Abstract
Blood testis barrier (BTB) is one of the tightest blood-barriers controlling the entry of substances into the intratubular fluid. Diabetes Mellitus (DM) is an epidemic metabolic disease concurrent with falling fertility rates, which provokes severe detrimental BTB alterations. It induces testicular alterations, disrupting the metabolic cooperation between the cellular constituents of BTB, with dramatic consequences on sperm quality and fertility. As Sertoli cells are involved in the regulation of spermatogenesis, providing nutritional support for germ cells, any metabolic alteration in these cells derived from DM may be responsible for spermatogenesis disruption, playing a crucial role in fertility/subfertility associated with this pathology. These cells have a glucose sensing machinery that reacts to hormonal fluctuations and several mechanisms to counteract hyper/hypoglycemic events. The role of DM on Sertoli/BTB glucose metabolism dynamics and the metabolic molecular mechanisms through which DM and insulin deregulation alter its functioning, affecting male reproductive potential will be discussed.
Keywords: blood-testis barrier, diabetes mellitus, Sertoli cells, glucose metabolism, male fertility
Introduction
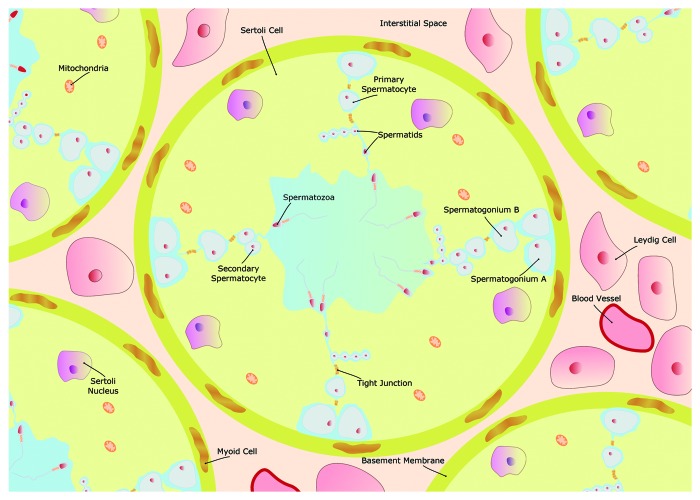
Spermatogenesis is a complex event that is dependent on blood-to-germ cells transport of glucose and other metabolic intermediates. However, this blood-to-germ cells communication is hindered by the presence of junctions that control the substances movement between these adjacent but metabolically separate compartments. This barrier is known as blood-testis barrier (BTB, also called Sertoli cell barrier)1,2 and, as it happens in other blood tissue barriers of the body, the free exchange of substances between the two distinct environments it creates (interstitial and adluminal compartments) is under tight control. In fact, the BTB is one of the tightest blood-tissue barrier3 and physically divides the seminiferous epithelium into basal and apical compartments, where different stages of germ cell development occur (Fig. 1). For instance, the spermatids maturation into mature spermatozoa (spermiogenesis) and the release of mature spermatozoa (spermiation) occur in the apical compartment where post-meiotic germ cells are located. Moreover, BTB suffers a restructuring, to facilitate the transit of primary preleptone spermatocytes while differentiating into leptotene and zygotene spermatocytes, at stages VIII-IX of the seminiferous epithelial cycle.4 Sertoli cells (SCs), the main component of BTB, are polarized epithelial cells that interact with each other through the establishment of tight junctions.5 They can alter their morphology, via adaptations of their apical and lateral portions, during the cycles of the seminiferous epithelium.6 These alterations are poorly understood although it is clear that BTB controls the access of plasma substances to the seminiferous epithelium adluminal compartment, maintaining different levels of substances between tubular luminal fluid and lymph or plasma. BTB alterations can be of diverse nature, including histological and/or biochemical modifications, and be a response to pathological conditions that may affect the main barrier functions: transport and permeability. Besides, the membrane fluidity and the cells surface charges regulate the transport rates across the barrier. Thus, any pathological condition that alters the normal functioning of these processes may compromise the male fertility potential.
Figure 1. Schematic illustration of the seminiferous tubule and the Sertoli/blood testis barrier (BTB). The BTB is a physical barrier between the interstitial space and the seminiferous tubule lumen, formed by Sertoli cells (SCs) and the tight connections between these cells. Outside the BTB is the basal compartment, where spermatogonial renewal occurs, and inside the BTB is the adluminal compartment, where meiosis, spermiogenesis and spermiation take place. Blood vessels and the Leydig cells, which produce testosterone, are located in the interstitial space. Adjacent to the basement membrane are several layers of modified myofibroblastic cells, termed peritubular cells. As a result of such particular organization, the establishment of a functional BTB is essential to create a special environment for the normal development of a fully efficient sperm.
One of the most prevalent chronic diseases in western societies is Diabetes Mellitus (DM). DM is described as a metabolic disorder resulting from defective insulin secretion, resistance to insulin, or both, and is rapidly rising worldwide.7,8 There are several types of DM, but the most important are type 1 diabetes mellitus (T1D) and type 2 diabetes mellitus (T2D). The processes responsible for both types are very distinct: T1D results from an absolute deficiency of insulin due to an autoimmune destruction of the pancreatic β cells, while T2D is characterized by impaired insulin secretion and increased insulin resistance. Both, T1D and T2D, are characterized by a hyperglycemic state that largely contributes to the progression of major vascular and endothelial alterations. Moreover, DM is also responsible for several comorbidities and complications such as dyslipidemia, hyperinsulinemia and hypoglycemia that cannot be disregarded when studying DM-related effects. As a metabolic disorder, DM induces structural and functional alterations in cells, tissues and organs and BTB is no exception. DM pandemic has grown rapidly not only in the Western World, but also in developing countries. It is predicted that the disease will have epidemic proportions within the next decades.9 Because of the increasing number of individuals developing DM at an early age, it is expected that they will experience more years of disease burden and a higher probability of developing serious DM-related complications. These complications will threaten life expectancy and reduce quality of life. Moreover, the individuals may have lower productivity during the prime years of their lives.10 Importantly, the increase incidence of DM has been associated with failing birth rates and fertility.11,12 Although the exact mechanisms by which DM affects male reproductive health remain obscure, this pathology has a significant impact on male reproductive function at multiple levels (as a result of dysfunctional spermatogenesis per se or on its endocrine control, or by impairing penile erection and ejaculation).13-15 Therefore, male infertility may become more widespread as DM rates rise.
Functional, Structural and Metabolic Organization of the Sertoli/Blood-Testis Barrier
In mammals, the BTB is a testis-specific structure composed by the Sertoli cell body and tight junctions, basal ectoplasmic specialization, basal tubulobulbar complex, desmosome-like junctions and gap junctions present alongside in the seminiferous epithelium. The BTB physically divides the seminiferous epithelium into basal and apical (or adluminal) compartments (Fig. 1). One of the most important components of BTB is the SC that is also one of the most important testicular cell types, as SCs number determines the testis size.16 Tight junctions are formed between the adjacent SCs, so that the passage of substances is under strict control and nothing larger than 1 KDa passes from the basal to the adluminal side of the seminiferous tubule.17 These tight junctions between SCs are crucial to define the apical and basal spaces in the seminiferous tubules. Among the several proteins involved in these processes (for an extensive review, see refs. 2,18–20), occludin and claudins play a crucial role being responsible for the functionality and dynamics of these BTB tight junctions.21-24 Its presence and regulation contribute to BTB functionality and is essential for BTB stability and selective passage of substances through the germinal epithelium.22,25,26 In sum, apart from the existence of an interstitial compartment containing interstitial cells, such as Leydig cells and testicular macrophages, and endothelial cells that serve as a first selective place to defend the testis against exogenous pathogens and environmental toxicants, such as heavy metals and xenobiotics (for a review, see refs. 27 and 28), the establishment of the BTB contributes to the creation of two distinct environments: (1) the basal compartment consisting of a tunica propria with peritubular cells and basement membrane and germ cells in the first step of spermatogenesis and (2) the apical (or luminal) compartment, containing mature spermatocytes and spermatids.
