Abstract
Objectives
Data on the incidence of symptomatic osteoarthritis (OA) are scarce. We estimated incidence of clinical hip, knee and hand osteoarthritis, and studied the effect of prevalent OA on joint-specific incident OA.
Methods
SIDIAP contains primary care records for >5 million people from Catalonia (Spain). Participants aged ≥40 years with an incident diagnosis of knee, hip or hand OA between 2006 and 2010 were identified using ICD-10 codes. Incidence rates and female-to-male Rate Ratios (RR) for each joint site were calculated. Age, gender and body mass index-adjusted Hazard Ratios (HR) for future joint-specific OA according to prevalent OA at other sites were estimated using Cox regression.
Results
3,266,826 participants were studied for a median of 4.45 years. Knee and hip OA rates increased continuously with age, and female-to-male RRs were highest at age 70-75 years. In contrast, female hand OA risk peaked at age 60-64 years, and corresponding female-to-male RR was highest at age 50-55.
Adjusted HR for prevalent knee OA on risk of hip OA was 1.35 (99%CI 1.28-1.43); prevalent hip OA on incident knee OA 1.15 (1.08-1.23). Prevalent hand OA predicted both incident knee and hip OA: HR 1.20 (1.14-1.26) and 1.23 (1.13-1.34) respectively.
Conclusions
The effect of age is greatest in the elderly for knee and hip OA, but around the menopause for hand OA. OA clusters within individuals, with higher risk of incident knee and hip disease from prevalent lower limb and hand OA.
BACKGROUND
According to national health survey statistics (Catalan Health Survey 2011), self-reported OA is highly common in the population of Catalonia (Spain): 46% and 21% of women and men aged 45 and over in the region respectively report suffering OA (1). Cohort studies carried out in Spain have shown a high prevalence of knee and hand OA of 10% and 6% respectively (2). OA has a huge impact on health care costs: in Spain, costs of knee and hip OA were of more than 4,700 million euro in 2007, equivalent to a 0.5% of the national gross national product that same year (3)
Despite this, national data on incidence of OA are lacking, and studies are scarce even in the international context. Although estimates of incident OA have been published for other populations, most of these reports focused on radiographic OA (4, 5), which is much more common but less clinically and economically relevant than symptomatic OA (6). Only one of these studies explored the epidemiology of hip, knee, and hand OA in the same cohort (7).
Women have consistently been shown to be at higher risk of hip, knee and hand OA, and some studies have even reported a lower joint space width and higher narrowing in females (8). Whether this increase in risk is constant with age or changes in relation with endogenous oestrogen production and menopause is unclear (9, 10).
We used a large population-based database to characterize the risk of incident OA related to age, gender and osteoarthritis at other joints by: (1.) estimating age and gender-specific incidence rates of clinically diagnosed knee, hip and hand OA in actual practice conditions, and (2.) studying the effect of a previous history of knee, hip and hand OA on risk of future incident joint-specific OA.
METHODS
Study design
Population-based, retrospective cohort study
Study population
General practitioners (GPs) are responsible for primary health care, long-term and most short-term drug prescriptions and referrals to both specialists and hospitals in Spain. The Spanish public healthcare system is universal, covering the practical totality of the population. The data for this study were obtained from the SIDIAP (Sistema d’Informació per al Desenvolupament de l’Investigació en Atenció Primària) Database. SIDIAP (www.sidiap.org) comprises of anonymised primary care electronic medical records of a highly representative sample of patients attending GPs in Catalonia (North-East Spain), covering a population of about 5 million patients (80% of the total population) from 274 primary care practices and with a total of 3,414 participating GPs. SIDIAP comprises of the clinical and referral events registered by primary care health professionals in medical records, comprehensive demographic information, prescription and corresponding pharmacy invoicing data, specialist referrals, primary care laboratory test results, hospital admissions, and their major outcomes (11). Health professionals gather this information using ICD-10 codes, and structured spreadsheets designed for the collection of relevant variables such as country of origin, gender, age, body mass index, smoking and drinking status, blood pressure measurements, etc. Encoding personal and clinic identifiers ensures the confidentiality of the information in the SIDIAP Database. All participants aged ≥40 years old SIDIAP with no history of OA at index joint on 01/01/2006 were eligible for this study. Study participants were then followed up until the end of study (31/12/2010), transfer out or death date, whatever came first.
