Summary
Background
Patients with Acute Myelogenous Leukemia (AML) are at risk for thrombotic complications. Risk to develop thrombosis is closely tied to leukemia subtype, and studies have shown an association between leukocytosis and thrombosis in AML M3.
Objective
To investigate the relative roles of cell count and the surface expression of tissue factor (TF) and phosphatidylserine (PS) in the procoagulant phenotype of AML cell lines.
Methods and Results
We evaluated the relative roles of cell count and the surface expression of TF and PS in the procoagulant phenotype of AML cell lines. The TF-positive AML M3 cell lines, NB4 and HL60, and AML M2 cell line, AML14, exhibited both extrinsic tenase and prothrombinase activity in a purified system and promoted experimental thrombus formation. In contrast, the TF-negative AML cell line, HEL, exhibited only prothrombinase activity and did not affect the rate of occlusive thrombus formation. In plasma, NB4, HL60 and AML14 shortened clotting times in a cell-count, PS- and TF-dependent manner. Exposure of cultured NB4, HL60, and AML14 cells to the chemotherapeutic agent daunorubicin increased their extrinsic tenase activity and PS expression. Clot initiation time inversely correlated with logarithm of PS index, defined as the product of multiplying leukocyte count with cell surface phosphatidylserine exposure.
Conclusion
Cultured AML cell lines promote coagulation in a cell count-, TF- and PS-dependent manner. We propose that leukemia cell PS index may serve as a biomarker for procoagulant activity and help identify patients with AML that may benefit from thromboprophylaxis.
Keywords: acute myelogenous leukemia, tissue factor, thrombosis, phosphatidylserine, blood coagulation, leukocyte count
Introduction
Surface expression of tissue factor (TF) has been identified on leukemic cells from some, but not all, patients with AML (1). TF expression has been shown to contribute to leukemic cell procoagulant activity (2–5). Several leukemia-specific oncogenic alleles, including the pathognomonic t(15;17) translocation in AML M3, have been shown to induce overexpression of TF (6, 7). Co-localization of fibrin with leukemia blast cells in marrow and peripheral vasculature suggests a causal relationship between the presence of AML cells and aberrant, intravascular blood coagulation (8, 9). Pathological intravascular coagulation may lead to thrombosis, however, not all patients whose leukemic cells express TF develop clinically evident signs of thrombosis (4, 10).
TF procoagulant activity is closely tied with phosphatidylserine (PS) exposure on the outer cell membrane of cells (11). PS is a charged constituent of a cell’s lipid membrane, and once exposed on the cell membrane outer leaflet facilitates the assembly and activation of coagulation factor complexes. Undifferentiated leukemic cells have been shown to express PS and exhibit procoagulant activity, which may be due to increased rates of apoptosis of peripheral AML blasts (12–14). The procoagulant phenotype of leukemia cells in vivo may be a result of genetic predisposition of AML cells to express TF, combined with physiological events that induce the exposure of PS on TF-expressing AML cells.
In this study, we characterize the prothrombotic and procoagulant phenotypes of four AML cell lines, NB4, HL60, AML14, and HEL as a function of cell count. The NB4 and HL60 cell lines were derived from different patients each with a diagnosis of AML M3 (15, 16). The AML14 cell line was derived from a patient with AML M2 (17). HEL cells have erythroleukemic characteristics (3). Our results demonstrate that extrinsic tenase activity, but not prothrombinase activity, corresponds with the ability of AML cells to drive coagulation and promote occlusive thrombus formation. Clot initiation time was shown to inversely correlate with the logarithm of PS index, which is the product of PS fluorescent intensity and labeled cell count. Determining the PS index may be useful as a biomarker for thrombophilia in AML.
Methods
Materials and Reagents
Daunorubicin hydrochloride (daunorubicin, Teva Parenteral Medicines, Inc, Irvine, CA) was obtained from the OHSU Doernboecher’s Children’s Hospital pharmacy. Fluorescein isothiocyanate (FITC)-conjugated bovine lactadherin, coagulation factors and immune-depleted plasmas were from Haematologic Technologies, Inc. (Essex Junction, VT). FITC-conjugated anti-TF antibodies were from Lifespan Biosciences (Seattle, WA). Equine fibrillar collagen was from ChronoLog (Havertown, PA). Spectrozyme FXa® and Spectrozyme TH® were from American Diagnostica (Stamford, CT). All other reagents were purchased from Sigma or obtained from previously mentioned sources (18).
Blood Collection and Preparation
All blood donations for coagulation studies were collected from healthy volunteers in accordance with Oregon Health and Science University Institutional Review Board Policy. Blood was collected by venipunture directly into sodium citrate (3.2% w/v) at a ratio of 9:1 v/v. To prepare plasma for clotting analysis, blood was subjected to centrifugation at 230×g for 10 min. Platelet rich plasma was pooled from 3 donors. Pooled plasma was centrifuged at 2150×g for 10 min, and platelet poor plasma (PPP) was collected and stored at −80°C.
Plasmas immunodepleted of FVII, FIX or FX (<1%) were replenished with FVII, FIX or FX at 30% to 300% of normal concentrations, respectively. Normal (100%) levels of FVII, FIX and FX were set at 0.5, 4.5 and 10 µg mL−1, respectively.
Cell Culture
Cell lines were from ATCC (Manassas, VA). AML cell lines were cultured in RPMI-1640 media containing 2 mM L-Glutamine, 10% fetal bovine serum, and 1×penicillin and streptomycin. In selected experiments, cells were treated with daunorubicin (0.2 µg mL−1) for two days. Cells were harvested by washing and suspending in HBSS from 107 to 3×102 mL−1.
Plasma Clotting Times
The time to clot plasma in the presence or absence of AML cells was measured as previously described (5). In selected experiments PPP was pretreated with buffer or anti-FXI antibodies (12.5 µg mL−1) and cells were pretreated with an anti-TF antibody (50 µg mL−1), or the PS blocking protein bovine lactadherin (200 nM) for 10 min at RT. Plasma was then mixed 1:1 with vehicle or cell suspensions (102 to 106 mL−1, final count) for 3 min at 37°C on a KC4 Coagulation Analyzer (Trinity Biotech, Wicklow, Bray Co., Ireland) before recalcification (8.3 mM, final Ca2+ concentration). The time for the plasma to clot (if less than 25 min) was recorded in duplicate and repeated 3 independent times.
