Abstract
Hippocampal neurons play a critical role in learning and memory; however, the effects of environmental mechanical forces on neurite extension and associated underlying mechanisms are largely unexplored, possibly due to difficulties in maintaining central nervous system neurons. Neuron adhesion, neurite length, and mechanotransduction are mainly influenced by the extracellular matrix (ECM), which is often associated with structural scaffolding. In this study, we investigated the relationship between substrate stiffness and hippocampal neurite outgrowth by controlling the ratios of polydimethylsiloxane (PDMS) base to curing agent to create substrates of varying stiffness. Immunostaining results demonstrated that hippocampal neurons have longer neurite elongation in 35∶1 PDMS substrate compared those grown on 15∶1 PDMS, indicating that soft substrates provide a more optimal stiffness for hippocampal neurons. Additionally, we discovered that pPKCα expression was higher in the 15∶1 and 35∶1 PDMS groups than in the poly-l-lysine-coated glass group. However, when we used a fibronectin (FN) coating, we found that pFAKy397 and pFAKy925 expression were higher in glass group than in the 15∶1 or 35∶1 PDMS groups, but pPKCα and pERK1/2 expression were higher in the 35∶1 PDMS group than in the glass group. These results support the hypothesis that environmental stiffness influences hippocampal neurite outgrowth and underlying signaling pathways.
Introduction
Ramón y Cajal first investigated neuronal morphology, including neurite length, dendrite morphology, and characterization of the dendritic arbor more than 100 years ago [1]. These discoveries greatly increased the understanding of overall nervous system appearance and function. Neurite outgrowth and branching are highly complex processes that determine where nerve terminals will contact each other [1]–[5]. Hippocampal neuron outgrowth goes through five stages with significant distinguishable landmarks, from seeding to completion [6]–[8]. The first day after plating, lamellipodia form and adhere to the substrate and minor neurite formation is observed [9]. After 3 days of culturing, neuronal axons appear, followed by neurite branching at day 5. As the maturation process of hippocampal neurons continues through 7 days of culturing, neurite spines and higher order branches are formed [6]–[9].
Peripheral neurons can sense and respond to different external cues, such as mechanical stretching, compression, vibration, and touch [10]. Peripheral nerve terminals can convert mechanical input into transductive electrical signals for further responses [10]. During neurite initiation, focal adhesion complexes are formed, and microtubules align to develop a tense bundle, which is accompanied by the emergence of actin filaments to initiate a growth cone [11], [12]. Extracellular matrix (ECM) components including collagen, laminin, and fibronectin, act on surface membrane receptors to increase cell adhesion and neurite outgrowth [13], [14]. This signal further acts on focal adhesion kinase to ERK1/2 pathways to trigger actin filament transformation and microtubule neurite outgrowth [15], [16]. The integrin influences focal-adhesion formation and cell migration [17], [18], and also binds phosphatidylinositol 4, 5-bisphosphate (PIP2), in order to regulate protein kinase Cα (PKCα) activity [19]. PKC activation has been proved to be need for focal-adhesion formation and cell spreading in the integrin-mediated signaling cascade [20], [21], and regulated by Rho-family GTPase and focal adhesion kinase (FAK) [22]. Such mechanisms have been clarified in non-neuronal cell cultures, including those of 3T3 fibroblasts, epithelial cells, and cancer cells [15], [16]; Recent studies suggest that neurite outgrowth begins immediately after neuronal adhesion [23], [24]. Neurite extension is enhanced by the activation of membrane mechanical receptors and ECM [24]–[26]. The mechanic receptors on the membrane surface further induce intracellular focal adhesion kinase to alter microtubule and initiate neurite extension. Furthermore, PKC, FAK, and ERK signaling pathways further activate gene transcription to stabilize neurite formation [27], [28].
Scientists have developed materials and chemical scientific methods to provide an elastic context capable of mimicking the physiological conditions of living organisms [29], [30]. Examining results of cultured embryonic stem cells on PDMS gel of varying elasticity showed that environmental stiffness can alter cellular behaviors [31]. Among synthetic polymeric materials, hydrogels composed of polyacrylamide (PA) have been used to verify the effects of substrate flexibility on hippocampal neurite branching [32]. Flanagan et al. reported that spinal cord neurons grown on a softer substrate, sans coating with extracellular matrix molecules, formed more than three times as many branches as neurons grown on stiffer gels [33].
Polydimethylsiloxane (PDMS) is a ligand-coated synthetic polymeric system that can be used to alter stiffness to make materials of physiologically equivalent Young’s moduli [34]. PDMS has the advantage of being highly suitable for neuron growth because of its considerable flexibility and relatively low modulus. Chou et al. provided evidence that neuronal cells exhibit different neurite outgrowth on different substrates of varying stiffness [35]. Accordingly, PDMS is also useful for determine stretch-activated action potential in DRG neurons grown on PDMS substrate that is stretched via mechanical stimulation [36]. When DRG neurons are cultured on PDMS substrate coated with poly-l-lysine, neurons show lower cell density and neurite outgrowth [37].
Here, we studied the effects of elasticity on hippocampal neurite development. We hypothesized that our engineered system could alter substrate stiffness by varying ratio of base to curing agent, allowing us determine the preferred elasticity for hippocampal neurite formation. Our results indicate that hippocampal neurons have longer neurite growth on glass coverslips than on soft PDMS substrate. Within our PDMS substrate groups, hippocampal neurites were longest when cultured on 35∶1 PDMS substrate, indicating that this particular PDMS substrate offers a highly suitable environment for hippocampal neurite formation and extension. Our findings demonstrate that neurite lengths were correlated with pPKCα, pFAKy397, pFAKy925, and pERK1/2.
Materials and Methods
Polydimethlysiloxane
Polydimethlysiloxane (PDMS, Sylgard 184) was purchased from Dow Corning (Taipei, Taiwan) and the ratios of base to curing agent were modulated to approximate physiologically relevant Young’s modulus. We altered the ratios of PDMS base to curing agent (15∶1 35∶1, and 50∶1) to establish elastic moduli of approximately 173, 88, and 17 kPa [37], [38]. We used this capacity to produce various PDMS substrates and to determine which had elasticity that was more physiologically relevant than conventional glass (∼ 70 GPa).
