Abstract
Necropsy of two llamas revealed numerous caseous nodules containing abundant acid-fast bacilli (AFB) in various organs. The AFB were identified by spoligotyping as Mycobacterium microti, vole type. Infection caused by M. microti should be considered in the differential diagnosis of debilitating diseases in New World camelids.
CASE REPORTS
Case 1.
An 8-year-old female llama (llama 1; weight, 60 kg) was presented to the Department of Fish and Wildlife Medicine, Institute of Veterinary Pathology, University of Bern, Bern, Switzerland, with a history of appetite loss over the previous few months, cachexia, and recumbency. According to its owner, the llama had always been healthy and gave birth to a sound cria every year. A blood sample was taken, and at the owner's request, the animal was immediately euthanized. The blood chemistry profile showed hypoproteinemia with hypoalbuminemia; increased urea, lactate dehydrogenase, and bilirubin levels; and decreased Fe levels (Table 1).
TABLE 1.
Blood chemistry profiles of llamas 1 and 2a
| Parameter | Normal valueb | Llama 1 | Llama 2 |
|---|---|---|---|
| Na (mmol/liter) | 147-158 | 153 | 143 |
| K (mmol/liter) | 4-5.4 | 2.9 | 3.5 |
| Ca (mmol/liter) | 2.26-2.57 | 2.11 | 1.99 |
| Mg (mmol/liter) | 0.8-1.13 | 0.87 | 0.67 |
| Cl (mmol/liter) | 107-134 | 103 | 108 |
| P (mmol/liter) | 1.35-2.75 | 1.55 | 2.3 |
| Fe (μmol/liter) | 17-31.6 | 10.2 | |
| Total protein (g/liter) | 57.3-72.2 | 50.5 | 46.7 |
| Albumin (g/liter) | 30.1-39.8 | 13.1 | 14.4 |
| BUN (mmol/liter) | 5.82-11.48 | 19.72 | 30.84 |
| Creatinine (μmol/liter) | 121-203 | 111 | 373 |
| Bilirubin, total (μmol/liter) | 0.2-1.4 | 1.9 | 0.1 |
| Glucose (mmol/liter) | 5.4-6.6 | 3.31 | |
| ASAT (IU) | 167-302 | 294 | 361 |
| AP (IU) | 46-107 | 61 | 1,180 |
| CK (IU) | 56-663 | 199 | 275 |
| γ-GT (IU) | 29.8-56.4 | 41 | 227 |
| GLDH (IU) | 6-44.2 | 9 | 109 |
| LDH (IU) | 200-785 | 1,400 | 2,511 |
| SDH (IU) | 1-2 | 2 | 3 |
Abbreviations: BUN, blood urea nitrogen; ASAT, Aspartate transaminase; AP, alkaline phosphatase; CK, creatine kinase; γ-GT, γ-glutamyltransferase; GLDH; glutamate dehydrogenase; LDH, lactate dehydrogenase; SDH, sorbitol dehydrogenase.
I. Hengrave, doctoral thesis, in preparation.
Case 2.
Four months later, a 4-year-old female llama (llama 2; weight, 130 kg) from a different owner was presented to the Clinic for Ruminants, University of Bern, with a history of appetite loss, muscle weakness, and incoordination of 3 weeks' duration. Upon admission, the llama presented in a moderate body condition and was recumbent. It showed open-mouth breathing and bruxism. A white film covered the tongue, and a slight swelling was apparent in the retropharyngeal region. The rectal temperature was 38.6°C (normal temperature, 37.5 to 38.9°C), the heart rate was 88 beats per min (normal heart rate, 60 to 90 beats per min), and the respiratory rate was 68 breaths per min (normal respiratory rate, 10 to 30 breaths per min). Examination of the digestive tract showed poor C1 filling, decreased stomach activity, and intestinal borborygmi. The white and red blood cell counts for llama 2 revealed normal total leukocyte and neutrophil counts, toxic neutrophils with a left shift (band neutrophil count, 1.04 × 109/liter; normal count, 0 to 0.4 × 109/liter), and monocytosis (1.91 × 109/liter; normal count, 0.2 × 109 to 0.94 × 109/liter). The packed cell volume (23%; normal volume, 27 to 35%), hemoglobin level (5.64 mmol/liter; normal level, 6.9 to 9.3 mmol/liter), and erythrocyte count (8.42 × 1012/liter; normal count, 9.7 × 1012 to 13.4 × 1012/liter) were reduced. Blood biochemistry analysis revealed hypoproteinemia with marked hypoalbuminemia, increased creatinine and urea levels, and elevated liver enzyme activities (Table 1). Parasitologic examination of feces detected Nematodirus sp., Strongylus sp., and Trichuris sp. eggs. The llama died shortly after hospitalization.
