Abstract
Objective:
Eradication of microorganisms present in the root canal system is paramount for the successful outcome of root canal therapy. The purpose of this study was to compare the of doxycycline absorbed from MTAD into root canal dentin after obturation with gutta-percha/AH26 and Resilon/RealSeal at different time intervals.
Materials and Methods:
Fifty-one extracted human teeth were instrumented. Thirty samples were obturated with either gutta-percha/AH26 or Resilon/self-etch RealSeal after final irrigation with MTAD. Fifteen samples were kept unobturated (positive control); six samples were obturated with either gutta-percha/AH26 or Resilon/self-etch RealSeal without MTAD irrigation (negative control).
After aging for 1, 3 or 6 weeks, dentin debri were collected, the Doxycycline compound was extracted and its amount was quantified using high performance liquid chromatography. The statistical significance of the change in Doxycycline concentrations was tested with two-way ANOVA.
Results:
The mean concentration of Doxycycline in dentin for one, three and six-week guttapercha/AH26 samples was 1.8±0.36, 1.22±0.22 and 0.67±0.11 respectively, whereas these concentrations in Resilon/RealSeal samples were 1.60±0.26, 0.80±0.14 and 0.59±0.01 respectively. Regarding the positive control group, these concentrations were 2.09±0.11, 1.54±0.12 and 0.72±0.07 respectively for 1, 3 and 6-week intervals. No Doxycycline was detected in negative control groups. The Doxycycline concentrations showed a significant difference forobturating materials (p=0.008). These concentrations were higher in the gutta-percha/AH26 samples than Resilon/RealSeal samples in each time interval.
Conclusion:
The remaining amount of Doxycycline bonded to dentin was higher when root canals were obturated with gutta-percha/AH26 compared to Resilon/RealSeal. The stability of Doxycycline showed a time dependent decrease.
Keywords: MTAD, Substantivity, Real Seal, AH26 Sealer, Resilon Sealer
INTRODUCTION
The outcome of endodontic therapy is influenced by the presence of bacteria in the root canal system at the time of obturation [1]. One of the strategies for preventing bacterial recolonization or eliminating the remaining bacteria after endodontic treatment is to apply a final irrigating solution with substantivity [2].
MTAD is an aqueous solution consisting of 3% doxycycline, 4.25% citric acid, and 0.5% polysorbate 80 detergent [3].
It is effective in removing endodontic smear layers [3] with less erosion [4], it eliminates microbes that are resistant to common endodontic irrigants and dressings [5] such as E. faecalis [6], it provides residual antimicrobial activity through the affinity of doxycycline towards dental hard tissues [7], and it subsequently releases without losing its antibacterial activity [8].
One study revealed that MTAD does not adversely affect the sealing ability of Resilon/Epiphany [9].
Its half-life in unobturated root canals has been reported to be 3.0 weeks (10). Some studies have shown that doxycycline has a significantly greater zone of inhibition than 6% NaOCl for P. micros, P. intermedia, and S. sanguis [11].
Resilon and RealSeal have been recently developed as an alternative root-filling material. RealSeal’s second generation is an acidic self-adhesive sealer [12, 13]. Self-adhesives have the advantage of reducing errors that might occur during bonding steps because they are less technique sensitive and require fewer steps and less time [13].
No studies have shown the changes in MTAD’s stability overtime. There is also no information about probable effects of obturation material on MTAD’s stability. The present study was aimed to compare the stability of doxycycline absorbed into root canal dentin after obturation with gutta-percha/AH26 or Resilon/Realseal after 1, 3 and 6-week time intervals.
MATHERIALS AND METHODS
Sample preparation
Fifty-one extracted human single rooted, single canal mandibular premolars with no carious lesion that were extracted due to periodontal problems were collected.
Straight and angulated radiographs were taken to confirm the root canal anatomy. Teeth with more than one canal or with calcified root canals were excluded. After disinfection and access cavity preparation, the root canals were instrumented with Protaper rotary instruments (Dentsply Maillefer, Ballaigues, Switzerland) in the following sequence: S1, S2, F1 and F2.
