Abstract
Human adenoviruses (HAdVs) are the major causes of a variety of acute illnesses. Virus isolation and neutralization tests are usually done to identify the causative virus, but these tests are labor-intensive and time-consuming, and standardized antisera are in limited supply. This study investigated a rapid and reliable method of virus identification based on PCR and phylogenetic analysis. The phylogenetic tree constructed by neighbor joining on the basis of the newly determined partial hexon sequences from 33 prototypes of HAdV-D and -E, along with 11 available prototypes of HAdV-A to -C and -F from GenBank, allowed HAdVs to be grouped into six distinct clusters. These clusters correspond closely to the six newly designated species, HAdV-A to -F. The partial hexon sequences of 57 isolates from patients with acute conjunctivitis obtained over 20 years plus those of 44 prototype strains were analyzed. Each isolate formed a monophyletic cluster along with its respective prototype strain, allowing serotype identification. Partial-hexon-based classification appears to be an effective tool for studying the molecular epidemiology of HAdVs.
Human adenoviruses (HAdVs) in the genus Mastadenovirus of the family Adenoviridae form a group of viruses with numerous serotypes (1, 9, 16, 31, 38, 42). HAdVs infect billions of people worldwide and cause various clinical manifestations, such as keratoconjunctivitis, upper and lower respiratory tract infections, hemorrhagic cystitis, and gastroenteritis (18, 21).
HAdVs were initially grouped into six subgenera (A to F) on the basis of several biochemical and biophysical criteria (1, 38). In 1999, reclassification of HAdVs on the basis of nucleotide and deduced amino acid sequences was approved by the International Committee on Taxonomy of Viruses, after which the 51 serotypes of HAdVs in the genus Mastadenovirus were grouped into six species, HAdV-A to HAdV-F (37).
In Japan, 4,528 cases of illness due to HAdVs were reported in 2001 to 2003 (Infectious Agents Surveillance Report [IASR] [http://idsc.nih.go.jp/iasr/index.html]). They were obtained from persons with epidemic conjunctivitis (821; 18.1%), upper and lower respiratory tract infections (615; 13.6%), and gastroenteritis (1,162; 25.7%) (IASR 23[7], 2002, and 24[6], 2003). HAdVs are major causative agents of keratoconjunctivitis and acute conjunctivitis in several countries, especially in East and Southeast Asia, including Japan (4, 5, 15, 19; J. C. Hierholzer, B. Guyer, D. M. O'Day, and W. Shaffer, Letter, N. Engl. J. Med. 290:1436, 1974). Among HAdVs, four strains, AdV-4, -8, -19, and -37, have been responsible for sporadic cases, as well as outbreaks of severe epidemic keratoconjunctivitis (EKC). These strains are also well known to be etiological agents of nosocomial infections (4, 22, 28, 40). Virus isolation, followed by a neutralization test, has usually been carried out for the purpose of serotyping (38). However, these procedures are complicated and time-consuming, and the standardized antisera are in limited supply. In addition, the neutralization tests with several serotype-specific antisera showed cross-reactions among AdV-15, AdV-22, and AdV-42 and among AdV-10, -13, -19, -30, and -37 (1). To address these problems, a method of PCR-restriction fragment length polymorphism (RFLP) analysis based on a partial hexon gene (956 bp) was developed (29). This method is extremely useful for rapid diagnosis, without viral isolation, of causative AdVs in patients with eye infections. However, we encountered difficulty in identifying the serotype of the HAdV isolates from patients with keratoconjunctivitis in Japan. These isolates were identified as AdV-4 and -8 by neutralization tests with type-specific antisera. However, when we compared the cleavage patterns by PCR-RFLP, the isolates showed patterns different from those of their respective prototype strains. The sequences of their PCR products showed several mutations at the cleaved site.
In this study, we determined the nucleotide sequences of the partial hexon genes (916 bp) of all prototype strains in HAdV-D and -E, which have not been available from GenBank. The database based on the hexon gene was constructed, including 11 available nucleotide sequences of prototype strains in HAdV-A to -C and -F, and it was used for phylogeny-based identification of AdV from patients with conjunctivitis.
MATERIALS AND METHODS
Virus strains.
Altogether, we obtained 33 prototype strains, including AdV-8 to -10, AdV-13, AdV-15, AdV-17, AdV-19, AdV-20, AdV-22 to -30, AdV-32, AdV-33, AdV-36 to -39, and AdV-42 to -50 from HAdV-D, and AdV-4 from HAdV-E, from the American Type Culture Collection or the National Institute of Infectious Diseases, Tokyo, Japan (Table 1). The AdV-19 isolate from a patient with EKC, which was identified as AdV-19a by genome typing, was used for determination of the hexon nucleotide sequences and as an example of AdV-19a in the database. These viruses were used directly for DNA extraction without further propagation. Eleven field isolates collected from EKC patients, including three isolates of AdV-4, three isolates of AdV-8, two isolates of AdV-19a, and three isolates of AdV-37, were propagated in either HEp-2 cells or HeLa cells (Table 2). These isolates were provided by K. Fujimoto, Sapporo City Institute of Public Health, and were identified by a neutralization test with type-specific antisera purchased from Denka Seiken Co., Ltd., Tokyo, Japan, and the National Institute of Infectious Diseases. These isolates have been well characterized genetically by genome typing. The 46 isolates, including the isolates untyped by PCR-RFLP, were from patients with EKC. They were propagated in LLC-MK2 or HeLa cells (Table 3).
TABLE 1.