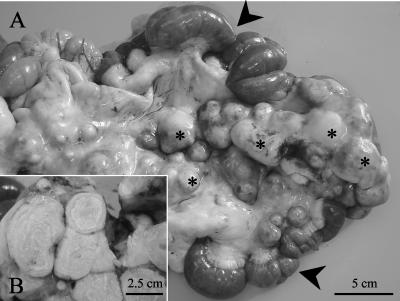
Necropsy was performed on both animals. Llama 1 was cachectic, while llama 2 had good fat stores. Gross lesions consisted of multiple confluent, yellowish, caseous nodules (diameters, up to 10 cm) with friable centers in the lungs (llamas 1 and 2), livers (llamas 1 and 2), spleen (llama 1), bronchial lymph nodes (llamas 1 and 2), hepatic lymph nodes (llamas 1 and 2), mediastinal lymph nodes (llama 2), and mesenteric lymph nodes (llama 2). Similar nodules of a smaller size were observed in the adjacent serosa. By examination of cut sections, these nodules were yellowish and firm with an onionskin-like structure and a partially mineralized center (Fig. 1). Additional findings for llama 2 included hydrothorax, ascites, hepatic lipidosis, splenomegaly, pulmonary edema and congestion, esophageal petechiae, intestinal hemorrhages, and cervical subcutaneous edema.
FIG. 1.
(A) Jejunum (arrowheads) with enlarged mesenteral lymph nodes (asterisks); (B) cut section of mesenteric lymph nodes, which are enlarged due to the presence of partially mineralized granulomas with onionskin-like structures.
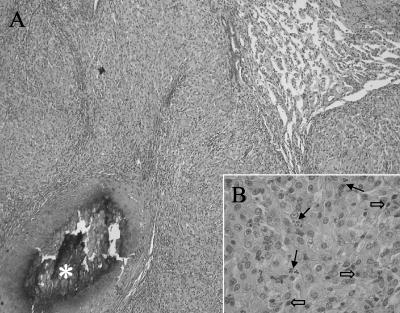
Histologically, the caseous nodules presented as granulomas composed of large numbers of closely packed, epithelioid macrophages admixed with various numbers of lymphocytes, plasma cells, and neutrophils (Fig. 2). The larger granulomas showed central necrosis with foci of mineralization and fibrous capsules of various thicknesses. Epithelioid macrophages contained eosinophilic, fine granular cytoplasms. Ziehl-Neelsen and Fite-Faraco staining revealed abundant acid-fast bacilli (AFB) within the epithelioid macrophages throughout all layers of the granulomas. They appeared as irregular long, straight, or curved structures without branches. Direct molecular testing of lung, liver, kidney, and lymph node specimens by the amplified Mycobacterium tuberculosis Direct Test (Gen-Probe, San Diego, Calif.) yielded M. tuberculosis complex. Only one culture medium (Mycobacteria Growth Indicator Tube; Becton Dickinson, Sparks, Md.) showed weak growth of AFB, while the solid media (Löwenstein-Jensen, Middlebrook 7H10/sel7H11) remained negative for growth. Due to the very sparse growth, biochemical identification of the AFB was not possible. The diagnosis of Mycobacterium microti infection was finally achieved by spacer oligonucleotide typing (spoligotyping [14]). Both strains showed a two-spacer spoligotype (spacers 37 and 38) (Fig. 3), which is a characteristic of M. microti, vole type (15).
FIG. 2.
Histologic features of the lung. (A) The lung parenchyma is obscured due to the presence of numerous granulomas with necrotic and partially mineralized centers (asterisk). Hematoxylin-eosin stain; magnification, ×81. (B) The granulomas are composed of epithelioid macrophages admixed with scattered neutrophils (solid arrows) and lymphocytes (open arrows). Hematoxylin-eosin stain; magnification, ×324.
FIG. 3.
Spoligotype patterns. Row 1, M. microti, vole type (isolated from llama 1); row 2, M. tuberculosis; row 3, M. bovis. The last two strains were isolated from human specimens and are shown for comparison.
