Abstract
Leptospirosis is considered an important reemerging infectious disease worldwide. The standard and most widespread method for the diagnosis of leptospirosis is the microscopic agglutination test (MAT). This test is laborious and time-consuming, and the interpretation of the results is subjective. In the present work we describe an application of flow cytometry (FCM) as a tool for the serological diagnosis of leptospirosis. The analysis is based on the sensitivity of FCM to the size and shape of the bacteria analyzed by measurement of light scatter parameters: forward scatter (FSC) and side scatter (SSC). The addition of positive serum to an infecting leptospiral serovar results in a shift of the light scatter parameter to a different location with higher FSC and SSC values, indicating the formation of leptospiral aggregates. By using immunofluorescent staining, we have shown that the large particles formed are the agglutinated leptospires. Quantification of the agglutination process has been achieved by calculating an agglutination factor (Af), based on changes in the light scatter parameters measured by FCM. Af enables us to determine the specificity of the serological reaction of the patient serum with each leptospiral serovar. In this work, 27 serum samples from 18 leptospirosis patients were tested by both the MAT and the FCM techniques, in which each serum sample was tested against 13 serovars. Twenty-six human serum samples derived from patients with a variety of other defined illnesses were used as negative controls and enabled us to define the Af threshold value as <9.3 for negative patients, while any value higher than that would be a positive result for leptospirosis. Compared to MAT, the FCM technique was found to be more specific and sensitive, especially in identifying the serogroup in the acute phase of the disease. The whole process was found to be rapid and took less than 1.5 h. Moreover, FCM analysis is objective and can be automated for the handling of large numbers of samples.
Leptospirosis is considered one of the most widespread zoonoses worldwide (18, 34). The disease is caused by spirochetes of the genus Leptospira. The genus Leptospira is classified serologically into two species, the pathogenic species Leptospira interrogans and the saprophytic species Leptospira biflexa. There are more than 200 serovars of L. interrogans and more than 60 serovars of L. biflexa (16).
Leptospirosis usually results from contact with the urine of infected animals (13). The diagnosis of leptospirosis is mainly based on serological tests, with the microscopic agglutination test (MAT) considered the standard methodology (8, 12). The serological test for Leptospira is based on the formation of bacterial aggregates resulting from the addition of serum samples to the Leptospira suspension. The agglutination leads to a significant change in the analyzed particles, as observed by dark-field microscopy by the MAT procedure.
A variety of serological tests other than MAT have been developed for the diagnosis of leptospirosis. Among them are the complement fixation test (33), several enzyme-linked immunosorbent assay formats (1, 29), the macroscopic slide agglutination test (14), the microcapsule agglutination test (9), the indirect hemagglutination assay (20), the dipstick assay (27), and other methods (3, 15, 22, 30). Each assay has its own advantages, drawbacks, and limitations (4, 18). Despite its widespread use, MAT has several limitations. The test is difficult to perform and control, the results are difficult to interpret, and it is time-consuming and labor-intensive (31). The interpretation of MAT results is subjective and may cause quality assurance difficulties. One of the disadvantages of serologic testing by MAT compared to that by other techniques is its low sensitivity, particularly with early acute-phase specimens (3, 5, 10). In this work, we describe the use of the flow cytometry (FCM) technique for the serological diagnosis of leptospirosis. It is shown that the diagnosis of leptospirosis and the definition of the serogroup involved are feasible, based on the changes in the light scatter parameters forward scatter (FSC) and side scatter (SSC). By the FCM technique, the sizes and the shapes of the cells can be determined by measurement of FSC and SSC (17, 26, 32). Whereas FSC is related to the cell size and the optical refraction index of the outer membrane of the cell, SSC is related to the cell's granularity. Analysis is possible due to the highly developed new generation of flow cytometric analyzers with the capability of observing particles with diameters of 0.5 μm, which is as small as a variety of bacterial species (2, 7, 11, 23-25, 28, 35).
FCM analysis was found to be objective, sensitive, and rapid. The duration of the whole process, i.e., the times for incubation of the sera, analysis, and interpretation of the results, was less than 1.5 h.
MATERIALS AND METHODS
Human sera and MAT.
