Abstract
We have developed and validated an oligonucleotide probe hybridization assay for human immunodeficiency virus type 1 (HIV-1) circulating recombinant form (CRF) CRF02_AG. In the p17 coding region of the gag gene, a CRF02_AG-specific signature pattern was observed. Five working probes were designed to discriminate CRF02_AG infections from infections by all other documented subtypes and CRFs in an enzyme-linked immunosorbent assay-based oligonucleotide probe hybridization assay. Nucleic acids were extracted from a panel of HIV-1-positive plasma samples from Cameroon, Bénin, Côte d'Ivoire, Kenya, Zambia, and Belgium and from blood spots from The Gambia. CRF02_AG (n = 147) and non-CRF02 (n = 100) samples were analyzed to evaluate and validate the oligonucleotide probe hybridization assay. The CRF02_AG-specific oligonucleotide probe hybridization assay has a high sensitivity and specificity, with good positive and negative predictive values in regions of high and low prevalence. A validation of the assay with West and West Central African samples indicated a sensitivity of 98.4% and a specificity of 96.7%. The oligonucleotide probe hybridization assay as a diagnostic tool will allow for rapid screening for CRF02_AG. This could be used to track the HIV epidemic in terms of documenting the real prevalence of CRF02_AG strains and will complement efforts in vaccine development. Moreover, this technology can easily be applied in laboratories in developing countries.
Genetic variation is the hallmark of retroviruses and is also apparent in human immunodeficiency virus type 1 (HIV-1). HIV-1 displays important genetic variability which is driven by the high error rate of the reverse transcriptase (23), the presence of viral RNA as a dimer, allowing recombination to occur (12), the high turnover rate of HIV-1 in vivo (24), and selective immune responses.
By genetic analyses, HIV strains collected from around the world have been shown to have substantial diversity. Representatives of different “pure” (nonrecombinant) subtypes, namely subtypes A, B, C, D, F, G, H, J, and K, and of 15 circulating recombinant forms (CRF), namely CRF01 to CRF15, were proposed based on a near-full-length genome analysis (http://hiv-web.lanl.gov/). The epidemiology of HIV-1 subtypes and CRFs is characterized by their differential distributions and varying levels of significance as driving causes of the pandemic on a regional and global basis. The largest proportion of HIV-1 infections in the year 2000 was due to subtype C strains (47.2%), followed by subtype A and CRF02_AG (27%) and subtype B strains (12.3%) (20).
Discrimination between subtype A and CRFs comprising fragments of subtype A is hampered by relatively small genetic distances between the parental subtype A virus and the respective subtype A fragments in CRFs. This often results in low-confidence classification when sequences of suboptimal length are phylogenetically (re)analyzed. In addition, it has not been possible to discriminate subtype A from CRF02_AG by env heteroduplex mobility assays (HMAs), and alternate experimental conditions are needed to discriminate between subtype A, CRF01_AE, and CRF02_AG by gag HMA (9).
The prototype strain of CRF02_AG, HIV-1 IbNG, was initially reported as a new subtype A isolate from Ibadan, Nigeria (11). An analysis of the full-length sequence revealed that HIV-1 IbNG was an A/G recombinant (4). Recent and retrospective molecular epidemiology studies indicated that in West and West Central Africa, CRF02_AG infections represent 50 to 70% of the circulating strains (1, 5, 15, 16, 17, 19, 21, 27, 28). In contrast to the high CRF02_AG prevalence in the West African and West Central African countries Cameroon, Gabon, and Equatorial Guinea, CRF02_AG infections are scarce in the Republic of Congo (26), the Democratic Republic of Congo (31), and Eastern and Southern African countries (3). Outside Africa, CRF02_AG has been introduced in Europe (8, 10, 18, 25, 29), and to a minor extent, in the United States (13).
To date, there have been few systematic large-scale attempts to characterize HIV isolates, and especially CRFs, emerging from different parts of the world. As such, our knowledge of the distribution of HIV strains in different populations and about changes in that distribution over time is rather limited. A major challenge in the design and evaluation of efficacious subtype-dependent candidate HIV-1 vaccines is the development of techniques for large-scale HIV genetic characterization to document the true prevalence rates of HIV subtypes and CRFs in developing countries.
Here we describe the design and potential use of a CRF02_AG-specific oligonucleotide probe hybridization assay for large-scale monitoring of the prevalence of CRF02_AG variants.
MATERIALS AND METHODS
Samples.
A reference panel of 25 plasmids, containing the complete gag gene of HIV-1 strains belonging to group M subtypes A to H, CRF01_AE, and CRF02_AG, was available (14). An evaluation panel of plasma samples (Bénin, n = 59; Cameroon, n = 53; Kenya, n = 50; Zambia, n = 10; Belgium, n = 80) were selected based on subtype information obtained in previous studies (9, 16, 17). A validation panel of plasma samples (Côte d'Ivoire, n = 30; Cameroon, n = 60) and dried blood spot samples (The Gambia, n = 10) were obtained from HIV-1-positive individuals from whom samples were generally taken in 2000-2001. As the “gold standard,” phylogenetic classification of the gag probe target fragment was used.