Upon diagnosis of M. microti infection, the state veterinarian quarantined both herds. All remaining llamas were tested twice at 6-week intervals by intradermal tuberculin testing of the axillary space with bovine and avian purified protein derivatives (9). The quarantine was lifted after the two consecutive tuberculin tests were negative. The owners and their families were also tested and were negative.
Epidemiological investigations revealed that llama 1 had been imported from South America in 1994, together with 83 other animals. At that time, the whole group was kept in quarantine for 2 months and tested for tuberculosis by intradermal skin tests. Seventy-eight llamas proved to be negative by the tuberculin skin test, while six animals had ambiguous results. Four weeks later these animals with questionable results were negative by a second tuberculin skin test. Llama 2 was an offspring of one of the imported animals (but not of llama 1) and was subsequently sold to a second owner. We could not determine if llama 1 or the dam of llama 2 was one of the animals that had exhibited an ambiguous skin test result.
In humans, tuberculosis is the leading infectious cause of death worldwide, with one-third of the global population infected with M. tuberculosis (7, 21). In animals, tuberculosis is of primary importance for both its impact on the economy and its zoonotic potential. The majority of tubercular diseases in ungulates are caused by Mycobacterium bovis (18), which can also infect Old World and New World camelids (1, 3, 5, 17, 24, 28). Among the latter, infections due to other mycobacteria, including M. tuberculosis, Mycobacterium paratuberculosis, Mycobacterium kansasii, and M. microti, have been described (2, 9, 13, 20, 22, 23). Mostly, animals in zoos are affected, while infections in their natural habitat in South America are rarely reported, suggesting that under natural conditions lamoids are not highly susceptible to infections with mycobacteria.
M. microti belongs to the M. tuberculosis complex (26), whose members (M. tuberculosis, M. bovis, the M. bovis BCG vaccine strain, Mycobacterium africanum, M. microti, and Mycobacterium canettii) share an identical 16S rRNA gene and show a >90% relatedness (85 to 89% relatedness for M. microti) at the DNA level (12). The natural hosts and reservoirs of M. microti, first discovered by Wells and Oxen (27), are small rodents such as voles, wood mice, and shrews (16). A postmortem study of snap-trapped field voles in the United Kingdom revealed a 21% prevalence of M. microti (4). Sporadic cases of M. microti infections were reported in cats, pigs, a ferret, a cow, a badger, and a captive vicuña (11, 15, 20). A very similar bacterium, the “Dassie bacillus,” was identified in an imported Cape hyrax with granulomatous lesions of the lung, liver, kidney, and spleen (6). In contrast to M. microti, the “Dassie bacillus” does not cause infection in mice.
M. microti has long been considered an unimportant pathogen in mammalian species other than small rodents. However, the application of molecular methods to the identification of members of the M. tuberculosis complex resulted in the recognition of increased numbers of M. microti infections in animals and humans, suggesting that the prevalence of M. microti infections has been underestimated (4). In 1998, the bacterium was first detected in humans with pulmonary tuberculosis in The Netherlands (8, 25). Later, three cases were diagnosed in Germany (10, 19) and two cases were diagnosed in Switzerland. Immunocompromised as well as immunocompetent patients were affected.
Since small rodents are the natural hosts of M. microti and circumstantial evidence revealed human-rodent interactions in some reported cases, rodents have been discussed as a source of infection for humans, but this has not been confirmed with certainty (25). Human-to-human transmission may occur, but no specific M. microti strain adapted to the human host has been identified (8). In a vicuña born in a zoo in Belgium, infection by direct or indirect contact with wild mice was assumed (20). In the two cases described herein, the source of infection could not be determined, and there is no information on the prevalence of M. microti in small rodents in Switzerland. Interestingly, one llama had been imported from South America 7 years earlier. At that time, however, tuberculin tests were negative. Since llama 2 was an offspring of an animal within the same herd as llama 1, llama-to-llama transmission cannot be excluded.
The gross and histological lesions in both llamas were similar to those reported in 1970 in a vicuña with a M. microti, llama type, infection (20). As in the vicuña, granulomas were disseminated in multiple organs of the two llamas and were not limited to the lung, as is described in humans (8, 10, 19). The increases in the levels of specific liver enzymes, such as glutamate dehydrogenase, γ-glutamyltransferase, and sorbitol dehydrogenase, as well as the hypoalbuminemia, can be explained by the severe lesions found in the liver. Elevated blood urea nitrogen and creatinine values were likely due to a prerenal uremia.
