Abstract
The melanoma differentiation-associated gene-7 (mda-7) is a known mediator of apoptosis in cancer cells but not in normal cells. We hypothesized that MDA-7 interferes with the prosurvival signaling pathways that are commonly altered in cancer cells to induce growth arrest and apoptosis. We also identified the cell signaling pathways that are antagonized by MDA-7 leading to apoptosis. Using an adenoviral expression system, mda-7 was introduced into the breast cancer cell lines SKBr3, MCF-7 and MDA-MB-468, each with a different estrogen receptor (ER) and HER-2 receptor status. Downstream targets of MDA-7 were assessed by reverse phase protein array analysis, western blot analysis and immunofluorescence confocal microscopy. Our results show that MDA-7-induced apoptosis was mediated by caspases in all cell lines tested. However, MDA-7 modulates additional pathways in SKBr3 (HER-2 positive) and MCF-7 (ER positive) cells including downregulation of AKT-GSK3β and upregulation of cyclin-dependent kinase inhibitors in the nucleus. This leads to cell cycle arrest in addition to apoptosis. In conclusion, MDA-7 abrogates tumor-promoting pathways including the activation of caspase-dependent signaling pathways ultimately leading to apoptosis. In addition, depending on the phenotype of the breast cancer cell, MDA-7 modulates cell cycle regulating pathways to mediate cell cycle arrest.
Keywords: MDA-7, IL-24, breast cancer and AKT
Introduction
Breast cancer is the most common malignancy among women in the United States with over 254 000 new cases diagnosed in 2009. Although the mortality rate has begun to decrease with the increased use of mammographic screening and improved adjuvant therapies, the death rate still ranks second among cancer related deaths in women.1 The treatment of breast cancer often involves a combination of surgery, radiation therapy, chemotherapy and hormone therapy. Targeted therapy against HER-2/neu and estrogen receptor (ER) are used in patients whose tumors express these proteins, however, a significant portion of these women will develop recurrence and ultimately die of metastatic disease. Resistance of breast cancers to radiation, hormone therapy and chemotherapy has led investigators to search for novel molecular-targeted therapies which are tumor specific.
The melanoma differentiation-associated gene 7 (mda-7), also known as interleukin-24, is a novel tumor suppressor gene, which encodes a 24 kDa cytokine. We have previously shown that adenoviral-mediated delivery of mda-7 can cause cell cycle arrest and induce apoptotic cell death in tumor cell lines without toxicity to normal cells.2–5 Adenoviral-mediated overexpression of the MDA-7 protein has also been shown to have synergistic effects on apoptotic cell death in breast cancer cells when combined with standard radiation therapy, chemotherapy or hormone therapy.6,7
Cancer is a disease that is characterized by uncontrolled cell proliferation, occurring through the aberrant regulation of molecular pathways responsible for cell cycle control and apoptosis. Normal cell division is precisely regulated by a multitude of protein groups, one of which includes the group known as the cyclin-dependent kinase inhibitors (CKIs). These CKIs act in concert with other proteins to prevent cell progression through the G1 to S phase regulatory check point of the cell cycle. The p21 and p27, members of the Cip/Kip family of CKIs, can interact with and inhibit the kinase activity of the CDK2-cyclin E complex, ultimately leading to cell cycle arrest.8
We hypothesized that overexpression of MDA-7 in breast cancer cell lines could interfere with the cell cycle aberrations and anti-apoptotic mechanisms typical of cancer cells. In this study we demonstrate that mda-7 exerts its tumor inhibitory effects via different pathways depending on the phentoype of the breast cancer cell. For example, in SKBr3 and MCF-7 cells, MDA-7 expression inhibits both anti-apoptotic and cell cycle pathways. In MDA-MB-468 cells, apoptotic proteins are greatly affected by MDA-7 overexpression, whereas there is little effect on proteins involved in proliferative pathways.
Materials and methods
Cell culture
All breast cancer cell lines (SKBr3, MDA-MB-468, MCF-7) were grown in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum, 100 U ml−1 penicillin, 100 μg ml−1 streptomycin and 2 mM L-glutamine (GIBCO/BRL, Life Technologies, Grand Island, NY). Cells were observed daily for contamination and harvested in the log phase of growth using 0.125% trypsin/1.3 mM EDTA (GIBCO) using standard procedures.
Adenoviral vectors
We utilized a replication-deficient human type 5 adenovirus (Ad5) carrying the mda-7 gene that has been previously described.6 The mda-7 gene was inserted in the E1 region of Ad5 linked to a CMV-IE promoter and followed by an SV40 polyadenylation sequence (Ad-mda7). Ad5 containing a luciferase reporter gene (Ad-Luc) or an empty vector were used as controls. DNA sequence analysis was used to confirm the identity of the viral stocks.
Adenoviral infections
Cell lines were infected with increasing multiplicity of infection of Ad-mda7 and Ad-luc (control). A multiplicity of infection of 3500 virus particles per cell (vp per cell) was utilized for experiments unless otherwise specified. Phosphate-buffered saline (PBS) served as a control. For each experiment, cells were counted and plated 24 h before infection with the adenoviral vectors. For assays involving apoptosis or cell cycle determination, infection was carried out in six-well plates where cells were plated at 5 × 104 cells per well. For assays involving western blot or reverse phase protein array (RPPA) analysis, infection was carried out in 100 mm plates with 1 × 106 cells per plate. For all infections, cells were incubated for 72 h with the adenoviral vectors and then harvested for their respective assays.