Complete set of partial hexon nucleotide sequences of HAdV-D and -E, along with available nucleotide sequences from GenBank
| Serotype | Strain | Species | GenBank accession no.a |
|---|---|---|---|
| AdV-8 | Trim | HAdV-D | AB099348 |
| AdV-9 | Hicks | HAdV-D | AB099349 |
| AdV-10 | J.J. | HAdV-D | AB099350 |
| AdV-13 | A.A. | HAdV-D | AB099351 |
| AdV-15 | CH38 | HAdV-D | AB099352 |
| AdV-17 | CH22 | HAdV-D | AB099353 |
| AdV-19 | AV-587 | HAdV-D | AB099354 |
| AdV-20 | AV-931 | HAdV-D | AB099355 |
| AdV-22 | AV-2711 | HAdV-D | AB099356 |
| AdV-23 | AV-2732 | HAdV-D | AB099357 |
| AdV-24 | AV-3153 | HAdV-D | AB099358 |
| AdV-25 | BP-1 | HAdV-D | AB099359 |
| AdV-26 | BP-2 | HAdV-D | AB099360 |
| AdV-27 | BP-4 | HAdV-D | AB099361 |
| AdV-28 | BP-5 | HAdV-D | AB099362 |
| AdV-29 | BP-6 | HAdV-D | AB099363 |
| AdV-30 | BP-7 | HAdV-D | AB099364 |
| AdV-32 | HH | HAdV-D | AB099365 |
| AdV-33 | D.J. | HAdV-D | AB099366 |
| AdV-36 | 275 | HAdV-D | AB099367 |
| AdV-37 | GW | HAdV-D | AB099368 |
| AdV-38 | LJ | HAdV-D | AB099369 |
| AdV-39 | D335 | HAdV-D | AB099370 |
| AdV-42 | 54/82 | HAdV-D | AB099371 |
| AdV-43 | 1309 | HAdV-D | AB099372 |
| AdV-44 | 1584 | HAdV-D | AB099373 |
| AdV-45 | 1590 | HAdV-D | AB099374 |
| AdV-46 | 1594 | HAdV-D | AB099375 |
| AdV-47 | 1601 | HAdV-D | AB099376 |
| AdV-48 | T85-844 | HAdV-D | AB099377 |
| AdV-49 | T87-677 | HAdV-D | AB099378 |
| AdV-50 | Wan | HAdV-D | AB099379 |
| AdV-4 | RI-67 | HAdV-E | AB099380 |
| AdV-12 | Huie | HAdV-A | X73487 |
| AdV-18 | DC | HAdV-A | Y17249 |
| AdV-3 | GB | HAdV-B | X76549 |
| AdV-7 | Gomen | HAdV-B | AF065065 |
| AdV-16 | CH79 | HAdV-B | X74662 |
| AdV-21 | AV-1645 | HAdV-B | AB053116 |
| AdV-34 | Compton | HAdV-B | AB052911 |
| AdV-2 | Adenoid6 | HAdV-C | J01917 |
| AdV-5 | Adenoid75 | HAdV-C | M73260 |
| AdV-40 | Dugan | HAdV-F | L19443 |
| AdV-41 | Tak | HAdV-F | X51783 |
Nucleotide sequence AB099348 to AB099380 were determined in this study.
TABLE 2.
Evaluation of the diagnostic method based on phylogenetic analysis to identify AdV serotypes
| Isolate/yr | Geographic origin | Typing by:
|
Genome type | Typing by phylogeny
|
Highest-scoring heterologous prototype
|
GenBank accession no. | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Neutralization test | PCR-RFLP | Type | Bootstrap (%) | Identity (%) | Type | Identity (%) | ||||
| TC-4822/84 | Sapporo | AdV-4 | Not AdV-1 to -51 | AdV-4a | AdV-4 | 100 | 96.4 | AdV-32 | 89.4 | AB098598 |
| TC-18040/91 | Sapporo | AdV-4 | Not AdV-1 to -51 | AdV-4a | AdV-4 | 100 | 96.4 | AdV-32 | 89.4 | AB098599 |
| 4439/98 | Sapporo | AdV-4 | Not AdV-1 to -51 | AdV-4a | AdV-4 | 100 | 96.4 | AdV-32 | 89.4 | AB098601 |
| TC-7223/86 | Sapporo | AdV-8 | AdV-8 | AdV-8A | AdV-8 | 100 | 99.9 | AdV-22 | 94.9 | AB099381a |
| TC-6821/86 | Sapporo | AdV-8 | AdV-8 | AdV-8B | AdV-8 | 100 | 99.8 | AdV-22 | 94.8 | AB099382a |
| TC-26218/96 | Sapporo | AdV-8 | AdV-8 | AdV-8E | AdV-8 | 100 | 99.3 | AdV-22 | 94.3 | AB099383a |
| 5363/96 | Sapporo | AdV-19 | Not AdV-1 to -51 | AdV-19a | AdV-19a | 100 | 100.0 | AdV-25 | 97.9 | AB099384a |
| 5464/96 | Sapporo | AdV-19 | Not AdV-1 to -51 | AdV-19a | AdV-19a | 100 | 100.0 | AdV-25 | 97.9 | AB099385a |
| 00402/90 | Sapporo | AdV-37 | AdV-37 | AdV-37/D6 | AdV-37 | 87 | 100.0 | AdV-25 | 98.8 | AB099386a |
| 20020/92 | Sapporo | AdV-37 | AdV-37 | AdV-37/D7 | AdV-37 | 87 | 100.0 | AdV-25 | 98.8 | AB099387a |
| 4056/99 | Sapporo | AdV-37 | AdV-37 | AdV-37/D10 | AdV-37 | 87 | 99.6 | AdV-25 | 98.6 | AB099388a |
Nucleotide sequence was determined in this study.
TABLE 3.