The clinical diagnosis of M. microti infection remains a challenge. The use of intradermal tuberculin testing for the diagnosis of tuberculosis in camelids is controversial due to the false-positive and false-negative results that have been reported (9). Information on the sensitivity of tuberculin testing of animals infected with M. microti is lacking. Only one case report of a human infection with M. microti describes a positive tuberculin skin test result with a 7-mm induration (19). The laboratory diagnosis of M. microti is hampered by its sparse growth (25). Therefore, molecular methods which are able to discriminate members within the M. tuberculosis complex are indicated. Molecular spoligotyping, for instance, has proved to facilitate both the detection and the identification of a M. microti infection without the need to culture this slowly growing organism (25).
Since lamoids are increasing in popularity and often kept in close contact with humans (e.g., as trekking animals and in children's zoos), they may represent a potential source of zoonotic infection. Therefore, an infection caused by M. microti should be considered a differential diagnosis in debilitating diseases with or without respiratory signs in all New World camelids.
Acknowledgments
We thank A. von Graevenitz for revising the manuscript.
REFERENCES
- 1.Barlow, A. M., K. A. Mitchell, and K. H. Visram. 1999. Bovine tuberculosis in llama (Lama glama) in the UK. Vet. Rec. 145:639-640. [DOI] [PubMed] [Google Scholar]
- 2.Belknap, E. B., D. M. Getzy, L. W. Johnson, R. P. Ellis, G. L. Thompson, and W. P. Shulaw. 1994. Mycobacterium paratuberculosis infection in two llamas. J. Am. Vet. Med. Assoc. 204:1805-1808. [PubMed] [Google Scholar]
- 3.Bush, M., R. J. Montali, L. G. Phillips, and P. A. Holobaugh. 1990. Bovine tuberculosis in a Bactrian camel herd: clinical, therapeutic, and pathologic findings. J. Zoo Wildl. Med. 21:171-179. [Google Scholar]
- 4.Cavanagh, R., M. Begon, M. Bennett, T. Ergon, I. M. Graham, P. E. W. de Haas, C. A. Hart, M. Koedam, K. Kremer, X. Lambin, P. Roholl, and D. van Soolingen. 2002. Mycobacterium microti infection (vole tuberculosis) in wild rodent populations. J. Clin. Microbiol. 40:3281-3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chartier, F., C. Chartier, M.-F. Thorel, and F. Crespeau. 1991. A new case of Mycobacterium bovis pulmonary tuberculosis in the dromedary (Camelus dromedarius) in Mauritania. Rev. Elev. Med. Vet. Pays Trop. 44:43-47. [PubMed] [Google Scholar]
- 6.Cousins, D. V., R. L. Peet, W. T. Gaynor, S. N. Williams, and B. L. Gow. 1994. Tuberculosis in imported hyrax (Procavia capensis) caused by an unusual variant belonging to the Mycobacterium tuberculosis complex. Vet. Microbiol. 42:135-145. [DOI] [PubMed] [Google Scholar]
- 7.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Global burden of tuberculosis. Estimated incidence, prevalence, and mortality by country. JAMA 282:677-686. [DOI] [PubMed] [Google Scholar]
- 8.Foudraine, N. A., D. van Soolingen, G. T. Noordhoek, and P. Reiss. 1998. Pulmonary tuberculosis due to Mycobacterium microti in a human immunodeficiency virus-infected patient. Clin. Infect. Dis. 27:1543-1544. [DOI] [PubMed] [Google Scholar]
- 9.Fowler, M. E. 1998. Tuberculosis, p. 169-171. In Medicine and surgery of South American camelids (llama, alpaca, vicuña, guanaco), 2nd ed. Iowa State University Press, Ames, Iowa.