The SCs are the main testicular cells present and responsible for the establishment of the BTB. They are responsible for water transport from the interstitial space into the lumen. This is a critical function of SCs as this fluid movements serves as vehicle for moving sperm from the testis to the epididymis.29 Moreover, SCs also control seminiferous epithelium fluid pH30,31 by secreting an iso-osmotic fluid through several membrane transporters.32 Their structure is very complex, with several cupshaped protrusions surrounding the various germ cell types that are distributed within the seminiferous epithelium.33 Each SC is in contact with multiple germ cells and intimate associations are established. There are two types of Sertoli-germ cell archoring junctions: desmosome-like junctions and ectoplasmic specializations (ESs) and both archoring junction types are known to mediate stable adhesion throughout spermatogenesis (for an extensive review, see ref. 34). This unique association between SCs and germ cells is the basis of the seminiferous epithelium cycle and each particular association is referred as a stage. The number of spermatogenesis stages in a particular mammalian species is defined by the number of morphologically recognizable associations established between SCs and germ cells within the testis.35 The fully differentiated SCs are often described as “nurse cells” because they are responsible for the physical and nutritional support of germ cell. They exhibit a well-organized cytoskeleton and deposit extracellular matrix components such as collagen and laminin.36 SCs also secrete anti-müllerian hormone (AMH), the c kit ligand and inhibin, among other specific products that are necessary for germ cell development and survival, in addition to secreting a series of peptides, nutrients and several metabolic intermediates.37,38 The metabolic cooperation between SC and developing germ cells involves the transference of various metabolic products, such as amino acids, carbohydrates, lipids, vitamins and metal ions.36 In fact, the close relationship between these somatic and germinative cell types is imperative for developing germ cells to receive an adequate level of energy substrates.39-42 Among the several factors and metabolic substances secreted by SCs, lactate plays a crucial role in the development of germ cells and therefore in the spermatogenic process.43
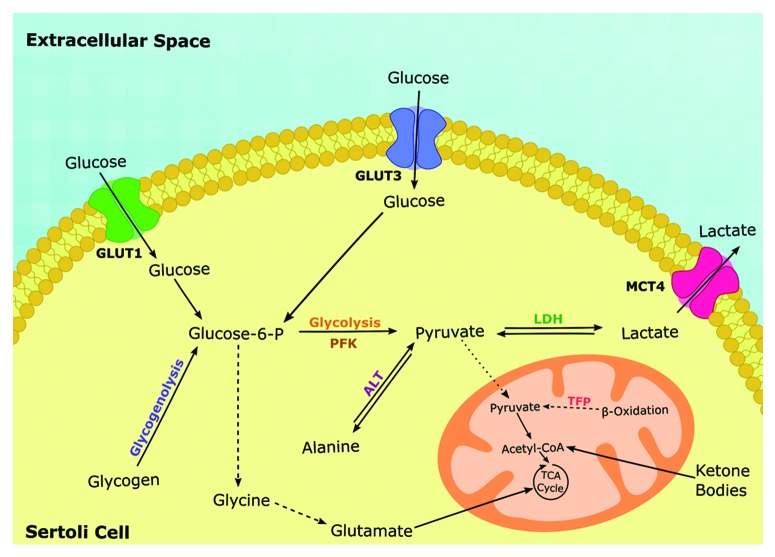
Like in other blood-tissue barriers, the glucose transport through the BTB is under strict control. Furthermore, the glucose metabolism in BTB has some unique characteristics that proven to be essential for a normal spermatogenesis. Glucose must cross the BTB and be metabolized or delivered to the several intra-barrier testicular cells and in the seminiferous epithelium. As discussed above, the SCs have functions that go far beyond the physical support of germ cells. They are responsible for lactate production from extracellular glucose, that is then exported to be metabolized by the developing germ cells.44 During this process, glucose has to permeate BTB through an energy independent process. This is achieved via facilitated diffusion mediated by glucose transporters (GLUTs) and is dependent of the GLUTs redistribution in plasma membrane and of GLUTs total levels.45 It has been reported that in SCs, glucose transporter 1 (GLUT1) and glucose transporter 3 (GLUT3) play a synergistic role in maintaining glucose uptake to assure lactate production.46-48 Recently, it has also been reported that glucose uptake and lactate production by SCs are under hormonal regulation.40,48-50 Therefore, the metabolic cooperation between testicular cells and the selective passage of metabolic substrates through BTB is exerted by several distinct and specialized mechanisms. The Sertoli cell barrier or BTB has a specific glucose sensing machinery that is under strict hormonal control, especially by sex hormones and FSH. These hormones receptors are located in SCs and are very sensitive to extracellular glucose levels (Fig. 2).51-54
Figure 2. Schematic illustration of Sertoli cells (SCs) main metabolic pathways. The SCs are capable of consuming a variety fuels including glucose, lactate, fatty acids and aminoacids. Nevertheless, SCs actively metabolize glucose being the majority of it converted in lactate and not oxidized in the TCA cycle. The extracellular lactate and pyruvate are transported via the members of the family of proton-linked plasma membrane transporters that carry molecules having one carboxylate group, the monocarboxylate transporters (MCT4), while glucose is imported via specific members of the family of membrane proteins called glucose transporters (GLUT1 and GLUT3). Once glucose enters the glycolytic pathway, it is decomposed to pyruvate which can (a) be converted into lactate via lactate dehydrogenase, (b) be converted into alanine via alanine transaminase or (c) be transported to the mitochondrial matrix, oxidized and decarboxylated by the pyruvate dehydrogenase forming the two carbon intermediate Acetyl-CoA which can enter the TCA cycle. The oxidation of these substrates is coupled with ADP phosphorylation via the electron transport chain to form ATP. Abbreviations: TCA, tricarboxylic acid; GLUT, glucose transporter; MCT, monocarboxylate transporter; ALT, alanine transaminase; LDH, lactate dehydrogenase; PFK, phosphofructokinase; TFP, trifunctional protein.
The structural organization of the BTB is very complex and therefore has a great influence in the functional status of the overall testicular metabolism. Future studies are needed to explore the mechanisms by which BTB disruption can alter the metabolic cooperation between the different testicular cell types, namely SCs and developing germ cells since this process is dependent of BTB maintenance and organization. Several substances and pathological conditions, such as DM, are known to alter BTB permeability, potentially compromising spermatogenesis.
Glucose and Lactate Transporters in Sertoli/Blood Testis Barrier
As discussed, the male reproductive health is highly dependent of glucose uptake and metabolization by testicular cells. Glucides are polar molecules. Although they can cross the lipidic bilayers by simple diffusion, they do it in a very inefficient manner. Therefore the cells take up glucose through carriers. There are two families of glucose transporters: the Sodium Dependent Glucose Transporters (SGLTs), also known as Solute Carrier Family 5 (SLC5) and the GLUTs, also known as Solute Carrier Family 2 (SLC2).55-57 These families are composed with a different number of transporters. The SGLTs family is composed by six different active transporters (SGLT1 to 6) while the GLUTs family is composed by 14 glucose transporter isoforms (GLUT1 to 14).57 GLUTs family can be divided into three subfamilies, namely class I (GLUT1-4), class II (GLUT5, 7, 9, 11) and class III (GLUT6, 8, 10, 12 and H+-coupled myo-inositol transporter). Several GLUTs are expressed in testicular cells and in spermatozoa (for extensive review see55). Positive immunoreaction for GLUT1, GLUT2 and GLUT3 was found in several testicular cells, including peritubular myoid cells.58 In SCs, the main component of BTB, it has been described at least four glucose transporters isoforms: GLUT1, GLUT2, GLUT3 and GLUT8 (Table 1).46,47,59 The presence of GLUT4 was also investigated and no positive immunoreaction was not detected in any testicular cell type, inclusive in peritubular myoid cells and SCs.58 Yet not all the glucose transporters identified are expected to contribute in the same manner to glucose transport. For instance, in the hippocampus, GLUT1 and GLUT3 are reported to be the most important in glucose uptake from the extracellular medium, since GLUT8 was not localized in the plasmatic membrane.60,61 Moreover, recent studies with GLUT8 knockout mice suggested that this transporter is mainly involved in the transport and recycling of glucose residues in the lysosomes membranes.62,63 These mechanisms that allow glucose incorporation are of extreme relevance since the tubular fluid has a low level of this sugar and SCs, which are responsible for the maintenance of this fluid homeostasis,32 need glucose and other metabolic intermediates to produce the metabolic substrates required for germ cells development.64 SCs have some particular features of glucose metabolism as they produce lactate at high rates49,50,64,65 that is then used for energy production by the developing germ cells, namely pachytene spermatocytes and round spermatids.43,65,66 The lactate export by SCs occurs through specific monocarboxylate transporters (MCTs). The SLC16A family of MCTs is composed of 14 members but only MCTs 1–4 are linked to lactate transport.67 These MCTs differ in their transport kinetics and their subcellular distribution determines the lactate shuttle across blood-tissues barriers. Although MCTs 1–4 transport monocarboxylates, they differ in substrate binding affinity and selectivity. For instance, MCT1 and MCT2 possess a high affinity for lactate and are expected to mediate the lactate uptake while MCT4 is involved in lactate export.68 Indeed, within the BTB, the SCs mainly express MCT4 to release lactate into the intratubular fluid that is subsequently taken up by differentiating germ cells via MCT1 and MCT2 (Table 1).69-72
Table 1. Expression of the glucose transporters (GLUT1, GLUT2, GLUT3, GLUT4 and GLUT8) and monocarboxylate transporters (MCT1, MCT2 and MCT4) in seminiferous tubular cells.
| Glucose transporters | Monocarboxylate transporters | |||||||
|---|---|---|---|---|---|---|---|---|
| Testicular cells | GLUT1 | GLUT2 | GLUT3 | GLUT4 | GLUT8 | MCT1 | MCT2 | MCT4 |
| Sertoli cells | + 46,47,59,58 | + 58 |
+ 47,58 |
- 58 |
+ 46 |
ND | ND | + 70-72 |
| Germ Cells | + 58 |
+ 58 |
+ 58 |
- 58 |
ND | + 69 |
+ 69 |
ND |
| Peritubular cells | + 58 |
+ 58 |
+ 58 |
- 58 |
ND | ND | ND | ND |
Legend: GLUT1: glucose transporter 1; GLUT2: glucose transporter 2; GLUT3: glucose transporter 3; GLUT4: glucose transporter 4; GLUT8: glucose transporter 8.; MCT1: monocarboxylate transporter 1; MCT2: monocarboxylate transporter 2; MCT4: monocarboxylate transporter 4; +: expression detected; - : expression not detected; ND : not determined; superscript numbers are references as indicated in references section.