Ex-vivo Occlusive Thrombus Formation Assay
Occlusive thrombus formation (times to occlusion) for flowing blood in a collagen-coated capillary tube were measured as previously described (19). In brief, glass capillary tubes (2.0 × 0.2 × 50 mm, VitroCom, Mountain Lakes, NJ) were coated with 100 µg mL−1 fibrillar collagen, washed and blocked with BSA prior to use.
Citrate-anticoagulated whole blood was mixed 9:1 v/v with cell suspension (106 cells mL−1, final cell count) and recalcified (7.5 mM Ca2+/3.75 mM Mg2+) immediately before adding to the capillary. Blood was driven at a constant pressure gradient, with an initial wall shear rates of ~300 s−1. The time for blood to cease flowing through the tube was recorded as the time to occlusion.
Chromogenic Measurement of Enzyme Activity
Activation of FX (tenase activity) and prothrombin (prothrombinase activity) was measured by using the initial-rate method of chromogenic substrate hydrolysis. Washed cells (106, final count) or buffer (0.1 M Tris, 0.1 M NaCl, 5 mM CaCl2, 0.1% BSA, pH=8.40) were incubated with factor VIIa (10 nM), or factor Va (10 nM) and factor Xa (15 nM) for 10 min prior to mixing with factor X (150 nM) and Spectrozyme FXa (400 nM), or prothrombin (200 nM) and Spectrozyme TH® (500 nM), respectively. Absorbance of 405 nm light was recorded at 1 min intervals for up to 2 hrs, and the slope of absorbance versus time measured and reported as activity (s−1).
Clot Initiation and Growth Assay
Measurements of the initiation of fibrin clot formation and growth rate were performed by pretreating plasma with an anti-FXI antibody (12.5 µg mL−1) for 10 min at RT prior to mixing with cells (106 to 102 mL−1). Next, the plasma mixture was recalcified (8.3 mM Ca2+) and the absorbance of 405 nm light was recorded at 1 min intervals for 2 hrs. The initial departure from baseline absorbance was recorded as the initiation time, and the slope measured and reported as the growth rate (s−1).
Flow Cytometry
Washed AML cells were incubated with vehicle, FITC-conjugated anti-TF antibody (30 µg mL−1) or FITC-conjugated bovine lactadherin (8.3 µg mL−1) for 30 min at RT. Cells were then washed twice in HBSS before measuring fluorescence with a FACS Calibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ).
Quantification of TF antigen
One million cells were lysed (50 mM Tris-HCl, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate pH=7.5) in the presence of protease inhibitors, for 2 hrs followed by centrifugation at 16,000×g for 10 min. TF antigen levels of the supernatant were determined with a TF ELISA (IMUBIND, American Diagnostica, Stamford, CT).
Statistics
Data is presented as mean and standard error. Flow cytometry results are presented as representative histograms from experiments performed in duplicate and repeated. Data were tested for normality using the Jarque-Bera test. For normally distributed data an ANOVA analysis followed by a Tukey’s post hoc analysis was used to assess statistical significant differences among the different experimental conditions. In the event the data was not normally distributed, we performed a Kruskal-Wallis test to assess statistically significant differences among the different experimental conditions. Significance required p<0.05.
Results
NB4, HL60, AML14 cells express TF and promote experimental thrombus formation
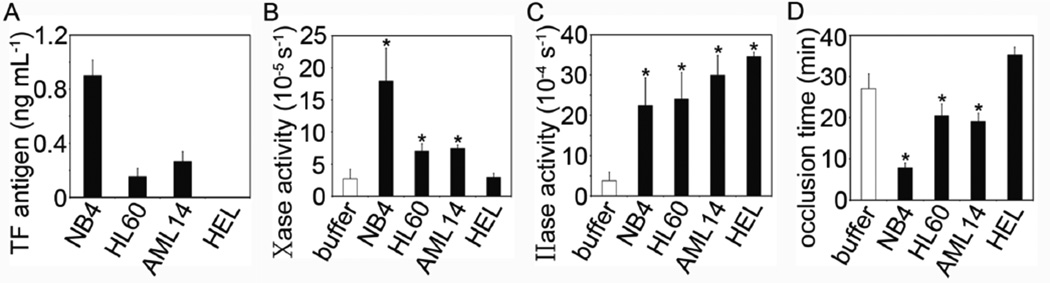
We first confirmed that NB4, HL60 and AML14 had detectable levels of TF antigen, while TF was not detected in HEL cells by ELISA (Fig 1A). While all cell lines demonstrated significant degrees of prothrombinase activity, only NB4, HL60 and AML14 showed significant extrinsic tenase activity relative to buffer (Fig 1B & C). We next measured the ability of AML cells to promote occlusive thrombus formation in whole blood. NB4, HL60 and AML14 cells significantly shortened the time to occlusion as compared to buffer, while HEL cells had no effect on time to occlusion (Fig 1D).
Figure 1.
(A) NB4, HL60, AML14 and HEL cells were lysed and TF antigen levels detected with an ELISA. (B) Cells were incubated with FVIIa (10 nM) and the activation of FX (150 nM) was measured by tracking 405 nm light absorbance in the presence of Spectrozyme Xa®. (C) Cells were incubated with FXa (15 nM) and FVa (10 nM) and the activation of FII (200 nM) measured by tracking 405 nm light absorbance in the presence of Spectrozyme TH®. (D) AML cells were spiked into whole blood, and the time to occlusion measured in an ex vivo occlusive thrombus formation assay. Data are mean ± SE (n = 3). * p<0.05 versus buffer.