Identification of clinical isolates from patients with EKC by phylogenetic analysis
| Isolate/yr | Geographic origin | Typing by phylogeny
|
Highest-scoring heterologous prototype
|
|||
|---|---|---|---|---|---|---|
| Type | Bootstrap (%) | Identity (%) | Type | Identity (%) | ||
| TC-4972/85 | Sapporo | AdV-4 | 100 | 96.4 | AdV-32 | 89.4 |
| TC-26823/96 | Sapporo | AdV-4 | 100 | 96.4 | AdV-32 | 89.4 |
| 4089/98 | Sapporo | AdV-4 | 100 | 96.4 | AdV-32 | 89.4 |
| TC-34148/02 | Sapporo | AdV-4 | 100 | 96.4 | AdV-32 | 89.4 |
| TC-7271/86 | Sapporo | AdV-8 | 100 | 99.8 | AdV-22 | 94.8 |
| TC-17640/91 | Sapporo | AdV-8 | 100 | 99.3 | AdV-22 | 94.3 |
| TC-17659/91 | Sapporo | AdV-8 | 100 | 99.3 | AdV-22 | 94.3 |
| TC-17870/91 | Sapporo | AdV-8 | 100 | 99.3 | AdV-22 | 94.3 |
| 20078/92 | Sapporo | AdV-8 | 100 | 99.3 | AdV-22 | 94.3 |
| TC-26219/96 | Sapporo | AdV-8 | 100 | 99.3 | AdV-22 | 94.3 |
| TC-26244/96 | Sapporo | AdV-8 | 100 | 99.3 | AdV-22 | 94.3 |
| 20026/92 | Sapporo | AdV-19a | 100 | 100.0 | AdV-25 | 97.9 |
| 4098/97 | Sapporo | AdV-19a | 100 | 100.0 | AdV-25 | 97.9 |
| 4394/97 | Sapporo | AdV-19a | 100 | 100.0 | AdV-25 | 97.9 |
| 4435/98 | Sapporo | AdV-19a | 100 | 100.0 | AdV-25 | 97.9 |
| 4481/98 | Sapporo | AdV-19a | 100 | 100.0 | AdV-25 | 97.9 |
| 4192/99 | Sapporo | AdV-19a | 100 | 100.0 | AdV-25 | 97.9 |
| 4206/99 | Sapporo | AdV-19a | 100 | 100.0 | AdV-25 | 97.9 |
| TC-33413/01 | Sapporo | AdV-19a | 100 | 100.0 | AdV-25 | 97.9 |
| TC-33429/01 | Sapporo | AdV-19a | 100 | 100.0 | AdV-25 | 97.9 |
| TC-5227/85 | Sapporo | AdV-37 | 90 | 100.0 | AdV-25 | 98.8 |
| TC-5972/85 | Sapporo | AdV-37 | 90 | 99.9 | AdV-25 | 98.9 |
| 00122/90 | Sapporo | AdV-37 | 90 | 100.0 | AdV-25 | 98.8 |
| 00178/90 | Sapporo | AdV-37 | 90 | 100.0 | AdV-25 | 98.8 |
| 00395/90 | Sapporo | AdV-37 | 90 | 99.9 | AdV-25 | 98.9 |
| 10025/91 | Sapporo | AdV-37 | 90 | 100.0 | AdV-25 | 98.8 |
| 10051/91 | Sapporo | AdV-37 | 90 | 99.9 | AdV-25 | 98.9 |
| 10356/91 | Sapporo | AdV-37 | 90 | 100.0 | AdV-25 | 98.8 |
| 10572/91 | Sapporo | AdV-37 | 90 | 100.0 | AdV-25 | 98.8 |
| 20051/92 | Sapporo | AdV-37 | 90 | 100.0 | AdV-25 | 98.8 |
| 30606/93 | Sapporo | AdV-37 | 90 | 99.9 | AdV-25 | 98.9 |
| 30882/93 | Sapporo | AdV-37 | 90 | 100.0 | AdV-25 | 98.8 |
| 30662/93 | Sapporo | AdV-37 | 90 | 100.0 | AdV-25 | 98.8 |
| 30663/93 | Sapporo | AdV-37 | 90 | 99.9 | AdV-25 | 98.9 |
| 40494/94 | Sapporo | AdV-37 | 90 | 100.0 | AdV-25 | 98.8 |
| 40510/94 | Sapporo | AdV-37 | 90 | 100.0 | AdV-25 | 98.8 |
| 40577/94 | Sapporo | AdV-37 | 90 | 100.0 | AdV-25 | 98.8 |
| 40711/94 | Sapporo | AdV-37 | 90 | 100.0 | AdV-25 | 98.8 |
| 5309/95 | Sapporo | AdV-37 | 90 | 100.0 | AdV-25 | 98.8 |
| 5617/95 | Sapporo | AdV-37 | 90 | 100.0 | AdV-25 | 98.8 |
| 5364/96 | Sapporo | AdV-37 | 90 | 100.0 | AdV-25 | 98.8 |
| 5970/96 | Sapporo | AdV-37 | 90 | 100.0 | AdV-25 | 98.8 |
| 4080/97 | Sapporo | AdV-37 | 90 | 100.0 | AdV-25 | 98.8 |
| 4206/97 | Sapporo | AdV-37 | 90 | 100.0 | AdV-25 | 98.8 |
| 4825/98 | Sapporo | AdV-37 | 90 | 100.0 | AdV-25 | 98.8 |
| 4190/99 | Sapporo | AdV-37 | 90 | 99.6 | AdV-25 | 98.6 |
| 5212/95a | Sapporo | AdV-8 | 100 | 96.7 | AdV-22 | 95.4 |
Representative unidentified isolate which caused nosocomial infection.
DNA extraction and PCR.
A partial hexon sequence was amplified as described previously (29). In brief, viral DNA was extracted from 100 μl of virus suspension by using the Sumitest EX-R&D kit (Genome Science Laboratories Co., Ltd., Fukushima, Japan) according to the manufacturer's instructions. The DNA was dissolved in 100 μl of TE buffer (10 mM Tris [pH 8.0], 1 mM EDTA). The 1,004 bp of the hexon gene was amplified with 50 pmol of a pair of primers, AdTU7 (positions 20,734 to 20,753; 5′-GCCACCTTCTTCCCCATGGC-3′) and AdTU4′ (positions 21,737 to 21,718; 5′-GTAGCGTTGCCGGCCGAGAA-3′). The positions of the primers for PCR were numbered according to the complete nucleotide sequence of the AdV-2 strain (GenBank accession no. J01917). Using 10 μl of the PCR product, nested PCR was performed to amplify the 956-bp DNA fragment with a pair of primers, AdnU-S′ (positions 20,743 to 20,762; 5′-TTCCCCATGGCNCACAACAC-3′) and AdnU-A (positions 21,698 to 21,679; 5′-GCCTCGATGACGCCGCGGTG-3′). PCR was carried out for 36 cycles in a Cetus 9600 thermal cycler (PE-Applied Biosystems, Foster City, Calif.). Each cycle consisted of denaturation at 94°C for 1 min, annealing at 50°C for 1 min, and primer extension at 72°C for 2 min. After the last cycle, the extension was continued at 72°C for 7 min. The PCR products were separated on 3% agarose gels and purified with a QIA Quick gel extraction kit (Qiagen, Valencia, Calif.).
PCR-RFLP analysis.
The 956 bp of PCR products from the hexon gene were separated on a 1% agarose gel and purified with a QIA Quick gel extraction kit. Three microliters (out of 10 μl) of purified DNA was digested with 5 U of the restriction endonucleases (REs) EcoT14I, HaeIII, and HinfI (Takara Shuzo Co., Ltd., Shiga, Japan). The digested DNA fragments were loaded onto 3% agarose gels and electrophoresed in Tris-acetate buffer (pH 8.0) with 1 mM EDTA at 100 V. The serotypes of AdVs were determined by comparison of the restriction patterns with those of the prototypes.
Genome typing.