Western blot analysis
Cells were washed twice with cold PBS and cell lysates were collected from cells after incubation of tissue culture with lysis buffer for 10 min. Protein concentrations were determined using the Bradford method using Bio-Rad Protein Assay Dye (Bio-Rad, Hercules, CA). Approximately 50 μg of protein was subjected to 10% SDS-polyacrylamide gel electrophoresis and then transferred to a nitrocellulose membrane. The following antibodies were used at a concentration of 1 μg ml−1: MDA-7 (Introgen Therapeutics, Houston, TX) and p21, p27, CDK2 and β-actin (Sigma-Aldrich Chemicals, St Louis, MO). Cyclin E (clone HE12, Santa Cruz Biotechnology, Santa Cruz, CA) was used at a concentration of 0.1 μg ml−1. Membranes were washed with Tris-buffered saline tween-20 incubated overnight at 4 °C with secondary horseradish peroxidase-conjugated antibodies (anti-goat immunoglobulin, Santa Cruz Biotechnology; anti-rabbit and anti-mouse immunoglobulin; Sigma-Aldrich Chemicals). After washing, the protein signal was detected using chemiluminescence.
Annexin V assay
All cell lines were evaluated for apoptosis using the ApoAlert Annexin V-fluorescein isothiocyanate (FITC) kit (BD Biosciences, San Jose, CA). Cells were thoroughly washed in PBS and centrifuged at 2000 r.p.m. for 5 min at 4 °C. All samples were then incubated with Annexin V-FITC reagent for 15 min at room temperature and subsequently washed. Propidium iodide (10 μg/ml) was added just before fluorescence-activated cell sorter analysis.
Cell cycle analysis
DNA content was analyzed using propidium iodide staining (Sigma-Aldrich Chemicals), followed by fluorescence-activated cell sorter analysis to identify the progression of cells through the cell cycle. Cells were prepared as a single-cell suspension of 1 × 106 cells per ml of PBS, fixed with cold 95% ethanol and centrifuged. The fixative was then decanted and the cells were washed two times with PBS. Staining with propidium iodide was performed at a final concentration of 50 μg ml−1 with RNAse at 1 mg ml−1 in PBS. Treated cells were then evaluated by fluorescence-activated cell sorter analysis following standard procedures.
Confocal microscopy
MCF-7, SKBr3 and MDA-MB-468 cells (4 × 105) were plated on glass coverslides in six-well plates and then treated with PBS, Ad-Luc and Ad-mda7. After 72 h, cells were washed 2 times with PBS and fixed with 3.7% paraformaldehyde/PBS for 15 min. Cells were subsequently permeabilized for 20 min at 4 °C with 0.2% Triton X-100 and blocked 1 h with 1% normal goat serum. Rabbit polyclonal anti-MDA-7, anti-CDK2, anti-p21, anti-p27 and anti-cyclin E were incubated overnight at 4 °C and developed with anti-rabbit fluorescein isothio-cyanate-conjugated secondary antibodies for 45 min at 37 °C. Cells were then imaged via confocal microscopy (Olympus FV500 confocal microscope, Olympus America Corporate Headquarters, Center Valley, PA). F-actin was detected using Alexa568-conjugated phalloidin (1:40; Molecular Probes, Invitrogen Corporation, Carlsbad, CA). Nuclei were stained using TO-PRO-3 (1:1000; Molecular Probes) or DAPI.
RPPA analysis
RPPA was performed as previously published.9 Briefly, the cells were washed twice with PBS and lysed with lysis buffer. Protein concentration was determined by Bradford Assay. A volume of 25–30 μl of lysate was transferred into a PCR 96-well plate. A volume of 10μl of 4 × sample buffer was added to each sample well. The plates were then covered and incubated at 95 °C for 5 min then centrifuged for 1 min at 2000 r.p.m. Protein cell lysates were serially diluted and transferred into 384-well plates and heated at 95 °C for 10 min. The lysate material was then printed onto nitrocellulose-coated glass slides (FAST Slides, Schleicher & Schuell BioScience, Keene, NH) with an automated robotic GeneTac arrayer (Genomic Solutions, Ann Arbor, MI). After printing, the slides were blocked and probed with 63 different primary antibodies followed by biotin-conjugated secondary antibodies. The signal intensity was determined using the DAKO signal amplification system (DAKO, Copenhagen, Denmark) and scanning the slides with ImageQuant (Molecular Dynamics, Sunnyvale, CA). The intensity was quantified using the MicroVigene automated RPPA module (VigeneTech, North Billerica, MA). Differences in loading were assessed and corrected for by normalizing the expression intensities. All samples were run in triplicate and the resulting intensities were averaged.
Statistical analysis
The statistical significance of the experimental results was evaluated using two independent, Student’s t-test. Significance was set at P<0.05. Bonferroni correction was used where necessary.