Ascertainment of clinically diagnosed osteoarthritis
Date of incident diagnoses of OA registered in the period from 1/1/2006 to 31/12/2010 in the study population were identified using ICD-10 codes for the following: knee OA (M17, M17.0, M17.1, M17.2, M17.3, M17.4, M17.5, and M17.9), hip OA (M16, M16.0, M16.1, M16.2, M16.3, M16.4, M16.5, M16.6, M16.7, and M16.9) and hand OA (M15.1, M15.2, M18, M18.0 to M18.5, and M18.9). Data on OA coding within SIDIAP have been validated against self-reported OA in the GLOW population-based cohort (12). SIDIAP does not contain data on laterality of the joint affected, and therefore joint site (eg knee) is considered here as a whole.
Validation of OA diagnosis in SIDIAP
Previous studies have shown that an OA diagnosis in SIDIAP is highly correlated with self-reported OA (sensitivity 71% (95% CI 69% to 73%), specificity 94% (95% CI 92% to 95%) in the GLOW cohort(12). To explore this further, we reviewed free text from the medical records of a random sample of 150 study participants with a newly registered OA code. We extracted any free text introduced in the primary care records in the 3 months before and after the date of an OA diagnosis. Then, we have used automatic natural language chain methods to anonymise the extracted text. Finally, one of the investigators, with a medical background and extensive knowledge of the Catalan and Spanish language (DPA) reviewed this text to identify: 1.mention of an X-Ray report, and 2.an alternative diagnosis.
Age and gender measurements
Age at cohort entry (on 1/1/2006 or first registration date in SIDIAP) was calculated based on date of birth, which was obtained from ID card or passport as well as gender.
Statistical analyses
Age (in 5-year groups) and gender-specific incidence rates (and 99% confidence intervals) in the study period were estimated. Age-specific female-to-male Rate Ratios (and 99% confidence intervals) were calculated using Poisson regression.
Survival analysis methods were used to model time from study initiation (1/1/2006) or date when the patient registered in one of the primary care practices covered by SIDIAP (whatever came last) to the date when the first of these events occurred: joint site OA diagnosis, date when the patient transferred out of the area, date of death, or date of end of study (31/12/2010). Cox regression modelling was used to compute adjusted (for age, gender and body mass index) hazard ratios (HR) and 99% confidence intervals for an incident clinical diagnosis of hip and knee OA according to prevalent joint-specific OA status. As a proportion of participants had missing information for one of the confounders (body mass index), we created a category for patients with missing values in order to avoid dropping these patients in the multivariable analysis. All the statistical analyses were carried out using Stata SE for Mac version 12.0.
RESULTS
After excluding SIDIAP participants aged <40 years, 3,266,826 participants were observed for a median (inter-quartile range) of 4.45 (4.19-4.98) years. Among these, 96,222, 30,350 and 37,590 incident cases of knee, hip and hand OA were identified respectively. In addition, 4,492 patients developed incident hip and hand OA, 14,171 knee and hand OA, 14,585 knee and hip OA, and 1,391 people developed incident OA at these three sites during the observation period. Baseline characteristics for these three groups and for patients with no prevalent or incident OA are shown in Table 1. New cases of hand OA were younger, more likely to be females, thinner, and had fewer co-morbidities than participants with newly diagnosed knee or hip OA.
TABLE 1. Baseline characteristics of newly diagnosed OA patients.
| Knee OA N = 96,222 |
Hip OA N = 30,350 |
Hand OA N = 37,590 |
No incident or preva-lent OA N = 2.955 m |
||
|---|---|---|---|---|---|
| Age | mean(SD) | 67.18 (11.17) |
67.79 (11.81) |
63.93 (11.65) |
61.30 (15.51) |
| Gender | n(%) males | 34243 (35.59) |
12698 (41.84) |
9,738 (25.91) |
1,472,916 (49.84) |
|
Body Mass
Index (BMI) N (%) excluding missings |
<18.5 kg/m2
(underweight) |
85 (0.11) | 72 (0.31) | 79 (0.29) | 126,374 (8.3) |
|
18.5 to <25
(normal weight) |
7795 (10.44) | 3756 (16.28) | 5720 (20.8) | 413,634 (27.2) |
|
|
25 to <30
(overweight) |
28259 (37.86) |
10009 (43.38) |
11577 (42.09) |
646,805 (42.6) |
|
|
≥30 kg/m2
(obese) |
38508 (51.59) |
9235 (40.03) | 10129 (36.83) |
332,792 (21.9) |
|
|
MISSING (%
out of total N) |
21,575 (22.42) |
7,278 (24.0) | 10,085 (26.83) |
1,435,751 (48.6) |
|
|
Co-morbid
conditions N (%) |
Myocardial
Infarction |
2,038 (2.12) | 786 (2.59) | 581 (1.55) | |
| Stroke | 3,172 (3.30) | 1208 (3.98) | 926 (2.46) | ||
| COPD | 6,684 (6.95) | 2604 (8.58) | 2155 (5.73) | ||
| DM | 17,901 (18.60) |
5577 (18.38) | 5199 (13.83) |
||
| HT | 53,377 (55.47) |
16252 (53.55) |
16928 (45.03) |
COPD = Chronic Obstructive Pulmonary Disease; DM = Type 2 Diabetes Mellitus; HT = Hypertension
Among the reviewed medical records extracts for 150 participants with an incident OA diagnosis, 59 (39.3%) had evidence of an X-Ray confirmation, and only 2 (1.3%) patients had an alternative diagnosis (1 polymyalgia rheumatica, and 1 senile rheumatoid arthritis).
