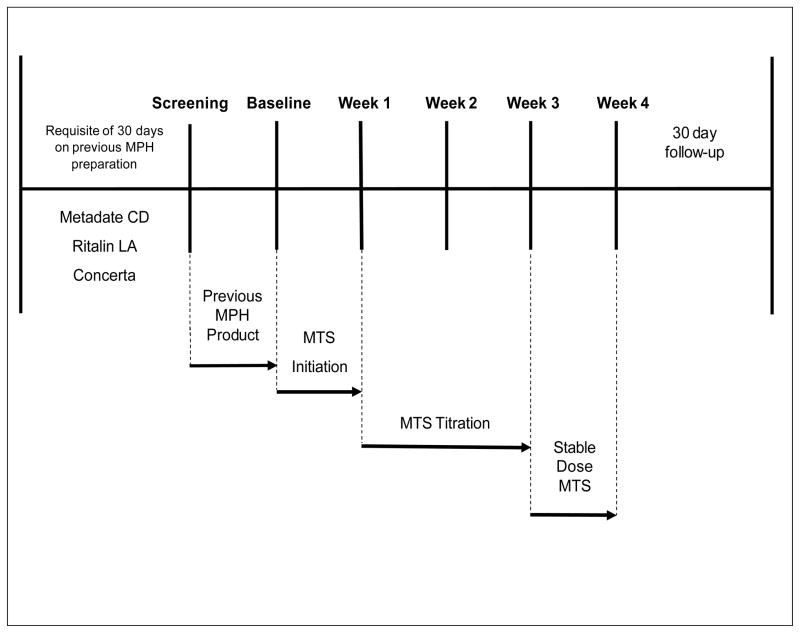
Subjects entered the screening visit, conducted by the study investigator, on their existing dose of oral ER-MPH and continued that medication until the baseline visit, 7 (±2) days later (Figure 1) at which time they were abruptly converted to MTS using a predefined dose-transition schedule (Table 1) based on their previous daily dose of oral ER-MPH. Subsequent study visits were scheduled weekly, 7 (±3) days apart. Patches were applied to alternating hips once daily in the morning and worn for up to 9 hours each day. Subjects remained on their initial MTS transition dose for 1 week and then entered a 2-week dose-adjustment period.
Figure 1.
Abrupt conversion study design. Week 1 was the initial effectiveness evaluation point, which assessed the accuracy and feasibility of the dose-transition schedule. Week 4 was the primary effectiveness evaluation point, the last measured change in ADHD-Rating Scale-IV total score from baseline. MPH = methylphenidate, MTS = methylphenidate transdermal system.
Table 1.
MTS dose-transition schedule
| Previous dose, mg/d
|
Converted MTS dose, mg/9-hour wear time | ||
|---|---|---|---|
| Concerta | Ritalin LA | Metadate CD | |
| 18 | 10 or 20 | 10 or 20 | 10 |
| 27 | 30 | 30 | 15 |
| 36 | 40 | 40 | 20 |
| 54 | 50 | 50 | 30 |
Table formulated by analyzing data provided in package inserts for the following:
Concerta, Alza Corporation, Palo Alto, CA USA; Ritalin LA, Novartis AG, Basel, Switzerland; or Metadate CD, UCB Inc., Atlanta, GA, USA
MTS = methylphenidate transdermal system
Titration to a higher dose or tapering to a lower dose of MTS was permitted based on tolerability and scores on the Clinical Global Impression-Severity (CGI-S) scale27. For titration purposes, response to MTS was categorized by the investigator into 1 of 4 conditions and associated actions: “Intolerable” (unacceptable tolerability profile); “Ineffective” (if the subject’s CGI-S score had become worse compared with baseline, or the subject had a CGI-S score of 4 [moderately ill] or worse); “Acceptable” (similar ADHD symptom control compared with the baseline ADHD-RS-IV score, with minimal side effects and CGI-S score of 3 [mildly ill] or better); or “Optimal” indicating superior ADHD symptom control (CGI-S score of 2 [borderline mentally ill] or 1 [not ill at all, normal]). “Intolerable” responses required the subject to be tapered to a lower dose, if available. “Ineffective” responses required increasing the subject’s MTS dose to the next available strength if tolerability permitted. “Acceptable” responses permitted the subject to be maintained on his or her current dose for the remainder of the dose optimization phase or, if in the investigator’s opinion there was potential for further symptom reduction, to try the next higher dose.
No further titration was permitted after the final dose-adjustment visit at the end of week 3, and subjects were maintained on their dose of MTS through the final week. Compliance with the treatment regimen was measured by patch counts at weekly study visits. Subjects were followed up for 30±3 days after the last dose of study medication to assess any new or ongoing adverse events (AEs).