RNA extractions and RT-PCR.
RNA extractions were performed as previously described (2). Reverse transcription-PCR (RT-PCR) (Access RT-PCR; Promega, Leiden, The Netherlands) was performed according to the manufacturer's recommendations (10 pmol of each primer, 10 mM dNTP mix, 25 mM MgSO4). The primers and primer positions (according to the HIV-1 HXB2 numbering) (HIV Sequence Database [http://hiv-web.lanl.gov/]) were as follows: H1GHMA101, 5′-TAGTATGGGCAAGCAGGGAG-3′ (HXB2 positions 890 to 909); and H1Gag1844, 5′-ACAGCATGCTGTCATCATTTCTTCTAGTG-3′ (HXB2 positions 1814 to 1843). The cycle protocol was 45 s at 48°C (cDNA reaction), followed by 2 min at 94°C; 40 cycles of 94°C for 30 s, 50°C for 30 s, and 68°C for 90 s; and 1 cycle for 7 min at 68°C. Amplified DNA (1 μl) was subjected to a second-round PCR using primers PGF1 (5′-ATAGAKRTAAAAGACACCAARGAAGC-3′) (HXB2 positions 1063 to 1088) and BHGHMA625 (5′-B-CATTCTGCAGCTTCCTCATTGAT-3′) (HXB2 positions 1402 to 1424; biotin labeled). Cycling conditions were 2 min at 94°C and 35 cycles of 94°C for 30 s, 52°C for 30 s, and 72°C for 60 s, followed by 1 cycle of 7 min at 72°C. Nested PCRs were carried out in a 50-μl reaction mixture containing 10 pmol of each primer, 20 mM dNTP mix, and 25 mM MgSO4.
Oligonucleotide probe hybridization assay procedure. (i) Streptavidin-coated MTP binding and detection.
The oligonucleotide probe hybridization assay was performed in streptavidin-coated 96-well microtiter plates (MTPs). All wash steps were done in a volume of 230 μl, with the plates being incubated for 2 min at room temperature, unless mentioned otherwise. All incubations at 37°C were done in an air incubator. All incubations at 65°C were done on a Multi-Blok Heater 2004-ICE (Lab-Line Instruments, Melrose Park, Ill.).
(ii) Tris buffer-based binding protocol.
MTPs were equilibrated by washing three times with Tris wash buffer (100 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.05% Tween 20). One hundred microliters of Tris binding buffer (500 mM NaCl, 10 mM Tris-HCl [pH 7.5], 1 mM EDTA) was added to each well. For each sample, 2 μl of PCR product 1 (∼100 to 250 ng) was added per well and per probe (combination) to be tested. Allowing for appropriate controls (see below), a maximum of 21 samples could be loaded per MTP. MTPs were incubated for 30 min at 37°C, followed by 15 min at room temperature. MTPs were then washed twice with Tris wash buffer.
(iii) Denaturation and hybridization.
Bound PCR products were denatured by the addition of 230 μl of 0.15 M NaOH and incubation at room temperature for 10 min. Washes with 0.15 M NaOH were repeated three times, with incubation times of 5, 2, and 2 min. MTPs were then washed three times with Tris neutralization buffer (100 mM Tris-HCl [pH 7.5]), followed by washing once with hybridization buffer (0.6 M NaCl, 20 mM NaxPO4 [pH 7], 1 mM EDTA, 1× Denhardt's solution, 1% Ficoll 400 [F-4375; Sigma-Aldrich, St. Louis, Mo.], 1% polyvinylpyrrolidone 360 [Sigma-Aldrich], 1% bovine serum albumin [B-4287; Sigma-Aldrich]). Three microliters of fluorescein isothiocyanate (FITC)-labeled probes (1 pmol/μl) was added to 100 μl of hybridization buffer in each well. MTPs were incubated for 2 h at 65°C on a Multi-Blok heater. The hybridization-probe solution was immediately discarded after hybridization.
(iv) Stringent washes.
MTPs were washed twice for 5 min each with 6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.1% sodium dodecyl sulfate buffer at 37°C in an air incubator and then were washed twice for 30 min each at 65°C with 1.8× SSC. The wash buffer was immediately discarded after each step.
(v) Tris buffer-based detection.