The metabolic cooperation established between SCs and the developing germ cells is highly dependent of GLUTs and MCTs functioning. In several pathological conditions, particularly in metabolic-related diseases, they play a crucial role in the development of the deleterious effects.
Diabetes Induces Important Alterations in Testicular Cells that Modulate Glucose Transport in Sertoli/Blood-Testis Barrier
DM is a metabolic disorder that has been associated with several comorbidities and long-term complications that result from the lesions and multiple processes that are originated in several tissues of the organism in response to the disease.73,74 The glucose deregulation is an important characteristic of DM and some of the most relevant side effects associated with this pathology are related with the hyper- and hypoglycemic events that occur, even if transiently, in diabetic individuals.75,76 The microvascular disease promoted by DM is a leading cause of blindness, renal failure, cardiovascular complications, increased atherosclerosis and nerve damage.77-80 Using biopsies from men with DM, it has been reported that testicular capillaries and lymphatic endothelia appeared structurally abnormal due to interstitial “matrix expansion.” Discrete ultrastructural lesions in apical SC cytoplasm were associated with spermatogenic disruption and the described morphological changes in the interstitial compartment suggested microvascular complications.81 It has also been described that testes from STZ-induced diabetic rats showed a reduction in seminiferous tubular diameter, an increase in the number of empty testicular tubules and also an increase in vascular density.82,83 Those authors suggested that the reported increased density on testicular vasculature might lead to increased scrotal temperature. Moreover they suggested that the possible increased temperature may be a cause of the observed germ cells apoptotic death. Furthermore, the increased wall thickness of small blood vessels in diabetics might lead to hypoxia-induced cellular damage.82,83
The effects of DM in human health are well known and have been object of research for several years. Contrarily, the effects of DM in male reproductive health did not caught much attention from scientists for many years. They were not considered a priority mainly because DM, and particularly T2D, was considered a late-onset disease. Therefore the effects of DM on male reproductive health were not considered as immediate or essential subjects of research. Nowadays this view has been challenged, especially because there is an increasing incidence of DM in young adolescents, that is expected to continue to rise, and the increasing number of DM to epidemic proportions is contemporaneous with failing birth.84
Male sexual dysfunctions such as decreased libido and impotence are associated with DM decreasing the male reproductive health and altering the sexual behavior with dramatic consequences to male fertility.85 Another important feature that is very prevalent in diabetic men and might contribute to reproductive failure is erectile dysfunction (ED), although it depends on several factors such as the individual age, the disease duration and also the control of blood glucose levels.86 The ejaculation of diabetic men has also been reported to be affected. In some diabetic men the semen passes backward into the bladder, a condition known as retrograde ejaculation.87,88 In patients suffering from diabetic autonomic neuropathy, the function of the urethral sphincter is often affected due to loss of innervation, which may be a cause for the reported retrograde ejaculation observed in those individuals.89 Additionally, DM can cause male subfertility or infertility by disrupting some important mechanisms, inducing abnormal sperm production and/or failure of the reproductive function.90-92 Although the effects of DM in male reproductive health is a matter of some controversy, several studies reported that sperm parameters, such as semen volume, sperm count, motility and morphology, are also altered in diabetic adolescents and men.93,94 Interestingly, the semen of diabetic men are reported to present higher fructose and glucose levels94 although the exact mechanisms of glucose transport through which it happens remain largely unknown.
The effects on male reproductive health are more pronounced when DM animal models are used. In rats it has been reported an increase in the number of damaged seminiferous tubules (e.g., increased seminiferous tubule wall thickness and SCs vacuolization) in the early stages of the disease. Moreover, it has been reported that rats with DM present a decrease in the gonadosomatic index, sperm concentration and motility, as well as decreased levels of serum testosterone and abnormal spermatogenesis.95-97 The endocrine deregulation caused by DM is well known. Although the absolute values for hormonal fluctuations may differ between studies, it is clear that DM induces a marked reduction in plasma testosterone levels, reaching values decreased more than one half. Moreover, a significant decrease in luteinizing hormone (LH) and follicle-stimulating hormone (FSH) was described in the plasma of diabetic individuals.98-101 Interestingly, insulin injections can restore FSH to normal levels98 while the synergistic treatment of insulin with testosterone can restore some alterations in the body and reproductive organ weights, as well as in sperm counts and motility.100 Besides these alterations in the endocrine system, testes of diabetic rats presented a seminiferous epithelium with abnormal histology and altered histo-architecture, as well as altered distribution of occludin, a key protein of the BTB tight junctions, which appeared not well organized or totally absent in portions of the seminiferous tubules.102 Evidences of BTB disruption in diabetic individuals were also described in human biopsies from diabetic individuals, where several morphological changes were detected in testicular cells. Those works reported an increased thickness of seminiferous tubule wall, germ cell depletion and SCs vacuolization and degeneration.103,104 Although none of these works studied the molecular mechanisms of glucose transport and metabolism, these morphological alterations will be detrimental to SCs functioning. It is expected that blood-to-germ cells substrate are altered, as SCs express several membrane transporters (e.g., GLUTs and MCTs) that are crucial for the transport of substances (e.g glucose) from the interstitial space (which is in close contact with the systemic circulation, since molecules can freely flow out of the blood stream into this space) into the cell and from the cellular cytoplasm to the adluminal compartment (e.g., lactate). The tight junctions between SCs prevent these molecules from entering the adluminal compartment directly from the interstitial space, while the transporters allow the “cellular passage” of the molecules. Severe alterations in the SCs functioning and morphology will affect the delivery of those molecules to the developing germ cells. Unfortunately, there is a lack of studies focused on glucose transport and metabolism in BTB of diabetic individuals or individuals subjected to diabetic conditions. Nevertheless one must note that, for instance, sex hormones are known as metabolic modulators of SCs metabolism49,50 and in diabetic individuals there is a severe deregulation of sex hormones levels. Hormonal deregulation induces important alterations in spermatogenesis, as the hormonal control of SCs regulates spermatogenesis.50,54 Indeed, sex hormones control lactate production by modulating lactate dehydrogenase A (LDHA), monocarboxylate transporter 4 (MCT4) and also GLUTs levels of both rat and human SCs.49,50 This tight and crucial control is altered by diseases that can promote sex hormone fluctuations, such as DM,15,105 with dramatic consequences to male fertility.
The mechanisms by which DM alters the metabolic cooperation in testis remain obscure, but there are clear evidences that blood-to-germ cells metabolites transport is altered and thus, spermatogenesis may become impaired. Although there are known morphological and functional alterations in BTB of diabetic male individuals, the exact meaning of those changes in carbohydrates metabolism is still unclear. This is a subject that should deserve special attention in the next few years since spermatogenesis depends of a correct metabolic cooperation between testicular cells.