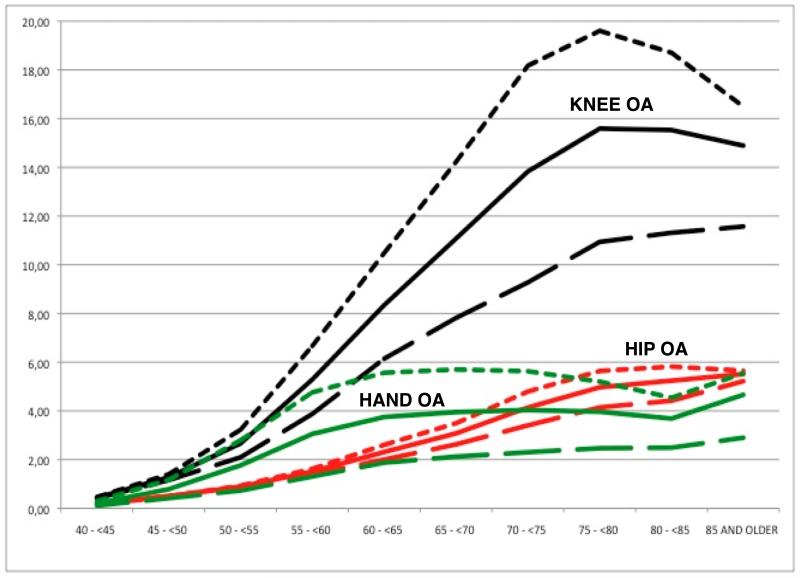
Incidence rates (IR) [99% Confidence Intervals] of knee OA were 6.5/1,000 person-years [6.4-6.6] overall, 8.3 [8.2-8.4] for female participants, and 4.6 [4.5-5.7] for males. Similarly, IR of hip and hand OA were: 2.1 [2.0-2.1] and 2.4 [2.4-2.4] overall, 2.4 [2.4-2.5] and 3.5 [3.5-3.6] for females, and 1.7 [1.7-1.8] and 1.3 [1.2-1.3] amongst male participants. Age and gender-specific IR of knee, hip and hand OA are shown in Figure 1 (and Supplementary Tables ST1, ST2 and ST3 respectively). In female participants, rates of knee and hip OA increased progressively with age, with a steepest slope in the ages 50 to 70 years, which then slowed down and peaked at 75-80 and at 80-85 years respectively, with a slight decrease in the final years of life (age 85 and older) [Figures 1a and 1b]. In contrast, IRs of hand OA in females increased more rapidly at earlier ages (40-45 years onwards) peaking at 60 years and then decreased in the elderly (>65) until reaching a trough at age 80-85 years to increase again in the last years of life (>85) [Figure 1c]. Rates of knee, hip and hand OA followed a more similar pattern in males: IRs increased continuously with age, and peaked only in the oldest ages (>85 years).
Figure 1.
Age and gender-specific Incidence Rates (/1,000 person-years) of knee OA (black), hip OA (red), and hand OA (green).
Solid = All population; Short dash line = women; Long dash line = men.