MTPs were blocked with antibody incubation buffer (100 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% [wt/vol] blocking reagent [1096176; Roche Diagnostics Belgium]) for 10 min at room temperature. The antibody incubation buffer was then discarded. One hundred microliters of diluted antibody (1/2,500; anti-fluorescein-AP antibody) (1426346; Roche Diagnostics Belgium) was transferred to each well. MTPs were incubated for 45 min at 37°C, followed by 15 min at room temperature. MTPs were washed four times with Tris wash buffer. The wash buffer was discarded. (Alternatively, the Tris wash buffer was not discarded at the last wash, and the MTPs were stored at 4°C overnight.) The hybridized oligonucleotide-antibody complex bound to the MTP was incubated with 190 μl of the colorimetric substrate para-nitrophenylphosphate (pNPP) (i.e., one Tris buffer tablet [T-8790; Sigma] and one pNPP tablet [N-2770; Sigma] dissolved at room temperature in 20 ml of sterile water) in each well. pNPP is hydrolyzed to p-nitrophenol upon addition to the complex, and p-nitrophenol is yellow and can be detected at 405 nm. At 405 nm, both absorbance and scattered light is measured, and at 650 nm, only scattered light is measured, so the latter was subtracted from the former to eliminate errors due to scattering. Absorbance readings were measured immediately by using an enzyme-linked immunosorbent assay reader, whereby the kinetics were monitored every 5 min over a period of 2 h. The Kincalc program was used to automatically calculate the optical density (OD) of each sample's reaction to the probe(s).
(vi) Controls on each MTP.
As positive and negative controls for probe reactivity, the probe target fragments of CRF02_AG and non-CRF02 samples, respectively, were processed as indicated above. For each sample, the reactivity of the PCR product with FITC-labeled PCR primer F-PGF1 was monitored as an indication of the quality and quantity of the PCR product. Wells which contained the probe and no PCR product, only a PCR product and no probe, or no PCR product and no probe were scored as negative. As a control for the binding of PCR products to the MTPs, FITC-labeled PCR products and no probe were added and NaOH treatment was omitted, which resulted in a high-level signal. As a control for denaturation, a FITC-labeled PCR product and no probe were added, which resulted in no signal.
Data analysis.
Cutoff values (CO) were determined from the 5th percentile of the OD distribution of true positives. The sensitivity was defined by the following equation: sensitivity = number of true positives/(number of true positives + number of false negatives), with true positives being CRF02_AG samples that reacted positively with one or more of the probes (OD > CO). The specificity was defined by the following equation: specificity = number of true negatives/(number of true negatives + number of false positives), with true negatives being non-CRF02_AG samples that did not react with any of the probes (OD < CO). The equation positive predictive value = number of true positives/(number of true positives + number of false positives) indicates the likelihood that a positive test result actually means that a CRF02_AG infection was identified. The equation negative predictive value = number of true negatives/(number of true negatives + number of false negatives) indicates the likelihood that a negative test result actually means that a non-CRF02_AG infection was identified.
Nucleotide sequence accession numbers.
The newly obtained nucleotide sequence data were deposited in the EMBL, GenBank, and DDBJ nucleotide sequence databases under the following accession numbers: AJ606488 to AJ606676.
RESULTS
Identification of a CRF02_AG-specific probe target region.
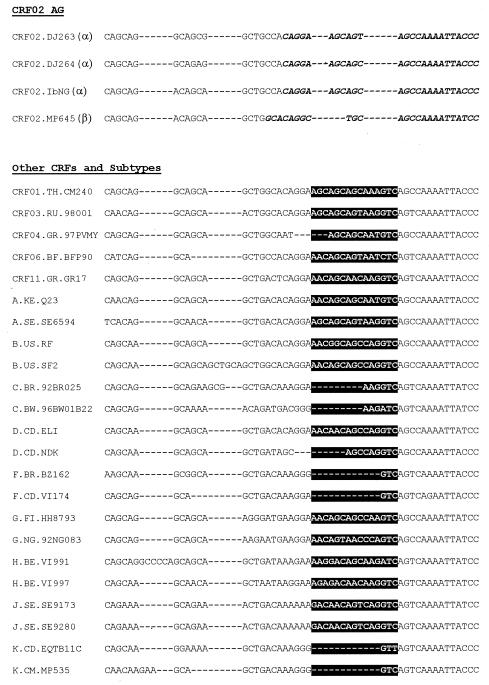
Based on sequences that are representative of all HIV-1 group M subtypes and CRFs, available from the Los Alamos National Laboratory HIV sequence database (http://hiv-web.lanl.gov/), a near-full-length genome alignment was generated and screened for CRF02_AG-specific signature patterns. A candidate probe target was identified in the HIV-1 gag p17 coding region (Fig. 1). Twenty-one of 2,330 sequences in the 1999 HIV-1 database (http://hiv-web.lanl.gov/) harboring the gag p17 probe target were described as CRF02_AG. Of these, 19 and 2, respectively, matched two different types of probe target (PAg17α and PAg17β), which were representative of two distinguished CRF02_AG subclades. Primers were designed to amplify a 360-bp probe target region by nested PCR. Five working probes were defined as follows (position numbers are according to HIV-1 strain HXB2 numbering [http://hiv-web.lanl.gov/]): PAg17α1, 5′-CAGGAAGCAGCAGCCAAAATTACCC-3′; PAg17α2, 5′-CAGGAAGCAGCAGTCAAAATTACCC-3′; PAg17α3, 5′-CAGGAAGCAGTAGCCAAAATTACCC-3′; PAg17α4, 5′-CAGGAAGCGGCAGCCAAAATTACCC-3′ (positions 1161 to 1187); and PAg17β, 5′-GCACAGGCTGCAGCCAAAATTACCC-3′ (positions 1163 to 1187).
