Abstract
It is well accepted that insulin-induced hypoglycemia can result in seizures. However, the effects of the seizures, as well as possible treatment strategies, have yet to be elucidated, particularly in juvenile or insulin-dependent diabetes mellitus (IDDM). Here we establish a model of diabetes in young rats, to examine the consequences of severe hypoglycemia in this age group; particularly seizures and mortality. Diabetes was induced in post-weaned 22-day-old Sprague-Dawley rats by streptozotocin (STZ) administered intraperitoneally (IP). Insulin IP (15 U/kg), in rats fasted (14–16 hours), induced hypoglycemia, defined as <3.5 mM blood glucose (BG), in 68% of diabetic (STZ) and 86% of control rats (CON). Seizures occurred in 86% of STZ and all CON rats that reached hypoglycemic levels with mortality only occurring post-seizure. The fasting BG levels were significantly higher in STZ (12.4±1.3 mM) than in CON rodents (6.3±0.3 mM), resulting in earlier onset of hypoglycemia and seizures in the CON group. However, the BG at seizure onset was statistically similar between STZ (1.8±0.2 mM) and CON animals (1.6±0.1 mM) as well as between those that survived (S+S) and those that died (S+M) post-seizure. Despite this, the S+M group underwent a significantly greater number of seizure events than the S+S group. 25% glucose administered at seizure onset and repeated with recurrent seizures was not sufficient to mitigate these continued convulsions. Combining glucose with diazepam and phenytoin significantly decreased post-treatment seizures, but not mortality. Intracranial electroencephalograms (EEGs) were recorded in 10 CON and 9 STZ animals. Predictive EEG changes were not observed in these animals that underwent seizures. Fluorojade staining revealed damaged cells in non-seizing STZ animals and in STZ and CON animals post-seizure. In summary, this model of hypoglycemia and seizures in juvenile diabetic rats provides a paradigm for further study of underlying mechanisms. Our data demonstrate that severe hypoglycemia (<2.0 mM) is a necessary precondition for seizures, and the increased frequency of these seizures is associated with mortality.
Introduction
The brain stores minimal glucose, mainly in the form of astrocytic glycogen [1]–[3] and is dependent upon a regular supply of glucose from circulating blood [4]. Hypoglycemia is the major limiting factor in the management of Type 1 diabetes [5]–[8] with patients experiencing an average of 2 episodes per week [9]. More severely, hypoglycemia can lead to seizures and coma, with generalized seizures being the major acute complication [10] occurring in children [11] and adolescents [12]. In addition, devastating effects, such as the “dead in bed” syndrome, possibly due to hypoglycemic seizures [13], occur approximately 3 times more frequently in young people with diabetes than in those without [14].
Studies evaluating the relationship between hypoglycemia and cognitive dysfunction have yielded conflicting results. Some studies have suggested that hypoglycemia results in cognitive decline [15]–[17] whereas others have demonstrated that cognition was not impaired long-term [18]–[20]. Perantie et al [21] showed a reduction particularly in spatial learning scores only after repeated hypoglycemic episodes with the initial episode occuring before the age of 5. A potential reason for this disparity in the literature, is that seizure incidence was not considered a variable when assessing the effects on cognition. Yet, seizures can exacerbate the effects of hypoglycemia-induced dysfuntion in humans [22] and in an animal model [23]. Very severe hypoglycemia (<1.0 mM) can result in neuronal injury [24]–[26] with seizures further exacerbating these effects [26]. Furthermore, recurrent hypoglycemic episodes diminish the premonitory symptoms of hypoglycemia (hypoglycemia unawareness) and the counterregulatory response to subsequent hypoglycemia (hypoglycemia-associated autonomic failure), thus jeopardizing patient safety [5], [8], [9], [27], [28].
Critically, both hyperglycemia and hypoglycemia can upset the balance between inhibition and excitation of neuronal networks and have been demonstrated to increase seizure susceptibility. A high-glucose medium is associated with reduced seizure threshold in vitro [29] and glucose deprivation can increase neuronal excitability [30], [31]. Abdelmalik et al [23] demonstrated that glucose deprivation alone can produce ictal activity in the hippocampus of a juvenile mouse and subsequently exacerbate synaptic dysfunction. The loss of high-energy substrates, such as glucose, leads to the release of excitatory amino acids such as glutamate that promote hyperexcitability and consequent excitotoxicity [3]. In addition, KATP channel expression decreases in overnight fasted rodents, therefore contributing to neuronal excitability and possibly to hypoglycemic seizures [32]. At basal expression levels, these channels play a neuroprotective role by hyperpolarizing neurons during energy shortages thus mitigating expenditure on action potential firing [31]. Previous animal studies of severe hypoglycemia (blood glucose < 1.0 mM) have shown that neuronal damage initially occurs in the cerebral cortex as well as in the CA1 and dentate gyrus regions of the hippocampus [26], [33] and subsequently in the basal ganglia and thalamus. Neurons in the brain stem, cerebellum, and spinal cord are generally spared, as are glial cells and white matter tracts. This pattern is also observed in human autopsy studies [33].
In the developing brain, excitation is predominant [34] and the increased propensity for seizures or seizure-like activity has been shown in several experimental models [35]. Age-related differences in seizure susceptibility may be due to functional differences in the substantia nigra pars reticulata (SNR). In adult rats, the SNR is functionally separated into the anterior anticonvulsant and posterior proconvulsant regions. However in rats, aged 15–21days, only the proconvulsant region is present. [36].
EEG abnormalities are more frequent in patients with IDDM than in the general population [37], [38] and are associated with hypoglycemia [39]. However, a clinical study revealed no clear EEG abnormalities during tonic-clonic seizures [40]. Similarly, severe seizure-like behaviours were not associated with electrographic abnormalities recorded in the cerebral cortex and hippocampus of nondiabetic adult rats [41]. While, subcortical structures such as the SNR are involved in the control of the motor component of hypoglycemic seizures [32], [42], the origin of these seizures remains unknown. Though other studies have used STZ to induce diabetes in young animals [43], [44], to our knowledge, hypoglycemic seizures have not been assessed in this age group; a population most susceptible to such events. Therefore, we have established an STZ model for Type 1 diabetes in juvenile rats to describe hypoglycemic seizures, establish seizure thresholds, evaluate the efficacy of anticonvulsants, and measure histological and electrophysiological correlates.
Materials and Methods
Animals
All studies were done in accordance with and approved by the Animal Research Council at the University Health Network (Toronto, Ontario, Canada). Male Sprague Dawley rats from Charles River Laboratories (21-day-old, weaned), weighing 40–60 g, were housed in pairs in a temperature-controlled environment with ad libitum access to water, a standard rat chow diet and under a 12-hour light/dark cycle. The rats were ear-punched for identification.
Induction of Diabetes
Streptozotocin (STZ) was dissolved in a citric acid buffer (0.1 mM sodium citrate (Fisher Scientific) buffered with 1 M citric acid (Fisher Scientific) to a pH of 4.5) making a 10 mg/ml solution just prior to injection. The 22-day-old rats were fasted overnight (14–16 hrs) and received IP injections with one of the following doses of STZ: 60 mg/kg, 75 mg/kg or 80 mg/kg, to induce diabetes. Controls (CON) were randomly selected and injected with the 0.1 mM sodium citrate buffer vehicle. Following STZ or citric buffer vehicle injections, body weights and tail vein blood glucose levels were measured (BG) using a Hemocue Glucose 201 glucometer (Hemocue, Vitaid). Measurements were made 2 days after STZ IP and every 4 days subsequently to confirm stable diabetes.
Induction of Hypoglycemia
Animals were fasted overnight (14–16 hours), administered insulin IP (15 units/kg; Humulin R; Eli Lilly and Company) and video-monitored for 5 hours to detect motor seizures or convulsive seizure-like events, these are referred to as “seizures” for the rest of this paper. Fasting BG was measured (see methods above) prior to insulin IP and hourly, after receiving insulin, leading up to seizures. The BG measures were conducted hourly, as the glucose meter (Hemocue) used was more accurate but required substantially more blood volume than over-the-counter glucose meters. “Lowest BG” was considered as the minimum blood sugar measured in the time period leading up to seizures or up to 3 hours in animals that did not seize. In some cases this measure was taken 15–30 mins prior, as blood samples were difficult to acquire when rats were lethargic. In a subset of animals (22 diabetic and 16 control rats), BG was measured at seizure onset before administering treatment to determine the BG threshold for seizures. After seizures, rats that appeared healthy as defined by resumption of normal grooming habits as well as eating and drinking, survived. Rats that continued seizing despite treatment, were not responsive and displayed agonal breathing (Table S1) were euthanized and brains were collected for histological analysis. These animals were categorized as non-surviving with seizures and mortality (S+M) in all further analyses.
Treatment of Seizures
The strategies that were employed to treat seizures are described in Table 1. CON rats were used in the glu treatment group and STZ rats were used in all three of the treatment groups.
Table 1. Treatment strategies that were employed to treat or attenuate seizures.
| Treatment | GLU | AC+1XGLU | C AC+MULTIPLE GLU |
| At Seizure Onset | 1 g/kg 25% glucose in saline | Diazepam 5 mg/kg+Phenytoin 50 mg/kg+25% glucose saline | Diazepam 5 mg/kg+Phenytoin 50 mg/kg+25% glucose saline |
| Subsequent Seizures | 0.5 g/kg glucose | Diazepam 2.5 mg/kg | 0.5 g/kg glucose for seizures or BG<2.5 mM |
Seizure Scoring
A seizure score was developed to characterize and quantify the SLEs observed in this model ( Table 2 ). The behaviours in this score chart were based on previous scores [45] and modified according to our observations. Treatment was administered to rodents who displayed obvious twitches (seizure score ≥ 2.5).
Table 2. Seizure score to characterize and quantify the observed seizures.
| Score | Behaviour |
| 0.5 | Head up or tail stiff |
| 1 | Rearing and Falling |
| 1.5 | Myoclonic jerk or hindlimbs/forelimbs stretched |
| 2.0 | Body extended with hindlimb or forelimb digging or body curled |
| 2.5 | Unilateral forelimb or hindlimb clonus |
| 3 | Ipsilateral or contralateral forelimb and hindlimb clonus |
| 3.5 | Bilateral forelimb or hindlimb clonus |
| 4 | Wild running |
| 4.5 | All limbs clonus |
| 5 | Head bent backwards forelimb clonus with tonus of hindlimbs |
| 5.5 | Partial barrel roll |
| 6 | Full barrell roll |
| 7 | Full tonic extension |
The seizure score that was obtained in 5-minute epochs was used for further analysis. Animals were subsequently grouped according to the types of seizures that they underwent. Two types of categorization were performed. Firstly, rats were segregated by whether or not the brainstem was recruited (defined by the loss of righting reflex) during the seizure (seizure score ≥ 4.0). Secondly, rats were also placed in three categories (1) partial seizures: only involving one limb (seizure score = 2.5); (2) partial to secondary generalization: the seizure spreads from one limb to other areas (3) generalization without prior evident partial seizures (seizure score ≥ 3.0).