All parents or other legal guardians (hereinafter referred to as “parents”) signed informed consent using documents and procedures approved by the appropriate institutional review board at each site. This trial was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation’s Good Clinical Practice Guidelines.
Statistical Methods
Assuming a 30% dropout rate, 175 subjects were needed to be enrolled to assure a final sample size of 122 study completers. Subjects who received at least 1 MTS patch application and who had at least 1 ADHD-RS-IV measurement on or after week 1 visit were included in the intent-to-treat (ITT) population. Study endpoint was considered the subject’s last visit, regardless of whether all 28 days of scheduled treatment were completed. All effectiveness analyses used the ITT population. Changes from baseline in effectiveness as measured by ADHD-RS-IV (18 items each rated from 0 to 3 with a total score of 0 to 54) and Conners’ Parent Rating Scale-Revised (CPRS-R) (80 items each rated from 0 to 3 with a total score of 0 to 240) were analyzed by paired t test. All statistical tests were conducted with the acceptable Type I error set at the 5% significance level. There was no adjustment for multiple testing. Descriptive statistics were calculated for Clinical Global Impression (CGI) and Parent Global Assessment (PGA) scales (ADHD severity and improvement rated from 0 to 7). The safety population was defined as all subjects who received at least 1 MTS patch application. Tolerability-related information was evaluated using descriptive statistics. Adverse events were considered to be treatment emergent if they began on or after the first patch application, or on or before 30 days after the final patch application in this study.
Effectiveness Assessments
The primary effectiveness variable was the change from baseline in the clinician-completed ADHD-RS-IV total score at week 4, and the change from baseline to the end of week 1 was the main secondary outcome. The ADHD-RS-IV was administered each week. Other outcome measures included CGI-S, CGI-Improvement (CGI-I)27, PGA, and CPRS-R28. Beginning at baseline and at each subsequent visit, the CGI-S assessment was completed by the investigator to measure the severity of mental illness. At weeks 1 and 4, the CGI-I rating scale was used to assess improvement relative to the ADHD symptoms at baseline (at which time the subjects were still taking oral ER-MPH). The PGA and CPRS-R assessments were completed at baseline and at each subsequent visit to capture parents’ opinions of their child’s symptom severity and improvement. CGI and PGA scores were dichotomized into 2 categories: improvement or no change (“very much improved”, “much improved”, “minimally improved”, or “no change”) and worsened (“minimally worse”, “much worse”, or “very much worse”).
Tolerability Assessments
Tolerability evaluations included subject and/or parent reporting of AEs, application site reactions, physical examinations, vital signs, ECGs, and laboratory assessments. Adverse events, coded using the Medical Dictionary for Regulatory Activities (MedDRA) Dictionary, Version 7.0, were recorded throughout the study and for 30 days after the last dose of study drug. Investigators categorized AEs according to intensity (“mild”, “moderate”, or “severe”) and relationship to study medication (“unrelated”, “possibly related”, or “probably related”). Events that were readily explained by other factors such as the subject’s underlying medical condition, concomitant therapy or accident and had no obvious temporal relationship with the investigational product were considered “unrelated”. Events in which there may have been some temporal relationship between the event and the administration of the investigational product, but with some ambiguity as to the cause were deemed “possibly related”. Events in which the temporal relationship between the event and the administration of the investigational product was compelling, and/or followed a known or suspected response pattern to that product, and could not have been explained by the subject’s medical condition, other therapies or accident were considered “probably related”.
Application site reactions were assessed on a scale ranging from 0 (no irritation) to 7 (strong reaction).
RESULTS
A total of 171 subjects (aged 6–12 years) were enrolled and 150 (88%) completed the 4-week study (Figure 2). All subjects received at least 1 MTS patch application; therefore, all are included in the safety population (n = 171). The ITT population was comprised of 164 subjects who received at least 1 MTS patch application and also had at least 1 ADHD-RS-IV measurement on or after week 1. Subject demographics and baseline characteristics are shown in Table 2.
Figure 2.
Disposition of subjects.
Table 2.