Hippocampal Primary Culture
Postnatal day 0 outbred ICR/CD1 mice (BioLASCO Taiwan Co., Ltd.) mice were used to obtain the hippocampal cultures. The usage of these animals was approved by the Institute of Animal Care and Use Committee of China Medical University, Taiwan, following the Guide for the Use of Laboratory Animals (National Academy Press). Mice were decapitated and brains were quickly removed. The bilateral hippocampi were dissected and placed in Neurobasal-A medium (Invitrogen, Carlsbad, CA, USA) at 4°C. The hippocampi were further transferred to 1.4 mL papain solution (∼100 units/mL papain, 0.5 mM EDTA, 0.2 mg/mL cysteine; Sigma, St. Louis, MO, USA) 37°C for 15 min for digestion. Following this, we added 0.263 mL DNasI (0.21%; Sigma, St. Louis, MO, USA) and 0.182 mL of MgCl2 (12.5 mM; Sigma, St. Louis, MO, USA) to the solution incubated at 37°C 5 min, then added 0.185 mL of horse serum. The cells were pelleted by centrifugation at 1500 rpm for 5 min. 4 mL of Neurobasal-A medium was added to the cell pellets. This solution/suspension was triturated with a 10 mL pipette approximately 50 times then passed through a 100 µm filter. The hippocampal neurons were then seeded on either glass coverslips or PDMS coated with poly-L-lysine (0.1%; Sigma, St. Louis, MO, USA), fibronectin (10 μg/mL; Sigma, St. Louis, MO, USA) or laminin (1 μg/mL; Millipore, Billerica, MA, USA) at a density of 1×105 cells/mL. Coverslips with hippocampal neurons were then placed in petri dishes with Neurobasal-A medium containing B-27 supplement (Invitrogen, Carlsbad, CA, USA) and 1% penicillin/streptomycin. The hippocampal neurons were incubated at 5% CO2 at 37°C for 1-day, 3-day, 5-day, and 10-day time spans for further experiments. All procedures were shown as Figure 1A.
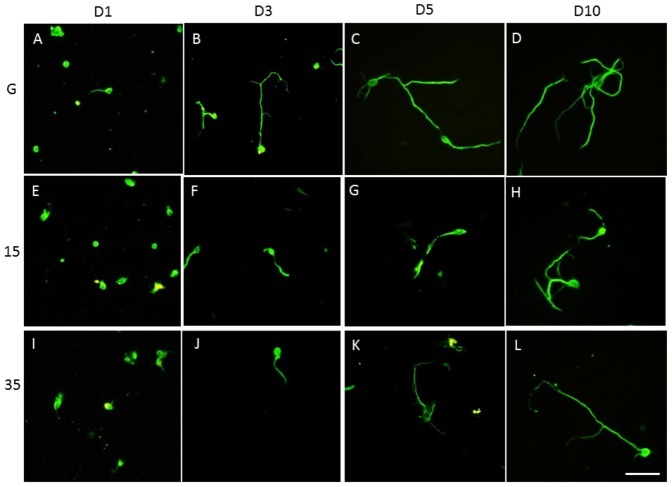
Figure 1. Schema of the experiment procedure and the Hippocampal primary cultures on glass, 15∶1 PDMS, and 35∶1 PDMS coated with poly-l-lysine, fibronectin or laminin over time followed staining with antibody or analyzing with western blot (A).
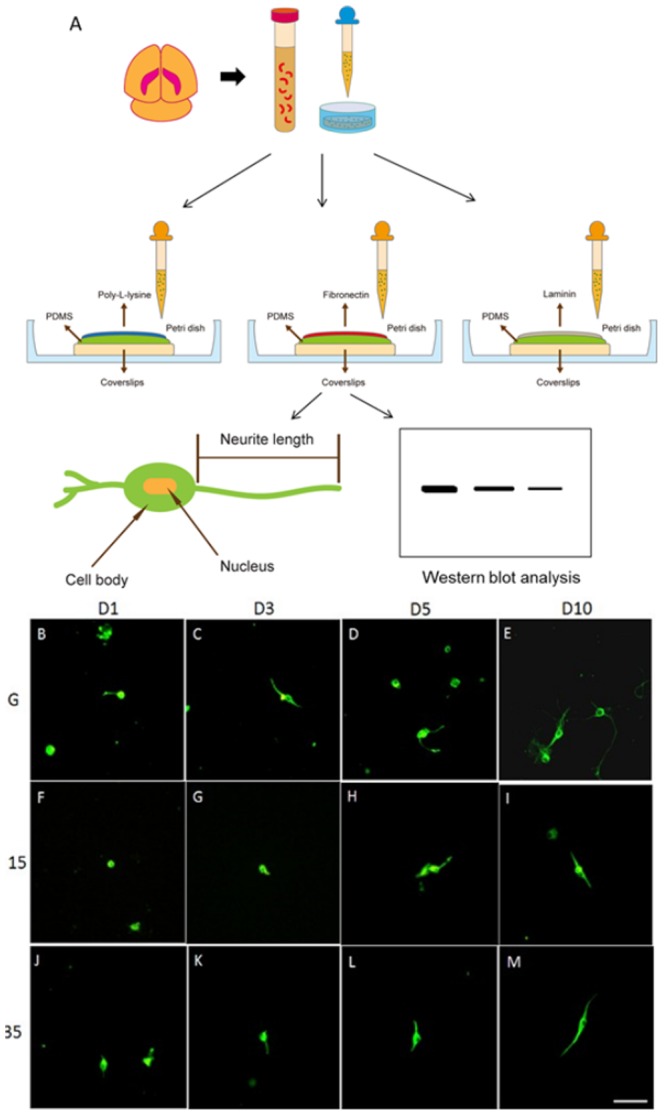
Hippocampal neurons had short neurites 1 to 3 days after seeding on poly- l-lysine-coated glass coverslips (B, C). Hippocampal neurons had longer neurites 5 and 10 days after being seeded on poly- l-lysine-coated glass coverslips (D, E). The neurites were shorter 1 and 3 days after seeding on 15∶1 PDMS (F, G). Hippocampal neurons had longer neurites 5 and 10 days after seeding on 15∶1 PDMS (H, I). Hippocampal neurites at day 1 and day 3 after seeding on 35∶1 PDMS (J, K). Hippocampal neurons had longer neurites at 5 and 10 days after seeding on 35∶1 PDMS (L, M). Tubulin (green) was used to determine neurite lengths (scale bar = 50 µm, n = 3).