Statistical Analyses
All statistical tests were performed with Sigma Stat software (11th version; Systat Software Inc.). Comparison of data between two groups was carried out via Students’ T-test. To compare proportions of the various treatment groups, a Chi-squared (n ≥ 5) or Fisher’s exact test (n < 5) was used. Significance was set at P<0.05. All error bars indicate ± SEM.
EEG Monitoring
17 STZ and 12 CON rats were implanted with intracranial electrodes using the methodology previously described [41]. Data acquisition and analysis were conducted using pCLAMP 9.0 (Molecular Devices, Inc., California U.S.). 2 STZ animals had electrodes in the left motor cortex and right hippocampal formation (CA1) and the remaining 15 STZ and 12 CON rats were implanted in the right hippocampal (CA1) and left mesencephalic reticular formation. Animals were allowed 5-7 days recovery prior to hypoglycemic insult and EEG recording. Continuous EEG recordings were obtained one hour prior to insulin administration to establish a baseline (after overnight fast). Rodents were then recorded for up to 5 hours after insulin IP or until seizures occured. Simultaneous video monitoring was performed on all animals.
Histology
48 hours after the hypoglycemic episode, 7 STZ and 5 CON rats, randomly selected, were transcardially perfused with 100 mL 0.1% Phosphate Buffered Saline (PBS) and 40 mL 4% paraformaldehyde. The brain was removed at either 48 hours post-hypoglycemic insult or in age-matched STZ and CON animals. Whole brains were cut along the coronal plane into 3 mm sections and embedded in paraffin. Subsequently, paraffin blocks were cut into coronal slices, 10 microns in thickness every 50 microns with a total of 3 slices taken from each block. Fluorojade C (emission: 450 nm, excitation 530 nm) staining was performed to mark degenerating neurons. Staining methods were in accordance with the manufacturer’s protocol (millipore.com) and cells that displayed fluorescence were counted as having undergone neurodegeneration.
Results
Optimization of STZ dosage in Juvenile Rodents
While the STZ model of diabetes induction has been widely demonstrated in adult rodents [46] and more recently in younger animals [43], [44], a model of hypoglycemic seizures in young diabetic animals has not been established. Though 40–60 mg/kg of STZ has been sufficient to induce diabetes in adult animals [47], younger animals require a more potent dose as their pancreatic β-cells are still developing. As such, dose optimization experiments were performed. Diabetes was confirmed through blood glucose (BG) measurement from a tail vein blood sample after STZ administration. Hyperglycemia (BG > 11.1 mM) was used as the inclusion criterion for diabetes. The dosage of 60 mg/kg failed to induce diabetes in any animal (n = 16). A 50% success rate was observed with the 75 mg/kg dose (n = 28). 80 mg/kg of STZ was 91% (n = 44) successful in inducing stable diabetes in rats, and was selected for future experiments.
A week after STZ administration, the diabetic (STZ) animals had a significantly decreased weight gain (p<0.005) body weight; 78.7±1.2 g (n = 40) than the age-matched control (CON) animals; 100.5±2.5 g, (n = 17).
Incidence of Hypoglycemia, Seizures and Mortality
To assess the prevalence of seizures and other concomitant effects of hypoglycemia, insulin IP (15 U/kg), was administered to overnight-fasted rats 1 week after STZ (diabetic rats, N = 63) or CON (controls, N = 23) induction. Overnight fasting, prior to receiving insulin, has been demonstrated to increase seizure incidence [32]. After insulin administration, the rats were video-monitored for 4-5 hours and hypoglycemia was confirmed through BG levels measured from the tail vein.
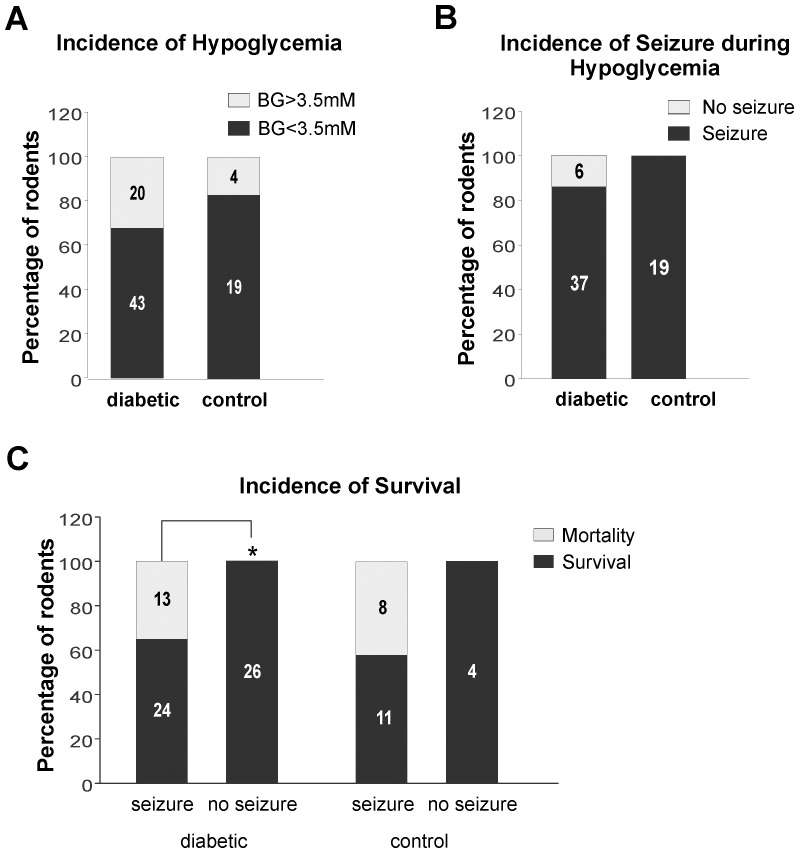
Both CON and STZ rats, attained hypoglycemia (BG < 3.5 mM). Incidence of hypoglycemia was 68% (n = 43/63) in STZ and 86% (n = 19/23) in CON rats ( Figure 1A ), which is a non-significant difference. In addition, there was no significant difference between the proportions of hypoglycemic CON (100%: n = 19/19) and STZ (86%: n = 37/43) rats that displayed convulsions ( Figure 1B ). Only 6 of the 26 non-seizing STZ rats and none of the non-seizing CON rats reached hypoglycemic levels. Mortality resulted in 35% (n = 13/37) of STZ and 42% (n = 8/19) of CON rats that exhibited seizures. Conversely, all non-seizing STZ (n = 26: p<0.005) and CON (n = 4) animals survived ( Figure 1C ).
Figure 1. Incidence of hypoglycemia, seizures and mortality after insulin IP (15 u/kg) in overnight-fasted PN 28-30 rats; 1 week after STZ (diabetic rats) or CON (controls).
A: Incidence of hypoglycemia in all animals injected with insulin; 68% of STZ (n = 43/63) and 83% of CON (n = 19/23); no statistically significant difference between both groups B: Incidence of seizures in rodents where hypoglycemia was confirmed (BG<3.5 mM); 86% of STZ (n = 37/43) and 100% of CON (n = 19/19); no significant difference exists between the two groups C: Rate of mortality after insulin administration and hypoglycemia; 35% (n = 13/37) of STZ and 42% (n = 8/19) of CON rats. 0% mortality observed in non-seizing STZ (n = 0/26) and CON (n = 0/4) rodents. (*) Denotes a statistically significant difference in survival between seizing and non-seizing rats in the STZ group (p<0.005).
Blood Glucose Decrease within the First Hour is Predictive of Seizure but not Mortality
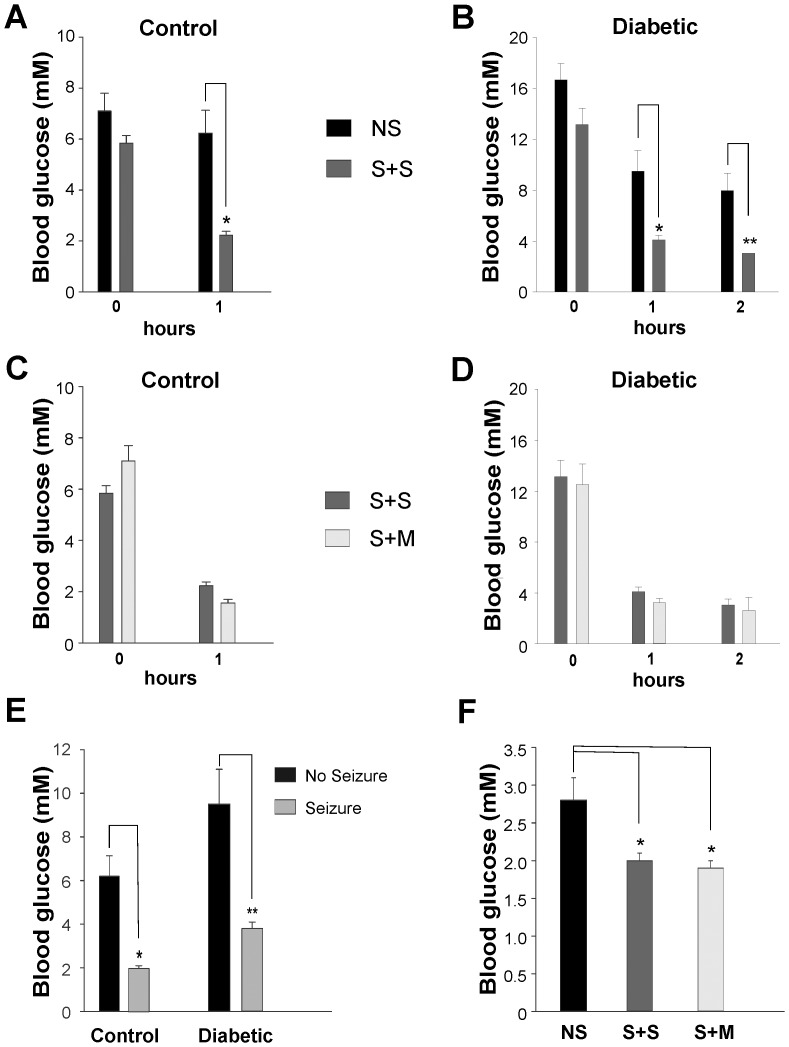
Blood glucose (BG) levels were measured prior to insulin administration and every subsequent hour until seizure onset. Fasting BG levels, prior to insulin administration, were significantly higher in STZ than in CON animals (p<0.001). Consequently, the latency to seizures was significantly increased in STZ (2.4±0.2 hours) compared to CON rats (1.1±0.1 hours, p<0.001). However, fasting BG levels were not a significant factor in CON ( Figure 2A ) or STZ rats ( Figure 2B ; Table 3 ), for predicting the incidence of seizures. Concurrently, prevalence of mortality was not significantly affected by fasting pre-insulin BG levels in CON ( Figure 2C ) or STZ rats ( Figure 2D ).