Subject demographics and baseline characteristics, ITT population
| Characteristic | Subjects (n=164) |
|---|---|
|
| |
| Age, years | |
| Mean (SD) | 9.4 (1.9) |
| Sex, n (%) | |
| Male | 117 (71) |
| Female | 47 (29) |
| Race, n (%) | |
| White | 129 (79) |
| African American | 19 (12) |
| Asian | 1 (0.6) |
| Other | 15 (9) |
| Weight (lb) | |
| Mean (SD) | 75.6 (26.0) |
| Height (in) | |
| Mean (SD) | 54.2 (5.2) |
| ADHD duration, years | |
| Mean (SD) | 3.6 (2.1) |
| ADHD subtype, n (%) | |
| Combined | 126 (77) |
| Inattentive | 34 (21) |
| Hyperactive/Impulsive | 4 (2) |
| ADHD-RS-IV Total Score | |
| Mean (SD) | 14.1 (7.5) |
| Median | 14.5 |
| CPRS-R Total Score | |
| Mean (SD) | 77.1 (45.4) |
| Median | 71.0 |
ITT = intent to treat, ADHD = attention-deficit/hyperactivity disorder, ADHD-RS-IV = ADHD-Rating Scale-IV, CPRS-R = Conners’ Parent Rating Scale-Revised
At the start of the study, subjects were receiving mean doses of 36.5 mg/d, 27.3 mg/d, and 26.7 mg/d of Concerta, Ritalin LA, and Metadate, respectively, and, based on the prospective dose-transition schedule (Table 1), were converted to MTS doses of 20 mg, 15 mg, and 15 mg, respectively. The mean final dose of MTS at the end of the dose-adjustment period for those previously receiving Concerta, Ritalin LA, and Metadate was 29.1 mg/d, 22.6 mg/d, and 20.0 mg/d, respectively (Table 3). Fifty-eight percent (95/164) of subjects remained on the dose to which they were initially converted. During titration, 4% (6/164) of patients required a lower patch size and 38% (63/164) increased the patch size. Note that the final doses of MTS stated in milligrams per day were considerably lower than those for the oral MPH products from which subjects had been switched, even after some MTS doses were titrated up.
Table 3.
Final MTS dose at end of study by ADHD medication at baseline, ITT population
| Baseline ADHD medication | Dose at Baseline | Final MTS Dose Subjects, n (%) |
|||
|---|---|---|---|---|---|
| 10 mg (n = 23) | 15 mg (n = 30) | 20 mg (n = 52) | 30 mg (n = 59) | ||
|
| |||||
| Concerta (n = 112) | 18 mg (n = 13) | 5 (38.5) | 5 (38.5) | 3 (23.1) | 0 |
| 27 mg (n = 24) | 1 (4.2) | 9 (37.5) | 10 (41.7) | 4 (16.7) | |
| 36 mg (n = 46) | 0 | 1 (2.2) | 26 (56.5) | 19 (41.3) | |
| 45 mg (n = 2) | 0 | 0 | 1 (50.0) | 1 (50.0) | |
| 54 mg (n = 27) | 0 | 0 | 1 (3.7) | 26 (96.3) | |
|
| |||||
| Ritalin LA (n = 26) | 10 mg (n = 3) | 2 (66.7) | 1 (33.3) | 0 | 0 |
| 20 mg (n = 9) | 4 (44.4) | 3 (33.3) | 2 (22.2) | 0 | |
| 30 mg (n = 9) | 0 | 3 (33.3) | 5 (55.6) | 1 (11.1) | |
| 40 mg (n = 2) | 0 | 1 (50.0) | 0 | 1 (50.0) | |
| 50 mg (n = 3) | 0 | 0 | 0 | 3 (100.0) | |
|
| |||||
| Metadate CD (n = 26) | 10 mg (n = 1) | 1 (100.0) | 0 | 0 | 0 |
| 15 mg (n = 1) | 1 (100.0) | 0 | 0 | 0 | |
| 20 mg (n = 12) | 8 (66.7) | 4 (33.3) | 0 | 0 | |
| 30 mg (n = 7) | 1 (14.3) | 3 (42.9) | 1 (14.3) | 2 (28.6) | |
| 40 mg (n = 3) | 0 | 0 | 2 (66.7) | 1 (33.3) | |
| 50 mg (n = 2) | 0 | 0 | 1 (50.0) | 1 (50.0) | |
Bolded data indicate subjects whose final dose of MTS was the same as the original MTS dose, as defined by the dose-transition schedule.