Immunofluorescent Microscopy and Image Analysis
After culturing hippocampal neurons on different substrates for different time spans, hippocampal neurons were washed with phosphate buffer saline (PBS) three times, fixed with 4% paraformaldehyde, and incubated with PBS containing 1% bovine serum albumin, 0.1% Triton X-100, and 0.02% sodium azide for blocking. The samples were further mixed with a primary antibody at 4°C overnight. The primary antibody used was anti-beta Tubulin (1∶500, Novus Biologicals, Littleton, CO, USA). The secondary antibody was incubated with Goat anti-Rabbit IgG DyLight488 (1∶1000, Jackson ImmunoResearch Laboratories, West Grove, PA). The stained hippocampal neurons were mounted with glycerol, sealed under a coverslip, and then examined using fluorescent microscope (Olympus, BX-51, Japan). The images were further analyzed using NIH ImageJ software (Bethesda, MD USA). Six primary cultures in total were employed, and twenty images were captured for each group. Neurite length was calculated from cell body to nerve terminal end as shown in Figure 1A.. Neurite outgrowth percentage was calculated by dividing the neurons with neurites by total neurons.
Western blot analysis
Cultured hippocampal neuron proteins were extracted on days 1, 3 and 5. Total proteins were prepared by homogenizing neurons in lysis buffer containing 50 mM Tris-HCl pH 7.4, 250 mM NaCl, 1% NP-40, 5 mM EDTA, 50 mM NaF, 1 mM Na3VO4, 0.02 % NaN3 and 1× protease inhibitor cocktail (Amresco, Solon, USA). The extracted proteins (30 µg per sample assessed by BCA protein assay) were subjected to 8% SDS-Tris glycine gel electrophoresis and transferred to a PVDF membrane. The membrane was blocked with 5% nonfat milk in TBS-T buffer (10 mM Tris pH 7.5, 100 mM NaCl, 0.1% Tween 20), incubated with anti-PKCα [pSer657] (1∶1000, Millipore, Billerica, MA, USA), pFAK [pTyr397] (1∶500, Novus Biologicals, Littleton, CO, USA), pFAK [pTyr925] (1∶500, Novus Biologicals, Littleton, CO, USA), and pERK1/2 [pThr202, pTyr204] (1∶500, Novus Biologicals, Littleton, CO, USA) in TBS-T with 1% bovine serum albumin, and incubated for 1 hour at room temperature. Peroxidase-conjugated anti-rabbit antibody and anti-mouse antibody (1∶5000, Jackson ImmunoResearch Laboratories, West Grove, PA) were used as a secondary antibody. The bands were visualized by an enhanced chemiluminescencent substrate kit (Thermo Scientific, Waltham, MA, USA) with LAS-3000 Fujifilm (Fuji Photo Film Co. Ltd). Where applicable, the image intensities of specific bands were quantified with NIH ImageJ software (Bethesda, MD, USA).
Statistical analysis
All statistic data are presented as the mean ± standard error. Statistical significance between glass and PDMS group was tested using the ANOVA test, followed by a post hoc Tukey’s test (p<0.05 was considered statistically significant).
Results
The hippocampus is known to play significant roles in memory processing but has rarely been associated with mechanical stimulation. Our recent studies reported that 35∶1 soft PDMS substrate could be used to mimic physiological conditions and examine mechanical responses among and within DRG neurons [36], [37]. Because external substrate elasticity imposed on DRG neurons can alter the responses of cytoskeletal elements, we were motivated to examine hippocampal neurite outgrowth. To identify the effects of substrate elasticity on hippocampal neurite outgrowth, we first verified neurite length by culturing hippocampal neurons on glass coverslips coated with poly-l-lysine (staining with microtubules for cytoskeleton). Poly-l-lysine is known to increase neuron attachment while accelerating cell growth [39]. In our glass coverslip group, hippocampal neurite appearance and total length were relatively short 1 day after seeding (Fig. 1B), yet after 3 days, the neurite length was longer (Fig. 1C). Neurite lengths increased from day 3 to day 5, and continued to lengthen even after 10 days of culturing on glass coverslips (Fig. 1D, E). When hippocampal neurons were cultured on 15∶1 PDMS substrate, neurites were short from day 1 to day 3 (Fig. 1F, G). Similar to the phenomena observed in the 15∶1 PDMS group, neurites were longer from day 5-10 but still significantly shorter compared with grown on stiff glass (Fig. 1H, I). Next, we examined neurite length of hippocampal neurons cultured on softer PDMS substrate. Our results showed that neurites were observed on days 1 and 3 after plating on softer 35∶1 PDMS (Fig. 1J, K), and neurite outgrowth increased 5 or 10 days after seeding (Fig. 1L, M).
ECM (including FN) is well known for its ability to enhance cell adhesion and support neurite outgrowth and axonal regeneration [40]. We sought to identify the effects of FN on hippocampal neurite outgrowth, particularly when cultured on soft PDMS substrate. Hippocampal neurite growth was initiated 1 day after seeding in our glass coverslip group, and outgrowth was quickly established by day 3 (Fig. 2A, B). Neurite lengths and branches increased significantly with time (5 to 10 days for neurons plated on glass coverslips) (Fig. 2C, D). When culturing hippocampal neurons on 15∶1 PDMS, neurites also developed from days 1–3 (Fig. 2E, F) to days 5–10 (Fig. 2G, H). Similarly, neurite formation proceeded properly with short neurite formation on days 1 and 3 after culturing neurons on 35∶1 PDMS (Fig. 2I, J). Hippocampal neurite formation increased with longer incubation, i.e., from 5 to 10 days (Fig. 2K, L).
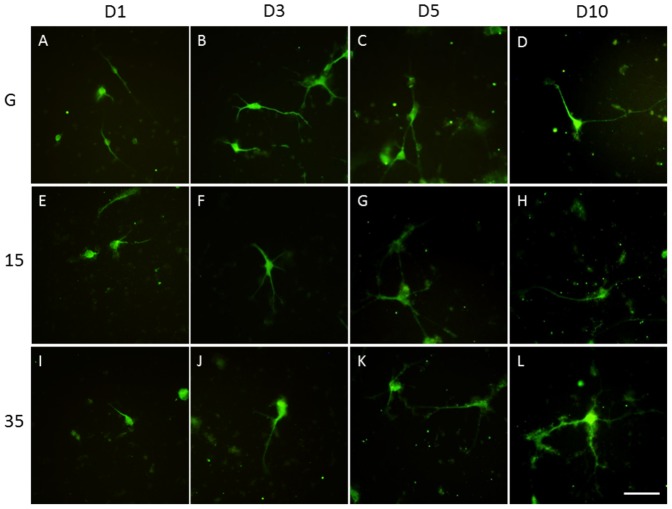
Figure 2. Hippocampal primary cultures on glass, 15∶1 PDMS, and 35∶1 PDMS coated with fibronectin over time.