Results
MDA-7 induces apoptosis in breast cancer cells
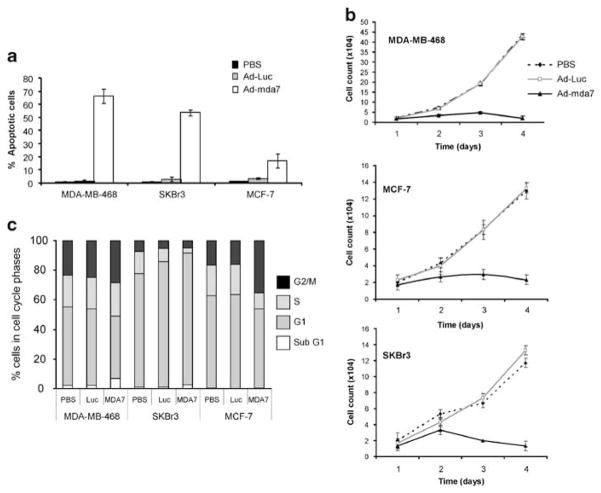
MDA-7 can induce apoptosis in a variety of cancer cell types, but not normal cells. To determine whether MDA-7 overexpression results in apoptosis in our panel of breast cancer cells (SKBr3, MCF-7 and MDA-MB-468), they were treated with a multiplicity of infection of 3500 vp per cell of Ad-mda7 and assessed for apoptotic state using Annexin V staining (Figure 1a). These three different cell lines were chosen as they have different phenotypes typical of different subtypes of breast cancer: SKBr3 is ER negative and HER-2 positive, MCF-7 is ER positive and HER-2 negative and MDA-MB-468 is triple receptor negative, devoid of ER, progesterone receptor and HER-2. Treatment of all cells with Ad-mda7 resulted in apoptosis, but to different degrees. Specifically, MDA-MB-468 cells were the most sensitive cells with 65.5% more of the Ad-mda7-treated cells undergoing apoptosis compared with Ad-Luc controls, whereas SKBr3 and MCF-7 cells were slightly less sensitive with 53 and 16% of the cells undergoing apoptosis (Figure 1a). Cell proliferation was also measured for each cell line under control (PBS and Ad-Luc) and Ad-mda7-treated conditions and the results showed that in all three cell lines, the cells did not proliferate in response to Ad-mda7 treatment, whereas the control cells proliferated logarithmically from days 2–4 post-treatment (Figure 1b). The percent apoptosis in response to Ad-mda7 treatment does not account for the lack of cell proliferation, therefore we asked whether the lack of cell proliferation was due to cell cycle changes. Cells were treated with the three conditions (PBS, Ad-Luc, Ad-mda7) and subjected to flow cytometric analysis by propidium iodide staining (Figure 1c). This analysis revealed that Ad-mda7 treatment of MCF-7 cells resulted in G2/M accumulation, whereas treatment of SKBr3 resulted in accumulation of cells in G1 phase of the cell cycle. There was no observable change in cell cycle profile in MDA-MB-468 cells as a result of MDA-7 expression. These results show that treatment of breast cancer cells with different phenotypes leads to different degrees of apoptosis, even though proliferation is inhibited in all cell lines. Therefore, in addition to changes in apoptosis, there are likely to be further alterations affecting the cell cycle pathway that need to be further examined.
Figure 1.
MDA-7 induces growth inhibition via cell cycle arrest and subsequent apoptosis in breast cancer cell lines. (a) MDA-MB-468, SKBr3 and MCF-7 cells were treated with Phosphate-buffered saline (PBS) or Ad-Luc or Ad-mda7 at a multiplicity of infection of 3500 vp per cell, stained with Annexin V and analyzed by flow cytometry. (b) The DNA content of the cells treated with PBS, Ad-Luc or Ad-mda7 was determined by propidium iodide staining and fluorescence-activated cell sorter analysis to assess the cell cycle distribution of the cells. (c) Growth curves were performed on each of the cell lines by counting cells every 24 h for 4 days post infection with Ad-Luc or Ad-mda7 or after mock infection with PBS. Error bars represent the standard error of the mean.
MDA-7 suppresses AKT in breast cancer cells
To examine the signaling pathways involved in MDA-7-mediated apoptosis and cell cycle alteration, we investigated how such treatment affects key proteins in tumor-promoting transduction pathways. We subjected the cell lysates of the Ad-mda7-treated cells to RPPA analysis, which allowed us to assess protein expression using 63 antibodies concurrently. Analysis of the data showed that there was a change in expression of more than two fold after Ad-mda7 treatment compared with the expression when treated with PBS of several proteins. Furthermore, we were able to assign the modulated proteins to the following pathways: caspase mediated apoptosis, cell cycle and AKT pathways.
AKT expression was reduced by 29.6-fold in MCF-7 cells after induction of MDA-7 expression in MCF-7 cells when compared with PBS-treated cells (Figure 2a). SKBr3 cells displayed a 6.5 fold decrease in AKT after MDA-7 expression as measured by RPPA analysis. MDA-MB-468 cells had little AKT expression in either the PBS, Ad-Luc or Ad-mda7 treated cells. Western blot analysis was used to validate the RPPA results. Densitometry was performed to evaluate the expression of AKT as a function of MDA-7 expression. The results showed that MDA-7 induced a decrease in AKT in all three cell lines (Figure 2b). Confocal fluorescence microscopy was used to further confirm the Ad-mda7 induced changes in levels and also to assess cellular localization of AKT (Figure 2c). Nuclear and cytoplasmic compartments were detected using TO-PRO3 and phallotoxin stains, respectively. The high AKT expression in the nucleus of control (PBS and Ad-Luc) treated MCF-7 cells was substantially decreased after infection with Ad-mda7. The reduction in AKT expression was apparent, but less striking in the SKBr3 and MDA-MB-468 cells with MDA-7 overexpression. The immunostaining results validated the RPPA data and collectively suggest that suppression of AKT could be an important mechanism utilized by MDA-7 to mediate growth inhibition in tumor cells.
Figure 2.
