Abstract
Evidence is rapidly accumulating that rare, recurrent copy number variants (CNVs) represent large effect risk factors for neuropsychiatric disorders. 22q11.2 Deletion Syndrome (22q11DS; Velo-Cardio-Facial Syndrome (VCFS) or DiGeorge Syndrome) is the most common known contiguous gene deletion syndrome, and is associated with diverse neuropsychiatric disorders across the lifespan. One of the most intriguing aspects of the syndrome is the variability in clinical and cognitive presentation: children with 22q11DS have high prevalence of autism spectrum (ASD), attention deficit, and anxiety disorders, as well as psychotic-like features, and up to 30% of adolescents and adults develop schizophrenia-like psychosis. Recently, cases of early-onset Parkinson’s Disease in adults have been reported, collectively suggesting a role for disrupted dopaminergic neurotransmission in the observed neuropsychiatric phenotypes. There is also some evidence that 22q11DS-associated ASD and schizophrenia represent two unrelated phenotypic manifestations, consistent with a neuropsychiatric pleiotropy model. This genetic lesion thus provides a unique model for the discovery of specific genomic risk and (potentially) protective factors for neuropsychiatric disease. Here we provide an overview of neuropsychiatric findings to date, which highlight the value of this syndrome in mapping the developmental trajectory of dimensional phenotypes that traverse multiple diagnostic categories. Potential sources of genetic variability that may contribute to the disorder’s heterogeneous presentation are reviewed. Because of its known genetic etiology, animal models can readily be developed that recapitulate specific aspects of the syndrome. Future research directions involve translational models and potential for drug screenable targets in the context of this human model system.
Keywords: Velocardiofacial/Di George Syndrome, schizophrenia, copy number variant, pleiotropy, neurodevelopment, dopamine
I. Introduction
22q11.2 Deletion Syndrome (22q11DS; OMIM #192430), also known as Velocardiofacial or DiGeorge Syndrome, is a neurogenetic disorder resulting from a hemizygous microdeletion of approximately 1.5 – 3 megabases (Mb) on the long arm of chromosome 22. With an estimated prevalence of 1/4000 live births, it represents one of the most common known recurrent copy number variants (CNVs). Its physical manifestations frequently include cleft palate, hypocalcemia, cardiac defects, and immune dysfunction (1; 2). 22q11DS is also associated with strikingly elevated risk for neuropsychiatric illness, particularly psychosis (3; 4); 25–30% of individuals with this syndrome develop schizophrenia or affective psychosis, making 22q11DS one of the greatest known risk factors for psychotic illness identified to date. Microdeletions of 22q11.2 account for up to 1–2% of schizophrenia cases and represent the only known recurrent CNV responsible for introducing new cases of schizophrenia in the population (5; 6). Moreover, non-psychotic psychiatric disorders and behavioral abnormalities are present from early childhood in 22q11DS (7; 8). This phenotypic variability implies that there may be distinct biological mechanisms that underlie the development of these psychiatric conditions. Several of the genes encoded in the deleted region are highly expressed in the brain, and known to affect early neuronal migration and cortical development (6; 9). As such, this syndrome provides a unique opportunity to connect genes to brain to behavior.