Hippocampal neurons had short neurites 1 and 3 days after seeding on fibronectin-coated glass (A, B). Hippocampal neurites were longer 5 and 10 days after seeding on glass coverslips coated with fibronectin (C, D). The neurites were shorter 1 and 3 days after seeding on 15∶1 PDMS (E, F). Hippocampal neurons had longer neurites at 5 and 10 days after seeding on 15∶1 PDMS (G, H). Hippocampal neurites at 1 and 3 days after seeding on 35∶1 PDMS (I, J). Hippocampal neurons had longer neurites at 5 and 10 days after seeding on 35∶1 PDMS (K, L). Tubulin (green) was used to determine neurite lengths (scale bar = 50 µm, n = 3).
Laminin is an ECM widely used for cell attachment and crucial for neuronal survival [41]. We cultured the hippocampal neurons on laminin to determine neurite outgrowth on different subtracts. Hippocampal neurite growth was initiated 1 day after seeding in our glass coverslip group, and outgrowth was quickly established by day 3 (Fig. 3A, B). Neurite lengths and branches increased significantly with time (5 to 10 days) for neurons plated on glass coverslips (Fig. 3C, D). When culturing hippocampal neurons on 15∶1 PDMS, neurites also developed from days 1–3 (Fig. 3E, F) to days 5–10 (Fig. 3G, H). Similarly, neurite formation proceeded properly with short neurite formation on days 1 and 3 after culturing neurons on 35∶1 PDMS (Fig. 3I, J). Hippocampal neurite formation increased with longer incubation, i.e., from 5 to 10 days (Fig. 3K, L).
Figure 3. Hippocampal primary cultures on glass, 15∶1 PDMS, and 35∶1 PDMS coated with laminin over time.
Hippocampal neurons had short neurites 1 and 3 days after seeding on laminin-coated glass (A, B). Hippocampal neurites were longer 5 and 10 days after seeding on glass coverslips coated with laminin (C, D). The neurites were shorter 1 and 3 days after seeding on 15∶1 PDMS (E, F). Hippocampal neurons had longer neurites at 5 and 10 days after seeding on 15∶1 PDMS (G, H). Hippocampal neurites at 1 and 3 days after seeding on 35∶1 PDMS (I, J). Hippocampal neurons had longer neurites at 5 and 10 days after seeding on 35∶1 PDMS (K, L). Tubulin (green) was used to determine neurite lengths (scale bar = 50 µm, n = 3).
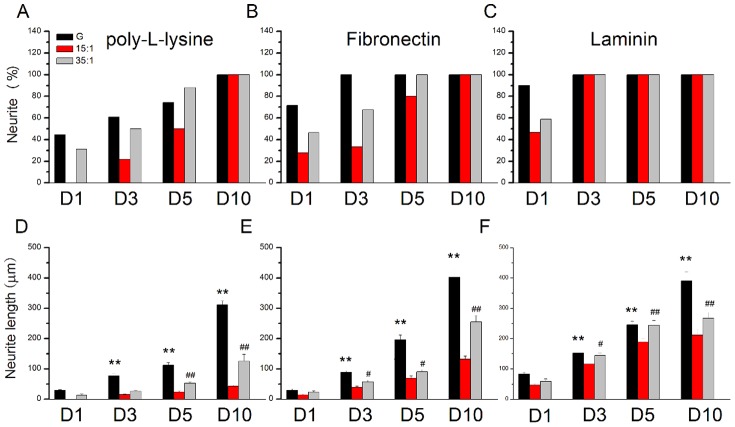
The results were quantified and plotted (Fig. 4A–F). Over time, the percentages of neurite initiation increased for cultures initiated on glass coverslips, from 1 to 10 days (Fig. 4A). Neurite length also increased over time, from 1 to 10 days, after culturing on solid glass substrate coated with poly-l-lysine. Similar phenomena were obtained for cultures in the soft PDMS groups (from 15∶1 to 35∶1 PDMS) (Fig. 4D). To determine the effects of ECM on hippocampal neurite performance, we coated FN to enhance focal adhesion. Our results show that the percentage of neurite formation was augmented when FN was used in all substrate groups from day 1 to day5 (Fig. 4B). Furthermore, hippocampal neurites showed a relative increase in length (after plating on FN-coated substrates) compared with the poly-l-lysine group (Fig. 4E). We also calculated the neurite initiation percentage of the cells cultured on coated with laminin (Fig. 4C). Furthermore, hippocampal neurites showed a relative increase in length (after plating on laminin-coated substrates) compared with the poly-l-lysine group, especially in the PDMS groups (Fig. 4F).
Figure 4. Quantification of the percentage of neurite outgrowth and neurite length on substrates with differing elasticity over time.
(A) Hippocampal neurons with neurite were determined for cultures on glass substrates, 15∶1 PDMS and 35∶1 PDMS substrates coated with poly- l-lysine. (B) Percentage of neurite outgrowth over time on substrates with varying elasticity, coated with fibronectin. (C) Percentage of neurite outgrowth over time on substrates with varying elasticity, coated with laminin. (D) Hippocampal neurons appear to have relatively short neurites when grown on 15∶1 PDMS substrates coated with poly- l-lysine. (E) The neurite lengths of hippocampal neurons were determined while culturing hippocampal neurons on glass substrates, 15∶1 and 35∶1 PDMS coated with fibronectin. Hippocampal neurite length increased when seeding on glass substrate, 15∶1and 35∶1 PDMS coated with fibronectin. (F) Hippocampal neurons appear to have relatively short neurites when grown on 15∶1 PDMS substrates coated with laminin. (*p<0.05 compared with glass substrate, **p<0.01 compared with a glass substrate, # p<0.05 compared with 35∶1 PDMS, ## p<0.01 compared with 35∶1 PDMS, n = 50 per groups). The error bars represent s.e.m.