Insulin (De)Regulation and Glucose Transport in the Sertoli/Blood-Testis Barrier
The management of blood glycaemia in individuals with DM is crucial and it may minimize the development of diabetes-related complications, although it does not entirely eliminate all the detrimental effects associated to this pathology.106,107 The extent of glycemic control is dependent of several factors such as the DM type, the severity and stage of the disease progression among others.108 Euglycemia is very difficult to attain and maintain with insulin therapy, insulin analogs and sensitizers (e.g., thiazolidinediones), insulin secretagogues (e.g., sulfonylureas) or with other antidiabetic drugs, such as α-glucosidase inhibitors.109,110 Additionally, diabetic individuals, especially those with T2D, become progressively more insulin-deficient throughout the years, becoming more vulnerable to the problems that arise from a poor glycemic control.111,112 On the other hand, diabetic individuals with intense insulin therapy gradually lose their sensitiveness to small variations in plasma glucose concentration and, as result, both hyperinsulinemia and hypoinsulinemia occur.113,114 The periods of hypoglycemia/hyperinsulinemia that occur in both T1D and T2D115,116 are responsible for several problems and compromise the physiological defenses against falling glucose plasma concentration.117,118 These events are crucial in diabetic individuals and although they are well studied for instance in liver and brain, their effect in BTB has been somewhat disregarded. There are only a few studies focused on insulin signaling and insulin regulation of BTB functioning. Within this barrier, SCs metabolic function in vitro is highly dependent on glucose53,119 and insulin concentration.120 Specific insulin receptors have been identified in these cells121 and it has been reported that insulin increases the rate of lactate production by SCs.120 More recently, we have studied the effect of insulin deprivation in cultured SCs to elucidate some of the metabolic mechanisms that are modulated by insulin.48,122 Insulin-deprived SCs presented unique alterations in glucose and acetate metabolism. In the first hours of insulin deprivation, glucose consumption was significantly decreased but after 48 h the insulin-deprived cells presented similar glucose consumption as cells cultured in the presence of insulin. Noteworthy, this identical glucose consumption and consequently lactate production was accompanied by a modulation in GLUTs levels, evidencing that SCs are capable of altering GLUT1 and GLUT3 levels under insulin-deprivation conditions. This may be a crucial mechanism to maintain lactate production, one of the most important SCs functions, within a physiological range in order to ensure adequate concentrations of this metabolite in the microenvironment where germ cell development occurs. The insulin deprivation conditions also downregulated lactate metabolism-associated gene transcript levels, such as LDHA, which is the most predominantly expressed LDH isoform in testis123 and is responsible for the conversion of pyruvate to lactate.124 MCT4, which is the main lactate exporter present in SCs was also downregulated.125 Importantly, SCs produce high amounts of acetate, which has a crucial role in the production of sub-products essential for germ cells high rate of lipids synthesis, that is also regulated by insulin.122 Insulin-deprived cells completely suppressed acetate production by downregulating Acetyl-CoA hydrolase gene transcript levels, thus suggesting a Krebs cycle stimulation to ensure SCs survival even while compromising lactate production and consequently germ cells development. All these in vitro adaptive mechanisms need further clarifications, but represent the first steps to elucidate the key mechanisms by which insulin deregulation that usually is faced by diabetic individuals can modulate BTB metabolic functioning and thus influence the male diabetic reproductive potential.
Contribution of Alternative Fuels to Sertoli/BTB Metabolic Cooperation in Diabetic Individuals
As previously discussed, euglycemia is difficult to achieve and maintain in diabetic individuals and as a result they often face hypo- and hypoglycemia episodes resulting from insulin therapy. These hypo- and hyperglycemia episodes are responsible for several deleterious effects such as alterations in proteins and membranes integrity, even if these periods are merely transient.126-128 Moreover, as discussed above, the BTB is responsible for the selective passage of ions, substances and metabolic intermediates to germ cells. DM alters the overall physiological cellular condition and modifies the metabolic environment surrounding the BTB. Indeed, the BTB cells metabolism is altered, particularly under conditions of prolonged glucose deprivation, as SCs need this substrate for lactate production that is then used by developing germ cells. When BTB faces hyperglycemia, alternative fuels are not expected to play any significant role as glucose is fully accessible and remains the main substrate for SCs. On the other hand, when diabetic individuals are subjected to hypoglycemia, even if transiently, the BTB must adapt its metabolism in order to maintain ATP and lactate production. Although the spermatogenesis maintenance in vivo relies mainly on glucose metabolism,129,130 under undesirable conditions, such as those provoked by DM, SCs can use alternative fuels to maintain lactate production. The main alternative fuels to SCs that can maintain spermatogenesis are monocarboxylic acids, fatty acids and ketone bodies42,131 (Fig. 2). It has been reported that β-oxidation pathway is used by SCs to produce ATP, especially by using free fatty acids or the recycling lipids of apoptotic spermatogenic cells and residual bodies that are phagocytized and degraded.132 In fact, lipids metabolism is crucial for spermatogenesis and mice with inactivated genes from lipid metabolism (hormone-sensitive lipase gene) are reported to present a compromised spermatogenesis.133 The metabolic plasticity exhibit by SCs is intriguing since they can also use unconventional fuels such as palmitate and ketone bodies.134 Moreover, SCs can also metabolize some aminoacids such as glutamine, alanine, leucine, glycine and valine.135 Special attention is required when analyzing the contribution of these alternative fuels to spermatogenesis, since BTB is not fully functional at birth and some of the mechanisms that control these fuels metabolism are dependent on the individual’s sexual maturity. For instance, higher oxidation of oleate to CO2 has been only reported for in vitro cultured testicular cells derived from immature animals.136
Although it has not been quantified yet, glycogen stores in the BTB secluded environment should not be overlooked. The presence of glycogen and glycogen phosphorylase activity in SCs was report a long time ago137,138 but since then no studies were done to completely elucidate these mechanisms. Glycogen may have an essential role in diabetic conditions. Glycogen storages use can be a valuable compensatory mechanism for the transient hypoglycemic periods. The functional alterations on BTB that arise in the testis regarding the use of alternative substrates in diabetic conditions should also be considered and fully investigated.
Conclusion
Diabetes Mellitus is a metabolic disease associated with subfertility and/or infertility. Nevertheless, not all diabetic men are infertile and there are conflicting results in the literature concerning the real impact of DM in the male reproductive health. The exact role for BTB in glucose dynamics during DM is also unknown, although the BTB plays a crucial role in the maintenance of spermatogenesis that is expected to be compromised by DM. This metabolic disease induces several alterations in the male reproductive tract but there is an urgent need for the elucidation of how individual components of BTB suffer the effect of DM, since these alterations may lead to an increased permeability that may end-up in infertility. Moreover, DM is associated with several comorbidities and thus it is very difficult to isolate the effects of each one when studying the mechanisms related with glucose dynamics through the BTB. SCs, the main cellular components of the BTB, are very sensitive to insulin fluctuations and DM is often associated with either hyper- or hypoinsulinemia. Therefore, the basic molecular mechanisms of glucose and insulin deregulation in SCs metabolism should deserve special attention, as they remain to be disclosed. There are several studies focused on the reproductive alterations of diabetic men or male animals, but the morphological integrity of BTB in diabetic males is still an intriguing issue. Interestingly, some studies also suggest a role for alternative substrates in SCs metabolism during diabetic conditions. The use of other metabolic substrates alternatively to glucose also induces important alterations in BTB permeability and alters the testicular physiology.
In conclusion, from a clinical perspective, the study of the sperm parameters and DNA integrity of diabetic individuals offers a more direct outcome of the disease, as they have a crucial importance for natural and assisted reproduction. However, BTB physiology is essential in the maintenance of the metabolic cooperation between testicular cells and should deserve special attention from researchers in order to identify possible mechanisms by which BTB is compromised in diabetic conditions and point toward possible sites for therapeutic intervention. In the next years this will certainly be a hot topic for those who are interested in DM and male reproductive health.
Acknowledgments
This work was supported by the Portuguese “Fundação para a Ciência e a Tecnologia”-FCT (PTDC/QUI-BIQ/121446/2010 and PEst-C/SAU/UI0709/2011) co-funded by Fundo Europeu de Desenvolvimento Regional-FEDER via Programa Operacional Factores de Competitividade-COMPETE/QREN. M.G.A. (SFRH/BPD/80451/2011) was financed by FCT. P.F.O. was financed by FCT through FSE and POPH funds (Programa Ciência 2008).