Human sera were sent from medical centers throughout Israel to the Israel Institute for Biological Research, the central reference laboratory for leptospirosis in Israel. Each serum sample was tested against 21 different serovars by MAT by the standard procedure (21). Agglutination was examined by dark-field microscopy at a magnification of ×100. The reported titer was calculated as the reciprocal of the highest dilution of serum that agglutinated at least 50% of the cells for each serovar used. A MAT-confirmed case was defined as a fourfold increase in antibody titer or a single titer ≥1:200, according to the case definition of the Centers for Disease Control and Prevention (6).
In the present study patients positive for leptospirosis were considered those suspected of having leptospirosis by the clinicians because they exhibited clinical symptoms typical of the disease. Their sera were sent to the Israel Institute for Biological Research and were found to be positive by MAT. Also, nine of these patients were known to work in areas of leptospirosis outbreaks from which L. interrogans serovar hardjo was isolated.
Sera from 26 patients whose paired sera were found to be negative by MAT served as negative controls: 4 serum samples from patients with murine typhus, 4 serum samples from patients with Mediterranean spotted fever, 4 serum samples from patients with Q fever, 5 serum samples from patients with syphilis, and 9 serum samples from patients with other clinical symptoms resembling leptospirosis.
Bacterial culture.
Twenty-one reference serovars of living leptospiral spirochetes were used; among these were 19 pathogenic serovars (L. interrogans) and 2 nonpathogenic (L. biflexa) serovars. Details about the serovars are listed in Table 1.
TABLE 1.
Reference serovars used in this study
| Species and serovar no. | Serovar | Origin |
|---|---|---|
| L. interrogans | ||
| 1 | Icterohaemorrhagiae M20 copenhageni | Human, Denmark, 1935 |
| 29 | Icterohaemorrhagiae RGA | ATCC 43642 |
| 2 | Canicola canicola Hond Utrecht (IV) | ATCC 23470 |
| 3 | Grippothyphosa grippo- thyphosa Moskva (V) | ATCC 23469 |
| 5 | Sejroe sejroe M-84 | Human, Denmark, 1937 |
| 6 | Szwajizak szwajizak | Human, Australia, 1951 |
| 7 | Mini sari | Human, Italy, 1941 |
| 9 | Hardjo hardjoprajitno | Human, Indonesia, 1938 |
| 11 | Pomona | ATCC 23478 |
| 12 | Bataviae bataviae | ATCC 23468 |
| 13 | Rachmati | ATCC 23603 |
| 14 | Borgpetersenii javanica | ATCC 23479 |
| 16 | Australis | ATCC 23605 |
| 17 | Cynopteri canalzonae | Panama Canal Zone |
| 18 | Pyrogenes | ATCC 23480 |
| 19 | Ballum castellonis | ATCC 23580 |
| 21 | Ballum mus 127 | Mus musculus, Denmark, 1943 |
| 23 | Tarassovi tarassovi | ATCC 23481 |
| 24 | Sejreo bratislava | Rome, 1970 |
| L. biflexa | ||
| 10 | Semaranga patoc I | Surface water, Italy |
| 15 | Andamana andamana | Human, Andamans, 1930 |
FCM.
FCM analysis was performed with a FACSCalibur analyzer (Becton Dickinson Immuno Cytometry Systems, San Jose, Calif.) equipped with a 15-mW argon laser as the excitation light source. The detectors used in this work are a photodiode for FSC (λ = 488/10 nm), a photomultiplier for SSC (λ = 488/10 nm), and a photomultiplier for green fluorescence (FL1; emission λ = 530/30 nm). The instrument settings included logarithmic amplifiers on all detectors. All experiments were performed for a fixed time of analysis (30 s). Acquisition and analysis were performed with CellQuest software.
Preparation of samples for FCM analysis.
Human sera were diluted (1:100) in saline-formaldehyde (0.14%) and filtered through a low-protein-binding 0.45-μm-pore-size syringe filter. Leptospiral organisms were grown for a week in EMJH medium (nos. 279410 and 279510; Becton Dickinson), counted with a Petroff-Hausser counting chamber under a dark-field microscope to confirm the presence of 1 × 108 to 2 × 108 bacteria/ml, and then harvested. Each serovar was diluted 1/10 in saline-formaldehyde (0.14%), incubated in an equal volume of serum at room temperature for 60 min, and analyzed by FCM.
Quantification of agglutination.
Quantification of the agglutination process based on the light scatter parameters was achieved by comparison of the agglutinated to the nonagglutinated Leptospira dot plots. Nonagglutinated leptospires could be observed as a subpopulation under region R2 on a dot plot of the light scatter parameters (FSC and SSC) (Fig. 1). Agglutinated leptospires could be observed as a subpopulation under region R3 on the same dot plot (Fig. 1).