Figure 2. Relationship of blood glucose (BG) decrease after insulin IP (15 u/kg) to seizure and survival.
A: No significant difference in BG of CON rats with or without seizures at 0hr (time of insulin administration). At 1 hr, BG in non-seizure (NS) group was significantly higher (*) than the seizure + survival (S+S); (p < 0.001) B: No significant difference in BG of STZ rats with or without seizures at 0hr (time of insulin administration). BG in NS group was significantly higher, at 1 hr (*) and 2hr (**) post-insulin, than the S+S group; (p < 0.001) C: No significant difference in BG of CON rats at 0hr or 1hr post-insulin in S+S and S+M groups D: No significant difference in BG of STZ rats at 0hr, 1hr or 2hr post-insulin in S+S and S+M groups E: BG levels at 1 hr post-insulin is significantly greater in CON; NS: 6.2±0.9 mM than CON with seizure: 2.0±0.1 mM (*), and in STZ; NS: 9.5±1.6 mM compared with STZ with seizure: 3.8±0.3 mM (**); (p<0.001) F: Lowest BG measured is significantly higher in the NS group (n = 6): 2.8±0.3 mM compared with either S+S (n = 24): 2.0±0.1 mM or S+M (n = 13): 1.9±0.1 mM groups (*); (p<0.002).
Table 3. Mean blood glucose level measured hourly related to outcome; (±S.E.M).
| N | 0 hr | N | 1 hr | N | 2hr | |
| NS | 27 | 16.7±1.3 | 18 | 9.5±1.6 | 21 | 8.0±1.4 |
| S+M | 16 | 12.5±1.7 | 10 | 3.2±0.4 | 6 | 2.6±1.0 |
| S+S | 27 | 13.2±1.3 | 26 | 4.1±0.4 | 17 | 3.1±0.5 |
NS: No Seizure.
S+M: Seizure + Mortality.
S+S: Seizure + Survival.
After insulin administration, both STZ and CON rats that eventually developed seizures had a significant drop in BG levels within the first hour compared to the NS groups (1hr BG in CON: NS = 6.2±0.9 mM, CON: seizure = 2.0±0.1 mM, STZ: NS = 9.5±1.6 mM, STZ: seizure = 3.8±0.3 mM, Figure 2E , p<0.001 for both comparisons).
In the STZ group, the 37 rats displaying seizures (lowest BG = 2.0±0.1 mM), reached significantly lower (p<0.002) BG levels than the 6 NS hypoglycemic animals (lowest BG = 2.8±0.3 mM). There was no significant difference in the lowest BG measured between seizing rats that survived (S+S = 2.0±0.1 mM) or died (S+M = 1.9±0.1 mM, Figure 2F ; Table 3 ). Control NS rodents did not achieve hypoglycemic levels.
Blood Glucose Threshold for Seizure
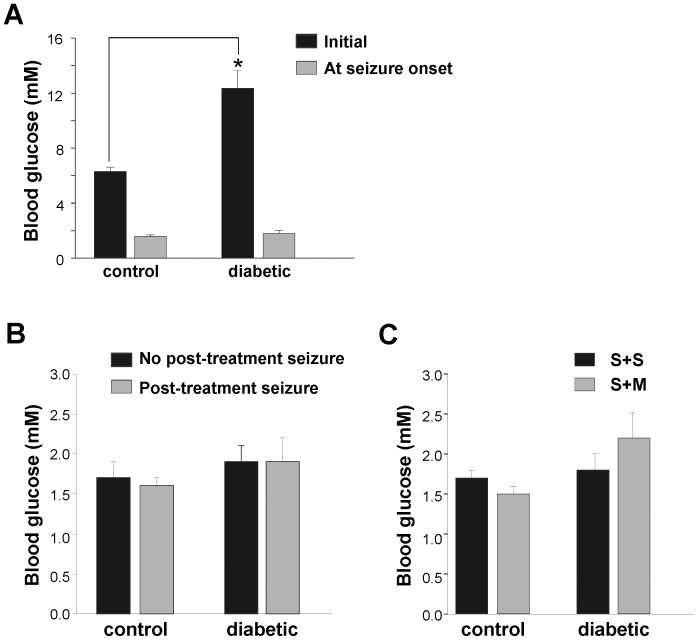
To establish the BG level for seizure threshold, BG at seizure onset (defined as a seizure score ≥2.5, refer to Table 1 ) was measured in 22 STZ and 16 CON rats. As mentioned above, prior to insulin administration, BG levels in STZ (12.4±1.3 mM) were significantly higher than in CON rats (6.3±0.3 mM; p<0.01) ( Figure 3A ). Despite this, at the onset of seizures, the BG levels were similar in STZ (1.8±0.2 mM) and CON rats (1.6±0.1 mM; Figure 3A ) due to the comparable decrease in the mean rates of BG in STZ (4.4±0.36 mM/hr) and CON rats (4.5±0.5 mM/hr) as well as the significantly higher latency to seizures in STZ (2.4±0.2 hours) than in CON (1.1±0.1 hours) rats (p<0.001).
Figure 3. Blood glucose (BG) threshold for seizures in CON vs. STZ rats and association with survival and seizures after treatment.
A. Initial BG (prior to insulin IP) is significantly lower in CON (6.3±0.3 mM; n = 16) than in STZ rats (12.4±1.3 mM; n = 22) (p<0.01). BG at seizure onset (observed seizure score ≥2.5) is similar in CON (1.6±0.1 mM) and STZ rats (1.8±0.2 mM) B: No significant association between BG levels at seizure onset and post-treatment seizures. BG levels glucose at seizure onset for CON: 1 seizure; 1.7 ±0.2 mM (n = 11) and >1 seizure; 1.6±0.1 mM (n = 5) and STZ: 1 seizure; 1.9±0.2 mM (n = 10), >1 seizure; 1.9±0.3 mM (n = 12) are not statistically different C: No significant difference between BG levels at seizure onset comparing survival and mortality. BG for CON: S+S; 1.7±0.1 mM (n = 11) and S+M; 1.5 ±0.1 mM (n = 5); STZ: S+S; 1.8±0.2 mM (n = 17) and S+M; 2.2±0.32 mM (n = 5) are not significantly different.
BG at seizure onset was not predictive of whether rats would undergo a single seizure (mitigated by glucose administration) or subsequent seizures (despite treatment). In CON rats, the BG levels were 1.7±0.2 mM (n = 11) and 1.6±0.1 mM (n = 5) in groups with single and multiple seizures, respectively. Likewise, in STZ rats, BG levels at seizure onset of rats with a single seizure and multiple seizures were 1.9±0.2 mM (n = 10) and 1.9±0.3 mM (n = 12), respectively ( Figure 3B ). Although the seizures occurred within a limited hypoglycemic range, the BG during recovery, measured hourly post-seizure, was variable (Figure S1 A-B). No significant difference was measured in the seizure onset BG levels of STZ surviving (1.8±0.2 mM, n = 17) and non-surviving rats (2.2±0.3 mM, n = 5). The BG of CON rats did not differ significantly between those that survived (BG 1.7±0.1 mM, n = 11) and those that did not (BG 1.5±0.1 mM, n = 5; Figure 3C ).
Continued Seizures are associated with mortality
In order to establish a seizure model, the observed seizure behaviours must first be described. Reliable video recordings were obtained in 22 STZ and 16 CON rats that displayed seizures. All rats were treated with glucose at seizure onset. Seizure scoring was performed to quantify the severity of the observed seizures ( Table 2 ). Prior to reaching seizure-inducing hypoglycemic levels, all rats that reached moderate hypoglycemia (<3.5 mM) became lethargic. In the absence of EEG recording, it was difficult to discern less severe seizure-like activity (seizure score: 0.5–2) from lethargic behavior. Therefore, only rats that reached a seizure score of ≥2.5 (single limb clonus) were treated and classified as having seized (see methods). Seizures in the STZ and CON animals exhibited variable progression, whether or not the animals survived (Figure S2 A-D). Notably, mortality occurred in all rats that reached a seizure score of 7.