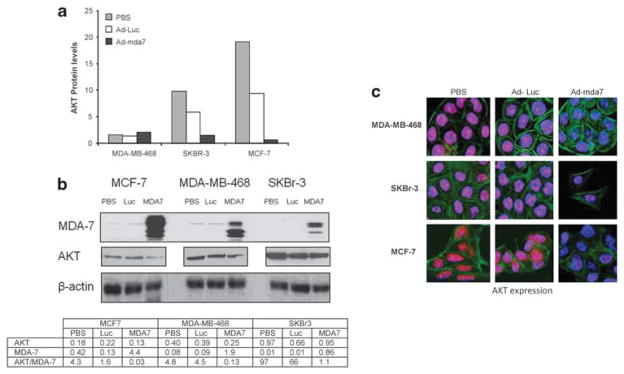
AKT is downregulated after overexpression of MDA-7. Lysates of MDA-MB-468, MCF-7 and SKBr3 cells were used to assess AKT protein expression after infection with Ad-Luc, Ad-mda7 or treatment with Phosphate-buffered saline (PBS) by (a) reverse phase protein array analysis and (b) western blot and densitometric analysis of AKT and MDA-7 expression. For western blots, the same 10% blot was used to determine MDA-7 and β-actin expression, whereas the same lysates were run on a separate 10% blot to determine AKT expression. There were not extra lanes between the lysates of the different cell lines on the AKT blot, therefore it is shown as separate panels despite being the same lysate. Densitometric values are normalized to actin expression. (c) Confocal microscopy was performed on the MDA-MB-468, MCF-7 and SKBr3 cells 72 h after treatment with PBS, Ad-Luc or Ad-mda7. Cells were stained with antibodies to detect AKT (red), the nucleus using TO-PRO-3 (blue) or the cytoskeleton using Alexa 568-conjugated phalloidin (green) and were imaged via confocal microscopy.
Apoptotic proteins are modulated by MDA7
We and others have shown that MDA-7 induces apoptosis in breast cancer cells. We analyzed our RPPA data to determine whether proteins involved in regulating apoptosis were among those that were affected by MDA-7 expression. The pro-apoptotic proteins cleaved poly (ADP-ribose) polymerase (PARP) and cleaved caspase 7 were both increased in response to MDA-7 overexpression. PARP protein was increased by 40.5, 8.6 and 16.6 fold in MCF-7, SKBr3 and MDA-MB-468 cells, respectively (Figure 3). Procaspase 7 exhibited a similar increase in expression with a 5.0, 7.1 and 4.2 fold increase in the MCF-7, SKBr3 and MDA-MB-468 cells, respectively (Figure 3). As a result, PARP cleavage and increased procaspase 7 could be partially responsible for the apoptosis observed in MDA-7-treated breast cancer cells. The X-linked mammalian inhibitor of apoptosis protein (XIAP) was decreased as a result of MDA-7 expression in both SKBr3 (9.1 fold) and MCF-7 (1.6 fold) cells compared with PBS-treated cells (Figure 3). These results suggest that the balance between apoptotic promoting and inhibiting proteins seems to be shifted towards cell death as a consequence of MDA-7 induction.
Figure 3.
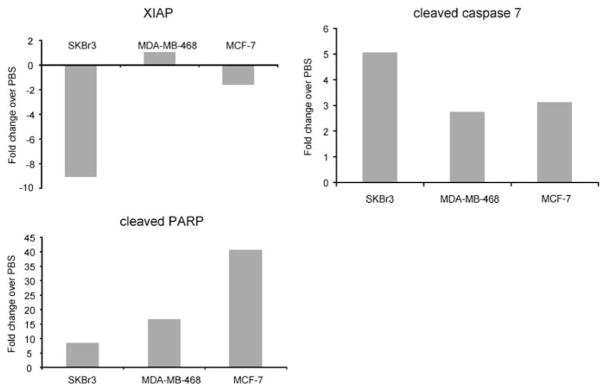
MDA-7 upregulates expression of proteins in the apoptotic pathway. Reverse phase protein array analysis was used to assess expression of proteins in the apoptotic pathway after MDA-7 overexpression in SKBr-3, MDA-MB-468 and MCF-7 cells. The fold change after MDA-7 expression over phosphate-buffered saline-treated control is shown for cleaved PARP, cleaved caspase 7 and XIAP in all three cell lines.
MDA-7 alters the expression of proteins regulating cell cycle progression
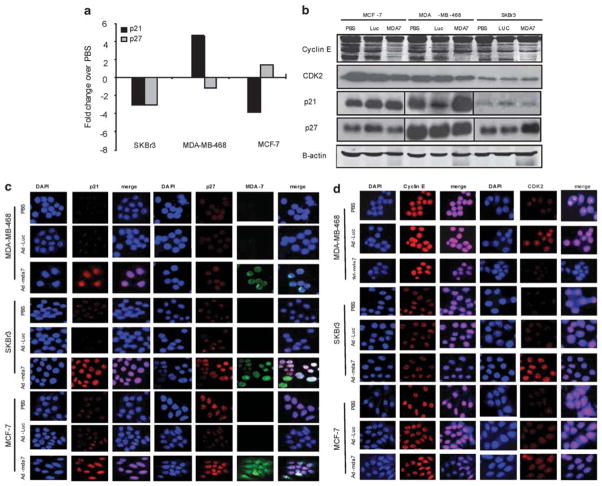
In addition to induction of apoptosis in all cell lines examined, MDA-7 resulted in cell cycle arrest in MCF-7 and SKBr3 cells (Figure 1). Therefore, we next examined whether MDA-7 altered the expression of proteins involved in regulating cell cycle progression. Lysates of SKBr3, MCF-7 and MDA-MB-468 cells infected with Ad-mda7, PBS or Ad-Luc were subjected to RPPA to assess p21 and p27 expression (Figure 4a). The RPPA results revealed that p21 and/or p27 increased in MDA-MB-468 and MCF-7, respectively, in response to Ad-mda7 treatment, whereas the levels of these inhibitors declined in SKBr3. These data were somewhat surprising because we had previously seen an accumulation of SKBr3 cells in G1, whereas MCF-7 and MDA-MB-468 cells accumulated in G2/M. Western blots were performed to further investigate the expression of p21 and p27 after Ad-mda7 treatment and the results revealed that in concordance with the RPPA data, the levels of these two proteins are increased (Figure 4b). We hypothesized that the levels of these inhibitors may not be the only determinant in their functionality and to more accurately assess their role in Ad-mda7-mediated cell cycle arrest it was critical to examine their cellular localization. Therefore, we performed confocal microscopy using fluorescent antibodies to p21 and p27 to assess the localization of these proteins (Figure 4c). Cytoplasmic sequestration of CKIs by AKT has been implicated as a mechanism for cell cycle deregulation in breast cancer.10,11 In all three cell lines, the intensity of p21 and p27 increased in the nucleus (as determined by DAPI staining) as a result of infection with Ad-mda7. Therefore, MDA-7 increases the expression of CKIs in the nucleus of cancer cells, where they can inhibit positive regulators of the cell cycle to halt cell cycle progression.