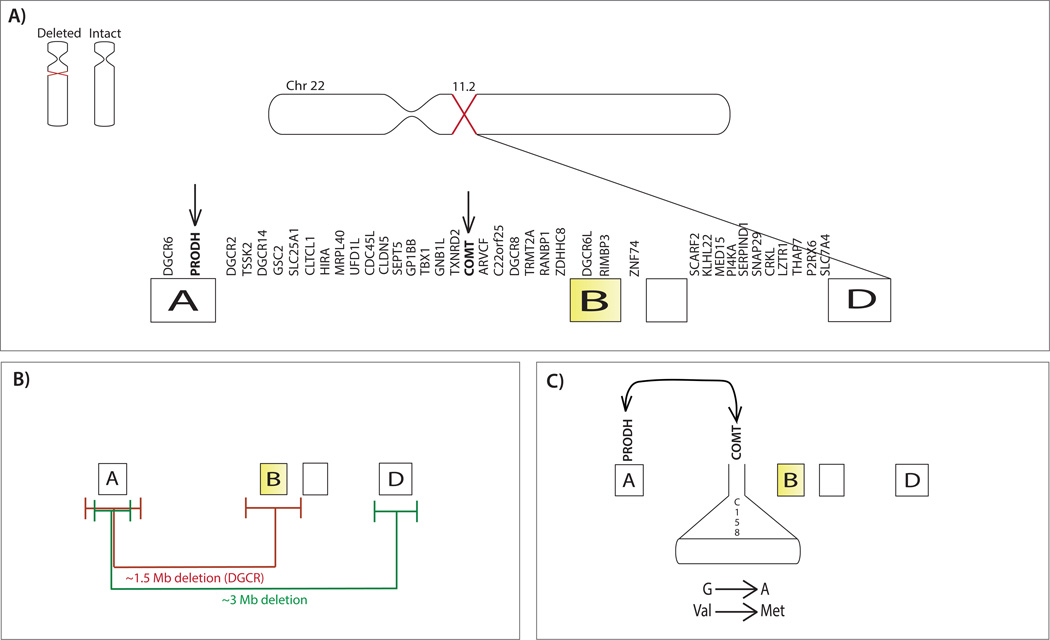
While about 85% of patients have approximately the same 3 Mb microdeletion, containing about 60 known genes (Figure 1), variability in the deletion size and breakpoint locations, as well as the characteristics of the intact chromosome (1; 6), may play an important role in the observed phenotypic variability in individuals with 22q11DS.
Figure 1. Chromosome 22q and potential sources of genetic variability.
(A) Hemizygous 22q11.2 deletion (light purple) and intact chromosome (light pink). To the right, the deleted segment of Chromosome 22 is shown (dark purple). Deletion breakpoints most commonly occur within the four distinct blocks of LCRs that lie in the deletion interval (termed A, B, C, D) (yellow) and deleted genes in the 22q11.2 locus are labeled above the segment (102). The genes COMT and PRODH are highlighted with arrows to indicate specific examples of genetic variability that are discussed in this review. (B) Breakpoint variability. The deleted segment is illustrated with the two most common deletion lengths, 3Mb (green line) and 1.5Mb deletions (red line), though other atypical deletions have been reported (see Supplemental Material). Error bars denote approximate variance in the deletion breakpoints for the 1.5 and 3Mb deletion lengths. The amount of variability in the deletion breakpoints may differ as a function of deletion size (102). The disorder is defined by a deletion in the DiGeorge critical region (DGCR), i.e. the region of the chromosome located between markers D22S36 and D22S788, which flank LCRs A and B. (C) Allelic variation within the intact chromosome and epistasis. The COMT Val158Met gene variant on the intact chromosome is illustrated as an example of the potential role of allelic variation, involving substitution of a methionine (Met) for valine (Val). As an example of episatasis, PRODH and COMT are illustrated to show the interactive role of the two gene products. Additional sources of variability include, but are not limited to: unmasking of autosomal recessive mutations via hemizygous deletion, parent of origin effects, and epigenetic effects.
Here we first review current literature on neuropsychiatric phenotypes across the lifespan in 22q11DS, and potential sources of genetic variability that may contribute to the heterogeneous presentation of the disorder. Next, we describe candidate endophenotypes relevant to neuropsychiatric risk, which can be assayed in both humans and animal models, helping us to bridge the gap between genetic and phenotypic variation. Finally, we suggest key directions for future research, involving new computational modeling methods and in vitro disease models, which show great promise for elucidating the molecular mechanisms underlying variable neuropsychiatric phenotypes of 22q11DS. Detailed coverage of medical comorbidities of 22q11DS and genetic association findings relevant to this locus in idiopathic neuropsychiatric disorders are outside the scope of this review, but are reviewed in detail elsewhere (10–12; also see Supplemental Material).