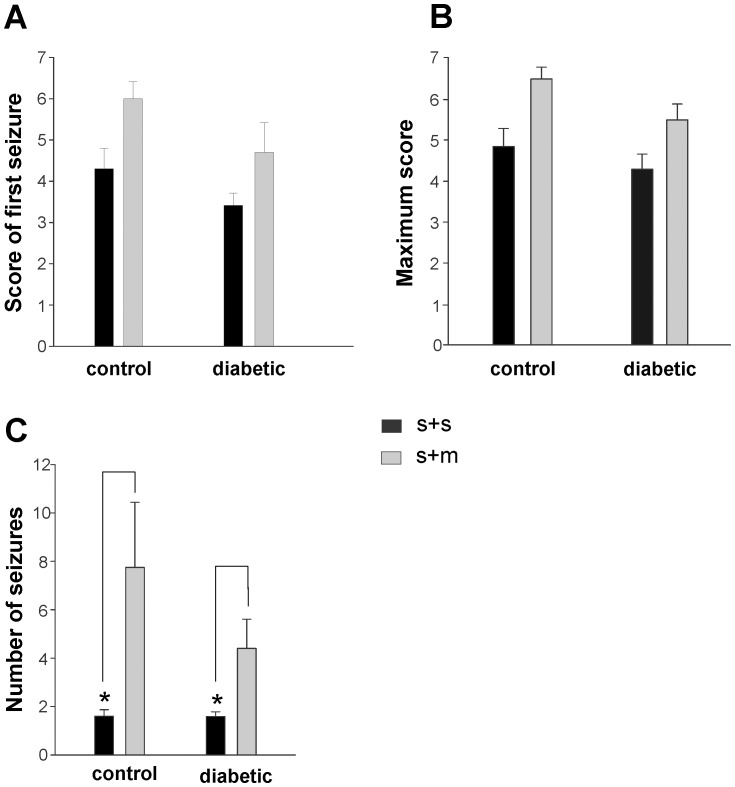
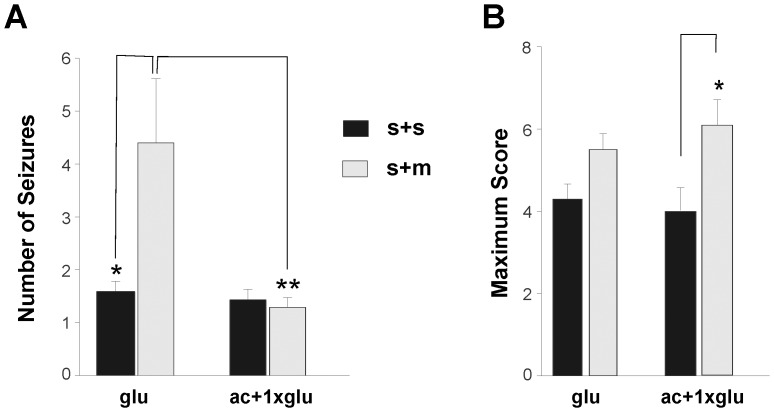
While it is evident that hypoglycemia and the resulting seizures are associated with mortality, it is not clear whether seizures were the sole cause ( Figure 3C ). Therefore, the analysis of seizure scores was used to isolate the effects of seizures on mortality. The severity of the first seizure, prior to treatment with glucose, was not significantly different in CON rats: S+S = 4.3±0.5 (n = 11) and S+M = 6.0±0.4 (n = 5) as well as STZ rats: S+S: 3.4±0.3 (n = 17) and S+M: 4.7±0.7 (n = 5) ( Figure 4A ). The most severe seizure, measured by the maximum seizure score, experienced by CON rats was statistically similar in S+S = 4.9±0.4 (n = 11) and S+M rats = 6.5±0.3 (n = 5, p>0.05), as was the case for the STZ rats: S+S = 4.3±0.4 (n = 17) and S+M = 5.5±0.4 (n = 5, p>0.05) ( Figure 4B ). Frequent BG measures could not be obtained to determine the duration of hypoglycemia. Instead, the period in which seizures (scores ranging from 0.5–7) occurred was used as an indication of hypoglycemia. These data were also statistically similar between S+M and S+S rats, whether or not they had diabetes (Figure S4). The S+M rats in both the STZ and CON groups trended toward a higher initial and maximum seizure score compared to the respective S+S rats. The lack of significance is potentially due to the low sample size of the S+M animals.
Figure 4. Comparing the association between seizure severity and mortality in CON and STZ rats.
A: No significant difference in the mean score of the first seizure treated in CON rats: S+S: 4.3±0.5 (n = 11) and S+M: 6.0±0.4 (n = 5) and STZ rats: S+S: 3.4±0.3 (n = 17) and S+M: 4.7±0.7 (n = 5) B: No significant difference in the mean maximum seizure score observed in CON rats: S+S: 4.9±0.4 (n = 11) and S+M: 6.5±0.3 and STZ rats: S+S: 4.3±0.4 (n = 17) and S+M: 5.5±0.4 (n = 5) C: A statistically significant difference in the mean number of seizures between (*) CON rats: S+S: 1.6±0.3 (n = 11) and S+M: 7.8±2.7 (n = 5; p<0.01) and between (*) STZ rats: S+S: 1.6±0.2 (n = 17) and S+M: 4.4±1.2 (n = 5; p<0.001).
A stronger predictor of mortality was the number of seizures that an animal underwent. The number of seizures observed was significantly higher in the CON rats: S+M = 7.8±2.7 (n = 5) compared with S+S = 1.6±0.3 (n = 11; p<0.01). This difference was also observed between STZ rats: S+S = 1.6±0.2 (n = 17) and S+M = 4.4±1.2 (n = 5); p<0.001 ( Figure 4C ).
Anticonvulsants Reduce Seizure Incidence but Not Mortality
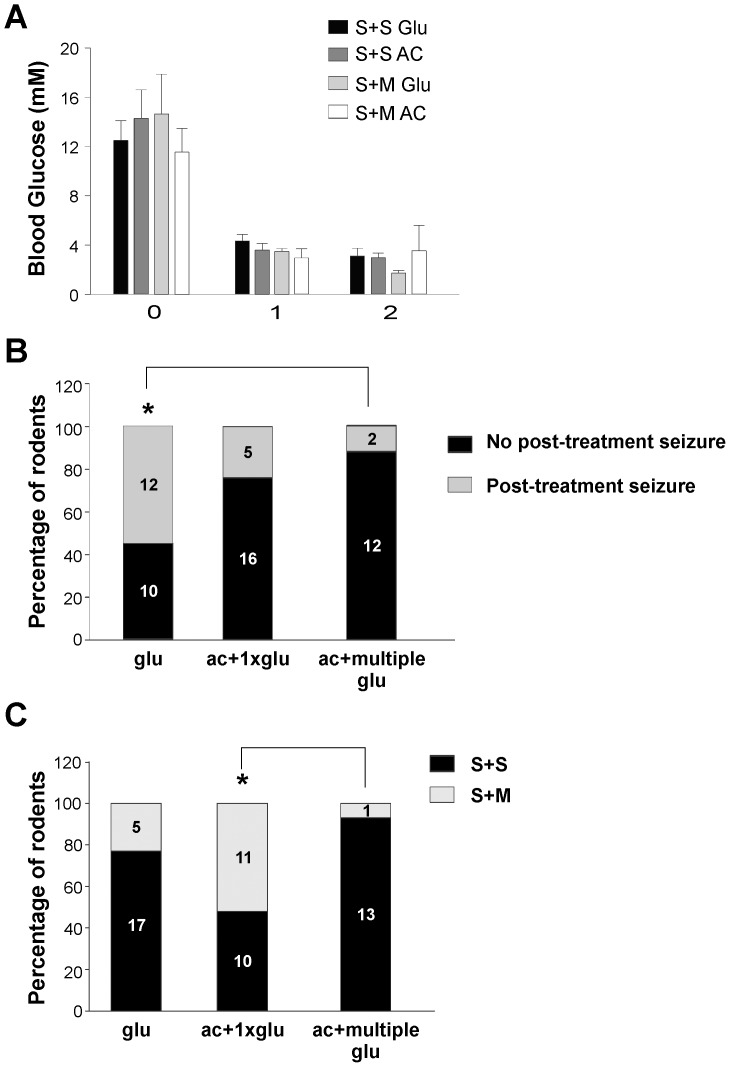
To evaluate the efficacy of glucose, the current treatment strategy, and whether outcomes differed with anticonvulsant treatment, the treatments described in Table 1 (glu, ac+1xglu, ac+multiple glu) were administered to STZ rats. The trend in BG decrease after insulin was similar in the treatment groups, indicating that the BG decline was not a confounding factor of treatment outcome ( Figure 5A ).
Figure 5. Efficacy of treatment strategies (see Table 1) in preventing subsequent seizures and mortality in STZ rats.
A: BG decrease is not significantly different in seizing animals regardless of treatment; glu: seizure+ survival (S+S Glu), ac+1xglu: S+S AC, glu: seizure + mortality (S+M Glu), ac+1xglu: S+M AC (Table 4). B: No significant difference in the incidence of seizures post-treatment in ac+1xglu: 24% (n = 5/21) compared with glu: 55% (n = 12/22) (p<0.05). (*) Significantly lower incidence of seizures post-treatment in ac+multiple glu: 15% (n = 2/14) compared with glu: 55% (n = 12/22) (p<0.02) C: (*) Significantly higher survival rate in ac+multiple glu: 93% (n = 1/14) compared with ac+1xglu: 48% (n = 10/21) (p<0.02). No significant difference in survival rate between ac+multiple glu: 93% (n = 13/14) and glu: 77% (n = 17/22).
Table 4. Mean blood glucose level measured hourly related to treatment and survival.
| N | 0 hr | N | 1 hr | N | 2hr | |
| S+S AC+1XGLU | 10 | 14.3±2.3 | 9 | 3.6±0.5 | 4 | 3.0±0.4 |
| S+S Glu | 17 | 12.5±1.6 | 17 | 4.3±0.5 | 13 | 3.2±0.6 |
| S+M AC+1XGLU | 11 | 11.6±1.9 | 5 | 3.0±0.7 | 3 | 3.5±2.1 |
| S+M Glu | 5 | 14.7±3.2 | 5 | 3.5±0.2 | 3 | 1.7±0.2 |
S+S Glu: Seizure + Survival; glucose treated.
S+S AC+1XGLU: Seizure + Survival; glucose and anticonvulsant treated.
S+M Glu: Seizure + Mortality; glucose treated.
S+M AC+1XGLU: Seizure + Mortality; glucose and anticonvulsant treated.
Treatment with glucose at seizure onset was not always successful in mitigating seizures. 55% (n = 12/22) of glu treated animals exhibited seizures after treatment compared with 24% of animals in the ac+1xglu treatment group (n = 5/21) ( Figure 5B ). In the ac+multiple glu group, repeated glucose administration at BG<2.5 mM, significantly reduced the incidence of post-treatment seizures compared to the glu group with only 15% of rodents (n = 2/14) undergoing subsequent seizures (p<0.02) ( Figure 5B ). There was not a significant difference between the ac+1xglu (n = 5/21) and the ac+multiple glu (n = 2/14).
Despite the success of anticonvulsant treatment in mitigating seizures with multiple glucose administrations, the impact on survival, ac+multiple glu: 93% (n = 13/14), compared with glu: 77% (n = 17/22) was not significant ( Figure 5C ). However, the survival rate of ac+multiple glu: 93% was significantly higher than ac+1xglu: 48% (n = 11/21; p<0.02). As the anticonvulsants appeared to be mitigating the motor component of the seizures ( Figure 5B ), despite continued hypoglycemia (Figure S3 A-B), improved survival rate was still dependent on continued glucose administration.