The viral DNA was extracted from the infected cells in a 75-cm2 plastic flask using 3 ml of Hirt lysis solution (10 mM Tris, 1 mM EDTA, 0.6% sodium dodecyl sulfate, pH 8.0) (17). Proteinase K was added at a final concentration of 50 μg/ml, and the samples were incubated at 37°C for 1 h. The cellular DNA was precipitated with 1 M NaCl (final concentration) overnight at 4°C. After phenol-chloroform extraction, the supernatant was treated with a mixture of ribonucleases A (25 mg/ml) and T1 (80 U/ml) (Sigma, St. Louis, Mo.), and phenol-chloroform extraction was performed. Viral DNA was precipitated with isopropanol and suspended in 50 μl of TE buffer. Each aliquot, containing 1 μg of viral genomic DNA, was digested with 5 U each of the REs BamHI, EcoRI, SmaI, and XhoI (Takara Shuzo, Kyoto, Japan). The digested viral DNA was loaded onto 1% agarose gels containing 1 μg of ethidium bromide/ml. The patterns of the fragments were analyzed by comparison with those of previously reported genome types (2, 3, 8, 10, 13, 14, 23, 27, 34, 36).
Sequence analysis.
The PCR products of PCR-RFLP described above were used to determine the nucleotide sequences. The nucleotide sequences were determined with a model 373A DNA auto sequencer (PE-Applied Biosystems) with fluorescent dideoxy chain terminators (PE-Applied Biosystems) and AdnU-S′ and AdnU-A. Two internal primers for sequencing, AdHxs (positions 21,151 to 21,170; 5′-GTRGCYCARTGYAACATGAC-3′) and AdHxaRe (positions 21,224 to 21,205; 5′-CCCTGGTAKCCRATRTTRTA-3′), were designed on the basis of the partial hexon gene sequences determined in this study.
Phylogenetic analysis.
The partial hexon nucleotide sequences of 33 prototype strains of HAdV-D and -E, not available from GenBank, were determined in this study (Table 1). The 11 partial hexon nucleotide sequences of a number of prototypes, including AdV-12 and -18 from HAdV-A; AdV-3, -7, -16, -21, and -34 from HAdV-B; AdV-2 and -5 from HAdV-C; and AdV-40 and -41 from HAdV-F, were available from GenBank (Table 1). A total of 57 sequences were analyzed and compared with the database, using SINCA software (Fujitsu Ltd., Tokyo, Japan). The evolutionary distances were estimated using Kimura's two-parameter method (25), and unrooted phylogenetic trees were constructed using the neighbor-joining method (30). Bootstrap analyses were performed by 1,000 resamplings of the data sets. Bootstrap values of ≥70% were considered statistically significant for the grouping (12).
Nucleotide sequence accession numbers.
The GenBank accession numbers of the nucleotide sequences presented in this study are AB099348 to AB099388.
RESULTS
Amplification of the 51 prototype strains.
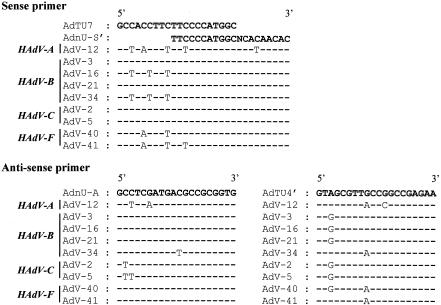
Comparison with 11 available nucleotide sequences from GenBank—2 from HAdV-A (AdV-12 and AdV-18), 5 from HAdV-B (AdV-3, -7, -16, -21, and -34), 2 from HAdV-C (AdV-2 and -5), and 2 from HAdV-F (AdV-40 and -41)—demonstrated that several short stretches (19 to 23 bases) in the hexon gene are highly conserved among HAdVs (Fig. 1). Twenty-base stretches in the hexon gene were selected for the first-PCR primers, AdTU7 and AdTU4′, and the second-PCR primers, AdnU-S′ and AdnU-A (29). These primers allowed the amplification of 956 bp of the genome encoding the 3′ one-third of the hexon of HAdVs. Previous studies indicated that the two sets of primers were capable of amplifying the genomes of several HAdVs known to cause eye infection. When the amplification was performed using the 51 HAdV prototype strains and the set of primers, all of the strains efficiently produced 956-bp fragments (data not shown). Amplification was specific to HAdVs; there was no cross-amplification with a variety of heterologous viruses, including influenza virus, mumps virus, measles virus, herpes simplex virus, and cytomegalovirus, nor did amplification of the genomes of enteric viruses, including Norwalk-like viruses and rotaviruses, occur (data not shown).
FIG. 1.
Alignment of hexon genes from various AdVs. The primer sequences were aligned, along with available nucleotide sequences of AdVs from GenBank.
The sensitivity of the PCR, as determined by a 956-bp band after staining, appeared to be 0.01 to 0.1 50% tissue culture infective dose/ml when 10-fold serial dilutions of the prototype strains of AdV-4, -8, -19, and -37 were tested (data not shown). These results indicated that the sets of primers were specific to HAdVs and capable of amplifying all prototype HAdV strains.
Phylogenetic analysis of prototype strains.
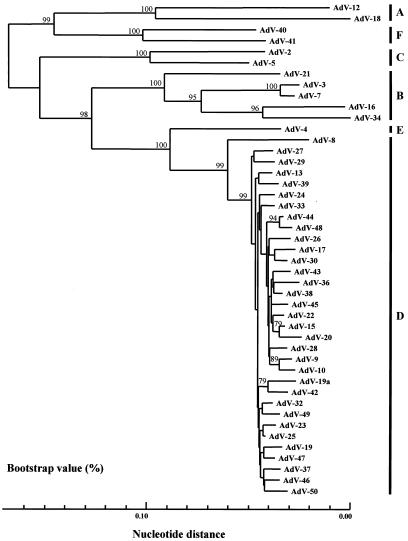
To date, only 11 hexon sequences (see above) are available from GenBank. No hexon sequences of the prototype strains from HAdV-D are available from GenBank. Therefore, we determined the nucleotide sequences of the prototype strains of 33 serotypes from HAdV-D and -E as described in Materials and Methods and obtained the complete set of the partial hexon nucleotide sequences of HAdV-D and HAdV-E (Table 1). An alignment of the partial hexon nucleotide sequences from 33 sequences from HAdV-D and -E and from 11 sequences from GenBank (i.e., 44 sequences in all) was performed with the SINCA genetic software program. It appeared that all of the prototype strains contained 916 bases and that no deletions or insertions occurred in any of them.