II. The Neuropsychiatric Phenotype of 22q11DS
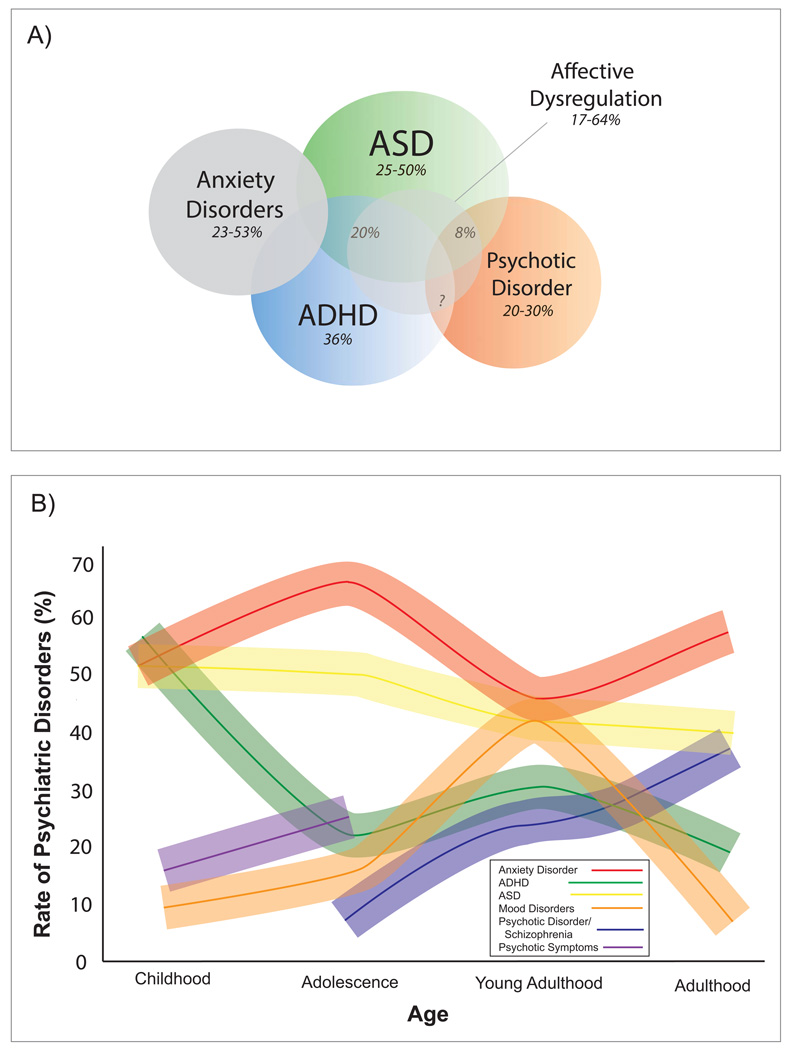
The most specific neuropsychiatric phenotype associated with 22q11DS is schizophrenia, as this is the only psychiatric condition that appears to be found at much higher frequency among 22q11.2 microdeletion carriers relative to other neurogenetic and developmental disorders associated with intellectual disability (5; 6). Nevertheless, ⅓ to ½ of children with the deletion are diagnosed with attention-deficit/hyperactivity disorder (ADHD), anxiety disorders (most commonly specific and social phobia), mood disorder, and autism spectrum disorders [ASDs; (1; 2; 7; 8;13; 14); see Figure 2A]. Indeed, at any given age at least 60% of individuals with 22q11DS meet diagnostic criteria for at least one psychiatric diagnosis, regardless of ascertainment method (3; 4; 14). Notably, psychopathology in 22q11DS encompasses emotional, behavioral and social disruptions in domains that cut across traditional diagnostic categories. For example, affective dysregulation, assessed dimensionally, may be part of a ‘core neuropsychiatric phenotype’ of 22q11DS (5; 6; 14).
Figure 2. Overlapping and Distinct Neuropsychiatric Phenotypes in 22q11DS.