Figure 4.
MDA-7 affects cell cycle regulatory proteins. MDA-MB-468, MCF-7 and SKBr-3 cells were infected with Ad-mda7, Ad-Luc, or phosphate-buffered saline and were subjected to (a) reverse phase protein array analysis for p21 and p27 expression. (b) Lysates of each infection condition were analyzed for expression of p21, p27, cyclin E and CDK2 by western blotting. Expression was confirmed and localization was assessed by confocal microscopy for (c) p21 and p27 and (d) cyclin E and CDK2. Nuclear location was determined by DAPI staining. Images of DAPI were merged with those of p21, p27, cyclin E or CDK2 to compare localization.
Cyclin E and CDK2 regulate the cell’s progression from late G1 to S phase of the cell cycle and their activity is negatively regulated by the CKIs, p21 and p27. To address whether the nuclear localization of CKIs mediated by MDA-7 subsequently leads to changes in expression of cyclin E and CDK2, we assessed whether their expression is altered using western blot analysis (Figure 4b). Cells infected with Ad-mda7 exhibited a decreased expression of cyclin E and CDK2 in SKBr3, MCF-7 and MDA-MB-468 cells. Confocal microscopy with immunofluorescence was used to further analyze any changes in the localization or expression of cyclin E and CDK2 (Figure 4d). Only modest decreases in CDK2 were observed in MCF-7 and MDA-MB-468 cells, suggesting that the growth inhibitory role of MDA-7 is likely to be the result of increased p21 and p27 nuclear levels.
MDA-7 modulates signaling pathways pertinent to cancer cell survival
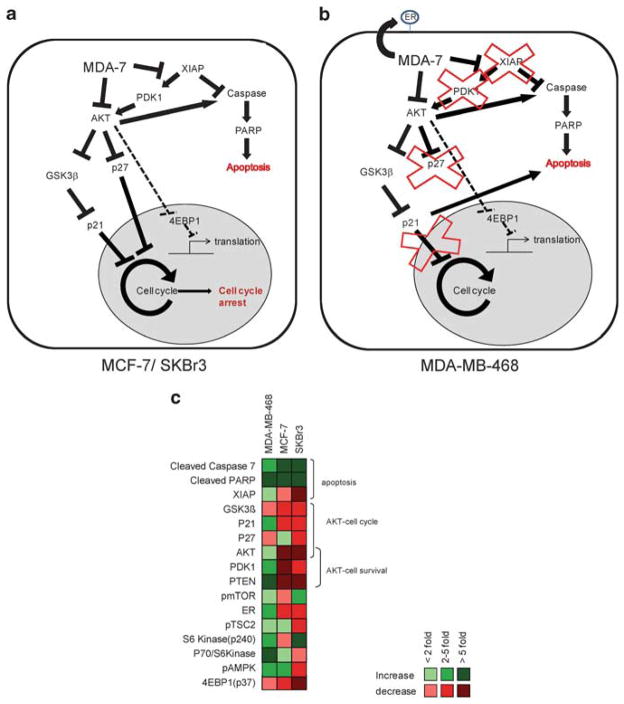
MDA-7 specifically inhibits growth in tumor cells; therefore by understanding the molecular interactions provoked by Ad-mda7 we may reveal molecular targets fundamental to tumorigenesis. Our data has shown that AKT is decreased by MDA-7 overexpression, especially in MCF-7 cells (Figure 2). AKT is upstream of apoptotic and cell cycle pathways, and therefore both the observed apoptotic and growth inhibitory effects mediated by MDA-7 could be attributed to AKT downregulation. Our RPPA data confirmed that proteins established as integral to the AKT survival pathways are downregulated as a result of MDA-7 expression. We also observed that PDK1, which is a kinase upstream of AKT, is down-regulated by 6.8 fold in MCF-7 cells and 3.2 fold in SKBr3 cells, whereas the tumor suppressor PTEN that also inhibits AKT is increased 54 fold in MDA-MB-468 cells (Figure 5). SKBr3 and MCF-7 cells also exhibit a greater than twofold decrease in the GSK3β, a downstream target of AKT, which is known to inhibit p21 and cyclin D.12 Therefore, these results suggest that in response to MDA-7, MCF-7 and SKBr3 utilize the PDK1-AKT-GSK3β pathway to induce cell cycle arrest. In contrast, the proteins in the PDK1-AKT-GSK3β pathway are not downregulated by MDA-7 expression in MDA-MB-468 cells and therefore are not a predominant mechanism of growth inhibition in MDA-MB-468 cells. These data suggest that the deregulated cell cycle pathways in the ER positive (MCF-7) and HER-2 positive (SKBr3) cells are modulated by MDA-7 to cause growth arrest whereas in MDA-MB-468 the apoptotic pathways are the dominant endpoint of MDA-7 activity.
Figure 5.