- 10.Horstkotte, M. A., I. Sobottka, C. K. Schewe, P. Schäfer, R. Laufs, S. Rüsch-Gerdes, and S. Niemann. 2001. Mycobacterium microti llama-type infection presenting as pulmonary tuberculosis in a human immunodeficiency virus-positive patient. J. Clin. Microbiol. 39:406-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huitema, H., and F. H. J. Jaartsveld. 1967. Mycobacterium microti infection in a cat and some pigs. Antonie Leeuwenhoek 33:209-212. [DOI] [PubMed] [Google Scholar]
- 12.Imaeda, T. 1985. Deoxyribonucleic acid relatedness among selected strains of Mycobacterium tuberculosis, Mycobacterium bovis, Mycobacterium bovis BCG, Mycobacterium microti, and Mycobacterium africanum. Int. J. Syst. Bacteriol. 35:147-150. [Google Scholar]
- 13.Johnson, C. T., C. E. Winkler, E. Boughton, and J. W. F. Penfold. 1993. Mycobacterium kansasii infection in a llama. Vet. Rec. 133:243-244. [DOI] [PubMed] [Google Scholar]
- 14.Kamerbeek, J., L. Schouls, A. Kolk, M. van Agterveld, D. van Soolingen, S. Kuijper, A. Bunshoten, H. Molhuizen, R. Shaw, M. Goyal, and J. van Embden. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35:907-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kremer, K., D. van Soolingen, J. van Embden, S. Hughes, J. Inwald, and G. Hewinson. 1998. Mycobacterium microti: more widespread than previously thought. J. Clin. Microbiol. 36:2793-2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lepper, A. W. D., and L. A. Corner. 1983. Naturally occurring mycobacterioses of animals, p. 418-444. In C. Ratledge and J. Stanford (ed.), Biology of mycobacteria, vol. 2. Academic Press, London, United Kingdom. [Google Scholar]
- 17.Mason, F. E. 1917. Tuberculosis in camels. J. Comp. Pathol. 30:80-84. [Google Scholar]
- 18.Montali, R. J., S. K. Mikota, and L. I. Cheng. 2001. Mycobacterium tuberculosis in zoo and wildlife species. Rev. Sci. Tech. Off. Int. Epizoot. 20:291-303. [DOI] [PubMed] [Google Scholar]
- 19.Niemann, S., E. Richter, H. Dalügge-Tamm, H. Schlesinger, D. Graupner, B. Königstein, G. Gurath, U. Greinert, and S. Rüsch-Gerdes. 2000. Two cases of Mycobacterium microti-derived tuberculosis in HIV-negative immunocompetent patients. Emerg. Infect. Dis. 6:539-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pattyn, S. R., F. A. Portaels, P. Kageruka, and P. Gigase. 1970. Mycobacterium microti infection in a zoo-llama, Lama vicugna (molina). Acta Zool. Pathol. Antverpiensia 51:17-24. [PubMed] [Google Scholar]
- 21.Raviglione, M. C., D. E. Snider, Jr., and A. Kochi. 1995. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA 273:220-226. [PubMed] [Google Scholar]
- 22.Ridge, S. E., J. T. Harkin, R. T. Badman, A. M. Mellor, and J. W. Larsen. 1995. Johne's disease in alpacas (Lama pacos) in Australia. Aust. Vet. J. 72:150-153. [DOI] [PubMed] [Google Scholar]
- 23.Stehman, S. M. 1996. Paratuberculosis in small ruminants, deer, and South American camelids. Vet. Clin. N. Am. Food Anim. Pract. 12:441-455. [DOI] [PubMed] [Google Scholar]
- 24.Thoen, C. O., W. D. Richards, and J. L. Jamagin. 1977. Mycobacteria isolated from exotic animals. J. Am. Vet. Med. Assoc. 170:978-990. [PubMed] [Google Scholar]
- 25.van Soolingen, D., A. G. M. van der Zanden, P. E. W. de Haas, G. T. Noordhoek, A. Kiers, N. A. Foudraine, F. Portaels, A. H. J. Kolk, K. Kremer, and J. D. A. van Embden. 1998. Diagnosis of Mycobacterium microti infections among humans by using novel genetic markers. J. Clin. Microbiol. 36:1840-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wayne, L. G., and G. P. Kubica. 1989. The mycobacteria, p. 1435-1457. In P. H. A. Sneath and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 2. The Williams & Wilkins Co., Baltimore, Md. [Google Scholar]
- 27.Wells, A. Q., and D. M. Oxen. 1937. Tuberculosis in wild voles. Lancet i:1221. [Google Scholar]
- 28.Zecha, B. C. 1990. Llamas infected with bovine tuberculosis maintained under domestic agricultural conditions, p. 523-525. In Proceedings of the 94th Annual Meeting of the U.S. Animal Health Association. U.S. Animal Health Association, Richmond, Va.