The root portion and apical foramen were covered with nail polish. Forty-five samples were selected and irrigated with MTAD (Dentsply Tulsa, Tulsa, OK) according to the manufacturer’s instructions: a 2-minute rinse with 3 ml of 1.3% NaOCl, a brief rinse with 1 ml of MTAD left for 5 minutes, and finally a 1-minute flush with 4 ml of MTAD.
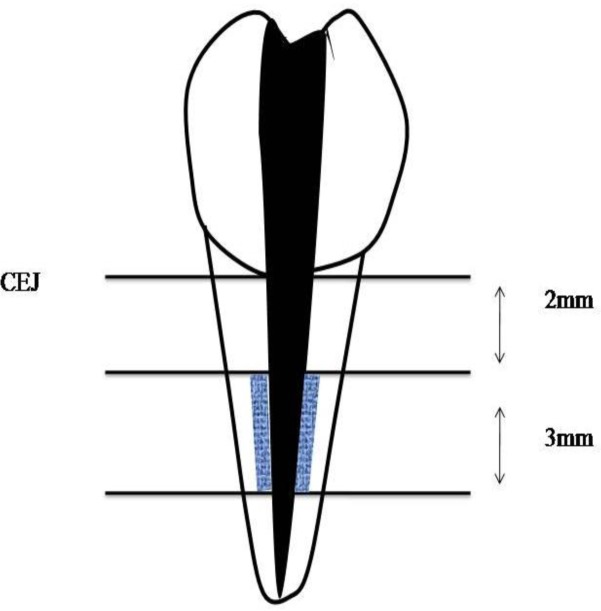
After drying all canals with paper points (Ariadent, Iran), 30 of the samples were randomly divided into two groups: A, obturation with gutta-percha (Gapadent Co, Ltd, Korea)/AH26 (Dentsply, DeTrey, Germany); B: obturation with Resilon/self-etch RealSeal (SybronEndo, USA). Fiftenn samples were kept unobturated (positive control); six samples were obturated with either gutta-percha/AH26 or Resilon/self-etch RealSeal (three samples for each group) without MTAD irrigation (negative control). After obturation, all teeth were restored with Coltosol (AriaDent, Iran). The samples were coded and placed in 37 degrees centigrade and 100% humidity. Then experimental samples in all groups were divided into three subgroups according to the tested time intervals: 1 week, 3 and 6 weeks.After aging, samples were split in half by a high speed diamond bur, obturation material was taken out with a spatula and dentin debris were collected in coded eppendorf tubes from 2 mm below the CEJ from both sides of the root canal by using a low speed round #7 bur to a width and depth equivalent to the bur’s radius and a length of 3 mm (Fig 1).
Fig 1.
location of dentinal debri collection
Recovery of active compound
0.02 gr of dentinal debris from each sample was measured by a digital Analytic Balance (Sartorius, Germany) and transferred to another coded eppendorf tube.
The recovery solution for the samples was 5% pH 7.5 EDTA (Merck, Germany), after adding 0.9 mL of the solution, the debris were vigorously shaken with Vortex mixer (Labnet international, USA) then 0.3 ml of 0.4% perchloric acid (Merck, Germany) in acetonitrile (Merck, Germany) was added to each sample. All samples were syringe-filtered with 13 mm 0.22 mm PTFE filters (MS®, USA) before HPLC analysis.
Quantification of doxycycline
Doxycycline was quantitatively analyzed according to the method described by Rasimick et al. [10] with some modifications. The separation was performed by High Performance Liquid Chromatography (HPLC) system (Waters Chromatography Division, Milford, MA, USA) with a C-8 column (Lichrospher 100RP8EC 5μm 250.46, Teknokroma, Spain). The protocol consisted of isocratic system with mobile phase including 49.5:49.5:0.5 (volume) water: acetonitrile: THF, respectively that was neutralized with NaOH to pH=2.5. The flow rate was 1 ml/min for 20 min and injection volume was 20 μL. Detection was carried out at a wavelength of 350 nm.
Linearity in the range of 0–60 μg/mL doxycycline was confirmed by using known dilutions of standard doxycycline hyclate (Merck, Germany) in mobile phase.
The limit of detection (LOD), S/N =3.3, and the limit of quantitation (LOQ), S/N=10, were 1.49 and 4.52 μg/ml, respectively.