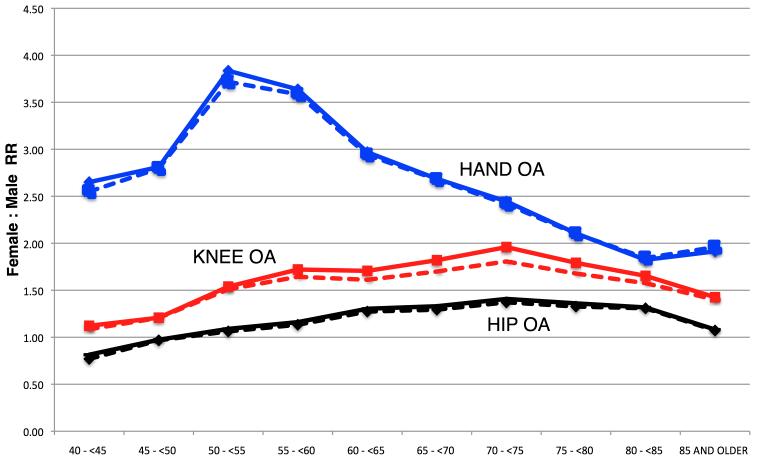
Risk of all three joint-specific OA appeared overall higher in females: adjusted RR 1.52 [1.47-1.54; p<0.001] for knee OA, 1.19 [1.15-1.23; p<0.001] for hip OA, and 2.50 [2.38-2.56; p<0.001] for hand OA. However, different age patterns for the gender effect were observed (figure 2). Women appeared at higher risk of knee OA from age 45 onwards (adjusted RR 1.21 [1.17-1.25]; p<0.001 for participants aged 45-50 years), and the gender-related excess risk increased progressively peaking at age 70-75 years (adjusted RR 1.81 [1.76-1.86]; p<0.001) to decrease slowly in the final stages of life. Hip OA followed a parallel pattern but starting at older ages: females had lower rates of hip OA than males in their 4th decade of life (adjusted RR 0.77 [0.73-0.81; p=0.006] in the 40-45 years), and risk was only increased for females aged 55 and older (adjusted RR 1.13 [1.06-1.20]; p=0.001 in those aged 55 to <60 years); this excess risk increased with age reaching a peak among participants aged 70-75 years (adjusted RR 1.37 [1.32-1.42]; p<0.001), and then slowly decreased to approximate male rates in the oldest ages. As for hand OA, the effect of gender was noticeable from the youngest ages (adjusted RR 2.55 [1.89-3.21]; p<0.001 in participants aged 40 to 45 years), peaked at age 50-55 (adjusted RR 3.72 [3.43-4.01]; p<0.001) and then decreased with age, more steeply in the range of 60 to 80 years [Figure 2].
Figure 2.
Age-specific unadjusted (solid) and multivariate adjusted (dash) female-to-male RRs for knee, hip and hand OA.
Results for Cox regression models on the effect of previous OA on incident hip and knee OA were as follows: previous hip or hand OA were related to incident knee OA (age and gender-adjusted HR 1.36 [1.28-1.45]; p<0.001, and 1.47 [1.40-1.54]; p<0.001 respectively) independent of BMI (further BMI-adjusted HR 1.15 [1.08-1.23]; p<0.001 for prevalent hip OA, and 1.20 [1.14-1.26]; p<0.001 for previous hand OA). Similarly, knee or hand OA predicted future hip OA, with age and gender-adjusted HR of 1.66 [1.58-1.75]; p<0.001 and 1.41 [1.30-1.53]; p<0.001 respectively. This stood for further adjustment for body mass index: HR 1.35 [1.28-1.43]; p<0.001 for previous knee OA, and HR 1.23 [1.13-1.34]; p<0.001 for a history of hand OA.
DISCUSSION
Key results
We report for the first time population-based estimates of incidence of clinically diagnosed knee, hip and hand OA among men and women aged 40 years and older in a Southern European/Mediterranean nation (Catalonia).
We show that the effects of age on both hip and knee OA risk in women follow similar patterns, increasing rapidly between age 50 and 75 years, and then decreasing in the oldest. Conversely, risk of incident hand OA peaks in women after typical menopause ages (55-60 years) and then plateaus, decreasing eventually in the elderly. Age-specific incidence of knee, hip and hand OA follow parallel trends in males, increasing continuously with age until the last stages of life. Gender-related excess risk for hip and knee OA is fairly stable with age, increasing slowly from 50 to 70-75 years, and then decreasing in the oldest ages. By contrast, the effect of female gender on risk of hand OA peaks around menopause, with more than 3.5-fold higher rates in women aged 50 to 60 years when compared to men of similar age. At older ages, the effect of age on hand OA risk attenuates, approximating to the effect of female gender on knee OA among those aged 80 and older. Finally, we demonstrate that a history of hand OA is associated with increased risk of hip and knee OA, and that previous knee OA is related to higher risk of hip OA and vice versa, independently of age, gender, and body mass index.