FIG. 1.
Dot plots of L. interrogans serovar icterohaemorrhagiae obtained by FCM. (A) Leptospira in phosphate-buffered saline; B(I) and B(II) light scatter (SSC-FSC) and fluorescence (SSC-FL1) dot plots, respectively, of L. interrogans serovar icterohaemorrhagiae in the presence of rabbit anti-icterohaemorrhagiae serum (30 min, 37°C) and the secondary antibody goat anti-rabbit immunoglobulin G-FITC (20 min, 37°C); (C) light scatter dot plots of Leptospira in the presence of rabbit anti-icterohaemorrhagiae serum (1:2,000) after 1 min (I), 5 min (II), and 10 min (III) of incubation at 37°C; (D) same as panel C after 35 min of incubation at 37°C with the serum dilution levels of 1:8,000 (I), 1:4,000 (II), and 1:2,000 (III). R1 (green), the region of FITC-stained agglutinated Leptospira; R2 (red), nonagglutinated Leptospira; R3 (pink), agglutinated Leptospira.
An arithmetic equation (equation 1) was developed in order to quantify the agglutination factor (Af):
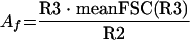 |
(1) |
where meanFSC(R3) is the mean FSC value of the events included in region R3, and R3 and R2 are the number of events in each region. This equation is based on three major parameters affecting the light scatter parameters of the agglutinated and nonagglutinated species on the FCM dot plots: (i) the decrease in the population in region R2, (ii) the increase in the population in region R3, and (iii) the increase in the mean FSC for region R3. The number of particles in region R3, after agglutination, is also dependent on the size of the bacterial cluster formed. In cases in which large particles are formed, fewer particles will be counted in region R3; however, the mean FSC will increase and will compensate for the low counts in region R3. The parameters R3, meanFSCR3, and R2 used in equation 1 are normalized to the values of nonagglutinated leptospires by measuring the values for the samples with different serovars before and after serum addition.
RESULTS
Determination of leptospiral agglutination by FCM.
The changes in light scatter parameters (FSC and SSC) were initially studied by monitoring the incubation of L. interrogans serovar icterohaemorrhagiae with hyperimmune rabbit anti-serovar icterohaemorrhagiae serum and fluorescent anti-rabbit immunoglobulin G-fluorescein isothiocyanate (FITC) conjugates.
Figure 1A shows the light scatter parameters of nonagglutinated Leptospira. Even though the leptospiral size is in the micron range, and hence their signals were at the limit of sensitivity of FCM, the Leptospira population could be observed by using logarithmic amplification of the FSC and SSC parameters. The Leptospira population was defined as region R2, in which a concentration dependency of the bacteria was observed (data not shown).
Upon addition of the specific rabbit antiserum and anti-rabbit immunoglobulin G-FITC conjugate, the majority of the Leptospira population shifted from region R2 to region R3 [Fig. 1B(I)], which reflected stronger FSC and SSC signals. The same events that reached the new SSC and FSC region (region R3) [Fig. 1B(I)] were also the events with higher fluorescence signals and were observed in region R1 on the SSC-FL1 dot plot [Fig. 1B(II)]. This correlation indicates that the larger particles observed by the light scatter parameters (FSC and SSC) in region R3 are the agglutinated leptospires; hence, they light up by specific immune staining, as shown in the SSC-FL1 dot plot (region R1). No agglutination was observed by performing the same experiment with the same experimental setup but with nonimmune rabbit serum (data not shown). The correlation between the events located in region R3 and those located in region R1 indicates that light scatter parameters are sufficient for the analysis of agglutinated Leptospira.
The shifts of the Leptospira signals from region R2 to region R3 were found to be dependent on the serum concentration and the incubation time (Fig. 1C and D, respectively). By the FCM technique, it was possible to observe agglutination of Leptospira in an incubation time shorter than 5 min with a serum dilution of 1:2,000. The titer in the same serum sample by end-point titration by MAT was 1:3,200 after incubation for 1 h, while by the FCM technique agglutination could be observed after 35 min of incubation, even when a dilution of 1:8,000 was used. Hence, compared to MAT, FCM analysis is more sensitive and rapid.