Glossary
Abbreviation:
- BTB
blood-testis barrier
- DHT
5α-dihydrotestosterone
- DM
diabetes mellitus
- E2
17β-estradiol
- ED
erectile dysfunction
- FSH
follicle-stimulating hormone
- GLUTs
glucose transporters
- GLUT1
glucose transporter 1
- GLUT3
glucose transporter 3
- GLUT8
glucose transporter 8
- LDHA
lactate dehydrogenase A
- LH
luteinizing hormone
- MCT4
monocarboxylate transporter 4
- SCs
Sertoli cells
- T1D
Type 1 diabetes mellitus
- T2D
Type 2 diabetes mellitus
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/tissuebarriers/article/23992
References
- 1.Mruk DD, Cheng CY. In search of suitable in vitro models to study germ cell movement across the blood-testis barrier. Spermatogenesis. 2012;2:6–10. doi: 10.4161/spmg.19878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mruk DD, Cheng CY. Tight junctions in the testis: new perspectives. Philos Trans R Soc Lond B Biol Sci. 2010;365:1621–35. doi: 10.1098/rstb.2010.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Setchell BP, Voglmayr JK, Waites GM. A blood-testis barrier restricting passage from blood into rete testis fluid but not into lymph. J Physiol. 1969;200:73–85. doi: 10.1113/jphysiol.1969.sp008682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russell L. Movement of spermatocytes from the basal to the adluminal compartment of the rat testis. Am J Anat. 1977;148:313–28. doi: 10.1002/aja.1001480303. [DOI] [PubMed] [Google Scholar]
- 5.Mruk DD, Cheng CY. Cell-cell interactions at the ectoplasmic specialization in the testis. Trends Endocrinol Metab. 2004;15:439–47. doi: 10.1016/j.tem.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Wang F, Zhang Q, Cao J, Huang Q, Zhu X. The microtubule plus end-binding protein EB1 is involved in Sertoli cell plasticity in testicular seminiferous tubules. Exp Cell Res. 2008;314:213–26. doi: 10.1016/j.yexcr.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 7.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–21. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 9.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–21. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 10.Day C. The rising tide of type 2 diabetes. Br J Diabetes Vasc Dis. 2001;1:37. doi: 10.1177/14746514010010010601. [DOI] [Google Scholar]
- 11.Hamilton BE, Ventura SJ. Fertility and abortion rates in the United States, 1960-2002. Int J Androl. 2006;29:34–45. doi: 10.1111/j.1365-2605.2005.00638.x. [DOI] [PubMed] [Google Scholar]
- 12.Lutz W. Fertility rates and future population trends: will Europe’s birth rate recover or continue to decline? Int J Androl. 2006;29:25–33. doi: 10.1111/j.1365-2605.2005.00639.x. [DOI] [PubMed] [Google Scholar]
- 13.Bener A, Al-Ansari AA, Zirie M, Al-Hamaq AO. Is male fertility associated with type 2 diabetes mellitus? Int Urol Nephrol. 2009;41:777–84. doi: 10.1007/s11255-009-9565-6. [DOI] [PubMed] [Google Scholar]
- 14.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–7. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 15.Grossmann M. Low testosterone in men with type 2 diabetes: significance and treatment. J Clin Endocrinol Metab. 2011;96:2341–53. doi: 10.1210/jc.2011-0118. [DOI] [PubMed] [Google Scholar]
- 16.Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 2003;125:769–84. doi: 10.1530/rep.0.1250769. [DOI] [PubMed] [Google Scholar]
- 17.Walker WH, Cheng J. FSH and testosterone signaling in Sertoli cells. Reproduction. 2005;130:15–28. doi: 10.1530/rep.1.00358. [DOI] [PubMed] [Google Scholar]
- 18.Weider K, Bergmann M, Brehm R. Connexin 43: its regulatory role in testicular junction dynamics and spermatogenesis. Histol Histopathol. 2011;26:1343–52. doi: 10.14670/HH-26.1343. [DOI] [PubMed] [Google Scholar]
- 19.Lee NP, Yeung WS, Luk JM. Junction interaction in the seminiferous epithelium: regulatory roles of connexin-based gap junction. Front Biosci. 2007;12:1552–62. doi: 10.2741/2168. [DOI] [PubMed] [Google Scholar]
- 20.Lui WY, Mruk D, Lee WM, Cheng CY. Sertoli cell tight junction dynamics: their regulation during spermatogenesis. Biol Reprod. 2003;68:1087–97. doi: 10.1095/biolreprod.102.010371. [DOI] [PubMed] [Google Scholar]
- 21.Morita K, Sasaki H, Fujimoto K, Furuse M, Tsukita S. Claudin-11/OSP-based tight junctions of myelin sheaths in brain and Sertoli cells in testis. J Cell Biol. 1999;145:579–88. doi: 10.1083/jcb.145.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gow A, Southwood CM, Li JS, Pariali M, Riordan GP, Brodie SE, et al. CNS myelin and sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell. 1999;99:649–59. doi: 10.1016/S0092-8674(00)81553-6. [DOI] [PubMed] [Google Scholar]
- 23.Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev. 2002;82:825–74. doi: 10.1152/physrev.00009.2002. [DOI] [PubMed] [Google Scholar]
- 24.Chung NPY, Mruk D, Mo MY, Lee WM, Cheng CY. A 22-amino acid synthetic peptide corresponding to the second extracellular loop of rat occludin perturbs the blood-testis barrier and disrupts spermatogenesis reversibly in vivo. Biol Reprod. 2001;65:1340–51. doi: 10.1095/biolreprod65.5.1340. [DOI] [PubMed] [Google Scholar]
- 25.Koval M. Claudins--key pieces in the tight junction puzzle. Cell Commun Adhes. 2006;13:127–38. doi: 10.1080/15419060600726209. [DOI] [PubMed] [Google Scholar]
- 26.Kaitu’u-Lino TJ, Sluka P, Foo CF, Stanton PG. Claudin-11 expression and localisation is regulated by androgens in rat Sertoli cells in vitro. Reproduction. 2007;133:1169–79. doi: 10.1530/REP-06-0385. [DOI] [PubMed] [Google Scholar]
- 27.Mruk DD, Su L, Cheng CY. Emerging role for drug transporters at the blood-testis barrier. Trends Pharmacol Sci. 2011;32:99–106. doi: 10.1016/j.tips.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su L, Mruk DD, Cheng CY. Drug transporters, the blood-testis barrier, and spermatogenesis. J Endocrinol. 2011;208:207–23. doi: 10.1677/JOE-10-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Setchell BP, Scott TW, Voglmayr JK, Waites GM. Characteristics of testicular spermatozoa and the fluid which transports them into the epididymis. Biol Reprod. 1969;1(Suppl 1):1–, 40-66. doi: 10.1095/biolreprod1.Supplement_1.40. [DOI] [PubMed] [Google Scholar]
- 30.Oliveira PF, Sousa M, Barros A, Moura T, Rebelo da Costa A. Intracellular pH regulation in human Sertoli cells: role of membrane transporters. Reproduction. 2009;137:353–9. doi: 10.1530/REP-08-0363. [DOI] [PubMed] [Google Scholar]
- 31.Oliveira PF, Sousa M, Barros A, Moura T, Rebelo da Costa A. Membrane transporters and cytoplasmatic pH regulation on bovine Sertoli cells. J Membr Biol. 2009;227:49–55. doi: 10.1007/s00232-008-9139-z. [DOI] [PubMed] [Google Scholar]
- 32.Rato L, Socorro S, Cavaco JE, Oliveira PF. Tubular fluid secretion in the seminiferous epithelium: ion transporters and aquaporins in Sertoli cells. J Membr Biol. 2010;236:215–24. doi: 10.1007/s00232-010-9294-x. [DOI] [PubMed] [Google Scholar]
- 33.Leblond CP, Clermont Y. Spermiogenesis of rat, mouse, hamster and guinea pig as revealed by the periodic acid-fuchsin sulfurous acid technique. Am J Anat. 1952;90:167–215. doi: 10.1002/aja.1000900202. [DOI] [PubMed] [Google Scholar]
- 34.Kopera IA, Bilinska B, Cheng CY, Mruk DD. Sertoli-germ cell junctions in the testis: a review of recent data. Philos Trans R Soc Lond B Biol Sci. 2010;365:1593–605. doi: 10.1098/rstb.2009.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hess RA, Renato de Franca L. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol. 2008;636:1–15. doi: 10.1007/978-0-387-09597-4_1. [DOI] [PubMed] [Google Scholar]