TF, PS and cell count regulate the procoagulant phenotype of NB4, HL60 and AML14 cells
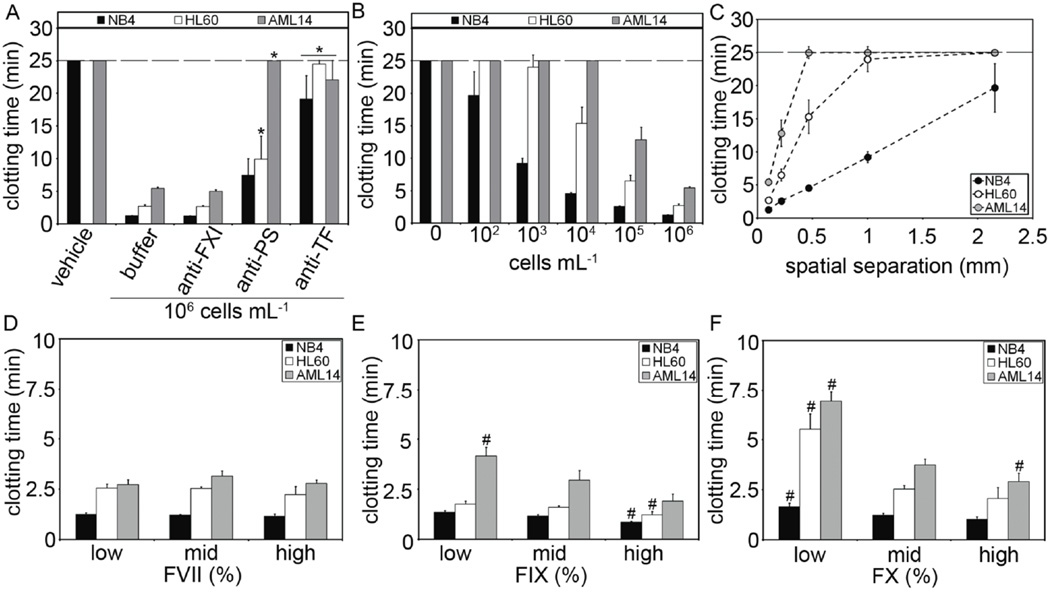
Our next experiments were designed to investigate the relative roles of TF, PS in the ability of NB4, HL60 and AML14 cells to induce clotting of plasma. We show that AML cells shortened clotting times from >25 minutes to <6 minutes (Fig 2A). Pretreating the cells with the PS-blocking protein, bovine lactadherin (anti-PS), prolonged clotting times of NB4 cells to ~5.6 min, HL60 cells to ~10 min, and AML14 cells to ~25 mins, although the increase of NB4 cells clotting time did not reach statistical significance (p<0.07). Pretreating the cells with a TF-blocking antibody prolonged the clotting times of NB4 cells to ~21 min, HL60 cells to ~25 min, and AML14 cells to ~22 min. In contrast, pretreating the plasma with an anti-FXI antibody failed to block the procoagulant effect of NB4, HL60 or AML14 cells. Taken together, our results suggest NB4, HL60 and AML14 cells are procoagulant in a TF- and PS-dependent manner, independent of the FXII/FXI pathway.
Figure 2.
Cells were incubated with buffer, anti-TF antibodies (50 µg mL−1) or bovine lactaderin (anti-PS, 200 nM) prior to mixing with human pooled plasma. An anti-FXI antibody (12.5 µg mL−1) was added in selected experiments. (A) Clotting times were measured following recalcification (8.3 mM Ca2+, final concentration). (B) Final cell count added to plasma was varied (102 to 106 mL−1, final count) and clotting times measured. (C) Clotting times were plotted against the calculated separation distance (spatial separation) of cells in plasma. (D-F) Purified FVII, FIX or FX were added to FVII, FIX or FX-immunodepleted plasmas, respectively. Final coagulation factor concentrations ranged from 30% to 300% of normal levels (30–50% low, 75–125% mid, 150–300% high). AML cells were added and clotting times measured. Data are mean ± SE (n = 3). * p<0.05 versus buffer; # p<0.05 versus mid coagulation factor concentrations.
Peripheral leukocyte counts in patients with AML range up to 108 mL−1 20). Previous studies have shown a relationship between the peripheral AML cell count and activation of coagulation in patients with AML (4, 21–23). Our next experiments were designed to investigate the role of cell count in the ability of NB4, HL60 and AML14 cells to shorten clotting times. Our data show that clotting times were inversely dependent upon cell count (Fig 2B). Our analysis showed that clotting times decreased as a function of mean separation distance between AML cells (spatial separation, cube root of plasma volume/cell; Fig 2C) consistent with previous work utilizing model TF and PS coated microspheres (5).
Previous studies have shown that normal variations of coagulation factor levels and can alter coagulation responses to distinct procoagulant stimuli (24, 25). Our next experiments were designed to investigate whether the concentration of coagulation factors in plasma had an effect on plasma clotting times of NB4, HL60 and AML14 cells. Varying coagulation factor VII levels had no effect on clotting times (Fig 2D). Increasing factor IX and factor X levels from 30–50% (low) to 150–300% (high) of normal levels significantly shortened clotting times by AML cells (2E,F). Our results show that the procoagulant phenotype of NB4, HL60 and AML14 cells were sensitive to the levels of plasma coagulation factor IX and X within the physiological range of variation.