Concerta, Alza Corporation, Palo Alto, CA USA; Ritalin LA, Novartis AG, Basel, Switzerland; or Metadate CD, UCB Inc., Atlanta, GA, USA
MTS = methylphenidate transdermal system, ADHD = attention-deficit/hyperactivity disorder, ITT = intent to treat
Effectiveness
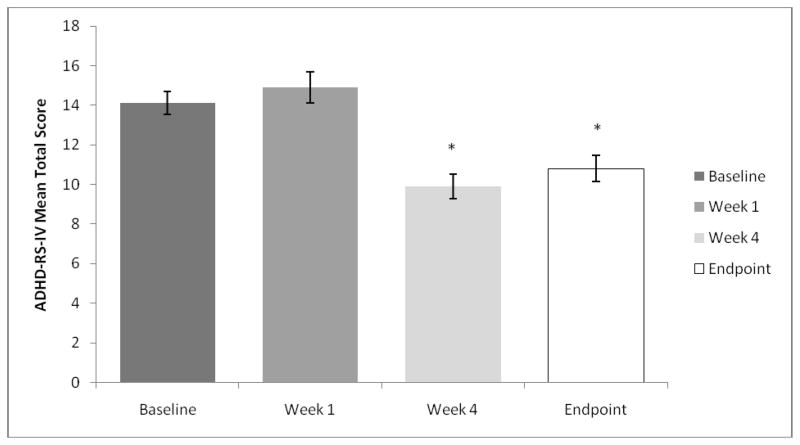
The mean ± SD ADHD-RS-IV total score at baseline (14.1±7.48) was similar to the mean ± SD ADHD-RS-IV total score at week 1 (14.9±9.84), indicating that on average there was little change in ADHD symptoms when subjects were converted to MTS from oral ER-MPH using the dose-transition schedule. In contrast, significant improvement was observed at week 4, when the ADHD-RS-IV mean ± SD total score (9.9±7.47) was significantly lower (p<0.0001 [n = 164]) than baseline after 42% (69/164) of the subjects had MTS dose-optimization adjustments, mostly upward (Figure 3).
Figure 3.
Mean ADHD-Rating Scale-IV (ADHD-RS-IV) total score in the intent-to-treat population compared with baseline. Study endpoint is defined as the last observation obtained post baseline.
*p<0.0001 for paired t-test of mean change from baseline.
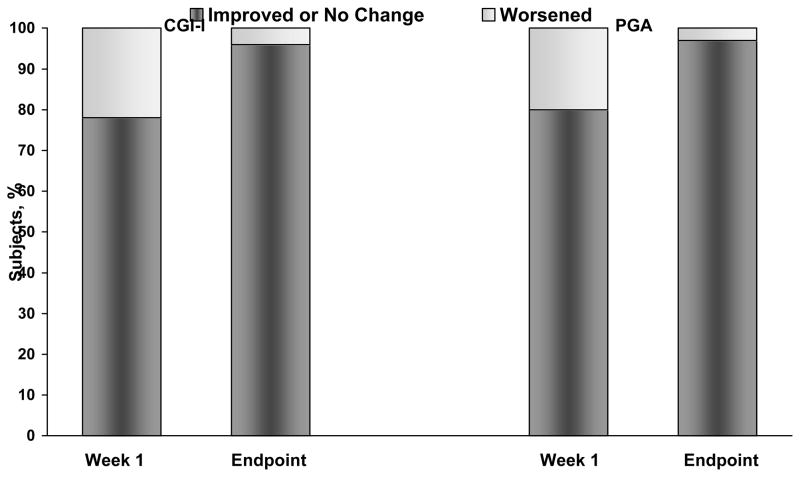
After 1 week of treatment, 78% (127/162) of subjects had a CGI-I score indicating improvement or no change, and 22% (35/162) worsened, demonstrating that, for the majority of subjects, symptom control did not deteriorate after abrupt conversion to MTS from oral ER-MPH. At week 4, 96% (144/150) of subjects’ scores were rated as improvement or no change and 4% (6/150) were rated as worsened relative to baseline. Furthermore, at week 4 the percentage of subjects reporting a CGI-I of “very much improved” and “much improved” (relative to baseline on ER-MPH) increased from 12% (19/162) and 15% (25/162) at week 1 to 31% (47/150) and 23% (34/150), respectively. Similarly, PGA scores reported by parents showed a general increase toward improvement in behavior compared to baseline (Figure 4). The CGI-S scores at week 1 demonstrated similar severity to baseline scores, but these improved by study endpoint. The CPRS-R mean (SD) total scores showed significant improvement (p<0.0001 [n = 164]) from baseline (77.1 [45.4]) at all measured assessments, including study endpoint (44.5 [33.3]).
Figure 4.
Percentage of subjects in each Clinical Global Impression-Improvement (CGI-I) and Parent Global Assessment (PGA) category at week 1 and study endpoint. Improvement or no change includes (“very much improved”, “much improved”, “minimally improved”, or “no change”). Worsened includes (“minimally worse”, “much worse”, or “very much worse”).
Tolerability
A total of 201 treatment-emergent adverse events (TEAEs) besides skin reactions were reported by 97 of the 171 subjects in the safety population during the study, and 94 of these 97 subjects (98%) reported events that were mild to moderate in intensity. The commonly reported TEAEs (≥3%) included headache, decreased appetite, insomnia, and upper abdominal pain (Table 4). Dose-related trends were noted for insomnia, anorexia, and upper abdominal pain where the highest incidence (6%, n = 4) occurred with the highest MTS dose. No other dose-related trends in the incidence of these events were noted. Insomnia and anorexia, reported by 1 and 2 subjects, respectively, resolved on MTS dose reduction.
Table 4.