FCM analysis of sera from suspected human leptospirosis cases.
Twenty-seven serum samples from 18 patients were examined by MAT and FCM analysis. Since a single titer ≥1:200 by MAT is considered positivity for leptospirosis, FCM analysis was performed with a serum dilution of 1:200. The sera were incubated for 60 min with each Leptospira serovar and then subjected to FCM analysis.
Quantification of the agglutination process, based on the light scatter parameters, was achieved by calculating Af, as described in the Materials and Methods section. Calculation of Af values for negative control sera enabled us to set a threshold that distinguished between negative and positive sera. In order to set the threshold, we measured the Af values for each of the 26 negative serum samples (described in Materials and Methods) against 13 serovars, resulting in a total of 338 tests. The average Af value was calculated to be 1.8 ± 1.5, and serum samples with Af values ≤9.3 (average plus five times the standard deviation) were considered negative for leptospirosis.
Figure 2A presents the results of a typical FCM analysis of a serum sample (from patient 2) positive by MAT and analyzed for 13 different Leptospira serovars. It was concluded from the FSC and SSC results that the serum sample was positive for serovars icterohaemorrhagiae copenhageni (serovar 1 in Fig. 2A), sejroe sejroe M84 (serovar 5), szwajizak szwajizak (serovar 6), and icterohaemorrhagiae RGA (ATCC 43642) (serovar 29). All of the same serovars except sejroe sejroe M84 were also found to be positive by MAT, with titers of 1:400, 1:400, and 1:200, respectively.
FIG. 2.
(A) Light scatter dot plots of 13 different Leptospira serovars (as presented in the Materials and Methods section and indicated in parentheses in each panel) in the presence of human serum sample 2/II. R2, nonagglutinated Leptospira; R3, agglutinated Leptospira. (B) Af for serum sample 2/II in comparison to that for a negative serum sample.
Figure 2B shows the Af values for each serovar, as calculated by equation 1, for a negative serum sample and for the leptospirosis-positive patient whose results are provided in Fig. 2A. It can be seen that for the leptospirosis-positive patient the Af values for four serovars were much higher than the negative Af values (positive or negative serum with negative results). The Af values for the sera with positive results for serovars icterohaemorrhagiae copenhageni (serovar 1 in Fig. 2A), sejroe sejroe M84 (serovar 5), szwajizak szwajizak (serovar 6), icterohaemorrhagiae RGA (ATCC 43642) (serovar 29), were 105, 44, 120, and 330, respectively. In this case, the results by both MAT and FCM indicate that serovar icterohaemorrhagiae is the predominant serogroup.
Table 2 summarizes the predominant serogroups obtained by MAT and FCM analyses of the 27 serum samples from 18 leptospirosis patients. In addition to exhibiting typical clinical symptoms of the disease, the sera of these patients were found to be positive for leptospires by MAT, which was performed against 21 serovars. Moreover, nine of these patients (patients 3, 5, 7, 8, 9, 15, 16, 17, and 18) were known to work in areas where leptospirosis outbreaks had occurred and from which serovar hardjo had been isolated.
TABLE 2.
FCM and MAT results for leptospirosis patients and negative controls
| Patient no./serum sample no. | Serogroup by MATa (titer) | Serogroup with highest positive (Af value) |
|---|---|---|
| 1/I | Icterob (1,600) | Ictero (300) |
| 2/I | Negative | Ictero (10) |
| 2/II | Ictero (400) | Ictero (330) |
| 3/I | Negative | Negative |
| 3/II | Hardjo (400) | Hardjo (40) |
| 4/I | Ictero (800) | Szwajizak (1,790), ictero (600) |
| 5/I | Hardjo (400) | Hardjo (650) |
| 6/I | Negative | Ballum (15,000) |
| 6/II | Ballum (400) | Ballum (5,600) |
| 7/I | Hardjo (400) | Hardjo (170) |
| 8/I | Hardjo (800) | Hardjo (24) |
| 9/I | Hardjo (400) | Hardjo (340) |
| 10/I | Ictero (800) | Ictero (330) |
| 11/I | Negative | Negative |
| 11/II | Ictero (800) | Szwajizak (65) |
| 11/II | Szwajizak (400) | Ictero (16) |
| 12/I | Negative | Negative |
| 12/II | Bataviae (400) | Bataviae (590) |
| 13/I | Negative | Negative |
| 13/II | Australis (200) | Australis (12) |
| 14/I | Canicola (3,200) | Ballum (400), canicola (205) |
| 14/II | Canicola (400) | Ballum (7,500) canicola (260) |
| 15/I | Hardjo (400) | Hardjo (215) |
| 15/II | Hardjo (800) | Hardjo (250) |
| 16/I | Negative | Hardjo (40) |
| 16/II | Hardjo (800) | Hardjo (610) |
| 17/II | Hardjo (3,200) | Hardjo (206) |
| 18/II | Hardjo (200) | Hardjo (36) |
| Negative controlsc | Negative | Negative (<9.3) |
Predominant serogroups.