The statistical significance of the change in doxycycline concentrations was tested with two-way ANOVA. (The interaction effect of the obturation material and time was insignificant; p=0.25). Post hoc analysis for pairwise comparison between time intervals was done by Tukey test. Correlation between time intervals and docycycline concentrations was calculated by Spearman correlation test. P-values less than 0.05 were considered as significant.
RESULTS
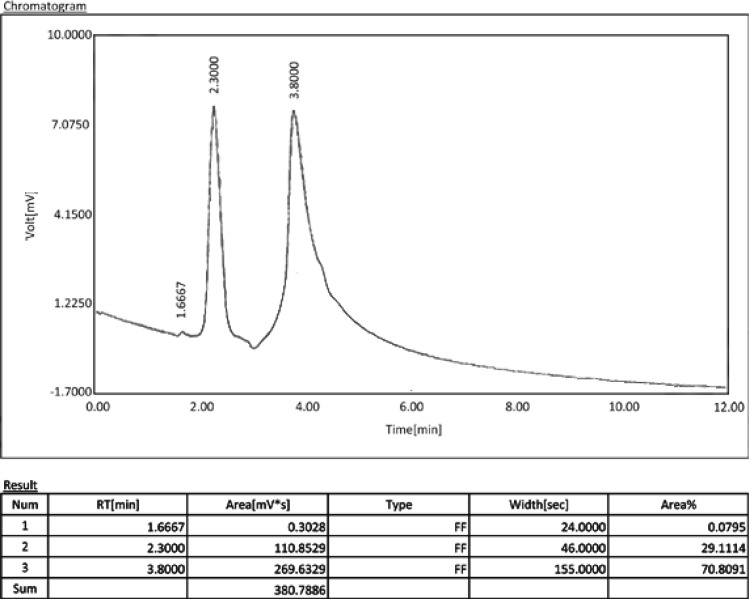
The comparative chromatograms of the extracted doxycycline in samples are illustrated in figure 2. The peak at the retention time of 3.8 min is contributed to oxytetracycline compared to the one in the standard chromatogram. The concentration of doxycycline in liquid is the concentration calculated based on the area under the curve from the recovered samples obtained from 0.02gr of dentinal debris. The concentration of doxycycline in dentin is its concentration in 1 gr dentin that was calculated by mathematical proportion from the concentration in liquid.
Fig 2a.
Chromatogram of a gutta-percha/AH26 sample. The peak at the retention time of 3.8 min. is contributed to doxycycline.
The mean concentration of doxycycline in dentin for one, three and six-week gutta percha/AH26 samples was 1.8±0.36, 1.22±0.22 and 0.67±0.11, respectively, whereas these concentrations in Resilon/RealSeal samples were 1.60±0.26, 0.80±0.14 and 0.59±0.01, respectively.
These concentrations were higher in the gutta-percha/AH26 samples than Resilon/RealSeal samples in each time interval.
A significant difference was seen between doxycycline concentrations in different time intervals regardless of the type of obturating material (p<0.001).
Regarding the positive control group, these concentrations were 2.09±0.11, 1.54±0.12 and 0.72±0.07, respectively for 1, 3 and 6-week intervals.No doxycycline was detected in the negative control groups.
The statistical analysis showed there was a significant difference for doxycycline concentrations between different obturating materials (p=0.008). No significant difference was seen between the doxycycline concentrations in the positive control group and gutta-percha/AH26 samples in each time interval (p>0.05), but a significant difference was seen between this concentration in the positive control and Resilon/RealSeal samples in each time interval (p<0.05). All groups showed a time dependent decrease in doxycycline concentrations (Spearman’s correlation coefficient=0.91, p<0.001).
The highest concentration of doxycycline was found in the one-week positive control samples and then in the one-week gutta percha/AH26 samples and the lowest concentration was found in six-week Resilon/RealSeal samples.
The mean concentration of doxycycline in dentin for one, three and six-week gutta percha/AH26 samples was 1.8±0.36, 1.22±0.22 and 0.67±0.11, respectively, whereas these concentration in Resilon/RealSeal samples were 1.60±0.26, 0.80±0.14 and 0.59±0.01, respectively.