Most commonly reported treatment-emergent adverse events (≥ 3% of Subjects), safety population
| TEAE | Subjects, n (%)(n = 171) |
|---|---|
|
| |
| Headache | 12 (7) |
| Decreased appetite | 11 (6) |
| Insomnia | 9 (5) |
| Upper abdominal pain | 7 (4) |
| Anorexia | 6 (4) |
| Nasopharyngitis | 6 (4) |
| Cough | 5 (3) |
| Pharyngolaryngeal pain | 5 (3) |
| Decreased weight | 5 (3) |
The total for each TEAE is based on the number of subjects reporting the TEAE
TEAE = treatment-emergent adverse event
Three subjects reported TEAEs other than skin reactions that led to study withdrawal. Two subjects reported 4 serious AEs that at follow up were reported to have resolved. A 7-year-old boy experienced “worsening of ADHD symptoms” and “condition aggravated” after receiving MTS 10 mg for 1 day; both events were considered unrelated to study treatment. A 12-year-old girl experienced “acute depression” and “suicide attempt” while receiving MTS 30 mg for 16 days; both events were considered possibly related to study treatment. The last subject, a 6-year-old girl, continued to experience anger/irritability after MTS study medication was discontinued. No deaths were reported during the study. At follow-up, no subject reported continuing AEs.
Skin Reactions
Four subjects withdrew from the study because of reactions at the application site that ranged from definite erythema with severe itching to normal appearance at the patch application site with moderate itching. However, at the last study visit, skin reaction scores of either 0 (no evidence of irritation) or 1 (minimal erythema) were reported for 70% (16/23), 83% (25/30), 73% (38/52), and 68% (39/57) of the subjects in the 10-, 15-, 20-, and 30-mg MTS treatment groups, respectively. Skin discomfort scores of either 0 (no discomfort) or 1 (mild discomfort) were reported for 96% (22/23), 87% (26/30), 92% (48/52), and 93% (53/57) of the subjects in the 10-, 15-, 20-, and 30-mg MTS treatment groups, respectively.
Fluctuations in blood pressure and pulse rate typically seen with stimulant therapy29 occurred in subjects throughout this study. Across all MTS treatment groups, 13 subjects had increases in systolic blood pressure (≥20 mm Hg), 63 subjects had increases in diastolic blood pressure (≥10 mm Hg), and 12 subjects had increases in pulse rate (≥110 bpm). There were no patterns or trends noted with any vital sign elevations. One subject experienced increased pulse rate as an AE, which was determined by the investigator to be mild and unrelated to MTS treatment. There were no reports of sudden death, stroke, or other serious cardiovascular events.
DISCUSSION
This study evaluated symptom control and tolerability in children with ADHD who were abruptly converted from a stable oral ER-MPH to MTS using a predefined dose-transition schedule. The majority of subjects (58%) in this study remained on the MTS dose defined by the schedule, 4% of subjects concluded the study on a lower dose, and 38% of subjects concluded the study on a higher dose, after systematic titration based on criteria for symptomatic improvement. Considering differences in absorption and first-pass liver metabolism between oral and transdermal administration routes, the bioavailability and utility of MTS may be altered compared with oral MPH products, so some subjects may require a lower dose of MTS. On average, the optimal nominal doses of MTS (amount absorbed in 9-hour wear time) were considerably below the nominal doses (amount ingested) of oral MPH from which subjects were switched, even after 38% were titrated upward. Therefore, MTS dose should be optimized based on individual patient response to therapy, starting at a smaller patch size than the oral dose.
Overall, subjects experienced continued control of ADHD symptoms during the week following abrupt conversion. The mean ADHD-RS-IV total score at week 1 was similar to the mean baseline score on oral ER-MPH, indicating that on average subjects’ ADHD symptoms did not worsen when they were switched to MTS. Additionally, clinician ratings on the CGI-I and CGI-S as well as parent ratings on the PGA at week 1 generally showed improvement or no change from baseline. Taken together, ADHD-RS-IV, CGI-I, CGI-S, PGA, and CPRS-R scores suggest that there was no significant worsening of subjects’ symptoms during the delivery system transition using the predefined dose-transition schedule in Table 1. These results appear to be in line with the large, 7-week, randomized clinical trial that was conducted in a naturalistic setting in which both MTS and osmotic-release oral system MPH (OROS, Alza Corporation, Palo Alto, CA, USA) were found to be significantly more effective than placebo as assessed by clinicians, parents21, and teachers22.