FIG. 1.
Alignment of the conserved signature specific for CRF02_AG with all other subtypes and CRFs comprising fragments of subtype A. Black boxes indicate amino acid insertions and deletions within different subtypes and CRFs compared to probes (in bold and italics) specific for CRF02_AG.
Optimization of the oligonucleotide probe hybridization assay with reference plasmids.
For all samples of the reference panel, positive PCR products were obtained. With all five probes and the experimental conditions described above, all CRF02_AG reference plasmids scored positive and all non-CRF02_AG reference plasmids scored negative. To maximize the number of samples that could be analyzed per plate, we used probe combinations PAg17α1 plus PAg17α2 and PAg17α3 plus PAg17α4, which were evaluated to be as sensitive as the separate probes (data not shown).
Evaluation of the CRF02_AG-specific oligonucleotide probe hybridization assay.
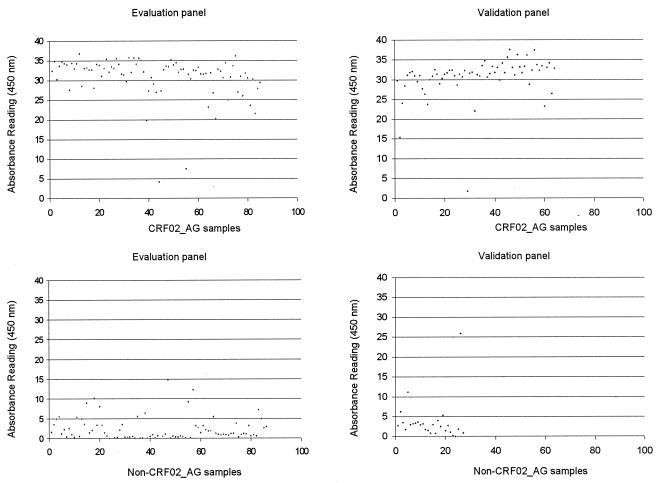
Plasma samples were selected from countries with a high CRF02_AG prevalence (Bénin and Cameroon) and from countries with a low CRF02_AG prevalence (Belgium, Kenya, and Zambia). The efficiency of PCR amplification for each cohort was as follows: Cameroon, 98% (52 of 53 samples); Zambia, 90% (9 of 10 samples); Benin, 89% (51 of 57 samples); Belgium, 85% (68 of 80 samples); and Kenya, 36% (18 of 50 samples). For evaluation of the CRF02_AG oligonucleotide probe hybridization assay, a panel of PCR-positive CRF02_AG (n = 85) and non-CRF02_AG (n = 71; 25 subtype A, 8 subtype B, 9 subtype C, 11 subtype D, 2 subtype F, 9 subtype G, 4 subtype H, 1 CRF01_AE, and 2 CRF06_cpx) samples were used (Table 1). As the gold standard, phylogenetic classification of the gag probe target fragment was used (30). Comparisons of the phylogenetic classification of the panel samples with the probe assay results (OD values) allowed calculations of means, medians, and 5th and 95th percentiles. From these, we determined cutoff values for positivity and negativity. A sample was considered to be CRF02_AG when it reacted with an OD of >15.0 with either a probe or probe combination. A sample was considered to be non-CRF02_AG when all probes and probe combinations reacted with OD values of <15.0. The OD distribution for the evaluation panel samples is depicted in Fig. 2. The overall sensitivity of the oligonucleotide probe hybridization assay evaluation panel was 97.6% (83 of 85 samples). A 100% specificity result was documented based on the fact that none of the 71 non-CRF02 (subtypes A to H, CRF01_AE, and CRF06_cpx) samples reacted with the CRF02_AG-specific probes. Overall, 63.5% (54 of 85) of the CRF02_AG samples reacted with both PAg17α probe combinations; 12.9% (11 of 85) only reacted with probe combination PAg17α3 plus PAg17α4; 1.2% only reacted with probe combination PAg17α1 plus PAg17α2. For West African countries, probe reactivity was only observed with probe combinations PAg17α1 plus PAg17α2 and/or PAg17α3 plus PAg17α4. In Cameroon, 61.7% (29 of 47) of the CRF02_AG samples reacted with PAg17α probes and 27.6% (13 of 47) of the samples reacted with probe PAg17β. For three Cameroonian CRF02_AG samples, reactivities to both probe PAg17α combinations and to PAg17β were observed. Two Cameroonian CRF02_AG samples did not react with any of the probes.