To assess the genetic relationships among these sequences, a phylogenetic tree based on the partial hexon nucleotide sequences was constructed. As shown in Fig. 2, the HAdVs were segregated into six major clusters, A to F. These clusters corresponded well to the six newly designated HAdV species, A to F (37). The genetic relationships of the 44 prototype strains within a cluster and between clusters were further analyzed by a one-by-one comparison. The nucleotide identities between the 44 prototypes and AdV-19a ranged from 74.1 to 99.2% (mean, 89.7%). In cluster D, the nucleotide identities ranged from 92.5 (between AdV-8 and AdV-19a) to 99.2% (between AdV-23 and AdV-25), with an average of 97.4%. AdV-19a showed only 96.9% nucleotide identity with the AdV-19 prototype AV-587, which was first reported in 1955 in Saudi Arabia (6), and did not form a monophyletic cluster with AdV-19. The nucleotide identities between the prototype strains of cluster D and the other clusters ranged from 74.6 to 90.5%: 74.6 (between AdV-23 and AdV-18) to 77.2% (between AdV-8 and AdV-12) in cluster A, 80.5 (between AdV-10 and AdV-16) to 84.6% (between AdV-42 and AdV-21) in cluster B, 79.7 (between AdV-10 and AdV-2) to 81.8% (between AdV-24 and AdV-5) in cluster C, 87.2 (between AdV-8 and AdV-4) to 90.5% (among AdV-30, AdV-38, and AdV-4) in cluster E, and 77.7 (among AdV-24, AdV-28, and AdV-41) to 79.6% (between AdV-17 and AdV-40) in cluster F. On the other hand, the nucleotide identities between the AdV-4 prototype strains of cluster E and the other clusters ranged from 74.1 to 90.5%: 74.1 (between AdV-4 and AdV-18) to 75.7% (between AdV-4 and AdV-12) in cluster A, 80.1 (between AdV-4 and AdV-34) to 83.0% (among AdV-4, AdV-7, and AdV-21) in cluster B, 81.4 (between AdV-4 and AdV-2) to 82.8% (between AdV-4 and AdV-5) in cluster C, 87.2 (between AdV-4 and AdV-8) to 90.5% (among AdV-4, AdV-30, and AdV-38) in cluster D, and 77.7% (among AdV-4, AdV-40, and AdV-41) in cluster F. These results indicated that phylogenetic analysis based on the partial nucleotide sequences is capable of distinguishing among prototype strains of HAdVs.
FIG. 2.
Phylogenetic analysis of AdV prototype strains and AdV-19a. The nucleotide sequences of a partial hexon region (916 bp) from 44 prototype strains and AdV-19a were analyzed by the neighbor-joining method. The numbers at the nodes are percentages of 1,000 bootstrap pseudoreplicates containing the cluster distal to the node.
Evaluation of the diagnostic method based on phylogenetic analysis to identify AdV serotypes.
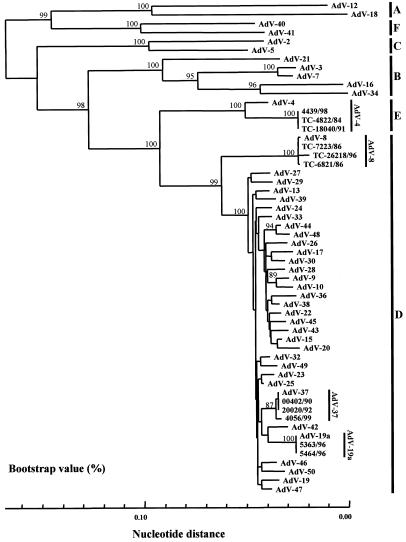
To evaluate the reliability of the phylogeny-based identification of HAdVs causing EKC, clinical isolates from EKC patients were subjected to the analysis. It has been reported that EKC is caused primarily by four serotypes, AdV-4, -8, -19a, and -37. These viruses present indistinguishable clinical manifestations. We used three isolates of AdV-4, three isolates of AdV-8, two isolates of AdV-19a, and three isolates of AdV-37. All isolates were obtained from outbreaks in Sapporo, in the northern part of Japan. The isolates were identified by the neutralization test with type-specific antisera and had all been well characterized genetically by genome typing and PCR-RFLP. When the partial nucleotide sequences of the three isolates of AdV-8 were compared with those of the 45 prototype strains, including AdV-19a, all isolates showed 99.3 to 99.9% nucleotide identity with the prototype strain of AdV-8 (Trim). The next-highest homology between isolate and prototype strains was 94.3 to 94.9% (Table 2). Thus, a difference of >5.0% was observed between homologous and heterologous prototype strains, allowing us to identify the isolates as AdV-8. When the two isolates of AdV-19 identified by the neutralization test were analyzed, they showed only 96.9% nucleotide identity with the AdV-19 prototype, AV-587. However, these isolates showed nucleotide sequences identical to those of AdV-19a. The second-highest nucleotide identity was seen between the isolates and AdV-25 (Table 2). When the three isolates of AdV-37 were analyzed, a similar result was obtained. All of the isolates had the highest identity with the prototype strain AdV-37 (GW), and at least a 1.0% difference was seen between AdV-37 and the next highest nucleotide identity, which was with AdV-25 (Table 2). Therefore, the phylogenetic analyses based on the partial hexon successfully separated three isolates of AdV-8, two isolates of AdV-19a, and three isolates of AdV-37 into distinct monophyletic clusters with their respective prototype strains. All isolates of AdV-8, -19, and -37 were segregated into cluster D, along with the prototype, with 99% bootstrap support (Fig. 3). When the three isolates of AdV-4 were analyzed, a similar result was obtained. All of the isolates had the highest identity with the prototype strain AdV-4 (RI-67), and a 7.0% difference was seen between AdV-4 and the next highest nucleotide identity, which was with AdV-32 (Table 2). Therefore, all AdV-4 isolates were segregated into cluster E with 100% bootstrap support. These results indicated that phylogeny-based clustering with the partial hexon nucleotide sequences can allow us to identify clinical isolates from EKC patients.
FIG. 3.
Phylogenetic analysis of AdV serotypes 4, 8, 19a, and 37. Nucleotide sequences of a partial hexon region of the isolates, along with those of prototype strains and AdV-19a, were determined and analyzed by the neighbor-joining method. The numbers at nodes are percentages of 1,000 bootstrap pseudoreplicates containing the cluster distal to the node. Three AdV-4 isolates form cluster E (HAdV-E) with the AdV-4 prototype strain. Three AdV-8 isolates, two AdV-19a isolates, and three AdV-37 isolates form cluster D (HAdV-D) with their respective prototype strains.
Identification of clinical isolates from patients with EKC by phylogenetic analysis.