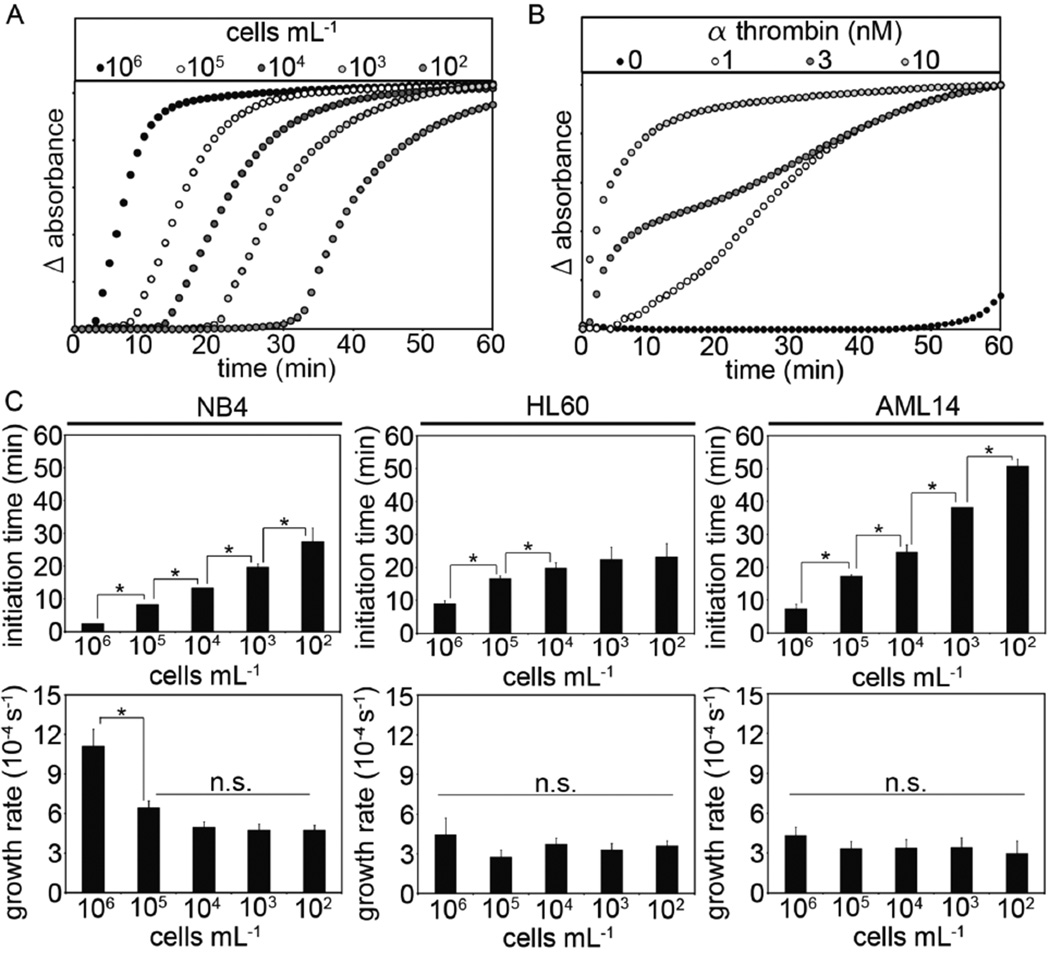
The kinetics of fibrin formation triggered by procoagulant cells is known to proceed by a linear growth rate following a significant time-lag (initiation time) (26). A representative fibrin formation curve following the addition of HL60 cells to plasma is shown in Figure 3A, while a reference curve for exogenously added thrombin-induced fibrin formation is shown in Figure 3B. Our results show that the initiation time was inversely dependent upon cell count for NB4, HL60 and AML14 cells. Clot growth rates were independent of cell count for HL60 and AML14 cells, while clot growth rates increased for NB4 cells above 104 cells mL−1 Fig 3C).
Figure 3.
AML cells were mixed with human pooled plasma (102 to 106 mL−1) prior to recalcification (8.3 mM, final Ca2+ concentration). A representative result for the clot formation assay following the addition of either (A) AML cells or (B) thrombin is shown. Initiation times were recorded as the time at which the absorbance of 405 nM light increased above baseline, and the growth rate measured as the slope of absorbance versus time. (C) Clot initiation times and clot growth rates were measured for NB4, HL60 and AML14 cells at 102 to 106 mL−1 final cell counts. Data are mean ± SE (n = 3). * p<0.05; n.s.=not significant.
Effects of daunorubicin on the procoagulant activity of NB4, HL60 and AML14 cells
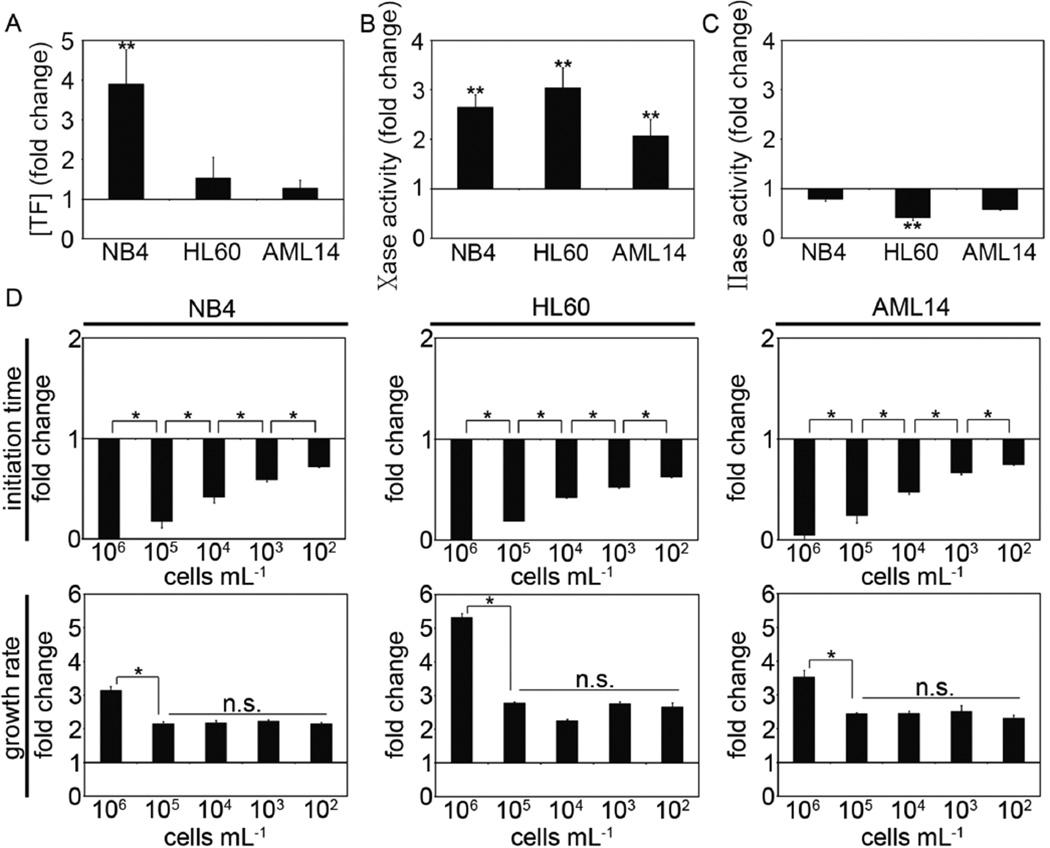
Daunorubicin is a component of induction chemotherapy in AML. In vitro, exposing AML cells to daunorubicin has been shown to increase the procoagulant activity of cultured cells (12, 27). Our next experiments were designed to investigate whether treatment of AML cells with daunorubicin changed their procoagulant phenotype. Our results show that TF antigen levels for NB4 cells significantly increased by approximately 4-fold following exposure to daunorubicin for two days, while changes in TF antigen levels of HL60 or AML14 cells remained unchanged (Fig 4A). The tenase activity of NB4 and HL60 cell increased ~3-fold following exposure to daunorubicin, while tenase activity of AML14 cells doubled (Fig 4B). Prothrombinase activity remained unchanged for NB4 and AML14 cells, and significantly decreased for HL60 cells following exposure to daunorubicin (Fig 4C). Clot initiation times for NB4, HL60 and AML14 cells shortened in a cell count-dependent manner, and clot growth rates increased for NB4, HL60 and AML14 cells following exposure to daunorubicin (Fig 4D). Daunorubicin treatment caused clot growth rates for NB4 cells to increase three-fold at cell counts of 106 mL−1, and double for cell counts from 105 to 102 mL−1. Clot growth rates for HL60 cells increased >5-fold over untreated cells at 106 cells mL−1, and ~3-fold at cell counts from 105 to 102 mL−1. Clot growth rates for AML14 cells increased >3-fold over untreated cells at 106 cells mL−1 and >2-fold for cell counts from 105 to 102 mL−1 following exposure to daunorubicin.
Figure 4.
NB4, HL60 and AML14 cells were treated with daunorubicin (0.2 µg mL−1) for 2 days. (A) TF antigen levels of treated cells were measured with an ELISA. (B) Cells were incubated with FVIIa (10 nM) and the activation of FX (150 nM) was measured by tracking 405 nm light absorbance in the presence of Spectrozyme Xa®. (C) Cells were incubated with FXa (15 nM) and FVa (10 nM) and the activation of prothrombin measured by tracking 405 nm light absorbance in the presence of Spectrozyme TH®. (D) Initiation time and growth rate were measured and shown as the fold-change relative to untreated cells. Data are mean ± SE (n = 3). ** p<0.05 versus untreated cells; ** p<0.05; n.s.=not significant.
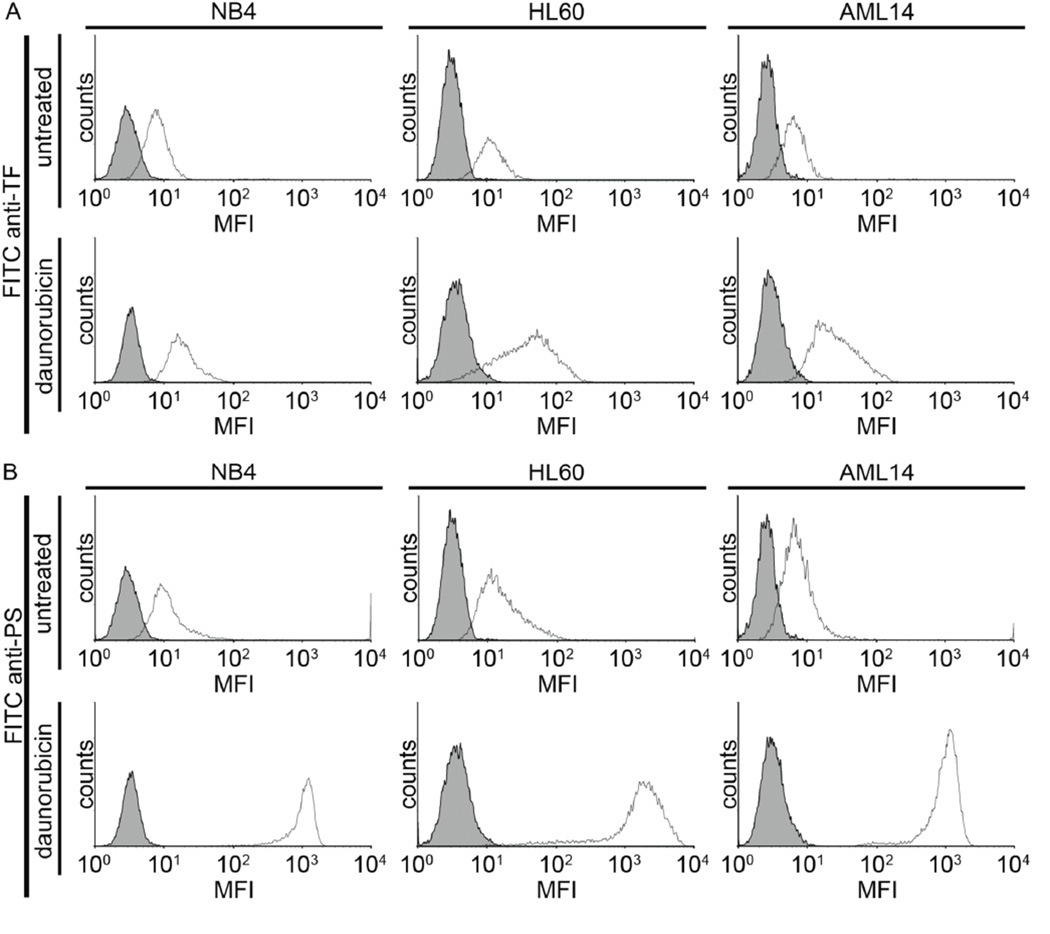
We next characterized the effect of daunorubicin on PS expression. Untreated AML cells were all weakly positive for TF and PS. Daunorubicin treatment resulted in a moderate increases in TF labeling (Fig 5A), yet dramatic increases in PS labeling (Fig 5B) for NB4, HL60 and AML14 cells. Taken together, we observed that exposure to daunorubicin caused an increase in TF and PS exposure, increased extrinsic tenase activity, shortened clot initiation times in a cell count-dependent manner, and increased clot growth rates for NB4, HL60 and AML14 cells.