TABLE 1.
Summary of the probe assay results for the evaluation panel
| Genetic subtype classification | No. of samples in cohort
|
No. of samples with positive probe assay result/no. tested | ||||
|---|---|---|---|---|---|---|
| Belgium | Benin | Cameroon | Kenya | Zambia | ||
| CRF02 | 1 | 37 | 47 | 82/85 | ||
| A | 14 | 8 | 1 | 2 | 0/25 | |
| B | 8 | 0/8 | ||||
| C | 3 | 6 | 0/9 | |||
| D | 9 | 2 | 0/11 | |||
| F | 2 | 0/2 | ||||
| G | 3 | 4 | 2 | 0/9 | ||
| H | 4 | 0/4 | ||||
| CRF01 | 1 | 0/1 | ||||
| CRF06 | 2 | 0/2 | ||||
| Total | 45 | 51 | 50 | 4 | 6 | 82/156 |
FIG. 2.
OD distribution of evaluation panel samples. Oligonucleotide probe hybridization assay results are depicted according to genetic subtype classifications of CRF02_AG and non-CRF02_AG samples. A sample was considered to be CRF02_AG if it reacted with an OD value of >15.0 with either a probe or probe combination. Only the highest OD value obtained with probe combination PAg17α1 plus PAg17α2 or PAg17α3 plus PAg17α4 or with probe PAg17β is indicated.
Validation of the CRF02_AG-specific oligonucleotide probe hybridization assay.
Samples from Côte d'Ivoire (n = 30), Cameroon (n = 60), and The Gambia (n = 10) had been sent under code for validation of the oligonucleotide probe assay. The efficiency of PCR amplification for each cohort was as follows: Côte d'Ivoire, 90% (27 of 30 samples); Cameroon, 95% (57 of 60 samples); and The Gambia, 80% (8 of 10 samples). For the oligonucleotide probe hybridization assay, CRF02_AG predictions for the validation panel of the different cohorts were as follows: Côte d'Ivoire, 92.6% (25 of 27 samples); Cameroon, 57.9% (33 of 57 samples); and The Gambia, 71.4% (5 of 7 samples). Subsequently, the code was revealed and the assay results were compared with the subtype classifications of the validation panel that were obtained by HMAs and/or sequencing results for gag and/or env gene fragments (Table 2). In cases of discrepant results, the probe target fragment was sequenced and analyzed. All but one CRF02_AG sample from Côte d'Ivoire were classified correctly as CRF02_AG infections by the oligonucleotide probe hybridization assay, although the CRF02_AG-specific probe target of the false-negative sample was conserved. No false-positive samples were documented. The samples from The Gambia were correctly identified as CRF02_AG or non-CRF02_AG. For the Cameroonian cohort, one false-positive sample was detected. Of 32 CRF02_AG samples, 9 (28.1%) were reactive with the PAg17β probe. For one Cameroonian CRF02_AG sample, reactivities to both probe PAg17α combinations and to PAg17β were observed. The OD distribution for the validation panel samples is depicted in Fig. 2. The overall sensitivity for the validation panel was 98.4% (62 of 63 samples), the specificity was 96.3%, the positive predictive value was 98.4%, and the negative predictive value was 96.3%.
TABLE 2.
Summary of the probe assay results for the validation panel
| Genetic subtype classification | No. of samples in cohort
|
No. of samples with positive probe assay result/no. tested | ||
|---|---|---|---|---|
| Cameroon | Côte d'Ivoire | The Gambia | ||
| CRF02 | 31 | 26 | 5 | 61/62 |
| A | 16 | 1 | 1/17 | |
| B | 0/0 | |||
| C | 0/0 | |||
| D | 2 | 1 | 0/3 | |
| F | 2 | 0/2 | ||
| G | 2 | 0/2 | ||
| H | 3 | 0/3 | ||
| CRF01 | 1 | 0/1 | ||
| CRF06 | 1 | 0/1 | ||
| Total | 57 | 27 | 7 | 62/91 |
DISCUSSION
The aim of this study was to develop an oligonucleotide probe hybridization assay for the identification of HIV-1 CRF02_AG infections. This assay was configured to distinguish CRF02_AG from all other subtypes and CRFs. The close genetic distance between subtype A fragments in CRF02_AG and subtype A strains hampered differentiation between CRF02_AG and subtype A by env HMAs (7) as well as by a one-tube real-time isothermal amplification subtyping method described by de Baar et al. (6). A DNA enzyme immunoassay genotyping method for the env gene developed by Plantier et al. (22) showed a sensitivity of 86.6% (13 of 15 samples) for identifying CRF02_AG infections. The newly developed oligonucleotide probe hybridization assay showed high signal reactions to probes, differentiating CRF02_AG from other subtypes (A to H) and CRFs (CRF01 and CRF06). The oligonucleotide probe hybridization assay was validated and had a sensitivity of 98.4%, a specificity of 96.7%, a positive predictive value of 98.4%, and a negative predictive value of 96.7%, which make the assay very reliable. The sensitivity of PCR amplification of the probe target region depended on the cohort studied.