Dose optimization of MTS resulted in statistically significant improvements in ADHD-RS-IV total score at week 4 compared with baseline (p<0.0001). Although the baseline score was obtained while subjects were on a stable dose of oral ER-MPH, the significant improvement with MTS cannot be cited as evidence for superiority of MTS over oral ER-MPH formulations because the study design did not control for critical confounds. Importantly, timing (which delivery system was used when) and placebo effect were not controlled in the current study. Furthermore, the quality of medication management (careful titration at weekly visits for MTS) was not controlled between delivery systems and seems the most likely explanation of the significant improvement (p<0.0001) at week 4, after optimal titration, but not at week 1, prior to titration. This is not the first illustration of the importance of careful titration based on comprehensive data collection: the MTA Cooperative Group reported significantly better 14-month symptom control from carefully titrated and managed stimulant medication than from routine community treatment, which in two-thirds of cases involved treatment with the same medication not as carefully managed13. Nevertheless, these results suggest that for some subjects whose response is not optimal, systematic titration to an optimized dose of MTS may be a viable alternative.
Adverse events associated with MTS after subjects’ abrupt conversion from oral ER-MPH were primarily mild to moderate, and, with the exception of application site reactions, were similar to AEs typically seen with oral ER-MPH therapy. Moreover, the application site reactions and other AEs reported in this abrupt conversion study were consistent with those reported in MTS pivotal, randomized, controlled trials20–25.
Similar to other stimulants, the AEs of insomnia and anorexia associated with MTS have been reported to resolve with repeated use, even without changes in wear time, in 40% to 60% of cases23,30. In this study, the reported statistics for insomnia may have been exaggerated because insomnia was recorded even if it was a single episode precipitated by a patch wear time >9 hours. Regardless of current wear time, if late-day side effects such as insomnia appear in patients, recurrence may be prevented by decreasing the patch wear time (which decreases the daily dose absorbed). Such adjustments are reported to be effective in a 4-week, multisite, randomized, double-blind, parallel-group, placebo-controlled, dose-titration study in which children (aged 6–12 years) with ADHD were randomized to receive either transdermal MPH or placebo starting at doses of 11 mg or 16 mg with a patch wear time of 12 hours31. Insomnia and anorexia occurred more often in subjects who started treatment with the 16-mg dose compared with the 11-mg dose (27.3% vs 5.0% for insomnia and 33.3% vs 27.5% for anorexia). Additionally, 33% (n = 22) of subjects who started with the 16-mg dose required wear-time reductions vs 23% (n = 9) of those who were initially treated with the 11-mg dose31. Reported AEs in that study were typical for MPH and similar to those noted in other MTS clinical studies20–22, and the majority of AEs that led to early study termination resolved on discontinuation of the study drug.
Limitations
The study was open-label and nonrandomized. As an open-label study, which lacks blinding, clinician- and parent-completed outcome measures (ADHD-RS-IV, CGI-I, and PGA) may have been susceptible to observer bias. The lack of a placebo arm may result in spurious attribution of improvement to the study treatment.
The study sample was restricted to subjects who were previously on a stable dose of oral ER-MPH with ADHD symptoms within 1.5 SDs above age/sex norms. Thus, these results may not be generalizable to subjects who are not on a stable dose of oral MPH or who have a poor response to oral MPH.
Although the mean symptom level was significantly better in subjects after 4 weeks on MTS than on the previously effective oral MPH (p<0.0001), the study was not designed to test for superiority of MTS over oral MPH because timing of delivery type and quality of management (titration, comprehensiveness of clinical evaluation, and frequency of visits) were not controlled.
The current study was of a relatively short duration and was designed to evaluate subject response after abrupt conversion from ER-MPH to MTS, thus the tolerability data presented herein should not be considered representative of the long-term tolerability of MTS or the tolerability of MTS in treatment-naïve children with ADHD. In addition, no structured AE reporting system was employed, and it has been demonstrated that spontaneous AE reporting may underestimate AEs recorded.
CONCLUSION
Although the predefined dose-transition schedule used in this study was successful for most subjects, it is recommended that MTS therapy start at the lowest dose with rapid titration to the optimal dose based on individual needs and response. Abrupt conversion from a stable dose of oral ER-MPH to MTS was accomplished without loss of symptom control. Most AEs were mild to moderate and, with the exception of application site reactions, were similar to AEs typically observed with oral ER-MPH therapy.