Regarding the positive control group, these concentrations were 2.09±0.11, 1.54±0.12 and 0.72±0.07, respectively for 1, 3 and 6-week intervals.
No doxycycline was detected in the negative control groups.
The statistical analysis showed there was a significant difference for doxycycline concentrations between different obturating materials (p=0.008).
These concentrations were higher in the gutta-percha/AH26 samples than Resilon/RealSeal samples in each time interval.
A significant difference was seen between doxycycline concentrations in different time intervals regardless of the type of obturating material (p<0.001). No significant difference was seen between the doxycycline concentrations in the positive control group and gutta-percha/AH26 samples in each time interval (p>0.05), but a significant difference was seen between this concentration in the positive control and Resilon/RealSeal samples in each time interval (p<0.05). All groups showed a time dependent decrease in doxycycline concentrations (Spearman’s correlation coefficient=0.91, p<0.001). The highest concentration of doxycycline was found in the one-week positive control samples and then in the one-week gutta percha/AH26 samples and the lowest concentration was found in six-week Resilon/RealSeal samples.
The mean concentrations of doxycycline found in our experimental groups are shown in Table 1.
Table 1.
Mean Concentration of Doxycycline
| Obturation Material | 1 week | 3 weeks | 6 weeks | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Concentration in Liquid (microg/ml) | Concentration in Dentin (mg/gr) | Concentration in Liquid (microg/ml) | Concentration in Dentin (mg/gr) | Concentration in Liquid (microg/ml) | Concentration in Dentin (mg/gr) | |
| Gutta Percha | 30.00±6.01 | 1.8 ±0.36 | 20.37±3.67 | 1.22±0.22 | 11.25±1.94 | 0.67±0.11 |
| Resilon | 26.70±4.36 | 1.60 ±0.26 | 13.43±2.33 | 0.80±0.14 | 9.841 ±1.64 | 0.59 ±0.01 |
| Positive Control | 34.94±1.9 | 2.09±0.11 | 25.82±2.11 | 1.54±0.12 | 12.02±1.32 | 0.72±0.07 |
DISCUSSION
Various methods for determination of doxycycline in-vitro and in-vivo have been reported such as microbiology, fluorimetry, TLC-fluorescence scanning densitometry, spectrophotometry, sequential injection chromatography (SIC) and HPLC [14].
High-performance liquid chromatography (HPLC) is a chromatographic technique used to separate a mixture of compounds with the purpose of identifying and quantifying the individual components of the mixture [15, 16].
Hplc separation is accomplished by means of sample mixture interacts with solid particles within the hplc column [15–17].
This technique offers several advantages over other techniques, including minimal sample manipulation before chromatography, rapid analysis and the simultaneous analysis of multiple compounds with good specificity, precision and accuracy [14].
Portenier et al [18] reported that the minimum concentration of doxycycline that provided a bactericidal effect against E. faecalis in the presence of dentin within 24 hours of contact, was 0.3% (3mg/ml). The measured concentration of doxycycline in the dentin in both groups of our study was lower. On the other hand, Newberry et al [19] reported that MTAD killed most strains of E. faecalis when diluted to 1:512. Upon dilution of MTAD to 1:512 the concentration of doxycycline would be 0.05mg; therefore, according to the results of the present study, the concentration of doxycycline in root canal dentin within 6 weeks might be able to kill most strains of E. faecalis which deserves further studies.
The decomposition and diffusion rate of the antimicrobial compounds is likely a function of pH [10]. It has been reported that acidic solutions and environments result in the dehydration of tetracycline [20, 21]. Acidic sealers like Epiphany SE might substantially affect the local pH of dentin and hence, the stability of doxycycline [10]. This might be one of the reasons for the lower concentration of doxycycline found in Resilon/RealSeal groups in the present study.
The bond strength of AH26 to dentin is related to the formation of covalent bonds between epoxide rings and exposed amino groups in the collagen network [22]. Doxycycline also has an amino group in its molecular structure [23]; Therefore, AH26 might be able to form covalent bonds with doxycycline too.
Although there is no study that documented the aforementioned reaction, in theory, the higher concentration of doxycycline in gutta-percha/AH26 samples might be due to this bonding. Further studies in this regard are recommended.