Four working probes (PAg17α1 to -α4) were sufficient to identify all CRF02_AG isolates in the West African cohort; in contrast, all five probes (PAg17α1 to -α4 and PAg17β1) were needed to identify CRF02_AG isolates in the West Central African cohort. This may indicate a further evolution of CRF02_AG in Cameroon or a founder effect by one CRF02_AG variant in West Africa.
The value of the probe assay for determining better estimates of the prevalence of CRF02_AG will complement efforts in vaccine development and evaluation. This assay will be a rapid and economical tool for use in the large-scale screening of this particular subtype at vaccine trial sites, especially in West Africa. The high sensitivity and specificity of the test also imply that there will be high positive and negative predictive values in regions of high and low prevalence.
A common drawback of using probes for genotyping in the context of HIV-1 is the huge diversity of viruses in place and time. The creation of subtype-specific probes that allow for the identification of a particular subtype by a difference of one point mutation at the probe target region has been evaluated for subtype C, with a disappointing sensitivity and specificity (results not shown). The CRF02_AG probes were designed based on a distinct signature whereby a deletion pattern compared to other subtypes and CRFs is conserved. We realize the shortcomings of the probe assay in terms of being representative of the results for the complete genome. There is a need for continuous evaluation of the probe assay, since as HIV diversity increases with time, new variants will arise that may require adaptation of the CRF02_AG probes that are used. Therefore, the aging of epidemics and cocirculation of other subtype strains may influence, through mutation and recombination, the representativeness of the probe assay result.
The actual future role of the CRFs in the global pandemic must be monitored. The probe assay as a diagnostic tool will allow rapid screening for CRF02_AG. This could be used for tracking of the HIV epidemic in terms of documenting the real prevalence of CRF02_AG virus infections. This finding may have implications on future vaccine, diagnostic, and treatment strategies, because with the extensive movement of people between continents, the chance of recombinants becoming epidemic outside of Africa increases.
Acknowledgments
We thank the Study Group on Heterogeneity of HIV Epidemics in African Cities for the contribution of plasma samples of HIV-1-infected individuals.
This work was supported by the Flanders Interuniversity Institute for Biotechnology (VIB). Part of this study was financially supported by the Japan Health Sciences Foundation. G.V.D.A. is a postdoctoral fellow of the FWO, Brussels, Belgium.
REFERENCES
- 1.Andersson, S., H. Norrgren, F. Dias, G. Biberfeld, and J. Albert. 1999. Molecular characterization of the human immunodeficiency virus (HIV)-1 and -2 in individuals from Guinea-Bissau with single or dual infections: predominance of a distinct HIV-1 subtype A/G recombinant in West Africa. Virology 262:312-320. [DOI] [PubMed] [Google Scholar]
- 2.Boom, R., C. J. Sol, M. M. Salimans, C. L. Jansen, P. M. Wertheim-van Dillen, and J. van der Noordaa. 1990. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 28:495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bredell, H., G. Hunt, A. Casteling, T. Cilliers, C. Rademeyer, M. Coetzer, S. Miller, D. Johnson, C. T. Tiemessen, D. J. Martin, C. Williamson, and L. Morris. 2002. HIV-1 subtype A, D, G, AG and unclassified sequences identified in South Africa. AIDS Res. Hum. Retrovir. 18:681-683. [DOI] [PubMed] [Google Scholar]
- 4.Carr, J. K., M. O. Salminen, J. Albert, E. Sanders-Buell, D. Gotte, D. L. Birx, and F. E. McCutchan. 1998. Full genome sequences of human immunodeficiency virus type 1 subtypes G and A/G intersubtype recombinants. Virology 247:22-31. [DOI] [PubMed] [Google Scholar]