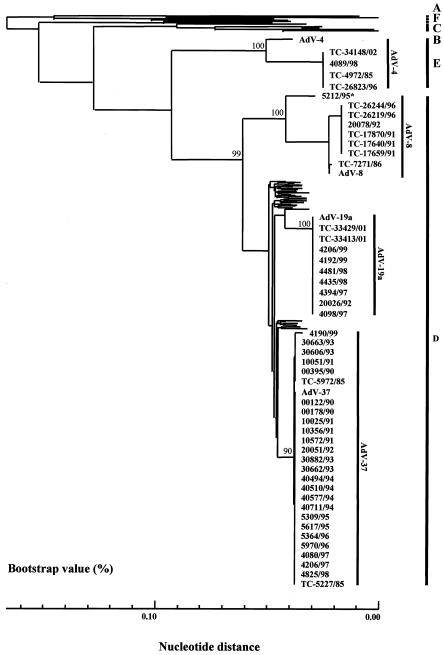
We applied this method for the rapid diagnosis of conjunctivitis, a major disease caused by the serotypes of HAdV-E and -D. These viruses have afflicted an increasing number of patients in Japan over the last 20 years. The nucleotide sequences from 46 isolates were analyzed, including 7 AdV-8, 9 AdV-19, and 26 AdV-37 strains of species D and 4 AdV-4 strains of species E. When the hexon nucleotide sequences of these isolates were compared with those of the 45 prototype strains, including AdV-19a, each isolate, with the exception of AdV-19, showed the highest nucleotide identity with its respective prototype. The nucleotide homologies of isolates with their prototype strains were 99.3 to 99.8% for AdV-8, 99.6 to 100.0% for AdV-37, and 96.4% for AdV-4. The isolates, with the exception of AdV-19, showed at least 1.0%-higher identity with the homologous prototype strain than with the highest heterologous strains (Table 3). The nucleotide sequence homologies between the AdV-19 isolates and AdV-19a were identical. The AdV-19 isolates showed at least 2.1%-higher identity with AdV-19a than with other prototype strains, including AdV-19 (Table 3). Accordingly, the isolates were designated AdV-19a. Thirty-three isolates of two serotypes, AdV-8 and AdV-37, were segregated into cluster D with their prototype strains. Nine isolates of AdV-19 were segregated into cluster D with AdV-19a (Fig. 4). Four isolates of AdV-4 and their prototype strain, RI-67, were grouped into cluster E (Fig. 4). These isolates formed distinct clusters with their respective prototype strains. We also applied this method to identify serologically unidentified isolates that caused nosocomial infections. The isolates showed cross-neutralization against the AdV-8 and the AdV-9 antisera. When the nucleotide sequences were analyzed, all of the isolates had identical nucleotide sequences. The nucleotide sequences of the unidentified isolates were compared with those of the 44 prototype strains of AdVs. The unidentified isolates showed 96.7% nucleotide identity with the prototype strain of AdV-8 (Trim). The next highest homology was 95.4% with AdV-22 (Table 3). The isolates were segregated into cluster D and formed a distinct cluster with the AdV-8 prototype strain with 100% bootstrap support. Therefore, we identified the isolates as variants of AdV-8 (Fig. 4). These results prove that phylogeny-based clustering with the partial hexon nucleotide sequences is a powerful tool for the identification of most HAdV isolates from patients with conjunctivitis.
FIG. 4.
Phylogenetic analysis of clinical isolates from patients with EKC. The results were obtained by the neighbor-joining method. The numbers at the nodes are percentages of 1,000 bootstrap pseudoreplicates containing the cluster distal to the node. The representative unidentified 5212/95* isolates formed a distinct cluster with the prototype strains of AdV-8 (Trim).
DISCUSSION
With the development of molecular biological techniques, the means of classifying and identifying most viruses, including HAdVs, hepatitis C virus, hepatitis B virus, transfusion-transmitted virus, human papillomavirus, influenza virus A, coronavirus, and enterovirus, shifted from serologic assays to genetic analyses (7, 20, 24, 26, 32, 33, 41). Most diagnostic laboratories and public health laboratories in Japan have this capability and expertise. Therefore, our newly developed procedure for the detection and typing of AdVs is appropriate for laboratories that have sequencing capability and software for phylogenetic analysis.
Genome typing has been used for the classification of HAdVs (1, 9, 16, 31, 42). This method, based on the cleavage pattern of the full genome of HAdV, is capable of distinguishing the prototype strains of 51 serotypes. Genome typing is a reliable method, although it requires several micrograms of viral DNA. In this method, it takes several weeks to prepare the viral DNA from infected cells.
To improve on this time, PCR- and RFLP-based rapid identification methods were developed by several researchers, including us (11). To identify the serotype of HAdV that causes conjunctivitis, we developed PCR-RFLP based on 956 bp of the 3′ one-third of the hexon gene digested with the REs EcoT14I, HaeIII, and HinfI (29). The pair of primer sequences used in PCR was well conserved among the available nucleotide sequences of HAdV from GenBank. PCR with the pair of primers amplified all prototype strains of 51 serotypes of HAdVs. We previously applied PCR-RFLP to 127 samples of conjunctival scrapings from patients with conjunctivitis and compared the results with those obtained by culture isolation and a neutralization test. The PCR gave positive results in 69 of 127 cases (54.3%), while 61 of 127 cases (48.0%) tested were positive by culture isolation. PCR products were further classified as AdV-3, -4, -8, -11, and -37 (29). Therefore, this PCR method is expected to amplify newly emerging HAdVs.
PCR-RFLP is useful for rapid identification of the serotypes of HAdVs from patients with conjunctivitis. Therefore, we analyzed the cleavage patterns of all prototype strains of HAdV-A to -F. However, no prototype strain showed a pattern identical to that of any of the isolates from nosocomial infections. Therefore, in order to analyze the genetic relationships between the isolates and prototype strains, we determined the nucleotide sequences of the PCR products for RFLP and those of the isolates. The phylogenetic analysis revealed that the isolates were variants of serotype AdV-4 or -8. Thus, the method is capable of directly amplifying adenoviral DNA from a clinical sample, and phylogenetic analysis based on the hexon gene can classify the HAdV. Indeed, we think that we could directly determine HAdVs by PCR using an eye swab and could identify the serotypes by phylogenetic analysis.
By our method, we could diagnose HAdV infections by PCR and type infectious HAdVs by phylogeny within a few days, in contrast to the 2 to 4 weeks or longer that an ordinary neutralization test requires. Therefore, this phylogeny-based system is considered highly useful when rapid diagnosis and typing are required, such as in the case of nosocomial infections. In fact, this method revealed that a new variant of AdV-8 caused severe nosocomial infection. HAdVs, such as AdV-4, -8, and -19, include a wide variety of variants (2, 3, 8, 10, 13, 14, 23, 27, 34-36, 39). The existence of variants makes it difficult to identify clinical isolates by RFLP, particularly when mutation by RE occurs on the cleavage site. However, this sort of mutation is advantageous for phylogeny-based classification, because it enables detailed molecular epidemiological study.