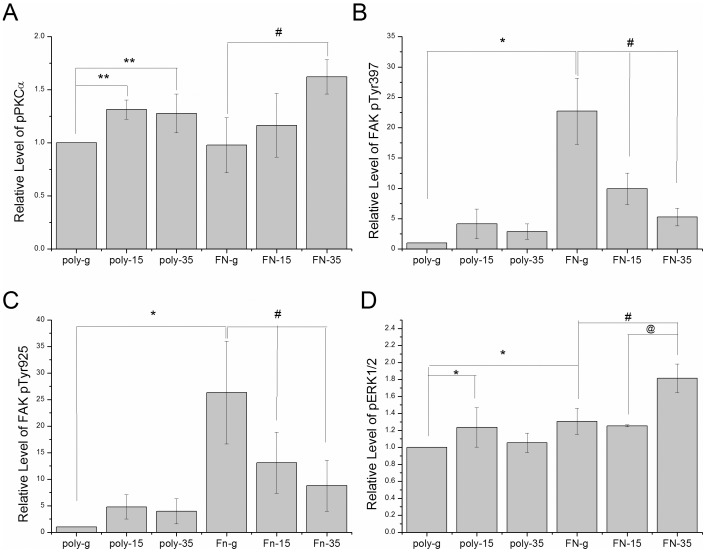
Understanding and accounting for the response of hippocampal neurite formation for cultures grown on softer substrates is critical in understanding the effects of mechanical environmental stimulation. Neurite length and extension proliferation is highly associated with mechanical force-activated kinases, such as pPKCα, pFAK397, pFAK925, and pERK1/2 [42]. We examined whether substrate stiffness altered focal adhesion and neurite elongation. We cultured hippocampal neurons on either glass coverslips or soft (15∶1 and 35∶1) PDMS coated with poly-l-lysine, a non-selective focal adhesion activator, to determine kinase expression. While culturing hippocampal neurons on substrates with varying stiffness, the expression of pPKCα was relatively increased with short neurite formation in the 15∶1 PDMS group (Fig. 5A; 131.46%±9.01% compared with glass groups, n = 3, P<0.05) and 35∶1 PDMS group (Fig. 5A; 127.74%±18.16% compared with glass groups, n = 3, P<0.05). We further discovered that pFAK397 and pFAK925 levels were not detectable in all groups (Fig. 5B, C). Our results also indicated that pERK1/2 was highest in the 15∶1 soft PDMS group (Fig. 5D; 123.59%±23.27% compared with glass groups, n = 3, P<0.05) and in 35∶1 PDMS group (Fig.5D; 105.48%±11.39% compared with glass groups, n = 3, P > 0.05). Similar results were observed in day 1 and day3. While culturing hippocampal neurons (day 1) on substrates coated with poly-L-lysine, the expression of pPKC α was relatively increased with short neurite formation in the 15∶1 PDMS group (Fig. S1; 196.88%±19.04% compared with glass groups, n = 3, P<0.05) and 35∶1 PDMS group (Fig. S1; 385.76%±13.87% compared with glass groups, n = 3, P<0.05). We further discovered that pFAK397 and pFAK925 levels were not detectable in all groups (Fig. S1). Our results also indicate that pERK1/2 was highest in the 15∶1 soft PDMS group coated with poly-L-lysine (Fig. S1; 164.89%±16.56% compared with glass groups, n = 3, P<0.05) and in 35∶1 PDMS group (Fig. S1; 186.86%±47.16% compared with glass groups, n = 3, P<0.05). While culturing hippocampal neurons (day 3) on substrates coated with poly-L-lysine, pPKC was also increased in both 15∶1 and 35∶1 groups (Fig. S2). All results were quantified and plotted as a bar chart in Figures S3 and S4. The group using glass coated with poly-L-lysine was used as a baseline.
Figure 5. pPKCα, pFAKy397, pFAKy925, and pERK1/2 were quantified 5 days after plating on substrates coated with poly-l-lysine.
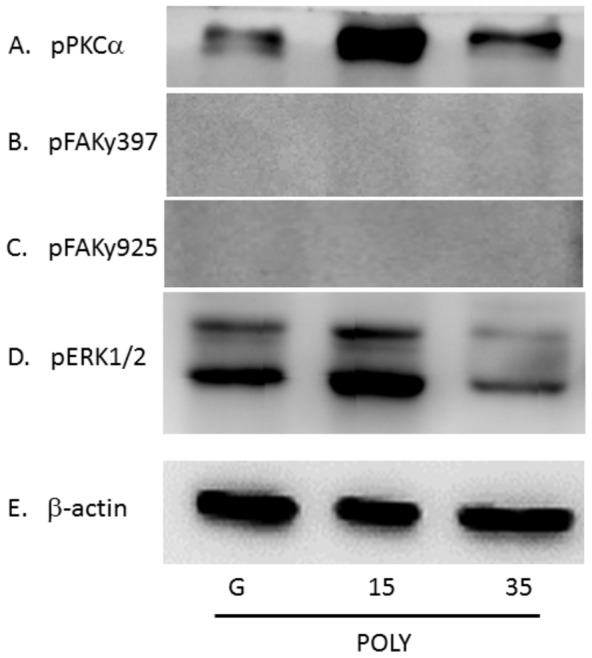
After culturing for 5 days, hippocampal neuron lysates were reacted with (A) pPKCα, (B) pFAKy397, (C) pFAKy925, (D) pERK1/2 antibodies, and (E) β-actin. Three groups shown: glass, 15∶1 PDMS, and 35∶1 PDMS. (n = 3)
Furthermore, we examined the above-mentioned pathways by culturing hippocampal neurons on FN-coated substrates. FN activates the highest expression of pPKCα in neurons in the 35∶1 PDMS compared with the glass groups (Fig. 6A; 35∶1 as 162.16%±16.1% compared with glass groups, n = 3, P<0.05). It is noteworthy that pFAK397 and pFAK925 were increased in glass group after seeding on FN-coated substrate (Fig. 6B, C). The level of pERK1/2 was simultaneously augmented with the same FN manipulation and demonstrated a proliferative increase in our 35∶1 PDMS groups (Fig. 6D; 181.34%±16.9% compared with glass groups, n = 3, P<0.05). The results were quantified and plotted as a bar chart in Figure 7, the group using glass coated with poly-l-lysine was used as a baseline.
Figure 6. pPKCα, pFAKy397, pFAKy925, and pERK1/2 were quantified 5 days after plating on substrates coated with fibronectin.