- 36.Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- 37.Griswold M. The central role of Sertoli cells in spermatogenesis. London: Academic Press, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Griswold M, McLean D. The Sertoli cell. In: Neill J, ed. Knobil and Neill’s physiology of reproduction. San Diego: Elsevier, 2006:949-75. [Google Scholar]
- 39.Riera MF, Meroni SB, Schteingart HF, Pellizzari EH, Cigorraga SB. Regulation of lactate production and glucose transport as well as of glucose transporter 1 and lactate dehydrogenase A mRNA levels by basic fibroblast growth factor in rat Sertoli cells. J Endocrinol. 2002;173:335–43. doi: 10.1677/joe.0.1730335. [DOI] [PubMed] [Google Scholar]
- 40.Riera MF, Meroni SB, Gómez GE, Schteingart HF, Pellizzari EH, Cigorraga SB. Regulation of lactate production by FSH, iL1beta, and TNFalpha in rat Sertoli cells. Gen Comp Endocrinol. 2001;122:88–97. doi: 10.1006/gcen.2001.7619. [DOI] [PubMed] [Google Scholar]
- 41.Erkkilä K, Aito H, Aalto K, Pentikäinen V, Dunkel L. Lactate inhibits germ cell apoptosis in the human testis. Mol Hum Reprod. 2002;8:109–17. doi: 10.1093/molehr/8.2.109. [DOI] [PubMed] [Google Scholar]
- 42.Rato L, Alves MG, Socorro S, Duarte AI, Cavaco JE, Oliveira PF. Metabolic regulation is important for spermatogenesis. Nat Rev Urol. 2012;9:330–8. doi: 10.1038/nrurol.2012.77. [DOI] [PubMed] [Google Scholar]
- 43.Mita M, Hall PF. Metabolism of round spermatids from rats: lactate as the preferred substrate. Biol Reprod. 1982;26:445–55. doi: 10.1095/biolreprod26.3.445. [DOI] [PubMed] [Google Scholar]
- 44.Jutte NH, Jansen R, Grootegoed JA, Rommerts FF, Clausen OP, van der Molen HJ. Regulation of survival of rat pachytene spermatocytes by lactate supply from Sertoli cells. J Reprod Fertil. 1982;65:431–8. doi: 10.1530/jrf.0.0650431. [DOI] [PubMed] [Google Scholar]
- 45.Klip A, Tsakiridis T, Marette A, Ortiz PA. Regulation of expression of glucose transporters by glucose: a review of studies in vivo and in cell cultures. FASEB J. 1994;8:43–53. doi: 10.1096/fasebj.8.1.8299889. [DOI] [PubMed] [Google Scholar]
- 46.Carosa E, Radico C, Giansante N, Rossi S, D’Adamo F, Di Stasi SM, et al. Ontogenetic profile and thyroid hormone regulation of type-1 and type-8 glucose transporters in rat Sertoli cells. Int J Androl. 2005;28:99–106. doi: 10.1111/j.1365-2605.2005.00516.x. [DOI] [PubMed] [Google Scholar]
- 47.Galardo MN, Riera MF, Pellizzari EH, Chemes HE, Venara MC, Cigorraga SB, et al. Regulation of expression of Sertoli cell glucose transporters 1 and 3 by FSH, IL1 beta, and bFGF at two different time-points in pubertal development. Cell Tissue Res. 2008;334:295–304. doi: 10.1007/s00441-008-0656-y. [DOI] [PubMed] [Google Scholar]
- 48.Oliveira PF, Alves MG, Rato L, Laurentino S, Silva J, Sá R, et al. Effect of insulin deprivation on metabolism and metabolism-associated gene transcript levels of in vitro cultured human Sertoli cells. Biochim Biophys Acta. 2012;1820:84–9. doi: 10.1016/j.bbagen.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 49.Rato L, Alves MG, Socorro S, Carvalho RA, Cavaco JE, Oliveira PF. Metabolic modulation induced by oestradiol and DHT in immature rat Sertoli cells cultured in vitro. Biosci Rep. 2012;32:61–9. doi: 10.1042/BSR20110030. [DOI] [PubMed] [Google Scholar]
- 50.Oliveira PF, Alves MG, Rato L, Silva J, Sá R, Barros A, et al. Influence of 5α-dihydrotestosterone and 17β-estradiol on human Sertoli cells metabolism. Int J Androl. 2011;34:e612–20. doi: 10.1111/j.1365-2605.2011.01205.x. [DOI] [PubMed] [Google Scholar]
- 51.Lam TK, Gutierrez-Juarez R, Pocai A, Rossetti L. Regulation of blood glucose by hypothalamic pyruvate metabolism. Science. 2005;309:943–7. doi: 10.1126/science.1112085. [DOI] [PubMed] [Google Scholar]
- 52.Hall PF, Mita M. Influence of follicle-stimulating hormone on glucose transport by cultured Sertoli cells. Biol Reprod. 1984;31:863–9. doi: 10.1095/biolreprod31.5.863. [DOI] [PubMed] [Google Scholar]
- 53.Riera MF, Galardo MN, Pellizzari EH, Meroni SB, Cigorraga SB. Molecular mechanisms involved in Sertoli cell adaptation to glucose deprivation. Am J Physiol Endocrinol Metab. 2009;297:E907–14. doi: 10.1152/ajpendo.00235.2009. [DOI] [PubMed] [Google Scholar]
- 54.Alves MG, Rato L, Carvalho RA, Moreira PI, Socorro S, Oliveira PF. Hormonal control of Sertoli cells metabolism regulates spermatogenesis. Cell Mol Life Sci. 2013;70:777–93. doi: 10.1007/s00018-012-1079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bucci D, Rodriguez-Gil JE, Vallorani C, Spinaci M, Galeati G, Tamanini C. GLUTs and mammalian sperm metabolism. J Androl. 2011;32:348–55. doi: 10.2164/jandrol.110.011197. [DOI] [PubMed] [Google Scholar]
- 56.Joost HG, Thorens B. The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members (review) Mol Membr Biol. 2001;18:247–56. doi: 10.1080/09687680110090456. [review] [DOI] [PubMed] [Google Scholar]
- 57.Scheepers A, Joost HG, Schürmann A. The glucose transporter families SGLT and GLUT: molecular basis of normal and aberrant function. JPEN J Parenter Enteral Nutr. 2004;28:364–71. doi: 10.1177/0148607104028005364. [DOI] [PubMed] [Google Scholar]
- 58.Kokk K, Veräjänkorva E, Wu XK, Tapfer H, Põldoja E, Pöllänen P. Immunohistochemical detection of glucose transporters class I subfamily in the mouse, rat and human testis. Medicina (Kaunas) 2004;40:156–60. [PubMed] [Google Scholar]
- 59.Ulisse S, Jannini EA, Pepe M, De Matteis S, D’Armiento M. Thyroid hormone stimulates glucose transport and GLUT1 mRNA in rat Sertoli cells. Mol Cell Endocrinol. 1992;87:131–7. doi: 10.1016/0303-7207(92)90241-W. [DOI] [PubMed] [Google Scholar]
- 60.Piroli GG, Grillo CA, Hoskin EK, Znamensky V, Katz EB, Milner TA, et al. Peripheral glucose administration stimulates the translocation of GLUT8 glucose transporter to the endoplasmic reticulum in the rat hippocampus. J Comp Neurol. 2002;452:103–14. doi: 10.1002/cne.10368. [DOI] [PubMed] [Google Scholar]
- 61.Reagan LP, Gorovits N, Hoskin EK, Alves SE, Katz EB, Grillo CA, et al. Localization and regulation of GLUTx1 glucose transporter in the hippocampus of streptozotocin diabetic rats. Proc Natl Acad Sci U S A. 2001;98:2820–5. doi: 10.1073/pnas.051629798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carayannopoulos MO, Chi MM, Cui Y, Pingsterhaus JM, McKnight RA, Mueckler M, et al. GLUT8 is a glucose transporter responsible for insulin-stimulated glucose uptake in the blastocyst. Proc Natl Acad Sci U S A. 2000;97:7313–8. doi: 10.1073/pnas.97.13.7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adastra KL, Frolova AI, Chi MM, Cusumano D, Bade M, Carayannopoulos MO, et al. Slc2a8 deficiency in mice results in reproductive and growth impairments. Biol Reprod. 2012;87:49. doi: 10.1095/biolreprod.111.097675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robinson R, Fritz IB. Metabolism of glucose by Sertoli cells in culture. Biol Reprod. 1981;24:1032–41. doi: 10.1095/biolreprod24.5.1032. [DOI] [PubMed] [Google Scholar]
- 65.Jutte NH, Grootegoed JA, Rommerts FF, van der Molen HJ. Exogenous lactate is essential for metabolic activities in isolated rat spermatocytes and spermatids. J Reprod Fertil. 1981;62:399–405. doi: 10.1530/jrf.0.0620399. [DOI] [PubMed] [Google Scholar]
- 66.Nakamura M, Okinaga S, Arai K. Metabolism of round spermatids: evidence that lactate is preferred substrate. Am J Physiol. 1984;247:E234–42. doi: 10.1152/ajpendo.1984.247.2.E234. [DOI] [PubMed] [Google Scholar]
- 67.Adijanto J, Philp NJ. The SLC16A family of monocarboxylate transporters (MCTs)--physiology and function in cellular metabolism, pH homeostasis, and fluid transport. Curr Top Membr. 2012;70:275–311. doi: 10.1016/B978-0-12-394316-3.00009-0. [DOI] [PubMed] [Google Scholar]