Rasimick et al. [10] analyzed the doxycycline concentration in teeth that were not obturated and showed that its concentration decreased over a 9-week period of time, which was consistent with our results.
The primary and most important factor in determining the long term success of an endodontic treatment is the presence and/or persistence of microorganisms [24]. There is strong evidence that all microorganisms cannot be removed after chemo-mechanical preparation of the root canal system [25]. However, it is possible to minimize the amount of bacteria present in the root canal [26]. Maintaining a good coronal seal after completion of obturation is also important to minimize bacterial colonization [27]. Microorganisms may penetrate into the root canal system via temporary restorations and any defects in the coronal tooth structure. Furthermore, reinfection of the root canal system can occur because of re-growth of residual microorganisms that have survived the endodontic treatment procedures [28]. A disinfection protocol that provides substantivity might improve the outcome of endodontic treatment [29] and optimize single visit disinfection [30]. The long term antibacterial efficacy of absorbed doxycycline in dentinal walls and its effects on success/failure of the treatment is not determined yet.
The possibility and mechanisms of leakage in root canals obturated with gutta-percha/AH26 [31–33] and Resilon/RealSeal [13, 31, 33] has been well documented. Because of the potential of leakage after obturation with either of these materials it might be beneficial to use irrigants with antimicrobial substantivity. According to outcomes of the present study, after using MTAD as the final irrigant, root canal obturation with non-acidic sealers like AH26 might be better in terms of stability of doxycycline.
Although the present study showed that the stability of doxycycline following MTAD irrigation decreased more in the presence of Resilon/RealSeal in the root canal space, the clinical relevance of the issue is still unclear. Besides, the stability of doxycycline showed a time dependent decrease that raises questions about the long-term antibacterial effectiveness of MTAD. Therefore, randomized clinical trials on the effects of MTAD on success/failure of the endodontic treatments are recommended.
CONCLUSION
The outcomes of this study showed that the stability of doxycycline following irrigation with MTAD was lower when the root canals were obturated with Resilon/RealSeal compared to gutta-percha/AH26. The stability of doxycycline showed a time dependent decrease.
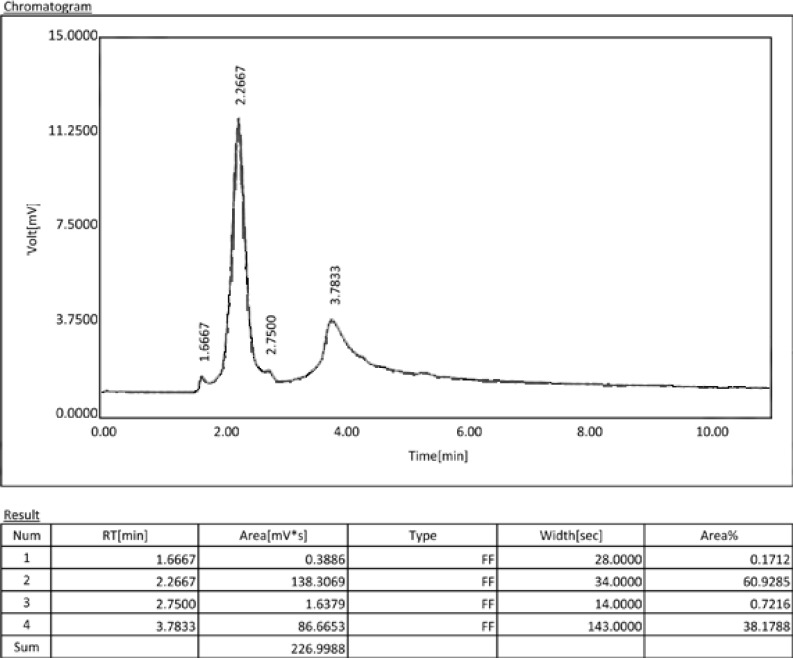
Fig 2b.
Chromatogram of a Resilon/RealSeal sample. The peak at the retention time of 3.8 min. is contributed to doxycycline
Acknowledgments
This study was supported by a grant from Tehran University of Medical Sciences and Health Services (grant no. 15658).