Anticonvulsants suppresses motor seizures
As demonstrated in Figure 5B , combining diazepam and phenytoin with glucose was more successful at mitigating convulsive behaviour than glucose alone. In the ac+1xglu-treated group the number of motor seizures were similar whether or not mortality resulted (S+S: 1.3±0.2; n = 8 and S+M: 1.4±0.2; n = 8) and significantly lower (p<0.05) than the glu-treated rats where mortality resulted (S+M: 4.4±1.2; n = 5) ( Figure 6A ). In ac+1xglu group, the 8 animals in the S+M group died despite having a reduced number of seizures, as glucose was necessary even though the anticonvulsants prevented the behavioural seizure manifestations. However, multiple glucose administrations (glu) alone were not sufficient to prevent continued seizures that eventually led to mortality ( Figure 6A ). These rats may have had a temporary increase in BG levels and returned to hypoglycemia. In the ac+multiple glu group only one animal died and could not be statistically compared to the other 2 groups (S+M: 3.0; n = 1).
Figure 6. Effects of treatments (see Table 1) on the seizure scores and mortality in STZ rats.
A: Mean number of seizures in (*) glu rats: S+S: 1.6±0.2 (n = 17) and S+M: 4.4±1.2 (n = 5) (p<0.001); ac+1xglu rats: S+S: 1.3±0.2 (n = 8) and S+M: 1.4±0.2 (n = 8). (**) Significant difference also exists between glu rats: S+M and ac+1xglu rats: S+M (p<0.05) B: Mean maximum seizure score attained in glu rats: S+S: 4.3±0.4 (n = 17) and S+M: 5.5±0.4 (n = 5); (*) ac+1xglu rats: S+S: 4.0±0.6 (n = 8) and S+M: 6.1±0.6 (n = 8) (p<0.01).
Interestingly, the maximum seizure score in the ac+1xglu-treated group was significantly higher in S+M rats: 6.1±0.6 (n = 8) compared to S+S rats: 4.0±0.6 (n = 8) (p<0.01). While anticonvulsants were able to transiently stop seizing, since the rats were not treated with continuous glucose, 5 rats eventually had more severe seizures. In contrast, the maximum score observed in the glu-treated rats that survived (4.3±0.4; n = 17) was statistically similar to those that died (5.5±0.4; n = 5) ( Figure 6B ). This was also the case in ac+multiple glu-treated rats: S+S: 4.0±0.4 (n = 13) and S+M: 5.5 (n = 1), though the sample size of the latter was not adequate for statistical analysis.
EEG seizure activity is not associated with motor seizures
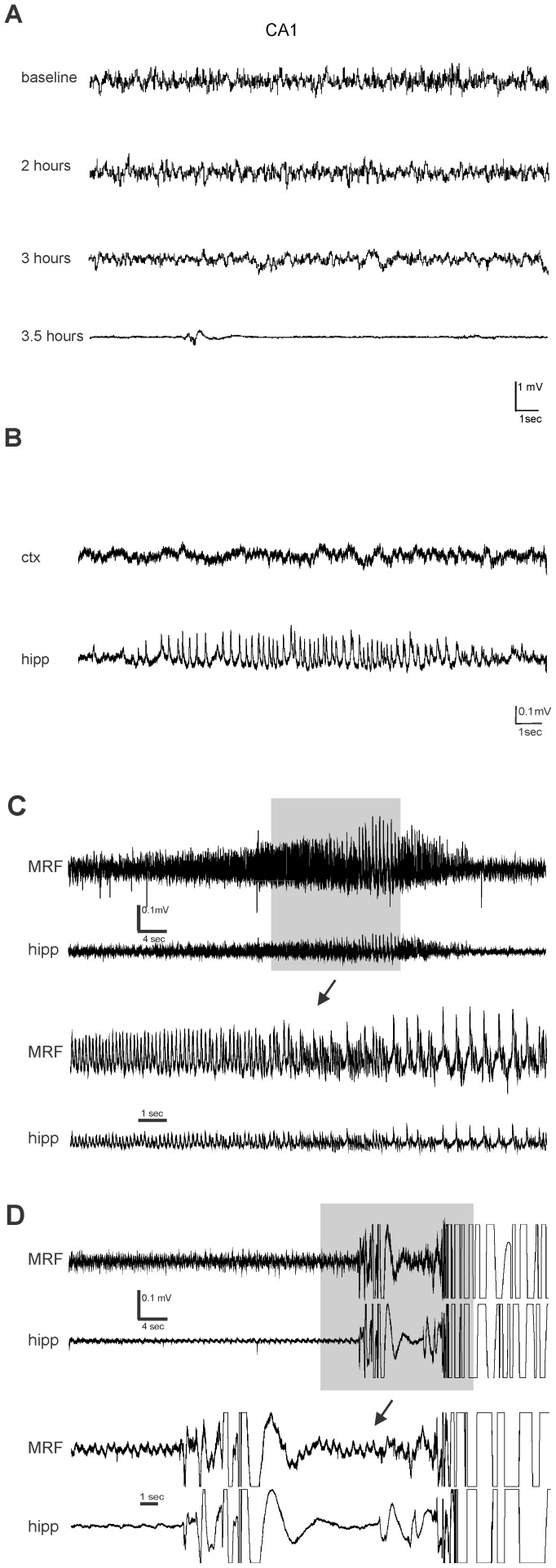
EEG recordings were obtained in 17 STZ and 12 CON rats. 11/12 CON and 13/17 STZ animals reached hypoglycemic levels following insulin administration of which 10 CON and 9 STZ rats had behavioral seizures. An example of baseline recording prior to insulin administration is shown in Figure 7A . 3 of the 13 hypoglycemic STZ rats showed significant suppression ( Figure 7A ) of EEG, characteristic of severe hypoglycemia (Auer 2004). However, behavioural seizures occurred independently of EEG suppression as only 2 out of 9 STZ and none of the CON rodents displayed such pronounced suppression prior to exhibiting convulsions. Burst suppression was characteristic of EEGs in all animals that reached hypoglycemic levels. Animals that seized showed intermittent, non-rhythmic spikes, often with a positive polarity. Two animals’ recordings also contained low-amplitude (50 uV) polyspike bursts. Brief electrographic seizures were recorded in the CA1 ( Figure 7B ) region in one animal and brainstem ( Figure 7C ) in another. Although, rats at this stage of hypoglycemia were lethargic and immobile, no overt convulsive behaviour was observed. The rat exhibiting the hippocampal electrographic seizure had previously received a “rescue” administration of glucose, diazepam and phenytoin after the prior incidence of a behavioural seizure. The other had not been treated as a behavioural seizure had not yet been observed.
Figure 7. EEG abnormalities during hypoglycemia is not associated with seizure behavior.
A: Representative EEG recording of hippocampus (CA1) at baseline (post-fasting; prior to insulin IP), 2 hours, 3 hours (slower waves) and 3.5 (EEG suppression) hours after insulin IP B: Electrographic seizure activity observed in CA1 (lower trace) after suppression of EEG activity. No ictal activity in cortex (upper trace) C: EEG recording of hippocampus and contralateral MRF obtained in STZ rat during hypoglycemia. Electrographic seizure activity observed after suppression of EEG activity. Lower trace illustrates magnification of the area in the gray box D: EEG of the rat in (C) during a behavioural seizure; ictal activity may be masked by movement artifact.
Consistent with previous studies [41], the behavioural manifestations classified as “seizures” were not associated with any characteristic recordings. Figure 7D illustrates motion artifact induced by seizure-like behavior, but no rhythmic or paroxysmal change in the EEG immediately before, or during the seizure-like behaviour.
Hypoglycemic seizures are associated with hippocampal and cortical damage
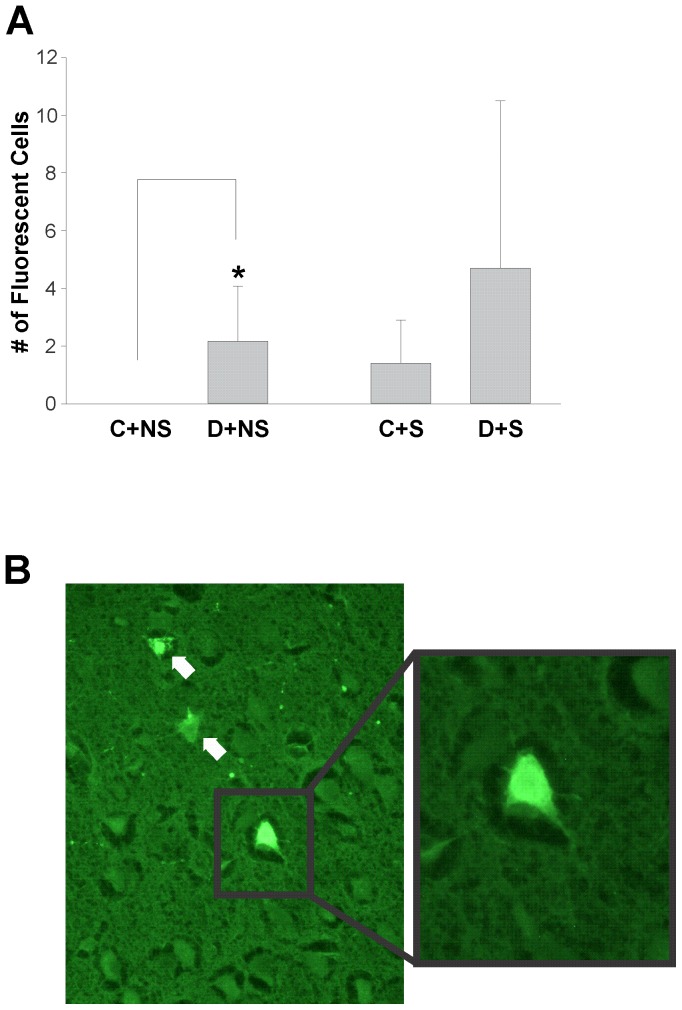
Fluorojade staining was performed to visualize degenerating cells and assess the effects of hypoglycemic seizures on the neurons of STZ and CON animals. The number of fluorescent cells was minimal in the four groups: CON and STZ rats dissected 48 hours after insulin-induced hypoglycemic seizures and age-matched non-seizing CON and STZ animals. STZ rats without seizures (D+NS: 2.17±1.9 cells; n = 6) exhibited a significantly higher number of damaged cells than CON rats (C+NS: 0±0; n = 5 cells; p<0.05). There was no significant difference between CON (C+S: 1.4±1.5 cells; n = 5) and STZ rats (D+S: n = 7; 4.7±5.8 cells) particularly due to the high variability in the number of damaged cells in the STZ animals. Two STZ rats displayed numerous (>10) fluorojade (+) cells while this others exhibited more minimal damage ( Figure 8 ). Consistent with previous literature, neurodegenaration was localized to the cortical and hippocampal regions [16], [26].