Ictero, Leptospira icterohaemorrhagiae serogroup.
Twenty-six serum samples; for details, see Materials and Methods.
For 15 patients, the highest Af values for the specific serogroup were measured by FCM analysis, and the results thus corresponded to the highest titers obtained by MAT. For example, for patients 1, 2, and 10, the highest Af values and MAT titers were for serogroup icterohaemorrhagiae, whereas for patients 3, 5, 7, 8, 9, 15, 16, 17, and 18 the highest values were for serogroup hardjo.
In some cases it was possible to identify leptospirosis in patients by FCM significantly before it was detected according to the appropriate titers by MAT. For example, the indication of positivity for leptospirosis by FCM appeared in the acute-phase sera of patients 2, 6, and 16, whereas by MAT it appeared only in the sera obtained later. These results demonstrate that the FCM technique can reliably be used as a tool for the diagnosis of leptospirosis at an early stage of the disease and for the identification of the infecting serogroup.
From Table 2 one can see that the Af value did not always correspond to the MAT titers. For example, the titer obtained by MAT was 1:400 for both patients 2 and 3, while the Af values were 330 and 40, respectively. Despite these differences, the predominant serogroup appeared to be the same by both methods. In three other patients (patients 4, 11, and 14), although the patients were found to be Leptospira positive by both the MAT and the FCM techniques, the predominant serogroups identified and defined by MAT and FCM were different. The predominant serogroups found by MAT were icterohaemorrhagiae, icterohaemorrhagiae, and canicola, respectively, whereas the predominant serogroups found by FCM were szwajizak, szwajizak, and ballum, respectively. However, the serogroups that were predominant by MAT also gave positive Af values. These differences can be explained by the differences in the natures of the two different methods and by the cross-reactivities of the serovars.
DISCUSSION
This paper has demonstrated a new methodology for serological testing for leptospirosis. The FCM technique was used for the diagnosis of leptospirosis by monitoring the agglutination of various serovars following incubation with human serum. By developing and applying an arithmetic equation to calculate Af, it was shown that FCM analysis could lead to an objective quantification of agglutination. This equation takes into account the changes in the light scatter parameters and the sizes of the populations of both the agglutinated and the nonagglutinated leptospires. Analysis of sera from 26 patients with other defined diseases, which were used as negative controls and each of which was tested against 13 Leptospira serovars, was used to define the threshold Af between negative and positive patients (Af = 9.3).
By implementation of this methodology, FCM analysis enabled the detection of the serogroup in all patients, and in three patients (patients 2, 6, and 16) serogroup detection occurred in the acute phase, when the MAT result was still negative.
However, the Af value did not always correspond to the MAT titers, and in 3 of 18 patients (patients 4, 11, and 14), the predominant serogroup determined by MAT differed from that determined by FCM. This can be explained by the differences in the natures of the two methods: microscopy takes into account only the presence or the absence of aggregates, whereas FCM measures light scatter parameters and the analysis is more precise, as it considers size, shape, and number of the aggregates. Moreover, FCM analysis can detect very small aggregates not visible by light microscopy, as can be found in the early stages of agglutination. This may lead to positive Af values and negative MAT results in the early stages of the disease. Moreover, it was recently shown (19) that serological analysis by MAT could not always predict and identify the infecting serovar in individual patients. The reasons for the poor predictive ability of MAT could emanate from the cross-reactivity between serogroups and from the paradoxical reaction of an acute-phase or an early-convalescent-phase serum sample. It is possible that analysis by FCM overcomes some of these problems as a result of its capability to analyze multiple parameters (FSC, SSC, and fluorescence) and will be able to improve the specificities and sensitivities of serological tests.