- 68.Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J. 1999;343:281–99. doi: 10.1042/0264-6021:3430281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mannowetz N, Wandernoth P, Wennemuth G. Basigin interacts with both MCT1 and MCT2 in murine spermatozoa. J Cell Physiol. 2012;227:2154–62. doi: 10.1002/jcp.22949. [DOI] [PubMed] [Google Scholar]
- 70.Oliveira PF, Alves MG, Rato L, Laurentino S, Silva J, Sá R, et al. Effect of insulin deprivation on metabolism and metabolism-associated gene transcript levels of in vitro cultured human Sertoli cells. Biochim Biophys Acta. 2012;1820:84–9. doi: 10.1016/j.bbagen.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 71.Oliveira PF, Alves MG, Rato L, Silva J, Sá R, Barros A, et al. Influence of 5α-dihydrotestosterone and 17β-estradiol on human Sertoli cells metabolism. Int J Androl. 2011;34:e612–20. doi: 10.1111/j.1365-2605.2011.01205.x. [DOI] [PubMed] [Google Scholar]
- 72.Rato L, Alves MG, Socorro S, Carvalho RA, Cavaco JE, Oliveira PF. Metabolic modulation induced by oestradiol and DHT in immature rat Sertoli cells cultured in vitro. Biosci Rep. 2012;32:61–9. doi: 10.1042/BSR20110030. [DOI] [PubMed] [Google Scholar]
- 73.McCall AL. Cerebral glucose metabolism in diabetes mellitus. Eur J Pharmacol. 2004;490:147–58. doi: 10.1016/j.ejphar.2004.02.052. [DOI] [PubMed] [Google Scholar]
- 74.Moreira PI, Oliveira CR. Mitochondria as potential targets in antidiabetic therapy. Handb Exp Pharmacol 2011:331-56. [DOI] [PubMed] [Google Scholar]
- 75.Cardoso S, Santos MS, Seiça R, Moreira PI. Cortical and hippocampal mitochondria bioenergetics and oxidative status during hyperglycemia and/or insulin-induced hypoglycemia. Biochim Biophys Acta. 2010;1802:942–51. doi: 10.1016/j.bbadis.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 76.Cardoso S, Carvalho C, Santos R, Correia S, Santos MS, Seiça R, et al. Impact of STZ-induced hyperglycemia and insulin-induced hypoglycemia in plasma amino acids and cortical synaptosomal neurotransmitters. Synapse. 2011;65:457–66. doi: 10.1002/syn.20863. [DOI] [PubMed] [Google Scholar]
- 77.Wei M, Gaskill SP, Haffner SM, Stern MP. Effects of diabetes and level of glycemia on all-cause and cardiovascular mortality. The San Antonio Heart Study. Diabetes Care. 1998;21:1167–72. doi: 10.2337/diacare.21.7.1167. [DOI] [PubMed] [Google Scholar]
- 78.Nakamura S, Makita Z, Ishikawa S, Yasumura K, Fujii W, Yanagisawa K, et al. Progression of nephropathy in spontaneous diabetic rats is prevented by OPB-9195, a novel inhibitor of advanced glycation. Diabetes. 1997;46:895–9. doi: 10.2337/diabetes.46.5.895. [DOI] [PubMed] [Google Scholar]
- 79.Willard AL, Herman IM. Vascular complications and diabetes: current therapies and future challenges. J Ophthalmol. 2012;2012:209538. doi: 10.1155/2012/209538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 81.Cameron DF, Murray FT, Drylie DD. Interstitial compartment pathology and spermatogenic disruption in testes from impotent diabetic men. Anat Rec. 1985;213:53–62. doi: 10.1002/ar.1092130108. [DOI] [PubMed] [Google Scholar]
- 82.Cai L, Chen S, Evans T, Deng DX, Mukherjee K, Chakrabarti S. Apoptotic germ-cell death and testicular damage in experimental diabetes: prevention by endothelin antagonism. Urol Res. 2000;28:342–7. doi: 10.1007/s002400000134. [DOI] [PubMed] [Google Scholar]
- 83.Anderson JE, Thliveris JA. Testicular histology in streptozotocin-induced diabetes. Anat Rec. 1986;214:378–82. doi: 10.1002/ar.1092140407. [DOI] [PubMed] [Google Scholar]
- 84.Pinhas-Hamiel O, Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr. 2005;146:693–700. doi: 10.1016/j.jpeds.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 85.Schoeffling K, Federlin K, Ditschuneit H, Pfeiffer EF. Disorders of sexual function in male diabetics. Diabetes. 1963;12:519–27. doi: 10.2337/diab.12.6.519. [DOI] [PubMed] [Google Scholar]
- 86.De Young L, Yu D, Bateman RM, Brock GB. Oxidative stress and antioxidant therapy: their impact in diabetes-associated erectile dysfunction. J Androl. 2004;25:830–6. doi: 10.1002/j.1939-4640.2004.tb02862.x. [DOI] [PubMed] [Google Scholar]
- 87.Greene LF, Kelalis PP. Retrograde ejaculation of semen dueto diabetic neuropathy. J Urol. 1967;98:696. [PubMed] [Google Scholar]
- 88.Ellenberg M, Weber H. Retrograde ejaculation in diabetic neuropathy. Ann Intern Med. 1966;65:1237–46. doi: 10.7326/0003-4819-65-6-1237. [DOI] [PubMed] [Google Scholar]
- 89.Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26:1553–79. doi: 10.2337/diacare.26.5.1553. [DOI] [PubMed] [Google Scholar]
- 90.Murray FT, Cameron DF, Orth JM. Gonadal dysfunction in the spontaneously diabetic BB rat. Metabolism. 1983;32(Suppl 1):141–7. doi: 10.1016/S0026-0495(83)80028-6. [DOI] [PubMed] [Google Scholar]
- 91.Agbaje IM, Rogers DA, McVicar CM, McClure N, Atkinson AB, Mallidis C, et al. Insulin dependant diabetes mellitus: implications for male reproductive function. Hum Reprod. 2007;22:1871–7. doi: 10.1093/humrep/dem077. [DOI] [PubMed] [Google Scholar]
- 92.Gazzaruso C, Coppola A, Giustina A. Erectile dysfunction and coronary artery disease in patients with diabetes. Curr Diabetes Rev. 2011;7:143–7. doi: 10.2174/157339911794940693. [DOI] [PubMed] [Google Scholar]
- 93.Barták V. Sperm quality in adult diabetic men. Int J Fertil. 1979;24:226–32. [PubMed] [Google Scholar]
- 94.Padrón RS, Dambay A, Suárez R, Más J. Semen analyses in adolescent diabetic patients. Acta Diabetol Lat. 1984;21:115–21. doi: 10.1007/BF02591100. [DOI] [PubMed] [Google Scholar]
- 95.Kühn-Velten N, Schermer R, Staib W. Effect of streptozotocin-induced hyperglycaemia on androgen-binding protein in rat testis and epididymis. Diabetologia. 1984;26:300–3. doi: 10.1007/BF00283654. [DOI] [PubMed] [Google Scholar]
- 96.Seethalakshmi L, Menon M, Diamond D. The effect of streptozotocin-induced diabetes on the neuroendocrine-male reproductive tract axis of the adult rat. J Urol. 1987;138:190–4. doi: 10.1016/s0022-5347(17)43042-4. [DOI] [PubMed] [Google Scholar]
- 97.Scarano WR, Messias AG, Oliva SU, Klinefelter GR, Kempinas WG. Sexual behaviour, sperm quantity and quality after short-term streptozotocin-induced hyperglycaemia in rats. Int J Androl. 2006;29:482–8. doi: 10.1111/j.1365-2605.2006.00682.x. [DOI] [PubMed] [Google Scholar]
- 98.Hutson JC, Stocco DM, Campbell GT, Wagoner J. Sertoli cell function in diabetic, insulin-treated diabetic, and semi-starved rats. Diabetes. 1983;32:112–6. doi: 10.2337/diabetes.32.2.112. [DOI] [PubMed] [Google Scholar]
- 99.Benitez A, Perez Diaz J. Effect of streptozotocin-diabetes and insulin treatment on regulation of Leydig cell function in the rat. Horm Metab Res. 1985;17:5–7. doi: 10.1055/s-2007-1013433. [DOI] [PubMed] [Google Scholar]
- 100.Seethalakshmi L, Menon M, Diamond D. The effect of streptozotocin-induced diabetes on the neuroendocrine-male reproductive tract axis of the adult rat. J Urol. 1987;138:190–4. doi: 10.1016/s0022-5347(17)43042-4. [DOI] [PubMed] [Google Scholar]
- 101.Scarano WR, Messias AG, Oliva SU, Klinefelter GR, Kempinas WG. Sexual behaviour, sperm quantity and quality after short-term streptozotocin-induced hyperglycaemia in rats. Int J Androl. 2006;29:482–8. doi: 10.1111/j.1365-2605.2006.00682.x. [DOI] [PubMed] [Google Scholar]
- 102.Ricci G, Catizone A, Esposito R, Pisanti FA, Vietri MT, Galdieri M. Diabetic rat testes: morphological and functional alterations. Andrologia. 2009;41:361–8. doi: 10.1111/j.1439-0272.2009.00937.x. [DOI] [PubMed] [Google Scholar]
- 103.Cameron DF, Murray FT, Drylie DD. Interstitial compartment pathology and spermatogenic disruption in testes from impotent diabetic men. Anat Rec. 1985;213:53–62. doi: 10.1002/ar.1092130108. [DOI] [PubMed] [Google Scholar]