Figure 8. Effects of diabetes and seizures on neuronal damage.
A: Significant difference in the number of fluorojade (+) cells between CON non-seizing (C+NS: 0±0; n = 5 animals) and STZ non-seizing groups (D+NS; 2.17±1.9 cells; n = 6; p<0.05). No significant difference in the number of cells between CON seizing (C+S: 1.4±1.5; n = 5 cells) and STZ seizing groups (D+S: 4.7±5.8 cells; n = 7) B: Fluorojade (+) cells in the cortical region of (D+S) rat magnified 40X (white arrows).
Discussion
This study has established a model of hypoglycemic seizures in young diabetic animals to examine seizure manifestations and consequences in this age group. As Type 1 diabetes is diagnosed primarily during childhood, these hypoglycemic episodes begin early in life and the associated neurological complications are particularly harmful [5], [13], [48]. Repeated hypoglycemic seizures in young children may also cause structural brain changes. Impaired awareness is particularly prevalent when the age of diabetes diagnosis is early (<6 years) and is associated with recurrent hypoglycemic episodes resulting in seizures or coma [48] though other studies have reported conflicting results [18], [20]. In addition, animal studies have demonstrated the increased susceptibility of the juvenile brain to neuronal excitability and seizures [34], [36], [49] and shorter latency to seizure generalization [45]. While there are several clinical studies that have assessed the effects of seizures such as EEG abnormalities [37] and increased cognitive impairmanet [50], the specific incidence of hypoglycemic seizures is unknown.
In our model, the drop in blood glucose (BG) within the first hour post-insulin administration was predictive of those animals that would attain severe hypoglycemia and progress to seizures ( Figure 2A /B). In STZ animals, the higher baseline fasting BG levels resulted in lower incidence of hypoglycemia and seizures ( Figure 1A /B). These higher initial BG levels in STZ rats combined with the similar rates of BG decline following insulin administration resulted in an increased latency to hypoglycemia and subsequent seizures in these animals.
Blood glucose measured at seizure onset provided evidence that there is a limited range of BG levels at which seizures occur and is an indication of a glycemic threshold for seizures. Despite expectations that the STZ animals would have increased seizure susceptibility [31], seizure-threshold BG levels were similar between the STZ and CON groups. A potential reason for this similarity is the brief period of diabetes in this study. Streptozotocin could only be administered in post-weaned (21-day old) animals and hypoglycemia was induced after a week in 28–29 day old rats. This age was chosen, as it is neuro-developmentally similar to young children, a population at risk for hypoglycemic episodes [51]. Additionally, Veliskova et al [45] reported the decreased latency to tonic-clonic seizures, associated with brainstem involvement, in this age group. As such, this age was maintained in our experiments to avoid additional variability in the results of the study. Recurrent hypoglycemic episodes occur more often in diabetic patients and therefore the resulting impaired counterregulation [9] may lower seizure threshold. These experiments were the first step in creating a model of hypoglycemic seizures in juvenile diabetic animals; not yet reported in the literature to our knowledge. Future experiments will evaluate the impact of recurrent hypoglycemic seizures in diabetic juvenile/younger mature animals chronically treated with insulin, as this is more representative of the clinical situation.
Mortality was only observed in animals post-seizure. Additionally, the animals that survived, despite having similar seizure scores, underwent a significantly lower number of seizures (CON: S+S = 1.6±0.3; n = 11, S+M = 7.8±2.7; n = 5 p<0.01 and STZ rats: S+S = 1.6±0.2; n = 17, S+M = 4.4±1.2; n = 5 p<0.001; Figure 4C ). This was further evidence for the association between seizures and mortality. Velisek et al, [32] showed that persistently low BG, despite multiple glucose administrations, was the potential cause of continued seizures. BG measured post-seizure demonstrated variable rates of recovery from seizures with some animals recovering only temporarily and returning to hypoglycemic levels (Figure S4). Further evidence for impaired recovery is that a single glucose dose was insufficient to ameliorate seizures in 12/22 rats. Four rats continued seizing despite multiple glucose treatments with mortality as an end result.
The behavioural presentation of seizures in this model suggests brainstem involvement due to the loss the righting reflex and rolling seizure manifestations [45]. Furthermore, mortality was always observed in rats that attained a seizure score of 7 (this score reflects full tonic extensions), suggesting brainstem involvement in the seizures where mortality resulted. Disruption in the functioning of brainstem regions may cause cardiac and respiratory changes that can lead to mortality [52]. Even animals that did not die naturally but had to be sacrificed (2 CON and 7 STZ rats; Table S1) due to their severe state displayed severe gasping/agonal breathing.
The survival rates were significantly higher in the ac+multiple glu compared with the ac+1xglu group. The correction of hypoglycemia with multiple administrations of glucose, where necessary, was beneficial for survival ( Figure 5A ). Anticonvulsant treatment at seizure onset coupled with multiple glucose administrations significantly reduced subsequent convulsions compared with the glu treated group ( Figure 5B ) with no significant effect on mortality. Interestingly, the BG levels remained lower, despite repeated glucose administrations (Figure S3 B), with decreased seizure occurrence potentially due to the presence of the anticonvulsants.
Diazepam, a GABA agonist, and phenytoin, which act on sodium channels, were only able to transiently stop seizures without glucose replenishment and the seizures eventually become more severe with mortality as an end result ( Figure 6B ). This temporary effect suggests that other seizure-causing mechanisms, such as enhanced NMDA activity, may trigger these seizures in the presence of continued hypoglycemia [42], [53]. This emphasizes the importance of repeated glucose replenishment to prevent both seizure severity and mortality. Even though anticonvulsants decreased the behavioural manifestations of seizures the rats may have continued to seize non-convulsively, particularly in the group only receiving a single dose of glucose treatment, as the energy substrate was not continuously replenished. This can have clinical implications as anticonvulsants may be administered to prevent continued seizures that exacerbate the neuronal damage caused by hypoglycemia [26], but it remains essential to replenish the energy substrate.
Consistent with previous literature, neuronal degeneration was negligible as BG levels were not maintained at the very severe hypoglycemic level of ≤ 1.0 mM with suppressed EEG, as was done experimentally by Auer [54]. Our results demonstrate that seizures occur at higher BG levels, ∼ 2.0 mM. Notably, there were significantly more fluorojade positive cells in non-seizing diabetic animals compared with non-seizing controls. Hyperglycemia can exacerbate neural damage caused by other pathologies such as seizures and ischemia [26], [55]–[57]. Yet, hyperglycemia per se has not been shown to cause neurodegeneration in adult animals [26]. Furthermore, Sasaki-Hamada et al [44] demonstrated that rats incurring diabetes at a juvenile age exhibited increased cognitive deficits compared to adult animals. Our data provide further evidence that studies on hypoglycemic seizures should be performed in a young diabetic model due to the differences in the outcome between adult and juvenile animals. However, due to the high variability of damage in seizing STZ rats, there was no significant difference between CON and STZ animals that seized.
As observed in previous studies in adult rodents [41], our EEG data provide further evidence that behavioral seizures in juvenile diabetic animals do not originate in the hippocampus. There were no apparent predictive EEG changes in the STZ and CON rats. Severe hypoglycemia (≤1.0 mM) as indicated by EEG isoelectricity [24] was not a requirement for convulsions; 18 of 19 rodents had behavioral seizures without pronounced suppression of EEG. There was slowing in the EEG waves prior to seizures in these animals, indicative of less severe hypoglycemia (>1.0 mM) [54]. It is not surprising that EEG isoelectricity is not associated with seizures since the BG of ≤1.0 mM, a BG level that is rarely reached by patients [58], was not a requirement for seizures. This was confirmed in Figure 3A where the mean BG at seizure onset was 1.6±0.1 mM in CON and 1.8±0.2 mM in STZ rats.
Electrographic seizures recorded in the hippocampus (+/–brainstem) were brief (10–30s), failed to spread to the cortex, and occurred in the context of EEG suppression that is associated with neuronal damage [54]. These observations suggest that the same mechanisms responsible for neurotoxicity may be crucial in sustaining the depolarization of susceptible neuronal networks, but the lack of adequate energy substrate could prevent spread to higher brain regions. In addition, the observed seizure-like behavior (some of which could be interpreted as primitive locomotion phenomenology) as seen in decerebrate preparations [59] suggests a brainstem or spinal cord generator. Further studies recording EEG activity from other brainstem and spinal regions are required to test this hypothesis.
There are several factors that potentially explain the mechanisms for seizures during hypoglycemia. The downregulation of KATP channels that impair potassium buffering has been postulated to enhance excitability [32]. The decrease in intracellular ATP impairs the activity of the Na+/K+-ATPase and thus the cell’s ability to restore intracellular potassium and extracellular sodium levels, leading to an enhanced glutamate receptor function [60]. This is further exacerbated by the increase in excitatory amino acids such as glutamate and aspartate as well as ammonia [54], [61] that has been correlated with the onset of seizures [25]. This increase in production of glutamate and aspartate is the result of accumulating oxaloacetate from an impaired Kreb’s cycle [54]. Additionally, increase in extracellular glutamate can saturate glutamate receptors in astrocytes as well as cause astrocytic swelling particularly in the cortex and hippocampus [62]. The ability of AP7, a glutamate receptor antagonist, to significantly mitigate hypoglycemic motor seizures is evidence for the crucial role of excitatory amino acids in these convulsions [42]. The increased excitability and possibly decreased inhibition can cause synchrony of neuronal firing, which can be propagated by electrical conduction through gap junctions [63]–[65].
In summary, hypoglycemic seizures due to exogenous insulin administration can occur in juvenile diabetic and non-diabetic animals. A blood glucose threshold of approximately 2mM was associated with the occurrence of seizures in both control and diabetic animals after an acute hypoglycemic event. Most importantly, mortality only occurred following seizures, and it was the frequency of seizures rather than blood glucose levels that were predictive of mortality. Treatment of seizures with glucose, helped prevent seizure-related mortality with no added protection from anticonvulsants. While electrographic seizures were recorded in two animals, it was not correlated with behavioural manifestations. Although there have been improvements in treatments such as better insulin, insulin pumps, and improved glucose monitoring techniques, hypoglycemic seizures remain a major problem in insulin-treated diabetic children and adolescents, and a better understanding of these is crucial for improved patient care. The description of the seizures and outcomes performed in our STZ-model is the first step toward accomplishing this goal.