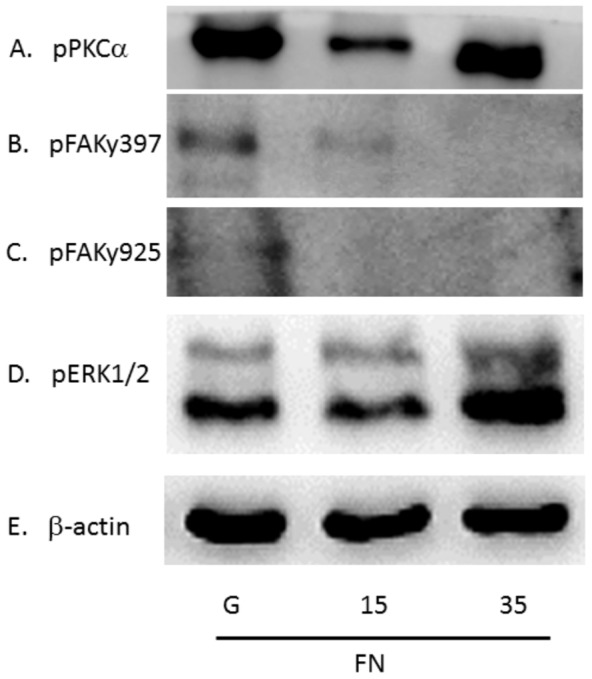
After culturing for 5 days, hippocampal neurons lysates were reacted with (A) pPKCα, (B) pFAKy397, (C) pFAKy925, (D) pERK1/2 antibodies, and (E) β-actin. Three groups shown: glass, 15∶1 PDMS, and 35∶1 PDMS. (n = 3)
Figure 7. Quantification of Western blot results (A) pPKCα, (B) pFAKy397, (C) pFAKy925, (D) pERK1/2 antibodies, Six groups shown: glass, 15∶1 PDMS, and 35∶1 coated with poly-L-lysine or fibronectin.
(*p<.05 compared to poly-g groups, # p< .05 compared to FN-g groups, @ p<.05 compared to FN-15 groups)
Discussion
The current study demonstrates the effects of soft matrix elasticity on hippocampal neurite formation and extension, which is highly regulated by ECM. To accomplish this, we employed PDMS substrate to control the stiffness and force for hippocampal neurite elongation. This approach was used to examine the cytoskeletal microtubule protein response of hippocampal neurons. Our findings are important because they elucidate the response of hippocampal neurons grown on various substrates of varying elasticity and indicate that neurite elongation is greatest on glass coverslips, which was verified by microtubule staining. Interestingly, hippocampal neurons seeded on PDMS substrate demonstrated shorter neurite length. From our array of PDMS substrates (15∶1, 35∶1, and 50∶1), the most suitable elasticity for hippocampal neuron growth was 35∶1 PDMS soft substrate. Among our three ratios of PDMS, 50∶1 PDMS was not suitable for cell growth and induced a low survival rate. (Data not shown). We also determined that pPKCα expression was relatively high for cultures grown on 15∶1 or 35∶1 PDMS compared with that for cultures grown on glass surfaces coated with poly-l-lysine. We found that pFAKy397 and pFAKy925 were expressed at low levels or not at all in neurons seeded on poly-l-lysine-coated substrate. We also determined that FN activated the highest expression levels of pPKCα and pERK1/2 in 35∶1 PDMS groups compared with glass groups. It is noteworthy that FAKs were induced in cultures grown in our glass group after seeding on FN-coated substrate. We consider that these results are significant and relevant to investigating cell–material interactions, cell mechanics, and biological engineering.
Previously published work reported that substrate stiffness can affect hippocampal neurites by altering gel rigidities from 0.02%–0.6% bis-acrylamide, and neuronal density can be changed by culturing on substrates with varying stiffness [43]. The aforementioned published work also showed that cell density can influence neurite branching when neurons are seeded on soft or stiff gels. One report described that the number of primary and secondary dendrites and branch and terminal points were higher in neurons cultured on stiff gels [32]. Our results indicated that hippocampal neurons cultured on solid glass coverslips exhibit longer neurite extension compared with those cultured on soft PDMS. Within our PDMS substrate groups, hippocampal neurons developed longer neurites on 35∶1 PDMS compared with 15∶1 or 50∶1. This implies that neurons are able to adhere to and initiate neurite growth on suitably rigid environments. Findings showing hippocampus cultured on FN coating groups with longer neurite length compared with poly-l-lysine groups suggest that FN can activate membrane mechanical receptors, possibly through integrin to enhance the focal adhesion complex and further increase neurite elongation, which is accompanied by increased pFAK, and pERK1/2 phosphorylation. Our findings are crucial because they elucidate the response of hippocampal neurite length on soft and firm substrate matrices and detail molecular mechanisms that support underlying growth and elongation mechanisms.
Both central and peripheral neurons have the ability to sense external environmental stiffness. Our previous study indicated that DRG neurons tend to attach and initiate neurite growth when cultured on a particular soft PDMS [37]. Flanagan et al. reported that mechanical forces have significant effects on spinal cord neuronal morphology and neurite branching [33]. They also suggested that neurons can respond to substrate stiffness and alter neurite branching. While neurons have been successfully cultured on softer substrates, spinal cord neurons, in particular, formed more than thrice more quickly and on softer substrates compared with stiffer gels [33].
Conclusions
It is well known that cells can respond to environmental stiffness and multiple molecular cues during neurite extension, but few studies have investigated the relevant signaling pathways. Here, we suggest that hippocampal neurons show improved attachment and neurite extension on substrates with specific stiffness (35∶1 PDMS). FN can induce hippocampal neurons to elongate neurite length, which is accompanied by increased pFAKs, and pERK1/2 phosphorylation compared to poly-L-lysine groups. These relative signaling pathways indicate crucial effects in hippocampal neuron regeneration and neurite development. This work is important in fields including neuroscience, biomaterials, and neuron–material interactions.
Supporting Information
pPKCα, pFAKy397, pFAKy925, and pERK1/2 were quantified 1 days after plating on substrates coated with poly-L-lysine or fibronectin. After culturing 1 day, hippocampal neuron lysates were reacted with (A) pPKCα, (B) pFAK397, (C) pFAK925, (D) pERK1/2 antibodies, and (E) β-actin. Three groups shown: glass, 15:1 PDMS, and 35:1 PDMS. (n = 3).