In conclusion, we have developed a new, rapid, and simple method of identifying HAdVs based on phylogenetic analysis of the partial hexon gene. PCR using a set of primers was efficient for amplifying all prototype strains. This method takes advantage of the divergence in relatively short hexon sequences both within and between serotypes, which in this investigation enabled the rapid identification of 58 isolates from four serotypes that appeared over a range of 20 years. Our method, in conjunction with the accumulation of database nucleotide sequences, should be of use not only for the rapid diagnosis and typing of HAdVs but also for global epidemiological study of these viruses.
REFERENCES
- 1.Adrian, T., G. Wadell, J. C. Hierholzer, and R. Wigand. 1986. DNA restriction analysis of adenovirus prototype 1 to 41. Arch. Virol. 91:277-290. [DOI] [PubMed] [Google Scholar]
- 2.Adrian, T., U. Wolf, H. J. Lauer, and R. Wigand. 1990. Restriction site mapping of adenovirus type 8 genome types. Res. Virol. 141:611-624. [DOI] [PubMed] [Google Scholar]
- 3.Allard, A., B. Albinsson, and G. Wadell. 1992. Detection of adenovirus in stools from healthy persons and patients with diarrhea by two-step polymerase chain reaction. J. Med. Virol. 37:149-157. [DOI] [PubMed] [Google Scholar]
- 4.Aoki, K., M. Kato, H. Ohtsuka, K. Ishii, N. Nakazono, and H. Sawada. 1982. Clinical and aetiological study of adenoviral conjunctivitis, with special reference to adenovirus type 4 and 19 infections. Br. J. Ophthalmol. 66:776-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aoki, K., R. Kawana, I. Matsumoto, G. Wadell, and J. C. de Jong. 1986. Viral conjunctivitis with special reference to adenovirus type 37 and enterovirus 70 infection. Jpn. J. Ophthalmol. 30:158-164. [PubMed] [Google Scholar]
- 6.Bell, S. D., Jr., T. Rondon Rota, and D. E. McComb. 1959. Adenoviruses isolated from Saudi Arabia. III. Six new serotypes. Am. J. Trop. Med. Hyg. 8:523-526. [DOI] [PubMed] [Google Scholar]
- 7.Bernard, H., S. Chan, M. M. Manos, C. Ong, L. L. Villa, H. Delius, C. L. Peyton, H. M. Bauer, and C. M. Wheeler. 1994. Identification and assessment of known and novel human papillomaviruses by polymerase chain reaction amplification, restriction fragment length polymorphisms, nucleotide sequence, and phylogenetic algorithms. J. Infect. Dis. 170:1077-1085. [DOI] [PubMed] [Google Scholar]
- 8.Chang, C. H., M. M. Sheu, C. L. Chern, K. H. Lin. W. L. Huang, and C. W. Chen. 2001. Epidemic keratoconjunctivitis caused by a new genotype of adenovirus type 8 (Ad8)—a chronological review of Ad8 in Southern Taiwan. Jpn. J. Ophthalmol. 45:160-166. [DOI] [PubMed] [Google Scholar]
- 9.De Jong, J. C., A. G. Wermenbol, M. W. Verweij-Uijterwaal, K. W. Slaterus, P. W. Dillen, G. J. J. Van Doornum, S. H. Khoo, and J. C. Hierholzer. 1999. Adenovirus from human immunodeficiency virus-infected individuals, including two strains that represent new candidate serotypes Ad50 and Ad51 of species B1 and D, respectively. J. Clin. Microbiol. 37:3940-3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Jong, J. C., M. Demazure, M. Legrand-Quillien, G. L. Lay, J. Colin, A. G. Wermenbol. M. W. Verweij-Uyterwaal, H. G. A. M. van der Avoort, and C. Chastel. 1992. New developments in the molecular epidemiology of adenovirus 8 keratoconjunctivitis. J. Med. Virol. 38:102-107. [DOI] [PubMed] [Google Scholar]
- 11.Elnifro, E. M., R. J. Cooper, P. E. Klapper, and A. S. Bailey. 2000. PCR and restriction endonuclease analysis for rapid identification of human adenovirus subgenera. J. Clin. Microbiol. 38:2055-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felsenstein, J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783-791. [DOI] [PubMed] [Google Scholar]
- 13.Fujii, S., N. Nakazono, H. Sawada, K. Ishii, M. Kato, K. Aoki, H. Ohtsuka, and K. Fujinaga. 1983. Restriction endonuclease cleavage analysis of adenovirus type 8: two new subtypes from patients with epidemic keratoconjunctivitis in Sapporo, Japan. Jpn. J. Med. Sci. Biol. 36:307-313. [DOI] [PubMed] [Google Scholar]
- 14.Fujii, S., N. Nakazono, K. Ishii, C. C. Lin, M. M. Sheu, C. W. Chen, and K. Fujinaga. 1984. Molecular epidemiology of adenovirus type 8 (Ad8) in Taiwan: four subtypes recovered during the period of 1980-1981 from patients with epidemic keratoconjunctivitis. Jpn. J. Med. Sci. Biol. 37:161-169. [DOI] [PubMed] [Google Scholar]
- 15.Guo, D. F., M. Shinagawa, K. Aoki, H. Sawada, S. Itakura, and G. Sato. 1988. Genome typing of adenovirus strains isolated from conjunctivitis in Japan, Australia, and the Philippines. Microbiol. Immunol. 32:1107-1118. [DOI] [PubMed] [Google Scholar]
- 16.Hierholzer, J. C., R. Wigand, L. J. Anderson, T. Adrian, and J. W. M. Gold. 1988. Adenovirus from patient with AIDS: a plethora of serotypes and a description of five new serotypes of subgenus D (Types 43-47). J. Infect. Dis. 154:804-813. [DOI] [PubMed] [Google Scholar]
- 17.Hirt, B. 1967. Selective extraction of polyoma DNA. J. Mol. Biol. 26:365-369. [DOI] [PubMed] [Google Scholar]
- 18.Horwitz, M. S. 2001. Adenoviruses, p. 2301-2326. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadephia, Pa.