Interpretation / put in context
Studies on incidence of OA are scarce, and it has been recognized that different definitions of disease make comparisons between them difficult. Most studies have reported estimated rates of either radiographic OA (usually defined by a Kellgren & Lawrence score≥2) (4, 13-17), or symptomatic OA (radiographic OA accompanied by typical OA symptoms), and some have studied self-reported OA (18). Although heterogeneity in case definition is always present, reliability problems have been raised particularly for radiographic studies (16), with studies on symptomatic OA offering more homogeneous and comparable results (19). Although we used a different definition of OA based on physician diagnosis, our estimates of incidence are comparable to other cohorts: as an example, incidence rates of symptomatic knee OA were 6/1,000 person-years in the study by Kannus et al (20) conducted in Finland, 10/1,000 in the Framingham cohort (21), 7.3/1,000 in the Norwegian population (18), 7.6/1,000 in Japan (17), and 6.5/1,000 in our data. Clinically diagnosed OA is most important with relation to patients’ quality of life and health care costs.
Only some of the incidence studies published had enough power to estimate age-specific IRs, and register-based studies similar to ours did not report joint-specific estimates (22, 23). The few papers reviewed demonstrated a continuously increasing risk of knee and hip OA after the age of 40 years for both genders, peaking at ages around 75-80 years, close to our results (7, 17, 24). By contrast, the differing patterns for hand OA in females versus males observed in our study have been shown in some studies, but not all: while Oliveria et al (7) showed continuously increasing risk until the age of 80 years, recent estimates from the Framingham cohort have suggested a peak in hand OA incidence around menopause, probably due to erosive hand OA, which appeared most commonly at the age of 50-55 years in that same population (25).
The effect of female gender on the risk of OA has been estimated in a number of studies (13, 26, 27) and even compiled in a meta-analysis (28), but the interaction with age has not been explored in the past. Our estimates of adjusted female-to-male age-specific risk ratios can hence not be compared to previous publications, but crude estimates can be calculated based on the information provided in some of the papers in press: while a gender effect would increase slowly in hip and knee OA (7, 17), the highest excess risk related to female sex seen around typical menopause ages (to-59 years) on hand OA is consistent with previous reports (7, 25). We speculate that this suggests a stronger effect of hormonal status on hand than on lower limb OA, which might at least partially explain the controversial results obtained from clinical trials on the effect of hormone replacement therapy on OA, which focused on knee and hip disease (29, 30). Alternatively, gender differences in health seeking behavior (eg women being more dissatisfied with hand deformity), or a higher awareness of hand OA in women patients by general practitioners could explain this finding.
Data on the association between prevalent OA at one joint and incident symptomatic disease at others are scarce. Contrary to our results, Felson and cols reported no association between previous hand OA and incident knee OA in the Framingham cohort (15), but other authors have demonstrated that there is an association between hand OA and both knee (31-33) and hip OA (31-34). Event a recent study has shown that hand joint space narrowing predicts magnetic resonance imaging-based knee OA traits (35). The effect size (HR) of the associations described are ostensibly attenuated when adjusted for BMI in our data, suggesting that mechanical loading is important but does not fully explain the pathogenesis of OA. Unobserved variables (genetics, joint shape, pain perception, etc) could explain this BMI-independent association.
Strengths and limitations
Strengths of our study are a big sample size, allowing us to study the interactions between gender and age on joint-specific OA risk, and the representativeness of the information gathered, which covers >80% of the population of the region. However, we are uncertain whether these findings are generalizable to other (eg Northern European, American) populations.
The impact of the disease is predominantly due to cases with clinically symptomatic disease seeking medical attention, which drives both the impact on patient symptoms as well as the pharmacy or surgery interventions and health care-related costs.
The main potential limitation of these data is the lack of validation of each individual diagnosis of OA, but previous cross-validation against classical cohort data have shown OA coding in SIDIAP to be highly specific (12). In addition, although X-Ray imaging is not required for the diagnosis of OA (36-38), we found evidence of radiological confirmation of the diagnosis in almost 40% of a random sample of 150 cases. Only about 1% of these patients had an alternative diagnosis close to the date of OA coding. All this suggests a high reliability of the recording of OA in SIDIAP.