(A) As a conceptual illustration of the variability of neuropsychiatric phenotypes in 22q11DS, we include autism spectrum disorder (ASD) (18; 103), attention-deficit/hyperactivity disorder (ADHD) (5), anxiety disorders, and psychosis (5), and estimated comorbidity rates across disorders based on existing literature (8; 103). It is important to note that comorbidity rates are not frequently reported in the literature; this is a critical issue for future research. Additionally, affective dysregulation is present in a substantial proportion of 22q11DS patients, regardless of diagnosis. (B) Developmental trajectories of psychiatric disorders with 22q11DS. As shown in the figure legend, each colored line portrays the estimated prevalence of a particular psychiatric disorder in 22q11DS patients throughout the lifespan. Shaded error bars for each line are illustrated to reflect variability across studies. Each percentage point on the line reflects data from published 22q11DS studies reporting on prevalence rates of anxiety disorder (5; 104), ADHD (5), ASD (18), mood disorder (5) psychotic disorder/schizophrenia (5; 77), and psychotic symptoms (17). In cross-sectional studies, rates of mood disorder (particularly depression) appear to peak in late adolescence and then decline, whereas rates of anxiety remain high through adulthood.
Some investigators have proposed that psychiatric diagnoses other than psychosis in 22q11DS represent nonspecific expressions of factors that affect brain development and function (6–8; 15); however, these distinct phenotypic manifestations may also represent genetic pleiotropy, in which the same genetic alteration can result in multiple physiological effects and phenotypic expressions. For instance, Vorstman et al. (8) found that, among patients diagnosed with schizophrenia in adulthood, only 8% had a probable ASD diagnosis in childhood, suggesting that ASD and schizophrenia may be distinct, pleiotropic manifestations of a 22q11.2 deletion. This phenomenon of distinct neuropsychiatric phenotypes is common to many CNVs that appear relevant to the etiology of idiopathic schizophrenia and autism (10–12; 15). It should be noted, however, that this study was conducted cross-sectionally, and thus the diagnosis of ‘probable ASD’ during childhood was made retrospectively (8). Prospective longitudinal studies are needed to confirm the intriguing possibility that 22q11DS-associated ASD and schizophrenia represent examples of true neuropsychiatric pleiotropy.
IIa. Developmental Trajectories of Neuropsychiatric Phenotypes
Although attention deficits (dimensionally assessed) characterize the vast majority of children with 22q11DS, clinical diagnoses of ADHD are not particularly stable over time (14). Nevertheless, a four-year longitudinal study (16) recently found that persistence of ADHD into adolescence in 22q11DS is predicted by childhood variables previously documented in the non-22q11DS ADHD literature, including higher rates of familial ADHD and history of childhood depression. These findings suggest that genetic background (i.e. family history) may also play a role in the variable neuropsychiatric phenotypes of 22q11DS.
The largest study of lifetime psychiatric diagnoses to date in 22q11DS (N=172; ages 5–54 years) combined data from 22q11DS cohorts from Israel and Switzerland (5), and found remarkably similar prevalence and developmental trends across countries. ADHD and anxiety disorders were the most common diagnoses during childhood (although notably, this study did not report on rates of ASDs), whereas rates of psychosis and mood disorders increased dramatically during adolescence and young adulthood. Additionally, while the average age at onset of overt psychotic disorder in 22q11DS is 19 to 26 years (13), earlier manifestations of psychotic-like symptoms characterize almost 1/3 of 22q11DS adolescents (17; 18), and 17% of pre-adolescent children (Figure 2B), suggesting a continuum of psychotic symptom severity in 22q11DS. Moreover, the socio-behavioral correlates of psychotic symptoms in 22q11DS youth - increased social withdrawal, reduced adaptability, and higher anxiety/depression - appeared strikingly similar to those reported in prospective studies of familial risk for schizophrenia (19). Additionally, consistent with epidemiologic studies in the general population (20), cognitive deterioration in adolescence is a dynamic phenotype that may be a potent predictor of psychosis in 22q11DS (21; 22, see Supplemental Material).
Collectively, these findings implicate early social and cognitive abnormalities as risk factors for subsequent development of overt psychotic disorder in 22q11DS; however, longer-term longitudinal follow-up studies are required to better understand the clinical significance and persistence of early psychotic symptoms in 22q11DS youth.
Most children with 22q11DS now survive into adulthood, but little is known about adult functioning. A recent study found significant functional impairment in over 75% of adults with 22q11DS (23). Variability in adaptive functioning was mediated primarily by cognitive abilities and the presence of psychotic disorder. Identification of remediable factors associated with better functioning is a key question for future research.