Figure 5.
NB4, HL60 and AML14 cells were treated with vehicle or daunorubicin (dauno, 0.2 µg mL−1) for 2 days. Cells were stained with FITC-conjugated anti-TF (30 µg mL−1) or FITC-conjugated bovine lactadherin (8.3 µg mL−1), washed and mean fluorescence intensity (MFI) measured via flow cytometry. Shaded histograms represent unstained cells. Representative histograms from 2 independent experiments performed in duplicate.
Correlation of clot initiation times with cell count, TF- and PS index
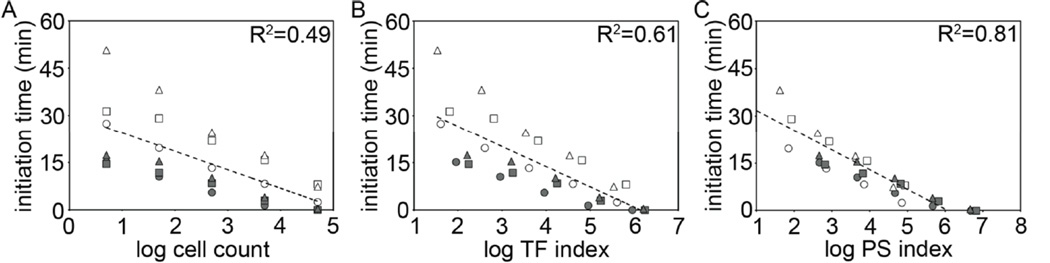
We analyzed the clot initiation times for all the clotting experiments performed with NB4, HL60 and AML14 cells in the plate reader. The logarithm of cell count alone did not correlate with clot initiation times (R2=0.49, p = 4.4 × 10−5, Fig 6A). The logarithm of TF index, the product of TF mean fluorescent intensity and cell count, resulted in an improved, but still weak correlation with clot initiation times (R2=0.61, p =1.0 × 10−6, Fig 6B). The logarithm of PS index, the product of PS mean fluorescent intensity and cell count, correlated strongly with clot initiation times for NB4, HL60 and AML14 cells in untreated and daunorubicin exposed conditions (R2=0.81, p = 2.4 × 10−11, Fig 6C). Taken together, our results suggest that PS index may provide a fluorescent labeling strategy to account for cell-type and cell count differences in procoagulant activity of TF-expressing AML cells.
Figure 6.
Correlation of clot initiation times with cell count, TF- and PS index. Mean clot initiation times were combined and plotted against the logarithm of (A) cell count, (B) TF index (anti-TF MFI × cell count) or (C) PS index (lactadherin MFI × cell count) for plate reader clotting experiments with untreated (blank) and daunorubicin-treated (shaded) NB4 (○), HL60 (△) and AML14 (□) cells. Regression analysis was performed using the method of least squares and represented by R2 values.
Discussion
Venous and arterial thrombosis significantly contributes to the morbidity and mortality of patients with acute myelogenous leukemia (AML) (28–32). The cumulative incidence of thrombosis in AML has been reported from 3–12%, with variation in thrombosis rates between AML subtypes (30, 32, 33). Anticoagulation is effective at preventing thrombosis in AML; however, it is not safe enough to be part of routine patient care due to thrombocytopenia during induction therapy (30, 34). A better understanding of the mechanism of thrombophilia in AML may help identify patients at high risk to develop thrombosis who may benefit from prophylactic anticoagulation.
Our results support a role for extrinsic tenase activity as a driver of the procoagulant phenotype of AML cell lines. Despite a common procoagulant mechanism, we found that NB4 cells shortened plasma clotting times and experimental thrombus formation times more than HL60 and AML14 cells. A prior study with HL60 cells observed that PS labeling correlated with experimentally increased procoagulant activity when controlling for cell count (27). Further, the majority of procoagulant activity of AML cells isolated from different patients with AML M3 was due to PS exposure (14). Through calculation of a PS index, we extend the potential for PS labeling to correlate with procoagulant activity across different cell types and counts, regardless of whether the cells had been treated with daunorubicin. As the cell lines utilized in this study were isolated from different patients with different AML subtypes, our results suggest a broader role for TF- and PS- expression to contribute to the coagulopathy in AML beyond AML M3. TF and PS have been shown to contribute to the procoagulant activity of freshly isolated AML cells from some patients across all AML subtypes, while marked leukocytosis has been associated with increased risk of thrombosis in AML M3 (21). Conversely, when evaluating thrombosis in patients with all AML subtypes, peripheral leukocyte count was not associated with thrombosis (30, 31). The TF expression and procoagulant activity of AML M3 cells are consistently found to be higher than cells from non-M3 subtypes (4). We demonstrate that the procoagulant phenotype of TF-positive AML cells is dependent upon the cell count. Thus, we hypothesize that the number of TF- and PS-expressing cells in the peripheral blood of patients with AML may predict risk to develop thrombosis. Along these lines, patients whose AML cells are TF-negative would not be expected to have an increased risk of thrombosis due to elevated leukocyte counts. For patients with TF-positive AML cells, low to moderate counts of peripheral AML cells, or peripheral AML cells with low PS exposure may initiate coagulation but fail to form sufficient fibrin or platelet activation to develop a clinically significant thrombus. Conversely, we propose that a high peripheral cell count and increased PS exposure of TF-positive AML cells would enhance clot formation and the risk to develop thrombosis. Anecdotal support for the former scenario is supported by the association of elevated leukocyte counts and thrombosis in AML M3, and the latter, by increased risk of thrombosis following administration of cytotoxic therapies.