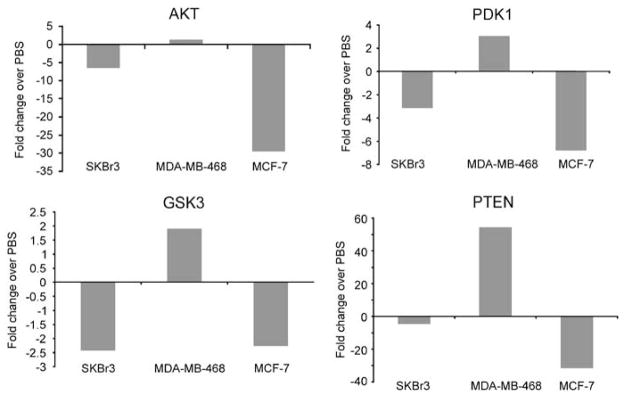
MDA-7 abrogates AKT signaling pathways in SKBr3 and MCF-7 cells. Reverse phase protein array analysis was performed on lysates of SKBr3, MDA-MB-468 and MCF-7 cells after infection with Ad-mda7, Ad-Luc or treatment with phosphate-buffered saline (PBS). Fold change in expression after MDA-7 expression compared with PBS treatment is shown for AKT, PDK1, GSK3β and PTEN.
Discussion
The replication incompetent adenoviral vector Ad-mda7 has been shown to induce apoptosis and growth inhibition in a range of cancer cell lines while exhibiting minimal or no toxicity to normal cells.2–5 This tumor specific apoptotic activity is evident regardless of the receptor status of the particular cell line studied.13,14 We have shown that MDA-7 is able to induce apoptosis in ER positive (MCF-7), HER-2 positive (SKBr3) or triple receptor negative (MDA-MB-468) breast cancer cells (Figures 1a and b). We noted the most significant apoptotic effect of MDA-7 was on the triple negative MDA-MB-468 cell line, whereas the effect of cell cycle arrest was limited to the ER positive or HER-2 positive cells (Figure 1c). However, the degree to which cells underwent apoptosis or cell cycle arrest did not correlate with the amount of MDA-7 protein expression in the cells. The cell lines tested are phenotypically distinct and are therefore differentially sensitive to the effects of the pleiotropic cytokine MDA-7. The observed apoptosis was a caspase-mediated response, evident by the MDA-7-induced increases in cleaved caspase 7 and cleaved PARP in all three cell lines tested. MDA-7 could be mediating these apoptotic effects through downregulation of XIAP in the SKBr3 and MCF-7 cells, but not in MDA-MB-468 cells (Figure 3) as no change in XIAP was detected in these cells after Ad-mda7 treatment. We noted that p21 was increased in MDA-MB-468 without any effect on cell cycle delay and therefore is likely contributing to apoptosis in these cells as well.
The cellular localization of p21 has a critical role in its ability to induce cell cycle arrest.15 Previous studies indicate that the localization of p21 into the cytoplasm renders it with a paradoxical anti-apoptotic effect.16,17 Zhou and Hung15 have demonstrated that AKT activation can lead to this mislocalization of p21, disrupting its potent growth-inhibitory effects. Furthermore, Medema et al.18 reported that activation of AKT with subsequent phosphorylation of its downstream targets resulted in decreased p27 expression in vivo. Our data using immuno-fluorescence confocal microscopic analysis clearly point to the increased expression of both p21 and p27 in all cell lines and both p21 and p27 are noticeably localized solely in the nucleus. Therefore our data suggest that treatment with Ad-mda7 results in relocalization and upregulation of p21 and p27. In addition, both ER positive and HER-2 positive cell lines (MCF-7 and SKBr3, respectively) exhibit cell cycle arrest after MDA-7 expression that can be attributed to an MDA-7-induced decrease in GSK3β expression. The observed arrest in MCF-7 and SKBr3 cells is in different cell cycle phases, presumably due to alterations in checkpoint protein reliability. Surprisingly, CDK2 and cyclin E levels did not decrease significantly after MDA-7 expression despite a G1 arrest in SKBr3 cells. It is likely that CDK2 and cyclin E are not binding as an active complex after MDA-7 expression and this cannot be appreciated through western blot or confocal microscopy. Therefore, the nuclear mechanisms of cell cycle arrest require further investigation.
The MDA-7 induced expression of cleaved caspases, change in localization of CKIs and decreased expression of GSK3β could all result from downregulation of AKT. Therefore, it was not surprising that we observed the downregulation of AKT after adenoviral-mediated over-expression of MDA-7. RPPA analysis showed marked decreases in AKT protein levels in MCF-7 and SKBr3 cell lines, whereas the MDA-MB-468 cells did not exhibit such a response. However, using immunofluorescence confocal microscopy and western blot analyses, we observed a remarkable decrease in AKT expression across all cell lines. It is likely that because the AKT levels in MDA-MB-468 cells are relatively low before MDA-7 expression, the differences in results of the assays are due to varying antibody sensitivities. Thus, MDA-7 acts through AKT to deactivate signaling pathways typically utilized by breast cancer cells, ultimately leading to apoptosis and cell cycle arrest. Interestingly, MDA-MB-468 cells, which have mutated PTEN and lack expression of the protein,19 exhibited increased PTEN expression after infection with Ad-mda7; however this was not the case in MCF-7 cells or SKBr3 cells. Therefore MDA-7 seems to work downstream of PTEN in these two cells lines and may target AKT directly.