(TIF)
pPKCα, pFAKy397, pFAKy925, and pERK1/2 were quantified 3 days after plating on substrates coated with poly-L-lysine or fibronectin. After culturing 3 days, hippocampal neuron lysates were reacted with (A) pPKCα, (B) pFAKy397, (C) pFAKy925, (D) pERK1/2 antibodies, and (E) β-actin. Three groups shown: glass, 15:1 PDMS, and 35:1 PDMS. (n = 3).
(TIF)
Quantification of western blot results (A) pPKCα, (B) pERK1/2 antibodies, and six groups shown: glass, 15:1 PDMS, and 35:1 coated with poly-L-lysine or fibronectin. (*P<.05 compared to poly-g groups, @@ P<.01 compared to FN-15 groups).
(TIF)
Quantification of western blot results (A) pPKCα, (B) pERK1/2 antibodies, and six groups shown: glass, 15:1 PDMS, and 35:1 coated with poly-L-lysine or fibronectin. (*P<.05 compared to poly-g groups, # P<.05 compared to FN-g groups).
(TIF)
Funding Statement
This work was supported by NSC 101-2320-B-039-014-MY3 and in part by the Taiwan Department of Health Clinical Trial and Research Center of Excellence (DOH102-TD-B-111-004). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ramón y Cajal S, DeFelipe J, Jones EG (1991) Cajal's degeneration and regeneration of the nervous system. New York: Oxford University Press. xvi, 769 p. p.
- 2. Elston GN (2003) Cortex, cognition and the cell: new insights into the pyramidal neuron and prefrontal function. Cereb Cortex 13: 1124–1138. [DOI] [PubMed] [Google Scholar]
- 3. Garcia-Lopez P, Garcia-Marin V, Freire M (2006) Three-dimensional reconstruction and quantitative study of a pyramidal cell of a Cajal histological preparation. J Neurosci 26: 11249–11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Spruston N (2008) Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci 9: 206–221. [DOI] [PubMed] [Google Scholar]
- 5. Lobato RD (2008) Historical vignette of Cajal's work "Degeneration and regeneration of the nervous system" with a reflection of the author. Neurocirugia (Astur) 19: 456–468. [DOI] [PubMed] [Google Scholar]
- 6. Santama N, Dotti CG, Lamond AI (1996) Neuronal differentiation in the rat hippocampus involves a stage-specific reorganization of subnuclear structure both in vivo and in vitro. Eur J Neurosci 8: 892–905. [DOI] [PubMed] [Google Scholar]
- 7. Fletcher TL, Banker GA (1989) The establishment of polarity by hippocampal neurons: the relationship between the stage of a cell's development in situ and its subsequent development in culture. Dev Biol 136: 446–454. [DOI] [PubMed] [Google Scholar]
- 8. Dent EW, Kwiatkowski AV, Mebane LM, Philippar U, Barzik M, et al. (2007) Filopodia are required for cortical neurite initiation. Nat Cell Biol 9: 1347–1359. [DOI] [PubMed] [Google Scholar]
- 9. Dehmelt L, Halpain S (2004) Actin and microtubules in neurite initiation: are MAPs the missing link? J Neurobiol 58: 18–33. [DOI] [PubMed] [Google Scholar]
- 10. Delmas P, Hao J, Rodat-Despoix L (2011) Molecular mechanisms of mechanotransduction in mammalian sensory neurons. Nat Rev Neurosci 12: 139–153. [DOI] [PubMed] [Google Scholar]
- 11. Korobova F, Svitkina T (2008) Arp2/3 complex is important for filopodia formation, growth cone motility, and neuritogenesis in neuronal cells. Mol Biol Cell 19: 1561–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mallavarapu A, Mitchison T (1999) Regulated actin cytoskeleton assembly at filopodium tips controls their extension and retraction. J Cell Biol 146: 1097–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Horacek MJ, Thompson JC, Dada MO, Terracio L (1993) The extracellular matrix components laminin, fibronectin, and collagen IV are present among the epithelial cells forming Rathke's pouch. Acta Anat (Basel) 147: 69–74. [DOI] [PubMed] [Google Scholar]
- 14. Letourneau PC, Condic ML, Snow DM (1994) Interactions of developing neurons with the extracellular matrix. J Neurosci 14: 915–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aplin AE, Stewart SA, Assoian RK, Juliano RL (2001) Integrin-mediated adhesion regulates ERK nuclear translocation and phosphorylation of Elk-1. J Cell Biol 153: 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, et al. (2004) FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol 6: 154–161. [DOI] [PubMed] [Google Scholar]
- 17. Woods A, Couchman JR (1994) Syndecan 4 heparan sulfate proteoglycan is a selectively enriched and widespread focal adhesion component. Mol Biol Cell 5: 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lydolph MC, Morgan-Fisher M, Hoye AM, Couchman JR, Wewer UM, et al. (2009) Alpha9beta1 integrin in melanoma cells can signal different adhesion states for migration and anchorage. Exp Cell Res 315: 3312–3324. [DOI] [PubMed] [Google Scholar]
- 19. Lim ST, Longley RL, Couchman JR, Woods A (2003) Direct binding of syndecan-4 cytoplasmic domain to the catalytic domain of protein kinase C alpha (PKC alpha) increases focal adhesion localization of PKC alpha. J Biol Chem 278: 13795–13802. [DOI] [PubMed] [Google Scholar]
- 20. Woods A, Couchman JR (1992) Protein kinase C involvement in focal adhesion formation. J Cell Sci 101 ( Pt 2): 277–290. [DOI] [PubMed] [Google Scholar]
- 21. Vuori K, Ruoslahti E (1993) Activation of protein kinase C precedes alpha 5 beta 1 integrin-mediated cell spreading on fibronectin. J Biol Chem 268: 21459–21462. [PubMed] [Google Scholar]
- 22. Bass MD, Humphries MJ (2002) Cytoplasmic interactions of syndecan-4 orchestrate adhesion receptor and growth factor receptor signalling. Biochem J 368: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hinsby AM, Lundfald L, Ditlevsen DK, Korshunova I, Juhl L, et al. (2004) ShcA regulates neurite outgrowth stimulated by neural cell adhesion molecule but not by fibroblast growth factor 2: evidence for a distinct fibroblast growth factor receptor response to neural cell adhesion molecule activation. J Neurochem 91: 694–703. [DOI] [PubMed] [Google Scholar]