Our study demonstrates that the logarithm of PS index (lactadherin labeling intensity × number of labeled cells) correlated with procoagulant activity across cell types and cell counts, supporting a role for PS-labeling in determining TF-positive AML cell procoagulant activity. PS is not sufficient to initiate coagulation; therefore, the role for PS-labeling to correlate with procoagulant activity of isolated cells would likely require a determination of TF antigen as well. Labeling of TF and PS together may increase specificity of a PS index. We propose that PS index has potential as a biomarker for thrombophilia in AML.
Acknowledgments
This work was supported by the OHSU Knight Cancer Institute and the National Institutes of Health (NIH) grants U54CA143906 (O.J.T.M), U54CA143798 (R.L.L), R01HL101972 (A.G. and O.J.T.M.), HL106919 (A.G), and the Oregon Clinical and Translational Research Institute (UL1TR000128). G.W.T was supported by a Joel Drillings Award for Cardiovascular Research from the American Heart Association (12PRE11930019). O.J.T.M. is an American Heart Association (AHA) Established Investigator (13EIA12630000). G.W.T is an Achievement Rewards for College Scientists scholar. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the AHA.
Footnotes
Disclosure of Conflicts of Interest
A.G. and Oregon Health & Science University have a significant financial interest in Aronora Inc, a company that may have a commercial interest in the result of this research. This potential conflict of interest has been reviewed and managed by the Oregon Health & Science University Conflict of Interest in Research Committee. The remaining authors declare no competing financial interests.
Authorship Contributions
Contribution: G.T. designed the project, performed research, analyzed data, and wrote the manuscript; O.R. performed research; R.L.L. contributed research tools/reagents and assisted in writing the manuscript, A.G. assisted in writing the manuscript and O.J.T.M. supervised the research and assisted in writing the manuscript.
References
- 1.Lopez-Pedrera C, Barbarroja N, Dorado G, Siendones E, Velasco F. Tissue factor as an effector of angiogenesis and tumor progression in hematological malignancies. Leukemia. 2006 Aug;20(8):1331–1340. doi: 10.1038/sj.leu.2404264. [DOI] [PubMed] [Google Scholar]
- 2.Falanga A, Iacoviello L, Evangelista V, Belotti D, Consonni R, D'Orazio A, et al. Loss of blast cell procoagulant activity and improvement of hemostatic variables in patients with acute promyelocytic leukemia administered all-trans-retinoic acid. Blood. 1995 Aug 1;86(3):1072–1081. [PubMed] [Google Scholar]
- 3.Marchetti M, Diani E, ten Cate H, Falanga A. Characterization of the thrombin generation potential of leukemic and solid tumor cells by calibrated automated thrombography. Haematologica. 2012 Aug;97(8):1173–1180. doi: 10.3324/haematol.2011.055343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanaka M, Yamanishi H. The expression of tissue factor antigen and activity on the surface of leukemic cells. Leuk Res. 1993 Feb;17(2):103–111. doi: 10.1016/0145-2126(93)90054-o. [DOI] [PubMed] [Google Scholar]
- 5.Tormoen GW, Rugonyi S, Gruber A, McCarty OJ. The role of carrier number on the procoagulant activity of tissue factor in blood and plasma. Phys Biol. 2011 Dec;8(6):066005. doi: 10.1088/1478-3975/8/6/066005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan J, Wang K, Dong L, Liu H, Chen W, Xi W, et al. PML/RARalpha fusion protein transactivates the tissue factor promoter through a GAGC-containing element without direct DNA association. Proc Natl Acad Sci U S A. 2010 Feb 23;107(8):3716–3721. doi: 10.1073/pnas.0915006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falanga A, Rickles FR. Management of Thrombohemorrhagic Syndromes (THS) in hematologic malignancies. Hematology Am Soc Hematol Educ Program. 2007:165–171. doi: 10.1182/asheducation-2007.1.165. [DOI] [PubMed] [Google Scholar]
- 8.Meyer JS, Boxer M. Leukemic cellular thrombi in pulmonary blood vessels. Subleukemic myelogenous leukemia following chloramphenicol-induced aplastic anemia. Cancer. 1973 Sep;32(3):712–721. doi: 10.1002/1097-0142(197309)32:3<712::aid-cncr2820320325>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 9.Takemori N, Hirai K, Onodera R, Uenishi H, Saito N, Takasugi Y, et al. Disseminated intravascular coagulation in a patient with acute myeloid leukemia. Ultrastructural evidence of hypercoagulation in bone marrow. Am J Clin Pathol. 1993 Jun;99(6):695–701. doi: 10.1093/ajcp/99.6.695. [DOI] [PubMed] [Google Scholar]
- 10.Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013 Jan;13(1):34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- 11.Shaw AW, Pureza VS, Sligar SG, Morrissey JH. The local phospholipid environment modulates the activation of blood clotting. J Biol Chem. 2007 Mar 2;282(9):6556–6563. doi: 10.1074/jbc.M607973200. [DOI] [PubMed] [Google Scholar]
- 12.Boles JC, Williams JC, Hollingsworth RM, Wang JG, Glover SL, Owens AP, 3rd, et al. Anthracycline treatment of the human monocytic leukemia cell line THP-1 increases phosphatidylserine exposure and tissue factor activity. Thromb Res. 2012 Feb;129(2):197–203. doi: 10.1016/j.thromres.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Weiss I, Svoboda K, Kwaan HC. Thrombogenic role of cells undergoing apoptosis. Br J Haematol. 2001 Nov;115(2):382–391. doi: 10.1046/j.1365-2141.2001.03095.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhou J, Shi J, Hou J, Cao F, Zhang Y, Rasmussen JT, et al. Phosphatidylserine exposure and procoagulant activity in acute promyelocytic leukemia. J Thromb Haemost. 2010 Apr;8(4):773–782. doi: 10.1111/j.1538-7836.2010.03763.x. [DOI] [PubMed] [Google Scholar]