Conclusions
The incidence of knee and hip OA increases continuously with age for both genders, whilst hand OA risk peaks around menopause in women. Related to this, the gender-related excess risk observed for hand OA is highest at the age of 50-55 years, with a maximum increase of almost 4-fold for female compared to male patients. Further investigations are required in order to clarify the potential mechanisms underlying this interaction.
In addition, we demonstrate a BMI-independent association between previous hand OA and incident knee and hip OA, supporting a systemic background for OA disease.
TABLE 2. Effect of prevalent hip and hand OA on risk of knee OA.
| INCIDENT KNEE OA | ||||
|---|---|---|---|---|
| Unadjusted IR/1,000 py [99%CI] |
Unadjusted HR [99%CI] |
Age and Gender- adjusted HR [99%CI] |
+ BMI-adjusted HR [99%CI] |
|
| REFERENCE | 6.44 [6.38-6.49] |
REF | REF | REF |
|
Previous
Hip OA N = 1,807 |
16.09 [15.15- 17.10] |
2.49 [2.44-2.54]; p<0.001 |
1.36 [1.28-1.45]; p<0.001 |
1.15 [1.08-1.23]; p<0.001 |
|
Previous
Hand OA N = 1,089 |
16.10 [15.39- 16.85] |
2.50 [2.44-2.55]; p<0.001 |
1.47 [1.40-1.54]; p<0.001 |
1.20 [1.14-1.26]; p<0.001 |
TABLE 3. Effect of prevalent knee and hand OA on risk of hip OA.
| INCIDENT HIP OA | ||||
|---|---|---|---|---|
| Unadjusted IR/1,000 py [99%CI] |
Unadjusted HR [99%CI] |
Age and Gender- adjusted HR [99%CI] |
+ BMI-adjusted HR [99%CI] |
|
| REFERENCE | 2.07 [2.04-2.10] |
REF | REF | REF |
|
Previous
Knee OA N = 2,699 |
5.98 [5.70-6.29] |
2.89 [2.82-2.95]; p<0.001 |
1.66 [1.58-1.75]; p<0.001 |
1.35 [1.28-1.43]; p<0.001 |
|
Previous
Hand OA N = 1,089 |
4.81 [4.45-5.20] |
2.32 [2.25-2.39]; p<0.001 |
1.41 [1.30-1.53]; p<0.001 |
1.23 [1.13-1.34]; p<0.001 |
REFERENCES
- 1.Enquesta de Salut de Catalunya [Catalonia Health Survey] Generalitat de Catalunya: 2011. [Google Scholar]
- 2.Carmona L, Ballina J, Gabriel R, Laffon A. The burden of musculoskeletal diseases in the general population of Spain: results from a national survey. Ann Rheum Dis. 2001 Nov;60(11):1040–5. doi: 10.1136/ard.60.11.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loza E, Lopez-Gomez JM, Abasolo L, Maese J, Carmona L, Batlle-Gualda E. Economic burden of knee and hip osteoarthritis in Spain. Arthritis Rheum. 2009 Feb 15;61(2):158–65. doi: 10.1002/art.24214. [DOI] [PubMed] [Google Scholar]
- 4.Cooper C, Snow S, McAlindon TE, Kellingray S, Stuart B, Coggon D, et al. Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis Rheum. 2000 May;43(5):995–1000. doi: 10.1002/1529-0131(200005)43:5<995::AID-ANR6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Kallman DA, Wigley FM, Scott WW, Jr., Hochberg MC, Tobin JD. The longitudinal course of hand osteoarthritis in a male population. Arthritis Rheum. 1990 Sep;33(9):1323–32. doi: 10.1002/art.1780330904. [DOI] [PubMed] [Google Scholar]
- 6.Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis. 2001 Feb;60(2):91–7. doi: 10.1136/ard.60.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oliveria SA, Felson DT, Reed JI, Cirillo PA, Walker AM. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum. 1995 Aug;38(8):1134–41. doi: 10.1002/art.1780380817. [DOI] [PubMed] [Google Scholar]
- 8.Lanyon P, Muir K, Doherty S, Doherty M. Age and sex differences in hip joint space among asymptomatic subjects without structural change: implications for epidemiologic studies. Arthritis Rheum. 2003 Apr;48(4):1041–6. doi: 10.1002/art.10886. [DOI] [PubMed] [Google Scholar]
- 9.Wluka AE, Cicuttini FM, Spector TD. Menopause, oestrogens and arthritis. Maturitas. 2000 Jun 30;35(3):183–99. doi: 10.1016/s0378-5122(00)00118-3. [DOI] [PubMed] [Google Scholar]