REFERENCES
- 1.Sjögren U, Figdor D, Persson S, Sundqvist G. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int Endod J. 1997 Sep;30(5):297–306. doi: 10.1046/j.1365-2591.1997.00092.x. [DOI] [PubMed] [Google Scholar]
- 2.Baca P, Mendoza-Llamas ML, Arias-Moliz MT, Gonzalez-Rodriguez MP, Ferrer-Luque CM. Residual effectiveness of final irrigation regimens on Enteroccus faecalis-infected root canals. J Endod. 2011 Aug;37(8):1121–3. doi: 10.1016/j.joen.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Torabinejad M, Khademi AA, Babagoli J, Cho Y, Johnson WB, Bozhilov K, et al. A new solution for the removal of the smear layer. J Endod. 2003 Mar;29(3):170–5. doi: 10.1097/00004770-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Torabinejad M, Cho Y, Khademi AA, Bakland LK, Shabahang S. The effect of various concentrations of sodium hypochlorite on the ability of MTAD to remove the smear layer. J Endod. 2003 Apr;29(4):233–9. doi: 10.1097/00004770-200304000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Shabahang S, Torabinejad M. Effect of MTAD on Enterococcus faecalis-contaminated root canals of extracted human teeth. J Endod. 2003 Sep;29(9):576–9. doi: 10.1097/00004770-200309000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Torabinejad M, Shabahang S, Aprecio RM, Kettering JD. The antimicrobial effect of MTAD: an in vitro investigation. J Endod. 2003 Jun;29(6):400–3. doi: 10.1097/00004770-200306000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Mohammadi Z, Shahriari S. Residual antibacterial activity of chlorhexidine and mtad in human dentin in vitro. J Oral Sci. 2008 Mar;50(1):63–7. doi: 10.2334/josnusd.50.63. [DOI] [PubMed] [Google Scholar]
- 8.Stabholz A, Kettering J, Aprecio R, Zimmerman G, Baker PJ, Wikesjo UM. Retention of antimicrobial activity by human root surfaces after in situ subgingival irrigation with tetracycline HCl or chlorhexidine. J Periodontol. 1993 Feb;64(2):137–41. doi: 10.1902/jop.1993.64.2.137. [DOI] [PubMed] [Google Scholar]
- 9.Shokouhinejad N, Sharifian MR, Aligholi M, Assadian H, Tabor RK, Nekoofar MH. The sealing ability of resilon and gutta-parcha following different smear layer removal methods: an ex vivo study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010 Jul;110(1):e45–9. doi: 10.1016/j.tripleo.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Rasimick BJ, Wan J, Musikant BL, Deutsch AS. Stability of doxycycline and chlorhexidine absorbed on root canal dentin. J Endod. 2010 Mar;36(3):489–92. doi: 10.1016/j.joen.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Carson KR, Goodell GG, McClanahan SB. Comparison of the antimicrobial activity of six irrigants on primary endodontic pathogens. J Endod. 2005 Jun;31(6):471–3. doi: 10.1097/01.don.0000148868.72833.62. [DOI] [PubMed] [Google Scholar]
- 12.Jin S, Jia W. Self-curing system for endodontic sealant applications. US Patent & Trademark Office; Jun 19, 2003. United States Patent Application 20030113686. [Google Scholar]
- 13.Kim YK, Grandini S, Ames JM, Gu LS, Kim SK, Pashley DH, et al. Critical review on methacrylate resin-based root canal sealers. J Endod. 2010 Mar;36(3):383–99. doi: 10.1016/j.joen.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 14.Mitic SS, Miletic GZ, Kostic DA, Naskovic Dokic DC, Arsic BB, Rasic ID. A rapid and reliable determination of doxycycline hyclate by HPLC with UV detection in pharmaceutical samples. J Serbian Chem Soc. 2008;73(6):665–71. [Google Scholar]
- 15.Dong MW. Modern HPLC for practicing scientists. Wiley-Interscience; 2006. [Google Scholar]
- 16.Edwards J. Characterization of Materials. Published Online, Copyright by John Wiley & Sons, Inc; 2012. [Google Scholar]