- 104.Sexton WJ, Jarow JP. Effect of diabetes mellitus upon male reproductive function. Urology. 1997;49:508–13. doi: 10.1016/S0090-4295(96)00573-0. [DOI] [PubMed] [Google Scholar]
- 105.Kalyani RR, Dobs AS. Androgen deficiency, diabetes, and the metabolic syndrome in men. Curr Opin Endocrinol Diabetes Obes. 2007;14:226–34. doi: 10.1097/MED.0b013e32814db856. [DOI] [PubMed] [Google Scholar]
- 106.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 107.Turner R, Holman R, Cull C, Stratton I, Matthews D, Frighi V, et al. UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. doi: 10.1016/S0140-6736(98)07019-6. [DOI] [PubMed] [Google Scholar]
- 108.Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes. 2008;57:3169–76. doi: 10.2337/db08-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.El-Kaissi S, Sherbeeni S. Pharmacological management of type 2 diabetes mellitus: an update. Curr Diabetes Rev. 2011;7:392–405. doi: 10.2174/157339911797579160. [DOI] [PubMed] [Google Scholar]
- 110.Mozersky RP. Pharmacologic management of diabetes mellitus. J Am Osteopath Assoc. 1999;99(Suppl):S15–9, S. [PubMed] [Google Scholar]
- 111.van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev. 2011;91:79–118. doi: 10.1152/physrev.00003.2010. [DOI] [PubMed] [Google Scholar]
- 112.Kilpatrick ES, Rigby AS, Atkin SL. The Diabetes Control and Complications Trial: the gift that keeps giving. Nat Rev Endocrinol. 2009;5:537–45. doi: 10.1038/nrendo.2009.179. [DOI] [PubMed] [Google Scholar]
- 113.Simonson DC, Tamborlane WV, DeFronzo RA, Sherwin RS. Intensive insulin therapy reduces counterregulatory hormone responses to hypoglycemia in patients with type I diabetes. Ann Intern Med. 1985;103:184–90. doi: 10.7326/0003-4819-103-2-184. [DOI] [PubMed] [Google Scholar]
- 114.Amiel SA, Sherwin RS, Simonson DC, Tamborlane WV. Effect of intensive insulin therapy on glycemic thresholds for counterregulatory hormone release. Diabetes. 1988;37:901–7. doi: 10.2337/diabetes.37.7.901. [DOI] [PubMed] [Google Scholar]
- 115.Cryer PE. Hypoglycemia: still the limiting factor in the glycemic management of diabetes. Endocr Pract. 2008;14:750–6. doi: 10.4158/EP.14.6.750. [DOI] [PubMed] [Google Scholar]
- 116.Heller SR, Kerr D, UK Hypoglycaemia Study Group Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia. 2007;50:1140–7. doi: 10.1007/s00125-007-0599-y. [DOI] [PubMed] [Google Scholar]
- 117.Dagogo-Jack SE, Craft S, Cryer PE. Hypoglycemia-associated autonomic failure in insulin-dependent diabetes mellitus. Recent antecedent hypoglycemia reduces autonomic responses to, symptoms of, and defense against subsequent hypoglycemia. J Clin Invest. 1993;91:819–28. doi: 10.1172/JCI116302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Segel SA, Paramore DS, Cryer PE. Hypoglycemia-associated autonomic failure in advanced type 2 diabetes. Diabetes. 2002;51:724–33. doi: 10.2337/diabetes.51.3.724. [DOI] [PubMed] [Google Scholar]
- 119.Hutson JC. Altered biochemical responses by rat Sertoli cells and peritubular cells cultured under simulated diabetic conditions. Diabetologia. 1984;26:155–8. doi: 10.1007/BF00281125. [DOI] [PubMed] [Google Scholar]
- 120.Oonk RB, Grootegoed JA, van der Molen HJ. Comparison of the effects of insulin and follitropin on glucose metabolism by Sertoli cells from immature rats. Mol Cell Endocrinol. 1985;42:39–48. doi: 10.1016/0303-7207(85)90005-X. [DOI] [PubMed] [Google Scholar]
- 121.Oonk RB, Grootegoed JA. Identification of insulin receptors on rat Sertoli cells. Mol Cell Endocrinol. 1987;49:51–62. doi: 10.1016/0303-7207(87)90063-3. [DOI] [PubMed] [Google Scholar]
- 122.Alves MG, Socorro S, Silva J, Barros A, Sousa M, Cavaco JE, et al. In vitro cultured human Sertoli cells secrete high amounts of acetate that is stimulated by 17β-estradiol and suppressed by insulin deprivation. Biochim Biophys Acta. 2012;1823:1389–94. doi: 10.1016/j.bbamcr.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 123.Blackshaw AW, Elkington JS. Developmental changes in lactate dehydrogenase isoenzymes in the testis of the immature rat. J Reprod Fertil. 1970;22:69–75. doi: 10.1530/jrf.0.0220069. [DOI] [PubMed] [Google Scholar]
- 124.Pesce A, Fondy TP, Stolzenbach F, Castillo F, Kaplan NO. The comparative enzymology of lactic dehydrogenases. 3. Properties of the H4 and M4 enzymes from a number of vertebrates. J Biol Chem. 1967;242:2151–67. [PubMed] [Google Scholar]
- 125.Galardo MN, Riera MF, Pellizzari EH, Cigorraga SB, Meroni SB. The AMP-activated protein kinase activator, 5-aminoimidazole-4-carboxamide-1-b-D-ribonucleoside, regulates lactate production in rat Sertoli cells. J Mol Endocrinol. 2007;39:279–88. doi: 10.1677/JME-07-0054. [DOI] [PubMed] [Google Scholar]
- 126.Desjardins M, Bendayan M. Ultrastructural distribution of glomerular basement membrane components in experimental diabetes. Diabetes Res. 1990;14:65–73. [PubMed] [Google Scholar]
- 127.Hirose K, Osterby R, Nozawa M, Gundersen HJ. Development of glomerular lesions in experimental long-term diabetes in the rat. Kidney Int. 1982;21:689–95. doi: 10.1038/ki.1982.82. [DOI] [PubMed] [Google Scholar]
- 128.Ditzel J, Junker K. Abnormal glomerular filtration rate, renal plasma flow, and renal protein excretion in recent and short-term diabetics. Br Med J. 1972;2:13–9. doi: 10.1136/bmj.2.5804.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mancine RE, Penhos JC, Izquierdo IA, Heinrich JJ. Effects of acute hypoglycemia on rat testis. Proc Soc Exp Biol Med. 1960;104:699–702. doi: 10.3181/00379727-104-25957. [DOI] [PubMed] [Google Scholar]
- 130.Zysk JR, Bushway AA, Whistler RL, Carlton WW. Temporary sterility produced in male mice by 5-thio-D-glucose. J Reprod Fertil. 1975;45:69–72. doi: 10.1530/jrf.0.0450069. [DOI] [PubMed] [Google Scholar]
- 131.Mason GF, Petersen KF, Lebon V, Rothman DL, Shulman GI. Increased brain monocarboxylic acid transport and utilization in type 1 diabetes. Diabetes. 2006;55:929–34. doi: 10.2337/diabetes.55.04.06.db05-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xiong W, Wang H, Wu H, Chen Y, Han D. Apoptotic spermatogenic cells can be energy sources for Sertoli cells. Reproduction. 2009;137:469–79. doi: 10.1530/REP-08-0343. [DOI] [PubMed] [Google Scholar]
- 133.Chung S, Wang SP, Pan L, Mitchell G, Trasler J, Hermo L. Infertility and testicular defects in hormone-sensitive lipase-deficient mice. Endocrinology. 2001;142:4272–81. doi: 10.1210/en.142.10.4272. [DOI] [PubMed] [Google Scholar]
- 134.Jutte NH, Eikvar L, Levy FO, Hansson V. Metabolism of palmitate in cultured rat Sertoli cells. J Reprod Fertil. 1985;73:497–503. doi: 10.1530/jrf.0.0730497. [DOI] [PubMed] [Google Scholar]
- 135.Kaiser GR, Monteiro SC, Gelain DP, Souza LF, Perry ML, Bernard EA. Metabolism of amino acids by cultured rat Sertoli cells. Metabolism. 2005;54:515–21. doi: 10.1016/j.metabol.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 136.Yount EA, Harris RA. Ketone body and acetate formation from oleate by isolated rat testicular cells. Arch Biochem Biophys. 1982;217:503–11. doi: 10.1016/0003-9861(82)90531-8. [DOI] [PubMed] [Google Scholar]
- 137.Leiderman B, Mancini RE. Glycogen content in the rat testis from postnatal to adult ages. Endocrinology. 1969;85:607–9. doi: 10.1210/endo-85-3-607. [DOI] [PubMed] [Google Scholar]
- 138.Slaughter GR, Means AR. Follicle-stimulating hormone activation of glycogen phosphorylase in the Sertoli cell-enriched rat testis. Endocrinology. 1983;113:1476–85. doi: 10.1210/endo-113-4-1476. [DOI] [PubMed] [Google Scholar]