Acknowledgments
Declaration of interest: This study was funded by Shire Development Inc., Wayne PA, USA. L. E. A. has received research support, speaker and/or consultant fees from Shire Development, Inc., Neuropharm, Targacept, Organon, Novartis, Autism Speaks, NIMH, and the Reach Institute/Ackerman Foundation. P. H. is an employee of and holds stock in Shire Pharmaceuticals, Inc. M. M. is an employee of Shire Pharmaceuticals, Inc. L. B-T. has received research support from Abbott Laboratories, Addrenex Pharmaceuticals, Alkermes, Inc., AstraZeneca LP, Berlex Laboratories, Bristol-Myers Squibb Company, Cephalon, Inc., Corcept Therapeutics, DOV Pharmaceutical, Inc., Eisai Pharmaceuticals, Eli Lilly and Company, Forest Laboratories, Inc., Johnson & Johnson Pharmaceutical Research & Development, New River Pharmaceuticals, Inc., Pfizer Pharmaceuticals, Saegis Pharmaceuticals, Inc., Shire Pharmaceutical Development, Inc., Takeda North America, and Wyeth Research. M. G. has received research support, speaker and/or consultant fees from Shire Pharmaceuticals, Inc., Abbott Laboratories, AstraZeneca LP, GlaxoSmithKline, Catreda, Eli Lilly and Company, Pfizer Pharmaceuticals, Wyeth Research, Sanofi, Johnson & Johnson Pharmaceutical Research & Development, Supernus, and Novartis. O. B. has received research support, speaker and/or consultant fees from Shire Pharmaceuticals, Inc., Ortho-McNeil Pharmaceuticals, Quintiles, Routledge Press, and the Problem Based Learning Institute. A. P. has no financial relationships to disclose. D. R. B. has no financial relationship to disclose. The authors would like to acknowledge the children and their families for study participation. The authors also acknowledge Laura Miesle, PharmD, of The JB Ashtin Group, Inc., Plymouth, Michigan, for assistance in the preparation of this manuscript and Shire Development Inc., Wayne, Pennsylvania, for funding this assistance.
Footnotes
DAYTRANA is a registered trademark of Shire Pharmaceuticals Ireland Limited, [0]Dublin, Ireland
DOT MATRIX is a registered trademark of Noven Pharmaceuticals, Miami, FL, USA
Previous Presentations
American Psychiatric Association; Toronto, Canada; 2006 May 24
American College of Clinical Pharmacy; St. Louis, Missouri; 2006 Oct 29
American Academy of Child and Adolescent Psychiatry; San Diego, California; 2006 Oct 27
U.S. Psychiatric and Mental Health; New Orleans, Louisiana; 2006 Nov 16
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. 4. Washington, DC: American Psychiatric Association; 2000. Text Revision. [Google Scholar]
- 2.Arnold LE. Contemporary Diagnosis and Management of ADHD. 3. Newtown, PA: Handbooks in Health Care Co; 2004. [Google Scholar]
- 3.American Academy of Pediatrics Committee on Quality Improvement, Subcommittee on Attention-Deficit/Hyperactivity Disorder. Clinical practice guideline: diagnosis and evaluation of the child with attention-deficit/hyperactivity disorder. Pediatrics. 2000;105:1158–70. doi: 10.1542/peds.105.5.1158. [DOI] [PubMed] [Google Scholar]
- 4.Biederman J. Attention-deficit/hyperactivity disorder: a selective overview. Biol Psychiatry. 2005;57:1215–20. doi: 10.1016/j.biopsych.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 5.Wilens TE, Biederman J, Spencer JS. Attention deficit/hyperactivity disorder across the lifespan. Annu Rev Med. 2002;53:113–31. doi: 10.1146/annurev.med.53.082901.103945. [DOI] [PubMed] [Google Scholar]
- 6.Biederman J, Mick E, Faraone SV. Normalized functioning in youths with persistent attention-deficit/hyperactivity disorder. J Pediatr. 1998;133:544–51. doi: 10.1016/s0022-3476(98)70065-4. [DOI] [PubMed] [Google Scholar]
- 7.Barkley RA, Fischer M, Edelbrock CS, Smallish L. The adolescent outcome of hyperactive children diagnosed by research criteria: I an 8-year prospective follow-up study. J Am Acad Child Adolesc Psychiatry. 1990;29:546–57. doi: 10.1097/00004583-199007000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Goodman DW, Ginsberg L, Weisler RH, et al. An interim analysis of the quality of life, effectiveness, safety, and tolerability (Q.U.E.S.T) evaluation of mixed amphetamine salts extended release in adults with ADHD. CNS Spectr. 2005;10(suppl 20):26–34. doi: 10.1017/s1092852900002418. [DOI] [PubMed] [Google Scholar]
- 9.Biederman J, Faraone SV, Spencer TJ, et al. Functional impairments in adults with self-reports of diagnosed ADHD: a controlled study of 1001 adults in the community. J Clin Psychiatry. 2006;67:524–40. doi: 10.4088/jcp.v67n0403. [DOI] [PubMed] [Google Scholar]