- 10.Nevitt MC, Cummings SR, Lane NE, Hochberg MC, Scott JC, Pressman AR, et al. Association of estrogen replacement therapy with the risk of osteoarthritis of the hip in elderly white women. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1996 Oct 14;156(18):2073–80. [PubMed] [Google Scholar]
- 11.Garcia-Gil Mdel M, Hermosilla E, Prieto-Alhambra D, Fina F, Rosell M, Ramos R, et al. Construction and validation of a scoring system for the selection of high-quality data in a Spanish population primary care database (SIDIAP) Inform Prim Care. 2012;19(3):135–45. doi: 10.14236/jhi.v19i3.806. [DOI] [PubMed] [Google Scholar]
- 12.Prieto-Alhambra D, Nogues X, Javaid MK, Wyman A, Arden NK, Azagra R, et al. An increased rate of falling leads to a rise in fracture risk in postmenopausal women with self-reported osteoarthritis: a prospective multinational cohort study (GLOW) Ann Rheum Dis. 2012 Jun 23; doi: 10.1136/annrheumdis-2012-201451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaisson CE, Zhang Y, McAlindon TE, Hannan MT, Aliabadi P, Naimark A, et al. Radiographic hand osteoarthritis: incidence, patterns, and influence of pre-existing disease in a population based sample. J Rheumatol. 1997 Jul;24(7):1337–43. [PubMed] [Google Scholar]
- 14.Duncan R, Peat G, Thomas E, Hay EM, Croft P. Incidence, progression and sequence of development of radiographic knee osteoarthritis in a symptomatic population. Ann Rheum Dis. 2011 Nov;70(11):1944–8. doi: 10.1136/ard.2011.151050. [DOI] [PubMed] [Google Scholar]
- 15.Felson DT, Zhang Y, Hannan MT, Naimark A, Weissman B, Aliabadi P, et al. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis Rheum. 1997 Apr;40(4):728–33. doi: 10.1002/art.1780400420. [DOI] [PubMed] [Google Scholar]
- 16.Hart DJ, Doyle DV, Spector TD. Incidence and risk factors for radiographic knee osteoarthritis in middle-aged women: the Chingford Study. Arthritis Rheum. 1999 Jan;42(1):17–24. doi: 10.1002/1529-0131(199901)42:1<17::AID-ANR2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 17.Muraki S, Akune T, Oka H, Ishimoto Y, Nagata K, Yoshida M, et al. Incidence and risk factors for radiographic knee osteoarthritis and knee pain in Japanese men and women: a longitudinal population-based cohort study. Arthritis Rheum. 2011 May;64(5):1447–56. doi: 10.1002/art.33508. [DOI] [PubMed] [Google Scholar]
- 18.Grotle M, Hagen KB, Natvig B, Dahl FA, Kvien TK. Obesity and osteoarthritis in knee, hip and/or hand: an epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet Disord. 2008;9:132. doi: 10.1186/1471-2474-9-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pereira D, Peleteiro B, Araujo J, Branco J, Santos RA, Ramos E. The effect of osteoarthritis definition on prevalence and incidence estimates: a systematic review. Osteoarthritis Cartilage. 2011 Nov;19(11):1270–85. doi: 10.1016/j.joca.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Kannus P, Jarvinen M, Kontiala H, Bergius L, Hyssy E, Salminen E, et al. Occurrence of symptomatic knee osteoarthrosis in rural Finland: a prospective follow up study. Ann Rheum Dis. 1987 Nov;46(11):804–8. doi: 10.1136/ard.46.11.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Felson DT, Zhang Y, Hannan MT, Naimark A, Weissman BN, Aliabadi P, et al. The incidence and natural history of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1995 Oct;38(10):1500–5. doi: 10.1002/art.1780381017. [DOI] [PubMed] [Google Scholar]
- 22.Sun J, Gooch K, Svenson LW, Bell NR, Frank C. Estimating osteoarthritis incidence from population-based administrative health care databases. Ann Epidemiol. 2007 Jan;17(1):51–6. doi: 10.1016/j.annepidem.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Kopec JA, Rahman MM, Sayre EC, Cibere J, Flanagan WM, Aghajanian J, et al. Trends in physician-diagnosed osteoarthritis incidence in an administrative database in British Columbia, Canada, 1996-1997 through 2003-2004. Arthritis Rheum. 2008 Jul 15;59(7):929–34. doi: 10.1002/art.23827. [DOI] [PubMed] [Google Scholar]