Supporting Information
Blood Glucose (BG) measured post seizure of glu-treated CON and STZ animals. A: BG post-seizure in CON rats at 1.0 hour (n = 8): 4.4±0.2 mM, 2.0 hours (n = 8): 5.5±0.5 mM B: BG post-seizure in STZ rats 1.0 hour (n = 11): 7.8±1.9 mM, 2.0 hours (n = 12): 13.7±2.9 mM, 3.5 hours (n = 5): 15.9.3±4.1 mM.
(TIF)
Evolution of seizures over the course of hypoglycemia (each trace represents a different animal). A: CON; S+S; n = 11 B: CON; S+M; n = 5 C: STZ; S+S; n = 17 D: STZ; S+M; n = 5.
(TIF)
Blood Glucose (BG) post seizure of glu and ac+multiple glu-treated STZ animals. A: BG post-seizure in glu rats 1.0 hour (n = 11): 7.8±1.9 mM, 2.0 hours (n = 12): 13.7±2.9 mM, 3.5 hours (n = 5): 15.9.3±4.1 mM B: BG post-seizure in ac+multiple glu rats at 0.5 hours (n = 12): 3.1±0.5 mM, 1.5 hours (n = 10): 3.2±0.4 mM, 2.5 hours (n = 6): 3.2±0.6 mM, 3.5 hours (n = 4): 6.3±3.3 mM.
(TIF)
Total duration in which the rodents demonstrated SLEs as an indication of hypoglycemia. No significant difference between glucose-treated control rats that seized and died; CON_GLU_S+M (n = 5): 124.3±31.5 and survived; CON_GLU_S+S (n = 11): 42.1±20.1 No significant difference between glucose-treated diabetic rats that seized and died; STZ_GLU_S+M (n = 5): 113.6±38.0 and survived; STZ_GLU_S+S (n = 17): 55.8±16.2 No significant difference between anticonvulsants+1 dose of glucose-treated diabetic rats that seized and died; STZ_ACGLU1_S+M (n = 8): 30.7±11.4, and survived; STZ_ACGLU1_S+S (n = 8): 36.1±12.0 Anticonvulsants+multiple glucose-treated diabetic rats that seized and survived; STZ_ACMULTGLU_S+S (n = 13): 97.04±26.2 could not be statistically compared with ones that seized and died; STZ_ACMULTGLU_S+M (n = 1).
(TIF)
Reasons for sacrificing 2 of 5 glu Treated CON rats, 2 of 5 glu treated STZ rats and 5 of 8 ac+1xglu treated STZ rats.
(DOC)
Funding Statement
This work is supported by the Juvenile Diabetes Research Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Brown AM, Baltan Tekkök S, Ransom BR (2004) Energy transfer from astrocytes to axons: The role of CNS glycogen. Neurochemistry International 45(4): 529–536. [DOI] [PubMed] [Google Scholar]
- 2. Gruetter R (2003) Glycogen: The forgotten cerebral energy store. Journal of Neuroscience Research 74(2): 179–183. [DOI] [PubMed] [Google Scholar]
- 3. McCall AL (2004) Cerebral glucose metabolism in diabetes mellitus. European Journal of Pharmacology 490(1–3): 147–158. [DOI] [PubMed] [Google Scholar]
- 4. Seaquist ER, Damberg GS, Tkac I, Gruetter R (2001) The effect of insulin on in vivo cerebral glucose concentrations and rates of glucose transport/metabolism in humans. Diabetes 2001 50(10): 2203–2209. [DOI] [PubMed] [Google Scholar]
- 5. Cryer P (2004) Diverse causes of hypoglycemia-associated autonomic failure in diabetes. The New England Journal of Medicine 350(22): 2272–2279. [DOI] [PubMed] [Google Scholar]
- 6. Blasetti A, Di Giulio C, Tocco AM, Verrotti A, Tumini S, et al. (2011) Variables associated with severe hypoglycemia in children and adolescents with type 1 diabetes: A population-based study. Pediatric Diabetes 12(1): 4–10. [DOI] [PubMed] [Google Scholar]
- 7. Katz ML, Volkening LK, Anderson BJ, Laffel LM (2012) Contemporary rates of severe hypoglycaemia in youth with type 1 diabetes: Variability by insulin regimen. Diabetic Medicine: A Journal of the British Diabetic Association 29(7): 926–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McCrimmon RJ (2012) Update in the CNS response to hypoglycemia. The Journal of Clinical Endocrinology and Metabolism 97(1): 1–8. [DOI] [PubMed] [Google Scholar]
- 9. McCrimmon RJ, Sherwin RS (2010) Hypoglycemia in type 1 diabetes. Diabetes 59(10): 2333–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lapenta L, Di Bonaventura C, Fattouch J, Bonini F, Petrucci S, et al. (2010) Focal epileptic seizure induced by transient hypoglycaemia in insulin-treated diabetes. Epileptic Disorders: International Epilepsy Journal with Videotape 12(1): 84–87. [DOI] [PubMed] [Google Scholar]
- 11. Davis EA, Keating B, Byrne GC, Russell M, Jones TW (1997) Hypoglycemia: Incidence and clincal predictors in a large population-based sample of children and adolescents with IDDM. Diabetes Care 20(1): 22–25. [DOI] [PubMed] [Google Scholar]
- 12. Svoren BM, Butler D, Levine BS, Anderson BJ, Laffel LM (2003) Reducing acute adverse outcomes in youths with type 1 diabetes: A randomized, controlled trial. Pediatrics 112(4): 914–922. [DOI] [PubMed] [Google Scholar]
- 13. Secrest AM, Becker DJ, Kelsey SF, Laporte RE, Orchard TJ (2010) Cause-specific mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes. Diabetes 59(12): 3216–3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heller SR (2002) Abnormalities of the electrocardiogram during hypoglycaemia: The cause of the dead in bed syndrome? International Journal of Clinical Practice Supplement (129): 27–32. [PubMed]
- 15. Yamada K, Rensing N, Izumi Y, De Erausquin G, Gazit V, et al. (2004) Repetitive hypoglycemia in young rats impairs hippocampal long-term potentiation. Pediatric Research 55(3): 372–379. [DOI] [PubMed] [Google Scholar]
- 16. Suh SW, Hamby AM, Swanson RA (2007) Hypoglycemia, brain energetics, and hypoglycemic neuronal death. Glia 55(12): 1280–1286. [DOI] [PubMed] [Google Scholar]
- 17. Hannonen R, Tupola S, Ahonen T, Riikonen R (2003) Neurocognitive functioning in children with type-1 diabetes with and without episodes of severe hypoglycaemia. Developmental Medicine and Child Neurology 45(4): 262–268. [DOI] [PubMed] [Google Scholar]
- 18. Musen G, Jacobson AM, Ryan CM, Cleary PA, Waberski BH, et al. (2008) DCCT Research Group. Impact of diabetes and its treatment on cognitive function among adolescents who participated in the Diabetes Control and Complications Trial. Diabetes Care 31(10): 1933–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Howorka K, Pumprla J, Saletu B, Anderer P, Krieger M, et al. (2000) Decrease of vigilance assessed by EEG-mapping in type I diabetic patients with history of recurrent severe hypoglycaemia. Psychoneuroendocrinology 25(1): 85–105. [DOI] [PubMed] [Google Scholar]
- 20. Frier BM (2011) Cognitive functioning in type 1 diabetes: The diabetes control and complications trial (DCCT) revisited. Diabetologia 54(2): 233–236. [DOI] [PubMed] [Google Scholar]
- 21. Perantie DC, Lim A, Wu J, Weaver P, Warren SL, et al. (2008) Effects of prior hypoglycemia and hyperglycemia on cognition in children with type 1 diabetes mellitus. Pediatric Diabetes 9(2): 87–95. [DOI] [PubMed] [Google Scholar]
- 22. Kaufman FR, Epport K, Engilman R, Halvorson M (1999) Neurocognitive functioning in children diagnosed with diabetes before age 10 years. Journal of Diabetes and its Complications 13(1): 31–38. [DOI] [PubMed] [Google Scholar]
- 23. Abdelmalik PA, Shannon P, Yiu A, Liang P, Adamchik Y, et al. (2007) Hypoglycemic seizures during transient hypoglycemia exacerbate hippocampal dysfunction. Neurobiology of Disease 26(3): 646–660. [DOI] [PubMed] [Google Scholar]
- 24. Auer RN, Olsson Y, Siesjo BK (1984) Hypoglycemic brain injury in the rat. correlation of density of brain damage with the EEG isoelectric time: A quantitative study. Diabetes 19833(11): 1090–1098. [DOI] [PubMed] [Google Scholar]
- 25. Lewis LD, Ljunggren B, Norberg K, Siesjo BK (1974) Changes in carbohydrate substrates, amino acids and ammonia in the brain during insulin-induced hypoglycemia. Journal of Neurochemistry 23(4): 659–671. [DOI] [PubMed] [Google Scholar]
- 26. Bree A, Puente E, Daphna Iken D, Fisher S (2009) Diabetes increases brain damage caused by severe hypoglycemia. American Journal of Physiology: Endocrinology and Metabolism 297(1): E194–E201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Inzucchi SE, Sherwin RS (2005) The prevention of type 2 diabetes mellitus. Endocrinology and Metabolism Clinics of North America 34(1): 199–219. [DOI] [PubMed] [Google Scholar]
- 28. Puente EC, Silverstein J, Bree AJ, Musikantow DR, Wozniak DF, et al. (2010) Recurrent moderate hypoglycemia ameliorates brain damage and cognitive dysfunction induced by severe hypoglycemia. Diabetes 59(4): 1055–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schwechter EM, Velísková J, Velísek L (2003) Correlation between extracellular glucose and seizure susceptibility in adult rats. Annals of Neurology 53(1): 91–101. [DOI] [PubMed] [Google Scholar]
- 30. Kirchner A, Veliskova J, Velisek L (2006) Differential effects of low glucose concentrations on seizures and epileptiform activity in vivo and in vitro. The European Journal of Neuroscience 23(6): 1512–1522. [DOI] [PubMed] [Google Scholar]
- 31. Ghasemi M, Shafaroodi H, Karimollah AR, Gholipour T, Nezami BG, et al. (2010) ATP-sensitive potassium channels contribute to the time-dependent alteration in the pentylenetetrazole-induced seizure threshold in diabetic mice. Seizure: European Journal of Epilepsy 19(1): 53–58. [DOI] [PubMed] [Google Scholar]