- 10.Designating September 7, 2004, as “National Attention Deficit Disorder Day”. S Res 370, 108th Cong, 2nd Session; 2004. [Google Scholar]
- 11.Prince JB. Pharmacotherapy of attention-deficit hyperactivity disorder in children and adolescents: update on new stimulant preparations, atomoxetine, and novel treatments. Child Adolesc Psychiatr Clin N Am. 2006;15:13–50. doi: 10.1016/j.chc.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Pliszka S. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:894–921. doi: 10.1097/chi.0b013e318054e724. [DOI] [PubMed] [Google Scholar]
- 13.The MTA Cooperative Group. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 1999;56:1073–86. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- 14.Wilens TE, Biederman J. The stimulants. Psychiatr Clin North Am. 1992;15:191–222. [PubMed] [Google Scholar]
- 15.Pelham WE, Gnagy EM, Burrows-Maclean L, et al. Once-a-day Concerta methylphenidate versus three-times-daily methylphenidate in laboratory and natural settings. Pediatrics. 2001;107:105. doi: 10.1542/peds.107.6.e105. [DOI] [PubMed] [Google Scholar]
- 16.Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366:237–48. doi: 10.1016/S0140-6736(05)66915-2. [DOI] [PubMed] [Google Scholar]
- 17.Greenhill LL, Pliszka S, Dulcan MK. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. AACAP Official Action. J Am Acad Child Adolesc Psychiatry. 2002;41(suppl):26S–49S. doi: 10.1097/00004583-200202001-00003. [DOI] [PubMed] [Google Scholar]
- 18.Daytrana [package insert] Wayne, PA: Shire US Inc; 2008. [Google Scholar]
- 19.McGough JJ, Wigal SB, Abikoff H, et al. A randomized, double-blind, placebo-controlled, laboratory classroom assessment of methylphenidate transdermal system in children with ADHD. J Atten Disord. 2006;9:476–85. doi: 10.1177/1087054705284089. [DOI] [PubMed] [Google Scholar]
- 20.Findling RL, Bukstein OG, Melmed RD, et al. A randomized, double-blind, placebo-controlled, parallel-group study of methylphenidate transdermal system in pediatric patients with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2008;69:149–59. doi: 10.4088/jcp.v69n0120. [DOI] [PubMed] [Google Scholar]
- 21.Bukstein OG, Vince BD, López FA, et al. Parent and teacher rated effects of MTS and OROS methylphenidate in ADHD. Poster presented at: Annual Meeting of the American Psychiatric Association; May 24, 2006; Toronto, Ontario, Canada. [Google Scholar]
- 22.Pelham WE, Burrows-MacLean L, Gnagy EM, et al. Transdermal methylphenidate, behavioral, and combined treatment for children with ADHD. Exp Clin Psychopharmacol. 2005;13:111–26. doi: 10.1037/1064-1297.13.2.111. [DOI] [PubMed] [Google Scholar]
- 23.Pelham WE, Manos M, Ezzell CE, et al. A dose-ranging study of a methylphenidate transdermal system in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2005;44:522–29. doi: 10.1097/01.chi.0000157548.48960.95. [DOI] [PubMed] [Google Scholar]
- 24.Wilens TE, Boellner SW, López FA, et al. Varying the wear time of the methyphenidate transdermal system in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2008;47:700–8. doi: 10.1097/CHI.0b013e31816bffdf. [DOI] [PubMed] [Google Scholar]
- 25.Arnold LE, Lindsay RL, López FA, et al. Treating attention-deficit/hyperactivity disorder with a stimulant transdermal patch: the clinical art. Pediatrics. 2007;120:1100–6. doi: 10.1542/peds.2007-0542. [DOI] [PubMed] [Google Scholar]
- 26.DuPaul GJ, Power TJ, Anastopolous AD, Reid R. ADHD Rating Scale-IV: Checklists, Norms, and Clinical Interpretation. New York, NY: Guilford Press; 1998. [Google Scholar]
- 27.Guy W. ECDEU Assessment Manual for Psychopharmacology-Revised. Rockville, Md: US Dept of Health, Education and Welfare; 1976. Clinical global impressions; pp. 218–22. DHEW Pub. No. ADM 76-338. [Google Scholar]
- 28.Connors CK. Connors’ Rating Scales – Revised: Technical Manual. New York, NY: Multi-Health Systems Inc; 1997. [Google Scholar]
- 29.Rapport MD, Moffitt C. Attention deficit/hyperactivity disorder and methylphenidate. A review of height/weight, cardiovascular, and somatic complaint side effects. Clin Psychol Rev. 2002;22:1107–31. doi: 10.1016/s0272-7358(02)00129-0. [DOI] [PubMed] [Google Scholar]
- 30.Greenhill LL, Pelham WE, Jr, López FA, et al. Once-daily transdermal methylphenidate improves teacher, parent, and CGI-I ratings. Poster presented at: 49th Annual Meeting of the American Academy of Child and Adolescent Psychiatry; October 22–27, 2002; San Francisco, CA. [Google Scholar]
- 31.Data on File. Study File N17-021. Shire Development Inc; Wayne, PA: [Google Scholar]