- 15.Lanotte M, Martin-Thouvenin V, Najman S, Balerini P, Valensi F, Berger R. NB4, a maturation inducible cell line with t(15;17) marker isolated from a human acute promyelocytic leukemia (M3) Blood. 1991 Mar 1;77(5):1080–1086. [PubMed] [Google Scholar]
- 16.Gallagher R, Collins S, Trujillo J, McCredie K, Ahearn M, Tsai S, et al. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood. 1979 Sep;54(3):713–733. [PubMed] [Google Scholar]
- 17.Paul CC, Tolbert M, Mahrer S, Singh A, Grace MJ, Baumann MA. Cooperative effects of interleukin-3 (IL-3), IL-5, and granulocyte-macrophage colony-stimulating factor: a new myeloid cell line inducible to eosinophils. Blood. 1993 Mar 1;81(5):1193–1199. [PubMed] [Google Scholar]
- 18.White-Adams TC, Berny MA, Patel IA, Tucker EI, Gailani D, Gruber A, et al. Laminin promotes coagulation and thrombus formation in a factor XII-dependent manner. J Thromb Haemost. 2010 Jun;8(6):1295–1301. doi: 10.1111/j.1538-7836.2010.03850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berny MA, Patel IA, White-Adams TC, Simonson P, Gruber A, Rugonyi S, et al. Rational Design of an Ex Vivo Model of Thrombosis. Cellular and Molecular Bioengineering. 2010 Jun;3(2):187–189. [Google Scholar]
- 20.Lichtman MA, Rowe JM. Hyperleukocytic leukemias: rheological, clinical, and therapeutic considerations. Blood. 1982 Aug;60(2):279–283. [PubMed] [Google Scholar]
- 21.Breccia M, Avvisati G, Latagliata R, Carmosino I, Guarini A, De Propris MS, et al. Occurrence of thrombotic events in acute promyelocytic leukemia correlates with consistent immunophenotypic and molecular features. Leukemia. 2007 Jan;21(1):79–83. doi: 10.1038/sj.leu.2404377. [DOI] [PubMed] [Google Scholar]
- 22.Scharf RE, Schneider W. Relationship of thrombin generation to peripheral blast cell count in patients with acute myeloblastic leukemia (AML) Eur J Haematol. 1990 May;44(5):273–276. doi: 10.1111/j.1600-0609.1990.tb00394.x. [DOI] [PubMed] [Google Scholar]
- 23.Stoffel N, Rysler C, Buser A, Gratwohl A, Tsakiris DA, Stern M. Leukocyte count and risk of thrombosis in patients undergoing haematopoietic stem cell transplantation or intensive chemotherapy. Thromb Haemost. 2010 Jun;103(6):1228–1232. doi: 10.1160/TH09-10-0700. [DOI] [PubMed] [Google Scholar]
- 24.Brummel-Ziedins KE, Pouliot RL, Mann KG. Thrombin generation: phenotypic quantitation. J Thromb Haemost. 2004 Feb;2(2):281–288. doi: 10.1046/j.1538-7933.2003.00576.x. [DOI] [PubMed] [Google Scholar]
- 25.Tormoen GW, Khader A, Gruber A, McCarty OJ. Physiological levels of blood coagulation factors IX and X control coagulation kinetics in an in vitro model of circulating tissue factor. Phys Biol. 2013 Apr 15;10(3):036003. doi: 10.1088/1478-3975/10/3/036003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jungi TW. A turbidimetric assay in an ELISA reader for the determination of mononuclear phagocyte procoagulant activity. J Immunol Methods. 1990 Oct 4;133(1):21–29. doi: 10.1016/0022-1759(90)90314-l. [DOI] [PubMed] [Google Scholar]
- 27.Langer F, Amirkhosravi A, Loges S, Meyer T, Eifrig B, Hossfeld DK, et al. An in vitro study on the mechanisms of coagulation activation in acute myelogenous leukemia (AML): role of tissue factor regulation by cytotoxic drugs and GM-CSF. Thromb Haemost. 2004 Nov;92(5):1136–1146. doi: 10.1160/TH04-04-0215. [DOI] [PubMed] [Google Scholar]
- 28.Oehadian A, Iqbal M, Sumantri R. Deep vein thrombosis in acute myelogenous leukemia. Acta Med Indones. 2009 Oct;41(4):200–204. [PubMed] [Google Scholar]
- 29.Falanga A, Marchetti M. Venous thromboembolism in the hematologic malignancies. J Clin Oncol. 2009 Oct 10;27(29):4848–4857. doi: 10.1200/JCO.2009.22.8197. [DOI] [PubMed] [Google Scholar]
- 30.De Stefano V, Sora F, Rossi E, Chiusolo P, Laurenti L, Fianchi L, et al. The risk of thrombosis in patients with acute leukemia: occurrence of thrombosis at diagnosis and during treatment. J Thromb Haemost. 2005 Sep;3(9):1985–1992. doi: 10.1111/j.1538-7836.2005.01467.x. [DOI] [PubMed] [Google Scholar]
- 31.Ziegler S, Sperr WR, Knobl P, Lehr S, Weltermann A, Jager U, et al. Symptomatic venous thromboembolism in acute leukemia. Incidence, risk factors, and impact on prognosis. Thromb Res. 2005;115(1–2):59–64. doi: 10.1016/j.thromres.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 32.Mohren M, Markmann I, Jentsch-Ullrich K, Koenigsmann M, Lutze G, Franke A. Increased risk of venous thromboembolism in patients with acute leukaemia. Br J Cancer. 2006 Jan 30;94(2):200–202. doi: 10.1038/sj.bjc.6602945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ku GH, White RH, Chew HK, Harvey DJ, Zhou H, Wun T. Venous thromboembolism in patients with acute leukemia: incidence, risk factors, and effect on survival. Blood. 2009 Apr 23;113(17):3911–3917. doi: 10.1182/blood-2008-08-175745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melillo L, Grandone E, Colaizzo D, Cappucci F, Valvano MR, Cascavilla N. Symptomatic venous thromboembolism and thrombophilic status in adult acute leukemia: a single-center experience of 114 patients at diagnosis. Acta Haematol. 2007;117(4):215–220. doi: 10.1159/000098700. [DOI] [PubMed] [Google Scholar]