- 19.Ishii, K., N. Nakazono, K. Fujinaga, S. Fujii, M. Kato, H. Ohtsuka, K. Aoki, C. W. Chen, C. C. Lin, M. M. Sheu, K. H. Lin, B. S. Oum, S. H. Lee, C. H. Chun, T. Yoshii, and S. Yamazaki. 1987. Comparative studies on aetiology and epidemiology of viral conjunctivitis in three countries of East Asia—Japan, Taiwan and South Korea. Int. J. Epidemiol. 16:98-103. [DOI] [PubMed] [Google Scholar]
- 20.Ishiko, H., Y. Shimada, M. Yonaha, O. Hashimoto, A. Hayashi, K. Sakae, and N. Takeda. 2002. Molecular diagnosis of human enteroviruses by phylogeny-based classification by use of the VP4 sequence. J. Infect. Dis. 185:744-754. [DOI] [PubMed] [Google Scholar]
- 21.Jackson, G. G., and R. L. Muldoon. 1973. Viruses causing common respiratory infection in man. IV. Reoviruses and adenovirus. J. Infect. Dis. 128:811-866. [DOI] [PubMed] [Google Scholar]
- 22.Jernigan, J. A., B. S. Lowry, F. G. Hayden, S. A. Kyger, B. P. Conway, D. H. M. Groschel, and B. M. Farr. 1993. Adenovirus type 8 epidemic keratoconjunctivitis in an eye clinic: risk factors and control. J. Infect. Dis. 167:1307-1313. [DOI] [PubMed] [Google Scholar]
- 23.Kanai, H. 2000. Genome analysis with restriction endonuclease recognizing 4- or 5-base pair sequences of adenovirus type. Jpn. J. Ophthalmol. 44:463-466. [DOI] [PubMed] [Google Scholar]
- 24.Kato, H., E. Orito, R. G. Gish, F. Sugauchi, S. Suzuki, R. Ueda, Y. Miyakawa, and M. Mizokami. 2002. Characteristics of hepatitis B virus isolates of genotype G and their phylogenetic differences from the other six genotypes (A through F). J. Virol. 76:6131-6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimura, M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequence. J. Mol. Evol. 16:111-120. [DOI] [PubMed] [Google Scholar]
- 26.Lemey, P., M. Salemi, L. Bassit, and A. Vandamme. 2002. Phylogenetic classification of TT virus groups based on the N22 region is unreliable. Virus Res. 85:47-59. [DOI] [PubMed] [Google Scholar]
- 27.Li, Q., and G. Wadell. 1998. The degree of genetic variability among adenovirus type 4 strains isolated from man and chimpanzee. Arch. Virol. 101:65-77. [DOI] [PubMed] [Google Scholar]
- 28.Nauheim, R. C., E. G. Romanowski, T. Araullo-Cruz, R. P. Kowalski, P. W. Turgeon, S. S. Stopak, and Y. J. Gordon. 1990. Prolonged recoverability of desiccated adenovirus type 19 from various surfaces. Ophthalmology 97:1450-1453. [DOI] [PubMed] [Google Scholar]
- 29.Saitoh-Inagawa, W., A. Oshima, K. Aoki, N. Itoh, K. Isobe, E. Uchio, S. Ohno, H. Nakajima, K. Hata, and H. Ishiko. 1996. Rapid diagnosis of adenoviral conjunctivitis by PCR and restriction fragment length polymorphism analysis. J. Clin. Microbiol. 34:2113-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 31.Schnurr, D., and M. E. Dondero. 1993. Two new candidate adenovirus serotypes. Intervirology 36:79-83. [DOI] [PubMed] [Google Scholar]
- 32.Simmonds, P. 1998. Variability of the hepatitis C virus genome. Curr. Stud. Hematol. Blood Transfus. 62:38-63. [DOI] [PubMed] [Google Scholar]
- 33.Stephensen, C. B., D. B. Casebolt, and N. Gangopadhyay. 1999. Phylogenetic analysis of a highly conserved region of the polymerase gene from 11 coronaviruses and development of a consensus polymerase chain reaction assay. Virus Res. 60:181-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka, K., N. Itoh, W. Saitoh-Inagawa, E. Uchio, S. Takeuchi, K. Aoki, E. Soriano, M. Nishi, R. B. Junior, C. M. Hasi, L. Tsuzuki-Wang, E. L. Durigon, K. E. Stewien, and S. Ohno. 2000. Genetic characterization of adenovirus strains isolated from patients with acute conjunctivitis in the city of Sao Paulo, Brazil. J. Med. Virol. 61:143-149. [PubMed] [Google Scholar]
- 35.Tanaka-Yokogui, K., N. Itoh, N. Usui, S. Takeuchi, E. Uchio, K. Aoki, M. Usui, and S. Ohno. 2001. New genome type of adenovirus serotype 19 causing nosocomial infections of epidemic keratoconjunctivitis in Japan. J. Med. Virol. 65:530-533. [PubMed] [Google Scholar]
- 36.Tsuzuki-Wang, L., K. Aoki, K. Isobe, S. Shiao, K. Toba, N. Kobayashi, Y. Noguchi, and S. Ohno. 1997. Genome analysis of adenovirus type 4 strains isolated from acute conjunctivitis in Japan. Jpn. J. Ophthalmol. 41:308-311. [DOI] [PubMed] [Google Scholar]
- 37.Van Regenmortel, M. H. V., C. M. Fauquet, D. H. L. Bishop, E. B. Carstens, M. A. Mayo, and D. J. McGeoch. 1999. Family Adenoviridae, p. 227-237. In M. H. V. Van Regenmortel et al. (ed.), Virus taxonomy: classification and nomenclature of viruses. Seventh Report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, Calif.
- 38.Wadell, G. 1984. Molecular epidemiology of human adenoviruses. Curr. Top. Microbiol. Immunol. 110:191-220. [DOI] [PubMed] [Google Scholar]
- 39.Wadell, G., and J. C. De Jong. 1980. Restriction endonucleases in identification of a genome type of adenovirus 19 associated with keratoconjunctivitis. Infect. Immun. 27:292-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warren, D., K. E. Nelson, J. A. Farrar, E. Hurwitz, J. Hierholzer, E. Ford, and L. J. Anderson. 1989. A large outbreak of epidemic keratoconjunctivitis: problems in controlling nosocomial spread. J. Infect. Dis. 160:938-943. [DOI] [PubMed] [Google Scholar]
- 41.Webster, R. G., W. J. Bean, O. T. Gorman, T. M. Chambers, and Y. Kawaoka. 1992. Evolution and ecology of influenza A virus. Microbiol. Rev. 56:152-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wigand, R., T. H. Adrian, and F. Bricout. 1987. A new human adenovirus of subgenus D: candidate adenovirus type 42. Arch. Virol. 94:283-286. [DOI] [PubMed] [Google Scholar]