- 24.Arden N, Nevitt MC. Osteoarthritis: epidemiology. Best Pract Res Clin Rheumatol. 2006 Feb;20(1):3–25. doi: 10.1016/j.berh.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Haugen IK, Englund M, Aliabadi P, Niu J, Clancy M, Kvien TK, et al. Prevalence, incidence and progression of hand osteoarthritis in the general population: the Framingham Osteoarthritis Study. Ann Rheum Dis. 2011 Sep;70(9):1581–6. doi: 10.1136/ard.2011.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008 Sep 15;59(9):1207–13. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson MG, Michet CJ, Jr., Ilstrup DM, Melton LJ., 3rd Idiopathic symptomatic osteoarthritis of the hip and knee: a population-based incidence study. Mayo Clin Proc. 1990 Sep;65(9):1214–21. doi: 10.1016/s0025-6196(12)62745-1. [DOI] [PubMed] [Google Scholar]
- 28.Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005 Sep;13(9):769–81. doi: 10.1016/j.joca.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Nevitt MC, Felson DT, Williams EN, Grady D. The effect of estrogen plus progestin on knee symptoms and related disability in postmenopausal women: The Heart and Estrogen/Progestin Replacement Study, a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2001 Apr;44(4):811–8. doi: 10.1002/1529-0131(200104)44:4<811::AID-ANR137>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 30.Cirillo DJ, Wallace RB, Wu L, Yood RA. Effect of hormone therapy on risk of hip and knee joint replacement in the Women’s Health Initiative. Arthritis Rheum. 2006 Oct;54(10):3194–204. doi: 10.1002/art.22138. [DOI] [PubMed] [Google Scholar]
- 31.Hirsch R, Lethbridge-Cejku M, Scott WW, Jr., Reichle R, Plato CC, Tobin J, et al. Association of hand and knee osteoarthritis: evidence for a polyarticular disease subset. Ann Rheum Dis. 1996 Jan;55(1):25–9. doi: 10.1136/ard.55.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cushnaghan J, Dieppe P. Study of 500 patients with limb joint osteoarthritis. I. Analysis by age, sex, and distribution of symptomatic joint sites. Ann Rheum Dis. 1991 Jan;50(1):8–13. doi: 10.1136/ard.50.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dahaghin S, Bierma-Zeinstra SM, Reijman M, Pols HA, Hazes JM, Koes BW. Does hand osteoarthritis predict future hip or knee osteoarthritis? Arthritis Rheum. 2005 Nov;52(11):3520–7. doi: 10.1002/art.21375. [DOI] [PubMed] [Google Scholar]
- 34.Hochberg MC, Lane NE, Pressman AR, Genant HK, Scott JC, Nevitt MC. The association of radiographic changes of osteoarthritis of the hand and hip in elderly women. J Rheumatol. 1995 Dec;22(12):2291–4. [PubMed] [Google Scholar]
- 35.Haugen IK, Cotofana S, Englund M, Kvien TK, Dreher D, Nevitt M, et al. Hand joint space narrowing and osteophytes are associated with magnetic resonance imaging-defined knee cartilage thickness and radiographic knee osteoarthritis: data from the Osteoarthritis Initiative. J Rheumatol. 2012 Jan;39(1):161–6. doi: 10.3899/jrheum.110603. [DOI] [PubMed] [Google Scholar]
- 36.Altman R, Alarcon G, Appelrouth D, Bloch D, Borenstein D, Brandt K, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 1991 May;34(5):505–14. doi: 10.1002/art.1780340502. [DOI] [PubMed] [Google Scholar]
- 37.Zhang W, Doherty M, Leeb BF, Alekseeva L, Arden NK, Bijlsma JW, et al. EULAR evidence-based recommendations for the diagnosis of hand osteoarthritis: report of a task force of ESCISIT. Ann Rheum Dis. 2009 Jan;68(1):8–17. doi: 10.1136/ard.2007.084772. [DOI] [PubMed] [Google Scholar]
- 38.Zhang W, Doherty M, Peat G, Bierma-Zeinstra MA, Arden NK, Bresnihan B, et al. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann Rheum Dis. 2010 Mar;69(3):483–9. doi: 10.1136/ard.2009.113100. [DOI] [PubMed] [Google Scholar]