Finally, one notable and understudied aspect of the 22q11DS phenotype in older adults is that of early-onset Parkinson’s Disease. This phenotype has now been described in multiple case reports (24; 25), suggesting that dopaminergic disruption in 22q11DS may be relevant to the expression of both psychosis and Parkinson’s Disease over the lifespan.
Collectively, these findings illustrate the substantial heterogeneity in the 22q11DS neuropsychiatric phenotype, which may be linked, at least in part, to underlying sources of genetic variability. The spectrum of associated psychopathology suggests a model of genetic pleiotropy, and additionally implies that schizophrenia and other neuropsychiatric disorders may share overlapping biological pathways (15; 26).
III. Sources of Genetic Variability
An initial approach to characterizing genetic variability in 22q11DS was to investigate the effect of the two most common deletion lengths, 1.5Mb and 3Mb, on clinical phenotypes. Early studies did not find evidence for effects of deletion size on severity of syndromic features (27; 28), and it has been argued that the 1.5Mb region contains all of the key genes responsible for the development of the syndrome and associated psychiatric risk (28; 29). However, more recent studies using high-resolution tiling arrays have noted considerably more variation in deletion breakpoints than previously observed (26), suggesting that more precise mapping may yield new insights regarding phenotypic differences between patients with seemingly similar microdeletions.
Close to 90% of cases of 22q11DS arise from de novo mutations, whereas approximately 10% of cases are inherited in an autosomal dominant fashion (26; 29). In de novo cases, the microdeletion occurs due to mispairing of low copy repeats (LCRs) during meiosis (1; 30). LCRs, or segmental duplications, are found throughout the genome; the 22q11 region contains several large (60- to 600-kb) clusters of such LCRs (26; 31). These regions tend to predict genomic instability, and often cause breakages implicated in various genetic disorders, via non-allelic homologous recombination (see Table 1).
Table 1.
Glossary
| Breakpoint | A specific site of chromosomal breakage associated with a chromosomal abnormality. |
| Copy number variant (CNV) | A type of genomic variation in which segments of DNA of more than 1,000 base pairs are duplicated or deleted, as genomic risk factor for common complex brain disorders. 22q11.2 microdeletion and duplication are examples of specific CNVs. |
| Endophenotype | A state-independent biomarker or cognitive marker of an illness (present whether or not the illness is active) that is heritable and present in unaffected relatives of subjects that have the illness (108). |
| Epistasis | Interactions between genes in which the contribution of one gene to a phenotype depends on the genotype at another locus. |
| Haploinsufficiency | The situation in which one copy of a gene is incapable of providing sufficient protein production to ensure normal function. |
| Hemizygosity | A genetic condition where there is only one copy of a gene in an otherwise diploid cell or organism. |
| Low copy repeats (LCRs) | Highly homologous sequence elements within the eukaryotic genome arising from segmental duplication. |
| Long-term potentiation (LTP) | A long-lasting enhancement in signal transmission between two neurons that results from stimulating them synchronously. |
| Non-allelic homologous recombination (NAHR) | A form of homologous recombination that occurs in two pieces of DNA that have similar sequences, often as a result of the presence of low copy repeats (LCRs). NAHR can occur within the same LCR or in an alternative LCR, and can result in a variety of chromosomal rearrangements, including deletion, duplication, translocation, and inversion. The presence of LCRs and resultant NAHR is believed to play a key role in molecular evolution in primates, as a mechanism involved in rapidly changing gene dosage (which may be advantageous) and even the creation of new genes (30). |
| Pleiotropy | The phenomenon whereby one gene influences multiple, independent phenotypes. |
| Prepulse inhibition (PPI) | A quantitative trait, readily measurable in humans and in mice, involving reduced magnitude of the startle reflex that occurs when the subject is presented with a weak stimulus, or prepulse, immediately before the startling stimulus is presented. |
| Single nucleotide polymorphism (SNP) | Genetic variation in a DNA sequence that occurs when a single nucleotide - A, T, C, or G - in a genome is altered, which can affect function of the gene product. |
Although the precise mechanisms are currently unknown, four possible genetic mechanisms which may play a role in the clinical heterogeneity of 22q11DS are: 1) breakpoint heterogeneity, which may impact gene expression via inclusion or exclusion of specific genes in LCR regions (see Figure 1 and Supplemental Material); 2) Allelic variation within the intact 22q11.2 chromosome, which may have substantial effects on amino acid translation as there is no compensating normal allele, potentially resulting in downstream effects on behavior (32); 3) Epistatic interactions (i.e., the phenomenon whereby one gene modifies the effects of another) within the intact chromosome (33); 4) Hemizygosity of microRNA (miRNA) genes. miRNAs are short, non-coding ribonucleic acid (RNA) molecules found in eukaryotic cells, and are an essential part of the cellular machinery for regulating gene expression and transcription. Hemizygosity of miRNA genes in the DiGeorge Critical Region (DGCR)- specifically, Dgcr8, mir-185 and mir-649- results in insufficiency of mature miRNAs, which can dramatically affect the target gene protein function (34; 35).