- 24. Kiryushko D, Berezin V, Bock E (2004) Regulators of neurite outgrowth: role of cell adhesion molecules. Ann N Y Acad Sci 1014: 140–154. [DOI] [PubMed] [Google Scholar]
- 25. Plantman S, Patarroyo M, Fried K, Domogatskaya A, Tryggvason K, et al. (2008) Integrin-laminin interactions controlling neurite outgrowth from adult DRG neurons in vitro. Mol Cell Neurosci 39: 50–62. [DOI] [PubMed] [Google Scholar]
- 26. Hattori N, Nomoto H, Fukumitsu H, Mishima S, Furukawa S (2007) Royal jelly-induced neurite outgrowth from rat pheochromocytoma PC12 cells requires integrin signal independent of activation of extracellular signal-regulated kinases. Biomed Res 28: 139–146. [DOI] [PubMed] [Google Scholar]
- 27. Ivankovic-Dikic I, Gronroos E, Blaukat A, Barth BU, Dikic I (2000) Pyk2 and FAK regulate neurite outgrowth induced by growth factors and integrins. Nat Cell Biol 2: 574–581. [DOI] [PubMed] [Google Scholar]
- 28. Wang X, Wang Z, Yao Y, Li J, Zhang X, et al. (2011) Essential role of ERK activation in neurite outgrowth induced by alpha-lipoic acid. Biochim Biophys Acta 1813: 827–838. [DOI] [PubMed] [Google Scholar]
- 29. Dadsetan M, Knight AM, Lu L, Windebank AJ, Yaszemski MJ (2009) Stimulation of neurite outgrowth using positively charged hydrogels. Biomaterials 30: 3874–3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hodgkinson GN, Tresco PA, Hlady V (2007) The differential influence of colocalized and segregated dual protein signals on neurite outgrowth on surfaces. Biomaterials 28: 2590–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Evans ND, Minelli C, Gentleman E, LaPointe V, Patankar SN, et al. (2009) Substrate stiffness affects early differentiation events in embryonic stem cells. Eur Cell Mater 18: 1–13 discussion 13–14. [DOI] [PubMed] [Google Scholar]
- 32. Previtera ML, Langhammer CG, Langrana NA, Firestein BL (2010) Regulation of dendrite arborization by substrate stiffness is mediated by glutamate receptors. Ann Biomed Eng 38: 3733–3743. [DOI] [PubMed] [Google Scholar]
- 33. Flanagan LA, Ju YE, Marg B, Osterfield M, Janmey PA (2002) Neurite branching on deformable substrates. Neuroreport 13: 2411–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Choi KM, Rogers JA (2003) A photocurable poly(dimethylsiloxane) chemistry designed for soft lithographic molding and printing in the nanometer regime. J Am Chem Soc 125: 4060–4061. [DOI] [PubMed] [Google Scholar]
- 35. Chou S-Y, Cheng C-M, Chen C-C, LeDuc PR (2011) Localized neurite outgrowth sensing via substrates with alternative rigidities. Soft Matter 7: 9871–9877. [Google Scholar]
- 36. Lin YW, Cheng CM, Leduc PR, Chen CC (2009) Understanding sensory nerve mechanotransduction through localized elastomeric matrix control. PLoS One 4: e4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cheng CM, LeDuc PR, Lin YW (2011) Localized bimodal response of neurite extensions and structural proteins in dorsal-root ganglion neurons with controlled polydimethylsiloxane substrate stiffness. J Biomech 44: 856–862. [DOI] [PubMed] [Google Scholar]
- 38. Cheng CM, Lin YW, Bellin RM, Steward RL Jr, Cheng YR, et al. (2010) Probing localized neural mechanotransduction through surface-modified elastomeric matrices and electrophysiology. Nat Protoc 5: 714–724. [DOI] [PubMed] [Google Scholar]
- 39. Beaudoin GM 3rd, Lee SH, Singh D, Yuan Y, Ng YG, et al. (2012) Culturing pyramidal neurons from the early postnatal mouse hippocampus and cortex. Nat Protoc 7: 1741–1754. [DOI] [PubMed] [Google Scholar]
- 40. Tonge DA, de Burgh HT, Docherty R, Humphries MJ, Craig SE, et al. (2012) Fibronectin supports neurite outgrowth and axonal regeneration of adult brain neurons in vitro. Brain Res 1453: 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen ZL, Indyk JA, Strickland S (2003) The hippocampal laminin matrix is dynamic and critical for neuronal survival. Mol Biol Cell 14: 2665–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Morgan MR, Humphries MJ, Bass MD (2007) Synergistic control of cell adhesion by integrins and syndecans. Nat Rev Mol Cell Biol 8: 957–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Previtera ML, Langhammer CG, Firestein BL (2010) Effects of substrate stiffness and cell density on primary hippocampal cultures. J Biosci Bioeng 110: 459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
pPKCα, pFAKy397, pFAKy925, and pERK1/2 were quantified 1 days after plating on substrates coated with poly-L-lysine or fibronectin. After culturing 1 day, hippocampal neuron lysates were reacted with (A) pPKCα, (B) pFAK397, (C) pFAK925, (D) pERK1/2 antibodies, and (E) β-actin. Three groups shown: glass, 15:1 PDMS, and 35:1 PDMS. (n = 3).
(TIF)
pPKCα, pFAKy397, pFAKy925, and pERK1/2 were quantified 3 days after plating on substrates coated with poly-L-lysine or fibronectin. After culturing 3 days, hippocampal neuron lysates were reacted with (A) pPKCα, (B) pFAKy397, (C) pFAKy925, (D) pERK1/2 antibodies, and (E) β-actin. Three groups shown: glass, 15:1 PDMS, and 35:1 PDMS. (n = 3).
(TIF)
Quantification of western blot results (A) pPKCα, (B) pERK1/2 antibodies, and six groups shown: glass, 15:1 PDMS, and 35:1 coated with poly-L-lysine or fibronectin. (*P<.05 compared to poly-g groups, @@ P<.01 compared to FN-15 groups).
(TIF)
Quantification of western blot results (A) pPKCα, (B) pERK1/2 antibodies, and six groups shown: glass, 15:1 PDMS, and 35:1 coated with poly-L-lysine or fibronectin. (*P<.05 compared to poly-g groups, # P<.05 compared to FN-g groups).
(TIF)