In addition, an intrinsic limitation of MAT is the subjective interpretation of the results and the difficulties in ensuring standardization between laboratories. The FCM methodology eliminates these drawbacks due to the accuracy and objectivity stemming from the nature of the FCM analysis. Another advantage of FCM is its rapidity, as the entire procedure, including incubation time and analysis, is completed in 1.5 h. Furthermore, the analysis can be automated and used to perform large numbers of tests.
In conclusion, FCM uses standard equipment, is available in many hospitals, and is used mainly for blood counts and other immunological purposes. This method can easily be used for the diagnosis of leptospirosis because of its sensitivity and objectivity and because automated procedures can be applied to FCM.
REFERENCES
- 1.Adler, B., A. M. Murphy, S. A. Locarnini, and S. Faine. 1980. Detection of specific antileptospiral immunoglobulins M and G in human serum by solid-phase enzyme-linked immunosorbent assay. J. Clin. Microbiol. 11:452-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez-Barrientos, A., J. Arroyo, R. Canton, C. Nombela, and M. Sanchez-Perez. 2000. Applications of flow cytometry to clinical microbiology. Clin. Microbiol. Rev. 13:167-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appassakij, H., K. Silpapojakul, R. Wansit, and J. Woodtayakorn. 1995. Evaluation of the immunofluorescent antibody test for the diagnosis of human leptospirosis. Am. J. Trop. Med. Hyg. 52:340-343. [DOI] [PubMed] [Google Scholar]
- 4.Bajani, M., D., D. A. Ashford, S. L. Bragg, C. W. Woods, A. Tin, R. A. Spiegel, B. A. Plikaytis, M. Phelan, P. N. Levett, and R. S. Weyant. 2003. Evaluation of four commercially available rapid serologic tests for diagnosis of leptospirosis. J. Clin. Microbiol. 41:803-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandao, A. P., E. D. Camargo, E. D. da Silva, M. V. Silva, and R. V. Abrao. 1998. Macroscopic agglutination test for rapid diagnosis of human leptospirosis. J. Clin. Microbiol. 36:3138-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 1997. Case definitions for infectious conditions under public health surveillance. Morb. Mortal. Wkly. Rep. 46(RR-10):49. [PubMed] [Google Scholar]
- 7.Clarke, R. G., and A. C. Pinder. 1998. Improved detection of bacteria by flow cytometry using a combination of antibody and viability markers. J. Appl. Microbiol. 84:577-584. [DOI] [PubMed] [Google Scholar]
- 8.Cole, J. R., Jr., C. R. Sulzer, and A. R. Pursell. 1973. Improved microtechnique for the leptospiral microscopic agglutination test. Appl. Microbiol. 25:976-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui, J. J., G. X. Xiao, T. Z. Chen, G. F. Zhu, T. Sato, M. Seki, S. Kobayashi, and Y. Arimitsu. 1991. Further evaluation of one-point microcapsule agglutination test for the diagnosis of leptospirosis. Epidemiol. Infect. 106:561-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cumberland, P. C., C. O. R. Everard, and P. N. Levett. 1999. Assessment of the efficacy of the IgM enzyme-linked immunosorbent assay (ELISA) and microscopic agglutination test (MAT). Am. J. Trop. Med. Hyg. 61:731-734. [DOI] [PubMed] [Google Scholar]
- 11.Davey, H. M., A. Jones, A. D. Shaw, and D. B. Kell. 1999. Variable selection and multivariable methods for the identification of microorganisms by flow cytometry. Cytometry 35:162-168. [DOI] [PubMed] [Google Scholar]
- 12.Faine, S. 1982. Guidelines for the control of leptospirosis. World Health Organization, Geneva, Switerland.
- 13.Faine, S., B. Adler, C. Bolin, and P. Perolat. 1999. Leptospira and leptospirosis. MediSci, Melbourne, Australia.
- 14.Galton, M. M., D. K. Powers, A. M. Hall, and R. G. Cornell. 1958. A rapid microscopic-slide screening test for the serodiagnosis of leptospirosis. Am. J. Vet. Res. 19:505-512. [PubMed] [Google Scholar]
- 15.Kawaoka, Y., M. Naiki, and R. Yanagawa. 1979. Radioimmunoassay system using a serovar-specific lipopolysaccharide antigen of Leptospira. J. Clin. Microbiol. 10:313-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kmety, E., and H. Dikken. 1993. Classification of the species Leptospira interrogans and history of its serovars. University Press Groningen, Groningen, The Netherlands.