It is evident that MDA-7 can ubiquitously induce apoptosis via caspase-mediated pathways. However, depending on the phenotype of breast cancer cells, MDA-7 can modulate distinct pathways to abrogate proliferation. The signaling pathways most likely to be exploited by MDA-7 in MCF-7 and SKBr3 cells cause cell cycle arrest in addition to apoptosis. In addition to driving apoptosis through downregulation of XIAP and upregulation of caspase proteins, MDA-7 acts via AKT and GSK3β and p27 to arrest the cell cycle in MCF-7 and SKBr3 cells. Indeed there is likely cross-talk between the two pathways; for example, AKT could link the apoptotic and cell cycle pathways. These pathways are summarized in Figure 6a. In the triple negative MDA-MB-468 cells, a different set of signals is supporting proliferation of the cells. Therefore, it appears that some of the pathways exploited by MDA-7 in the MCF-7 and SKBr3 cells are not intact in MDA-MB-468 cells. For example, cell cycle arrest is not observed in MDA-MB-468 cells; therefore, the activity of MDA-7 is shifted to intensify the apoptotic response. Figure 6b summarizes the similarities and differences observed in the triple negative MDA-MB-468 cells compared with the ER positive and HER-2 positive cell lines.
Figure 6.
MDA-7 interferes with tumor promoting pathways. (a) A model is proposed to specify the pathways modulated by MDA-7 in the ER + (MCF-7) and HER-2/neu positive (SKBr3) cells. In both cell lines, apoptotic pathways are upregulated and cell cycle arrest is induced. AKT downregulation could be responsible for both outcomes; however XIAP and PDK1 are also affected by MDA-7 and could be involved in mediating the growth inhibitory effects. (b) A model is also proposed to show the differences observed upon MDA-7 expression in the triple negative (MDA-MB-468) cells. We did not observe a cell cycle arrest associated with MDA-7 in MDA-MB-468 cells, nor did we observe any change in p27, PDK1 or XIAP (shown by an ‘x’ through these pathways). Therefore, all the activity of MDA-7 is directed towards proapoptotic effects. (c) Proteins that were detected as having a greater than twofold change in expression by reverse phase protein array analysis are shown in a heat map. Green represents an increase in expression after Ad-mda7 infection compared with control, whereas red represents a decrease. Lighter shades of red or green indicate less than a twofold change, whereas darker shades indicated a greater than fivefold change in the protein. Proteins that showed at least a twofold change in one of the cell lines tested could be grouped into the pathways leading to apoptosis (cleaved caspase 7, cleaved PARP and XIAP), AKT-mediated cell cycle arrest (GSK3β, p21, p27 and AKT) and AKT-mediated cell survival (AKT, PDK1 and PTEN). There were several proteins involved in the mammalian target of rapamycin (mTOR) pathway that were also modulated by MDA-7: pmTOR (mTOR phosphorylated at Ser 2481), estrogen receptor (ER), pTSC2 (tuberin phosphorylated at Thr1462), S6 kinase phosphorylated at Ser 240, the p70/S6Kinase complex, pAMPK (AMP-activated protein kinase phosphorylated at Thr 172) and 4E-binding protein 1 (4E-BP1) phosphorylated at Thr 37.
We analyzed proteins involved in the mammalian target of rapamycin pathway, a pathway controlled by AKT, after MDA-7 expression and although we observed changes in proteins upstream (AMPK, TSC1 and TSC2) and downstream of mammalian target of rapamycin (p70 and S6 Kinase) (Figure 6c), no consistent changes leading to downregulation of the prosurvival pathways could be identified other than downregulation of Thr 37-phosphorylated eukaryotic initiation factor 4E-binding protein 1 (4E-BP1) in all three cell lines. The 4E-BP1 inhibits 5′-cap-dependent mRNA translation by binding and inactivating eIF4E. Thr 37 is phosphorylated by mammalian target of rapamycin and when 4E-BP1 is in a hyperphosphorylated form, it does not inhibit translation of mRNA, some of which encode proteins involved in cell cycle regulation.20 Thus, our RPPA data provides a framework for studying proteins of the AKT/mammalian target of rapamycin pathway as potentially mediating MDA-7-induced growth inhibition and differentiation, but require further validation.
Given the vast array of downstream targets that the AKT pathway may activate, coupled with the diverse set of pathways through which MDA-7 can act, novel treatments approaches can be formulated. Aberrant AKT-induced signal cascades have been implicated in multidrug-resistant tumors including those that over-express HER-2/neu.21,22 Many traditional chemotherapeutics exert their effects via activation of apoptotic and cell cycle pathways.23 As tumors evolve, these pathways may become disrupted consequently leading to chemo-resistance.24 Our group has previously reported that MDA-7 can sensitize tumor cells to the effects of radiotherapy, chemotherapy and hormonal therapy in effect bolstering the current treatment protocols for those tumors that prove to be resistant to conventional therapies.6,7 MDA-7 modulates many key proteins involved in apoptosis and cellular growth including AKT, thus providing a means of overriding or restoring aberrant pathways and restoring sensitivity of cells to chemotherapy, radiotherapy and hormonal therapy. We observed that MDA-7 expression reestablishes ER expression in MDA-MB-468 cells, possibly affecting the phenotype of the cell altering the pathways that the tumor cell is utilizing for survival and perhaps providing a means of targeting these triple receptor negative cells. The further characterization of intracellular signaling pathways particular to cancer cells will help elucidate novel molecular targets for the future of cancer therapy.