- 32. Velisek L, Veliskova J, Chudomel O, Poon K, Robeson K, et al. (2008) Metabolic environment in substantia nigra reticulata is critical for the expression and control of hypoglycemia-induced seizures. The Journal of Neuroscience 28(38): 9349–9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Auer RN, Hugh J, Cosgrove E, Curry B (1989) Neuropathologic findings in three cases of profound hypoglycemia. Clinical Neuropatholog 8(2): 63–68. [PubMed] [Google Scholar]
- 34. Rakhade SN, Jensen FE (2009) Epileptogenesis in the immature brain: Emerging mechanisms. Nature Reviews.Neurology 5(7): 380–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ben-Ari Y, Holmes GL (2006) Effects of seizures on developmental processes in the immature brain. Lancet Neurology 5(12): 1055–1063. [DOI] [PubMed] [Google Scholar]
- 36. Moshe SL, Garant DS, Sperber EF, Veliskova J, Kubova H, et al. (1995) Ontogeny and topography of seizure regulation by the substantia nigra. Brain & Development 17 Suppl: 61–72 [DOI] [PubMed] [Google Scholar]
- 37. Tupola S, Salonen I, Hannonen R, Verho S, Saar P, et al. (2004) Comparison of regional cerebral perfusion, EEG and cognitive functions in type 1 diabetic children with and without severe hypoglycaemia. European Journal of Pediatrics 163(6): 335–336. [DOI] [PubMed] [Google Scholar]
- 38. Verrotti A, Scaparrotta A, Olivieri C, Chiarelli F (2012) Seizures and type 1 diabetes mellitus: Current state of knowledge. European Journal of Endocrinology/European Federation of Endocrine Societies 167(6): 749–758. [DOI] [PubMed] [Google Scholar]
- 39. Snogdal LS, Folkestad L, Elsborg R, Remvig LS, Beck-Nielsen H, et al. (2012) Detection of hypoglycemia associated EEG changes during sleep in type 1 diabetes mellitus. Diabetes Research and Clinical Practice 98(1): 91–97. [DOI] [PubMed] [Google Scholar]
- 40. Lahat E, Barr J, Bistritzer T (1995) Focal epileptic episodes associated with hypoglycemia in children with diabetes. Clinical Neurology and Neurosurgery 97(4): 314–316. [DOI] [PubMed] [Google Scholar]
- 41. del Campo M, Abdelmalik PA, Wu CP, Carlen PL, Zhang L (2009) Seizure-like activity in the hypoglycemic rat: Lack of correlation with the electroencephalogram of free-moving animals. Epilepsy Research 83(2–3): 243–248. [DOI] [PubMed] [Google Scholar]
- 42. Velísková J, Chudomel O, Poon KL, Marshall B, Velisek L (2007) The involvement of the substantia nigra pars reticulata in hypoglycemic seizures. Epilepsia 48(s5): 106–108. [DOI] [PubMed] [Google Scholar]
- 43. Iwai T, Suzuki M, Kobayashi K, Mori K, Mogi Y, et al. (2009) The influences of juvenile diabetes on memory and hippocampal plasticity in rats: Improving effects of glucagon-like peptide-1. Neuroscience Research 64(1): 67–74. [DOI] [PubMed] [Google Scholar]
- 44. Sasaki-Hamada S, Sacai H, Oka JI (2012) Diabetes onset influences hippocampal synaptic plasticity in Streptozotocin-treated rats. Neuroscience 227: 293–304. [DOI] [PubMed] [Google Scholar]
- 45.Velísková J (2006) Behavioural characterization of seizures in rats. In Asla PitKäen PA, (Philip A.) Schwartzkroin, Solomon L. Moshé (Ed.), Models of seizures and epilepsy (1st ed., pp. 601); Elsevier Academic Press.
- 46. Like AA, Rossini AA (1976) Streptozotocin-induced pancreatic insulitis: New model of diabetes mellitus. Science 193(4251): 415–417. [DOI] [PubMed] [Google Scholar]
- 47. Szkudelski T (2001) The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiological Research/Academia Scientiarum Bohemoslovaca 50(6): 537–546. [PubMed] [Google Scholar]
- 48. Trang TL, Gallego PH, Davis EA, Timothy WJ (2009) Impaired Awareness of Hypoglycemia in a Population-Based Sample of Children and Adolescents With Type 1 Diabetes. Diabetes Care 32 (10): 1802–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Velísková J, Claudio OI, Galanopoulou AS, Lado FA, Ravizza T, et al. (2004) Seizures in the developing brain. Epilepsia 45(s8): 6–12. [DOI] [PubMed] [Google Scholar]
- 50. Becker DJ, Ryan CM (2000) Hypoglycemia: a Complication of Diabetes Therapy in Children. Trends Endocrinol Metab 11(5): 198–202. [DOI] [PubMed] [Google Scholar]
- 51. Ennis K, Tran PV, Seaquist ER, Rao R (2008) Postnatal Age Influences Hypoglycemia-induced Neuronal Injury in the Rat Brain. Brain Res 1224: 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Moseley B, Bateman L, Millichap JJ, Wirrell E, Panayiotopoulos CP (2012) Autonomic epileptic seizures, autonomic effects of seizures and SUDEP. Epilepsy & Behavior 26(3): 375–385. [DOI] [PubMed] [Google Scholar]
- 53. Suh SW, Gum ET, Hamby AM, Chan PH, Swanson RA (2007) Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. The Journal of Clinical Investigation 117(4): 910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Auer R (2004) Hypoglycemic brain damage. Metabolic Brain Disease 19(3–4): 169–175. [DOI] [PubMed] [Google Scholar]
- 55. Lin B, Ginsberg MD, Busto R (1998) Hyperglycemic exacerbation of neuronal damage following forebrain ischemia: microglial, astrocytic and endothelial alterations. Acta Neuropathol 96: 610–620. [DOI] [PubMed] [Google Scholar]
- 56. Kondo F, Asanuma M, Miyazaki I, Kondo Y, Tanaka K, et al. (2001) Progressive cortical atrophy after forebrain ischemia in diabetic rats. Neurosci Res 39: 339–346. [DOI] [PubMed] [Google Scholar]
- 57. Moreira T, Cebers G, Pickering C, Ostenson CG, Efendic S, et al. (2007) Diabetic Goto-Kakizaki rats display pronounced hyperglycemia and longer-lasting cognitive impairments following ischemia induced by cortical compression. Neuroscience 144: 1169–1185. [DOI] [PubMed] [Google Scholar]
- 58. Cryer P (2012) Severe hypoglycemia predicts mortality in Diabetes. Diabetes Care 35(9): 1814–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Whelan PJ (1996) Control of Locomotion in the Decerebrate Cat. Progress in Neurobiology 49(5): 481–515. [DOI] [PubMed] [Google Scholar]
- 60. Marinelli S, Federici M, Giacomini P, Bernardi G, Mercuri NB (2001) Hypoglycemia enhances ionotropic but reduces metabotropic glutamate responses in substantia nigra dopaminergic neurons. Journal of Neurophysiology 85(3): 1159–1166. [DOI] [PubMed] [Google Scholar]
- 61. Cardoso S, Carvalho C, Santos R, Correira S, Santos MS, et al. (2011) Impact of STZ-induced hyperglycemia and insulin-induced hypoglycemia in plasma amino acids and cortical synaptosomal neurotransmitters. Synapse 65 (6): 457–466. [DOI] [PubMed] [Google Scholar]
- 62. Han BC, Koh SB, Lee EY, Seong YH (2004) Regional difference of glutamate-induced swelling in cultured rat brain astrocytes. Life Sciences 76(5): 573–583. [DOI] [PubMed] [Google Scholar]
- 63. Perez-Velazquez JL, Carlen PL (2000) Gap junctions, synchrony and seizure. Trends Neurosci 23: 68–74. [DOI] [PubMed] [Google Scholar]
- 64.Somjen G. Ions in the brain: Normal function, seizures and stroke 2004: Oxford University Press.
- 65. Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C (2008) Astroglial Metabolic Networks Sustain Hippocampal Synaptic Transmission. Science 322(5907): 1551–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Blood Glucose (BG) measured post seizure of glu-treated CON and STZ animals. A: BG post-seizure in CON rats at 1.0 hour (n = 8): 4.4±0.2 mM, 2.0 hours (n = 8): 5.5±0.5 mM B: BG post-seizure in STZ rats 1.0 hour (n = 11): 7.8±1.9 mM, 2.0 hours (n = 12): 13.7±2.9 mM, 3.5 hours (n = 5): 15.9.3±4.1 mM.
(TIF)
Evolution of seizures over the course of hypoglycemia (each trace represents a different animal). A: CON; S+S; n = 11 B: CON; S+M; n = 5 C: STZ; S+S; n = 17 D: STZ; S+M; n = 5.
(TIF)
Blood Glucose (BG) post seizure of glu and ac+multiple glu-treated STZ animals. A: BG post-seizure in glu rats 1.0 hour (n = 11): 7.8±1.9 mM, 2.0 hours (n = 12): 13.7±2.9 mM, 3.5 hours (n = 5): 15.9.3±4.1 mM B: BG post-seizure in ac+multiple glu rats at 0.5 hours (n = 12): 3.1±0.5 mM, 1.5 hours (n = 10): 3.2±0.4 mM, 2.5 hours (n = 6): 3.2±0.6 mM, 3.5 hours (n = 4): 6.3±3.3 mM.
(TIF)
Total duration in which the rodents demonstrated SLEs as an indication of hypoglycemia. No significant difference between glucose-treated control rats that seized and died; CON_GLU_S+M (n = 5): 124.3±31.5 and survived; CON_GLU_S+S (n = 11): 42.1±20.1 No significant difference between glucose-treated diabetic rats that seized and died; STZ_GLU_S+M (n = 5): 113.6±38.0 and survived; STZ_GLU_S+S (n = 17): 55.8±16.2 No significant difference between anticonvulsants+1 dose of glucose-treated diabetic rats that seized and died; STZ_ACGLU1_S+M (n = 8): 30.7±11.4, and survived; STZ_ACGLU1_S+S (n = 8): 36.1±12.0 Anticonvulsants+multiple glucose-treated diabetic rats that seized and survived; STZ_ACMULTGLU_S+S (n = 13): 97.04±26.2 could not be statistically compared with ones that seized and died; STZ_ACMULTGLU_S+M (n = 1).
(TIF)
Reasons for sacrificing 2 of 5 glu Treated CON rats, 2 of 5 glu treated STZ rats and 5 of 8 ac+1xglu treated STZ rats.
(DOC)