- 5.Cham, F., L. Heyndrickx, W. Janssens, G. Van der Auwera, K. Vereecken, K. De Houwer, S. Coppens, H. Whittle, and G. van der Groen. 2000. Study of HIV type 1 gag/env variability in The Gambia, using a multiplex DNA polymerase chain reaction. AIDS Res. Hum. Retrovir. 16:1915-1919. [DOI] [PubMed] [Google Scholar]
- 6.de Baar, M. P., E. C. Timmermans, M. Bakker, E. de Rooij, B. van Gemen, and J. Goudsmit. 2001. One-tube real-time isothermal amplification assay to identify and distinguish human immunodeficiency virus type 1 subtypes A, B, and C and circulating recombinant forms AE and AG. J. Clin. Microbiol. 39:1895-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delwart, E. L., E. G. Shpaer, J. Louwagie, F. E. McCutchan, M. Grez, H. Rubsamen-Waigmann, and J. I. Mullins. 1993. Genetic relationships determined by a DNA heteroduplex mobility assay: analysis of HIV-1 genes. Science 262:1257-1261. [DOI] [PubMed] [Google Scholar]
- 8.Esteves, A., R. Parreira, T. Venenno, M. Franco, J. Piedade, J. Germano De Sousa, and W. F. Canas-Ferreira. 2002. Molecular epidemiology of HIV type 1 infection in Portugal: high prevalence of non-B subtypes. AIDS Res. Hum. Retrovir. 18:313-325. [DOI] [PubMed] [Google Scholar]
- 9.Heyndrickx, L., W. Janssens, L. Zekeng, R. Musonda, S. Anagonou, G. Van der Auwera, S. Coppens, K. Vereecken, K. De Witte, R. Van Rampelbergh, M. Kahindo, L. Morison, F. E. McCutchan, J. K. Carr, J. Albert, M. Essex, J. Goudsmit, B. Asjo, M. Salminen, A. Buvé, Study Group on Heterogeneity of HIV Epidemics in African Cities, and G. van der Groen. 2000. Simplified strategy for detection of recombinant human immunodeficiency virus type 1 group M Isolates by gag/env heteroduplex mobility assay. J. Virol. 74:363-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holguin, A., B. Rodes, and V. Soriano. 2000. Protease gene analysis of HIV type 1 non-B subtypes in Spain. AIDS Res. Hum. Retrovir. 16:1395-1403. [DOI] [PubMed] [Google Scholar]
- 11.Howard, T. M., D. O. Olaylele, and S. Rasheed. 1994. Sequence analysis of the glycoprotein 120 coding region of a new HIV type 1 subtype A strain (HIV-1IbNg) from Nigeria. AIDS Res. Hum. Retrovir. 10:1755-1757. [DOI] [PubMed] [Google Scholar]
- 12.Jetzt, A. E., H. Yu, G. J. Klarmann, Y. Ron, B. D. Preston, and J. P. Dougherty. 2000. High rate of recombination throughout the human immunodeficiency virus type 1 genome. J. Virol. 74:1234-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krogstad, P., S. H. Eshleman, Y. Geng, J. B. Jackson, M. Wantman, B. Korber, D. Lang, A. Wiznia, G. Johnson, S. Nachman, and P. Palumbo. 2002. Mother-to-child transmission in United States of subtypes D and A/G human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 18:413-417. [DOI] [PubMed] [Google Scholar]
- 14.Louwagie, J., F. E. McCutchan, M. Peeters, T. P. Brennan, E. Sanders-Buell, G. A. Eddy, G. van der Groen, K. Fransen, G. M. Gershy-Damet, R. Deleys, and D. S. Burke. 1993. Phylogenetic analysis of gag genes from 70 international HIV-1 isolates provides evidence for multiple genotypes. AIDS 7:769-780. [DOI] [PubMed] [Google Scholar]
- 15.Montavon, C., C. Toure-Kane, F. Liegeois, E. Mpoudi, A. Bourgeois, L. Vergne, J. L. Perret, A. Boumah, E. Saman, S. Mboup, E. Delaporte, and M. Peeters. 2000. Most env and gag subtype A HIV-1 viruses circulating in West and West Central Africa are similar to the prototype AG recombinant virus IbNg. J. Acquir. Immune Defic. Syndr. 23:363-374. [DOI] [PubMed] [Google Scholar]
- 16.Nkengasong, J. N., C. C. Luo, L. Abuouya, D. Pieniazek, C. Maurice, M. Sassan-Morokro, D. Ellenberger, D. J. Hu, C. P. Pau, T. Dobbs, R. Respess, D. Coulibaly, I. M. Coulibaly, S. Z. Wiktor, A. E. Greenberg, and M. Rayfield. 2000. Distribution of HIV-1 subtypes among HIV-seropositive patients in the interior of Côte d'Ivoire. J. Acquir. Immune Defic. Syndr. 23:430-436. [DOI] [PubMed] [Google Scholar]
- 17.Nyambi, P., L. Heyndrickx, K. Vereecken, S. Burda, K. De Houwer, S. Coppens, M. Urbanski, C. Williams, P. Ndumbe, and W. Janssens. 2002. Predominance of infection with HIV-1 circulating recombinant form CRF02_AG in major Cameroonian cities and towns. AIDS 16:295-296. [DOI] [PubMed] [Google Scholar]