- 17.Gilman JJ. Materials Science and Technology. Maney Publishing; London, UK: 2006. [Google Scholar]
- 18.Portenier I, Waltimo T, Orstavik D, Haapasalo M. Killing of Enterococcus faecalis by MTAD and chlorhexidine digluconate with or without cetrimide in the presence or absence of dentine powder or BSA. J Endod. 2006 Feb;32(2):138–41. doi: 10.1016/j.joen.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 19.Newberry BM, Shabahang S, Johnson N, Aprecio RM, Torabinejad M. The antimicrobial effect of biopure MTAD on eight strains of Enterococcus faecalis: an in vitro investigation. J Endod. 2007 Nov;33(11):1352–4. doi: 10.1016/j.joen.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Khalil SA, Daabis NA, Naggar VF, Motawi MM. The in vitro adsorption of some antibiotics on antacids. Pharmazie. 1976;31(2):105–9. [PubMed] [Google Scholar]
- 21.Williams A, Lemke L. Foye’s Principles of Medicinal Chemistry. Lippincott Williams & Wilkins; Philadelphia: 2002. [Google Scholar]
- 22.Lee KW, Williams MC, Camps JJ, Pashley DH. Adhesion of endodontic sealers to dentin and gutta-percha. J Endod. 2002 Oct;28(10):684–8. doi: 10.1097/00004770-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006 Feb;54(2):258–65. doi: 10.1016/j.jaad.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Chandra A. Discuss the factors that affect the outcome of endodontic treatment. Aust Endod J. 2009 Aug;35(2):98–107. doi: 10.1111/j.1747-4477.2009.00199.x. [DOI] [PubMed] [Google Scholar]
- 25.Nair PN, Sjogren U, Krey G, Kahnberg KE, Sundqvist G. Intraradicular bacteria and fungi in root-filled, asymptomatic human teeth with therapy-resistant periapical lesions: a long-term light and electron microscopic follow-up study. J Endod. 1990 Dec;16(12):580–8. doi: 10.1016/S0099-2399(07)80201-9. [DOI] [PubMed] [Google Scholar]
- 26.Allard U, Stromberg U, Stromberg T. Endodontic treatment of experimentally induced apical periodontitis in dogs. Endod Dent Traumatol. 1987 Oct;3(5):240–4. doi: 10.1111/j.1600-9657.1987.tb00630.x. [DOI] [PubMed] [Google Scholar]
- 27.Ray HA, Trope M. Periapical status of endodontically treated teeth in relation to the technical quality of the root filling and the coronal restoration. Int Endod J. 1995 Jan;28(1):12–8. doi: 10.1111/j.1365-2591.1995.tb00150.x. [DOI] [PubMed] [Google Scholar]
- 28.Sundqvist G, Figdor D, Persson S, Sjögren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998 Jan;85(1):86–93. doi: 10.1016/s1079-2104(98)90404-8. [DOI] [PubMed] [Google Scholar]
- 29.Mohammadi Z, Abbott PV. Antimicrobial substantivity of root canal irrigants and medicaments: a review. Aust Endod J. 2009 Dec;35(3):131–9. doi: 10.1111/j.1747-4477.2009.00164.x. [DOI] [PubMed] [Google Scholar]
- 30.Siqueira JF, Jr, Rocas IN. Optimising single-visit disinfection with supplementary approaches: a quest for predictability. Aust Endod J. 2011 Dec;37(3):92–8. doi: 10.1111/j.1747-4477.2011.00334.x. [DOI] [PubMed] [Google Scholar]
- 31.Kokorikos I, Kolokouris I, Economides N, Gogos C, Helvatjoglu-Antoniades M. Long-term evaluation of the sealing ability of two root canal sealers in combination with self-etching bonding agents. J Adhes Dent. 2009 Jun;11(3):239–46. [PubMed] [Google Scholar]
- 32.Warneke S, Arenskotter M, Tenberge KB, Steinbuchel A. Bacterial degradation of poly(trans-1,4-isoprene) (gutta percha) Microbiology. 2007 Feb;153(Pt 2):347–56. doi: 10.1099/mic.0.2006/000109-0. [DOI] [PubMed] [Google Scholar]
- 33.Yigit DH, Gencoglu N. Evaluation of resin/silicone based root canal sealers. Part I: physical properties. Digest J Nanomaterials Biostructures. 2012;7(1):107–15. [Google Scholar]