Acknowledgments
This work was supported in part by a grant from the Susan G. Komen Breast Cancer Foundation, PDF0504074, awarded to KKH.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.American Cancer Society. Breast Cancer Facts & Figures 2009–2010. American Cancer Society Inc; Atlanta: 2009. [Google Scholar]
- 2.Chada S, Ramesh R, Mhashilkar AM. Cytokine- and chemokine-based gene therapy for cancer. Curr Opin Mol Ther. 2003;5:463–474. [PubMed] [Google Scholar]
- 3.Gopalan B, Litvak A, Sharma S, Mhashilkar AM, Chada S, Ramesh R. Activation of the Fas-FasL signaling pathway by MDA-7/IL-24 kills human ovarian cancer cells. Cancer Res. 2005;65:3017–3024. doi: 10.1158/0008-5472.CAN-04-3758. [DOI] [PubMed] [Google Scholar]
- 4.Mhashilkar AM, Schrock RD, Hindi M, Liao J, Sieger K, Kourouma F, et al. Melanoma differentiation associated gene-7 (mda-7): a novel anti-tumor gene for cancer gene therapy. Mol Med. 2001;7:271–282. [PMC free article] [PubMed] [Google Scholar]
- 5.Saeki T, Mhashilkar A, Chada S, Branch C, Roth JA, Ramesh R. Tumor-suppressive effects by adenovirus-mediated mda-7 gene transfer in non-small cell lung cancer cell in vitro. Gene Ther. 2000;7:2051–2057. doi: 10.1038/sj.gt.3301330. [DOI] [PubMed] [Google Scholar]
- 6.Chada S, Mhashilkar AM, Liu Y, Nishikawa T, Bocangel D, Zheng M, et al. mda-7 gene transfer sensitizes breast carcinoma cells to chemotherapy, biologic therapies and radiotherapy: correlation with expression of bcl-2 family members. Cancer Gene Ther. 2006;13:490–502. doi: 10.1038/sj.cgt.7700915. [DOI] [PubMed] [Google Scholar]
- 7.Kawabe S, Nishikawa T, Munshi A, Roth JA, Chada S, Meyn RE. Adenovirus-mediated mda-7 gene expression radiosensitizes non-small cell lung cancer cells via TP53- independent mechanisms. Mol Ther. 2002;6:637–644. [PubMed] [Google Scholar]
- 8.Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 9.Tibes R, Qiu Y, Lu Y, Hennessy B, Andreeff M, Mills GB, et al. Reverse phase protein array: validation of a novel proteomic technology and utility for analysis of primary leukemia specimens and hematopoietic stem cells. Mol Cancer Ther. 2006;5:2512–2521. doi: 10.1158/1535-7163.MCT-06-0334. [DOI] [PubMed] [Google Scholar]
- 10.Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, Hung MC. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol. 2001;3:245–252. doi: 10.1038/35060032. [DOI] [PubMed] [Google Scholar]
- 11.Liang J, Zubovitz J, Petrocelli T, Kotchetkov R, Connor MK, Han K, et al. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med. 2002;8:1153–1160. doi: 10.1038/nm761. [DOI] [PubMed] [Google Scholar]
- 12.Liang J, Slingerland JM. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle. 2003;2:339–345. [PubMed] [Google Scholar]
- 13.Chada S, Mhashilkar AM, Ramesh R, Mumm JB, Sutton RB, Bocangel D, et al. Bystander activity of Ad-mda7: human MDA-7 protein kills melanoma cells via an IL-20 receptor-dependent but STAT3-independent mechanism. Mol Ther. 2004;10:1085–1095. doi: 10.1016/j.ymthe.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 14.Sarkar D, Lebedeva IV, Gupta P, Emdad L, Sauane M, Dent P, et al. Melanoma differentiation associated gene-7 (mda-7)/IL-24: a ‘magic bullet’ for cancer therapy? Expert Opin Biol Ther. 2007;7:577–586. doi: 10.1517/14712598.7.5.577. [DOI] [PubMed] [Google Scholar]
- 15.Zhou BP, Hung MC. Novel targets of Akt, p21(Cipl/WAF1), and MDM2. Semin Oncol. 2002;29(Suppl 11):62–70. doi: 10.1053/sonc.2002.34057. [DOI] [PubMed] [Google Scholar]
- 16.Asada M, Yamada T, Ichijo H, Delia D, Miyazono K, Fukumuro K, et al. Apoptosis inhibitory activity of cytoplasmic p21(Cip1/WAF1) in monocytic differentiation. EMBO J. 1999;18:1223–1234. doi: 10.1093/emboj/18.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porter AG. Protein translocation in apoptosis. Trends Cell Biol. 1999;9:394–401. doi: 10.1016/s0962-8924(99)01624-4. [DOI] [PubMed] [Google Scholar]
- 18.Medema RH, Kops GJ, Bos JL, Burgering BM. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature. 2000;404:782–787. doi: 10.1038/35008115. [DOI] [PubMed] [Google Scholar]
- 19.DeGraffenried LA, Fulcher L, Friedrichs WE, Grunwald V, Ray RB, Hidalgo M. Reduced PTEN expression in breast cancer cells confers susceptibility to inhibitors of the PI3 kinase/Akt pathway. Ann Oncol. 2004;15:1510–1516. doi: 10.1093/annonc/mdh388. [DOI] [PubMed] [Google Scholar]
- 20.Gingras AC, Kennedy SG, O’Leary MA, Sonenberg N, Hay N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev. 1998;12:502–513. doi: 10.1101/gad.12.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knuefermann C, Lu Y, Liu B, Jin W, Liang K, Wu L, et al. HER2/PI-3K/Akt activation leads to a multidrug resistance in human breast adenocarcinoma cells. Oncogene. 2003;22:3205–3212. doi: 10.1038/sj.onc.1206394. [DOI] [PubMed] [Google Scholar]
- 22.Yu D, Jing T, Liu B, Yao J, Tan M, McDonnell TJ, et al. Overexpression of ErbB2 blocks Taxol-induced apoptosis by upregulation of p21Cip1, which inhibits p34Cdc2 kinase. Mol Cell. 1998;2:581–591. doi: 10.1016/s1097-2765(00)80157-4. [DOI] [PubMed] [Google Scholar]
- 23.Kerr JF, Winterford CM, Harmon BV. Apoptosis. its significance in cancer and cancer therapy. Cancer. 1994;73:2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 24.Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153–164. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]