- 18.Ortiz, M., L. Munoz, A. Bernal, A. Rodriguez, A. Zorraquino, J. Vadillo, A. Salas, A. Moreno, and A. Garcia-Saiz. 2000. Molecular characterization of non-B HIV type 1 subtypes from Africa in Spain. AIDS Res. Hum. Retrovir. 16:1967-1971. [DOI] [PubMed] [Google Scholar]
- 19.Ortiz, M., I. Sanchez, M. P. Gonzalez, M. I. Leon, N. Abeso, E. Asumu, and A. Garcia-Saiz. 2001. Molecular epidemiology of HIV type 1 subtypes in equatorial Guinea. AIDS Res. Hum. Retrovir. 9:851-855. [DOI] [PubMed] [Google Scholar]
- 20.Osmanov, S., C. Pattou, N. Walker, B. Schwardlander, J. Esparza, and the W.H.O.-UNAIDS Network for HIV Isolation and Characterization. 2002. Estimated global distribution and regional spread of HIV-1 genetic subtypes in the year 2000. J. Acquir. Immune Defic. Syndr. 29:184-190. [DOI] [PubMed] [Google Scholar]
- 21.Peeters, M., E. Esu-Williams, L. Vergne, C. Montavon, C. Mulanga-Kabeya, T. Harry, A. Ibironke, D. Lesage, D. Patrel, and E. Delaporte. 2000. Predominance of subtype A and G HIV type 1 in Nigeria, with geographical difference in their distribution. AIDS Res. Hum. Retrovir. 16:315-325. [DOI] [PubMed] [Google Scholar]
- 22.Plantier, J. C., L. Vergne, F. Damond, S. Mboup, E. MPoudi-Ngole, L. Buzelay, I. Farfara, D. Brand, M. Peeters, F. Brun-Vezinet, E. Delaporte, and F. Barin. 2002. Development and evaluation of a DNA enzyme immunoassay method for env genotyping of subtypes A through G of human immunodeficiency virus type 1 group M, with discrimination of the circulating recombinant forms CRF01_AE and CRF02_AG. J. Clin. Microbiol. 40:1010-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Preston, B. D. 1997. Reverse transcriptase fidelity and HIV-1 variation. Science 275:228-229. [DOI] [PubMed] [Google Scholar]
- 24.Ramratnam, B., S. Bonhoeffer, J. Binley, A. Hurley, L. Zhang, J. E. Mittler, M. Markowitz, J. P. Moore, A. S. Perelson, and D. D. Ho. 1999. Rapid production and clearance of HIV-1 and hepatitis C virus assessed by large volume plasmapheresis. Lancet 354:1782-1785. [DOI] [PubMed] [Google Scholar]
- 25.Stanojevic, M., A. Papa, E. Papadimitriou, S. Zerjav, D. Jevtovic, D. Salemovic, T. Jovanovic, and A. Antoniadis. 2002. HIV-1 subtypes in Yugoslavia. AIDS Res. Hum. Retrovir. 18:519-522. [DOI] [PubMed] [Google Scholar]
- 26.Taniguchi, Y., J. Takehisa, B. Bikandou, I. Mboudjeka, M. Y. N′Doundou-N′Kodia, M. Obengui M'Pandi, P. M'Pele, Y. Harada, E. Ido, M. Hayami, H. Ichimura, and H. J. Parra. 2002. Genetic subtypes of HIV type 1 based on the vpu/env sequences in the Republic of Congo. AIDS Res. Hum. Retrovir. 18:79-83. [DOI] [PubMed] [Google Scholar]
- 27.Tebit, D. M., L. Zekeng, L. Kaptue, M. Salminen, H. G. Krausslich, and O. Herchenroder. 2002. Genotypic and phenotypic analysis of HIV type 1 primary isolates from western Cameroon. AIDS Res. Hum. Retrovir. 18:39-48. [DOI] [PubMed] [Google Scholar]
- 28.Toure-Kane, C., C. Montavon, M. A. Faye, P. M. Gueye, P. S. Sow, I. Ndoye, A. Gaye-Diallo, E. Delaporte, M. Peeters, and S. Mboup. 2000. Identification of all HIV type 1 group M subtypes in Senegal, a country with low and stable seroprevalence. AIDS Res. Hum. Retrovir. 16:603-609. [DOI] [PubMed] [Google Scholar]
- 29.Ustina, V., K. Zilmer, L. Tammai, M. Raukas, A. Andersson, E. Lilja, and J. Albert. 2001. Epidemiology of HIV in Estonia. AIDS Res. Hum. Retrovir. 17:81-85. [DOI] [PubMed] [Google Scholar]
- 30.Van de Peer, Y., and R. De Wachter. 1994. TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput. Appl. Biosci. 10:569-570. [DOI] [PubMed] [Google Scholar]
- 31.Vidal, N., M. Peeters, C. Mulanga-Kabeya, N. Nzilambi, D. Robertson, W. Ilunga, H. Sema, K. Tshimanga, B. Bongo, and E. Delaporte. 2000. Unprecedented degree of human immunodeficiency virus type 1 (HIV-1) group M genetic diversity in the Democratic Republic of Congo suggests that the HIV-1 pandemic originated in Central Africa. J. Virol. 74:10498-10507. [DOI] [PMC free article] [PubMed] [Google Scholar]