- 17.Koch, A. L., B. R. Robertson, and D. K. Button. 1996. Deduction of the cell volume and mass from forward scatter intensity of bacteria analyzed by flow cytometry. J. Microbiol. Methods 27:49-61. [Google Scholar]
- 18.Levett, P. N. 2001. Leptospirosis. Clin. Microbiol. Rev. 14:296-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levett, P. N. 2003. Usefulness of serologic analysis as a predictor of the infecting serovar in patients with severe leptospirosis. Clin. Infect. Dis. 36:447-452. [DOI] [PubMed] [Google Scholar]
- 20.Levett, P. N., and C. U. Whittington. 1998. Evaluation of the indirect hemagglutination assay for diagnosis of acute leptospirosis. J. Clin. Microbiol. 36:11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayers, D. M. 1985. Manual of laboratory methods for the diagnosis of leptospirosis. Technical note no. 30. Pan American Zoonoses Center, World Health Organization, Geneva Switzerland.
- 22.Mayers, D. M. 1987. Serodiagnosis of human leptospirosis by counterimmunoelectrophoresis. J. Clin. Microbiol. 25:897-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nebe von Caron, G., P. Stephens, and A. R. Badley. 1999. Bacterial detection and differentiation by cytometry and fluorescent probes. Proc. R. Microsc. Soc. 34:321-327. [Google Scholar]
- 24.Phillips, A. P., and K. L. Martin. 1988. Limitation of flow cytometry for the specific detection of bacteria in mixed populations. J. Immunol. Methods 106:109-117. [DOI] [PubMed] [Google Scholar]
- 25.Pinder, A. C., and R. G. McClelland. 1994. Rapid assay for pathogenic Salmonella organisms by immunofluorescence flow cytometry. J. Microsc. 176:17-22. [DOI] [PubMed] [Google Scholar]
- 26.Shapiro, H. M. 1985. Practical flow cytometry. Alan R. Liss, Inc., New York, N.Y.
- 27.Smits, H. L., Y. V. Ananyina, A. Chereshsky, L. Dancel, A. F. R. F. Lai, H. D. Chee, P. N. Levett, T. Masuzawa, Y. Yanagihara, M. A. Muthusethupathi, E. J. Sanders, D. M. Sasaki, H. Domen, C. Yersin, T. Aye, S. L. Bragg, G. C. Gussenhoven, M. G. Goris, W. J. Terpstra, and R. A. Hartskeerl. 1999. International multicenter evaluation of the clinical utility of a dipstick assay for detection of Leptospira-specific immunoglobulin M antibodies in human serum specimens. J. Clin. Microbiol. 37:2904-2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stopa, P. J. 2000. The flow cytometry of Bacillus anthracis spores revisited. Cytometry 41:237-244. [DOI] [PubMed] [Google Scholar]
- 29.Terpstra, W. J., G. S. Ligthart, and G. J. Schoone. 1985. ELISA for the detection of specific IgM and IgG in human leptospirosis. J. Genet. Microbiol. 131:377-385. [DOI] [PubMed] [Google Scholar]
- 30.Torten, M., E. Shenberg, and J. van der Hooden. 1966. The use of immunofluorescence in the diagnosis of human leptospirosis by genus-specific antigen. J. Infect. Dis. 116:537-543. [DOI] [PubMed] [Google Scholar]
- 31.Turner, L. H. 1968. Leptospirosis. II. Serology. Trans. R. Soc. Trop. Med. Hyg. 62:880-889. [DOI] [PubMed] [Google Scholar]
- 32.Vorauer-Uhl, K., A. Wagner, N. Borth, and H. Katinger. 2000. Determination of liposome size distribution by flow cytometry. Cytometry 39:166-171. [PubMed] [Google Scholar]
- 33.Wolf, J. W. 1954. The laboratory diagnosis of leptospirosis. Charles C Thomas, Publisher, Springfield, Ill.
- 34.World Health Organization. 1999. Leptospirosis worldwide. Wkly. Epidemiol. Rec. 74:237-242. [PubMed] [Google Scholar]
- 35.Zahavy, E., M. Fisher, A. Bromberg, and U. Olshevsky. 2003. Detection of frequency resonance energy transfer pair on double-labeled microsphere and Bacillus anthracis spores by flow cytometry. Appl. Environ. Microbiol. 69:2330-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]