Additionally, findings are mixed regarding the contribution of parent of origin for the 22q11.2 deletion to phenotypic variability. One study reported that 11 of 12 de novo cases with psychosis had a maternal origin of the deletion (5). While other studies have found quantitative endophenotypes – gray matter volume and language abilities - linked to maternal origin of the deletion (36; 37), another research group found no evidence for an effect of parental origin of the deletion on schizophrenia risk (38).
Recessive mutations in the intact chromosome, which may be unmasked by hemizygous deletion at 22q11.2, present another potential source of genetic variability. This mechanism has been shown to contribute to a variety of genetic disorders (39–41), although to our knowledge has not yet been investigated in 22q11DS
Finally, epigenetic effects refer to inherited changes in phenotype or gene expression resulting from mechanisms other than alterations in the underlying DNA sequence, such as DNA methylation (42; 43). Epigenetic effects are not well understood in 22q11DS and may be an important source of phenotypic variability requiring further study in large samples, and in animal models.
IV. Endophenotypes in Mice and Men
Analysis of quantitative traits that lie intermediate between these levels of analysis, such as changes in molecular and cellular properties, brain structure and function, and cognition, may better elucidate the pathophysiologic mechanisms linking structural genetic variation to distal psychiatric phenotypes. Such “deep phenotyping” approaches in the context of a known genetic model such as 22q11DS allow us to map a relatively homogeneous biological pathway to the development of complex neuropsychiatric disorders.
Notably, the mouse genome contains a region on chromosome 16 that is homologous to the 22q11.2 region in humans, and thus genetic techniques for selective deletion and/or over-expression of genes within the syntenic region in mice allow us to pinpoint genes contributing to specific behavioral phenotypes (see Supplemental Material). Below we provide examples across multiple levels of analysis, based on both human and animal studies.
IVa. Structural Neuroanatomy
Humans
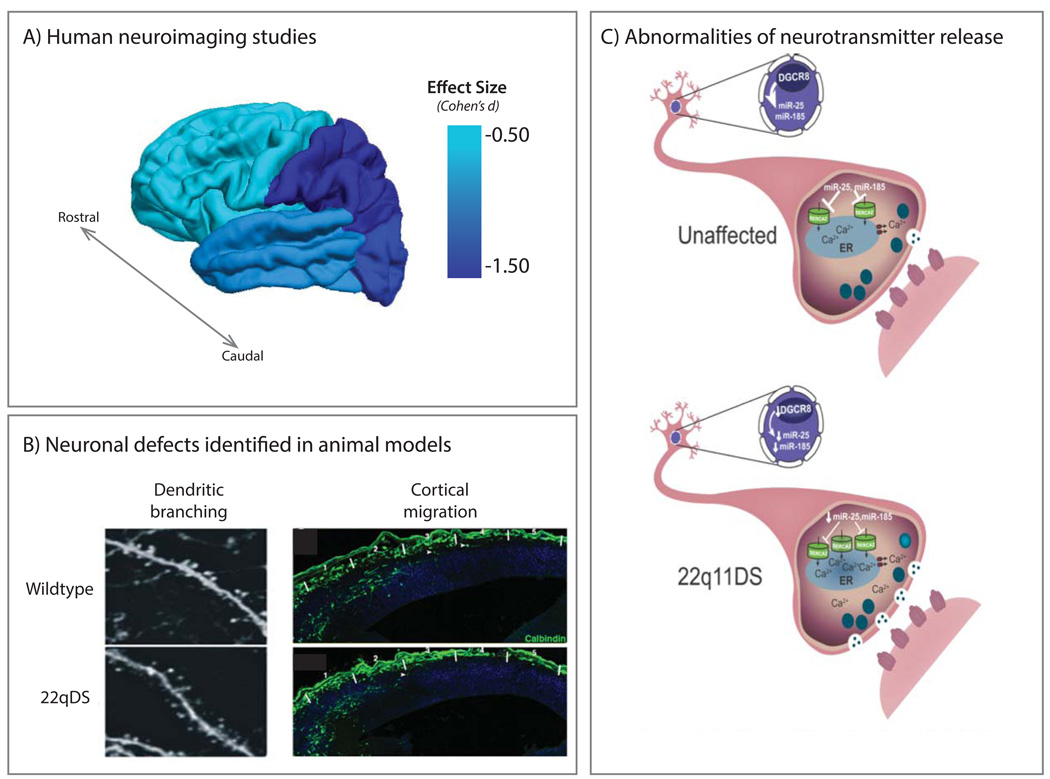
Collectively, human studies suggest global brain volumetric reduction in 22q11DS, particularly in the parietal lobes (44; 45), as well as significant thinning of midline brain regions (46). Interestingly, there appears to be a rostro-caudal gradient of volumetric reduction in 22q11DS, with caudal regions such as the occipital lobe and cerebellum showing greater reductions, while the frontal lobe is relatively preserved, at least in children (44; 47) (Figure 3). This gradient is conserved subcortically, where the caudate is more reduced in posterior regions than anterior (48), as is the thalamus (49) and corpus callosum (50; 51). Genetic influences are likely to play a role in this rostro-caudal gradient, particularly genes that encode neurodevelopmental morphogens involved in establishing the anterior-posterior axis (52). With increasing age, there appears to be differential reduction in fronto-temporal regions, which may also be relevant to increased vulnerability to psychosis onset in adolescence in 22q11DS (see Section V).
Figure 3. Neuroanatomic Abnormalities in 22q11DS.
A) Effect sizes for lobar gray matter reduction in children with 22q11DS relative to typically developing controls, constructed from a meta-analysis of structural MRI studies (45). This effect tends to follow a rostral-caudal gradient. Although not displayed in the figure, effect sizes from subcortical and midline structures are variable, ranging from −0.86 (hippocampus) to −0.20 (amygdala). B) Irregular dendritic branching and abnormal interneuron cortical migration found in 22q11DS murine models (reprinted with permission from Fenelon et al, 2011 (105) and Meechan et al, 2009 (9)). The Dgcr8 gene, within the 22q11.2 locus, is a key component of the microprocessor complex critical for miRNA production. As shown (left panel), Dgcr8+/− mice show reduced width of basal dendrites of pyramidal neurons in the medial prefrontal cortex (mPFC) compared to wildtype. Although basic synaptic transmission is normal in the mPFC of Dgcr8+/− mice, short-term synaptic plasticity is impaired, suggesting a neural substrate for cognitive impairment in 22q11DS. Right panel shows abnormal cortical migration of 22q11DS interneurons in the LgDel mouse model (9). While the frequency of calbindin-labeled interneurons did not differ between wildtype and LgDel mice, there is an abberant distribution, indicating disrupted interneuron migration, in the cortex of LgDel mice. C) Model of sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA2)-dependent mechanism of synaptic dysfunction in Dgcr8+/− mice, described in Earls et al., 2012 (58). SERCA2 upregulation leads to elevated endoplasmic reticulum Ca2+, increasing neurotransmitter release and increased long-term potentiation (LTP) in an age-dependent manner. MicroRNAs miR-25 and miR-185 are known regulators of SERCA2 and are absent in Dgcr8+/− mice; their restoration rescues LTP, suggesting that miRNA-dependent SERCA2 dysregulation may contribute to learning and neuropsychiatric phenotypes in 22q11DS.
Mice
Studies in mice suggest that anomalous cortical neurogenesis may underlie structural abnormalities observed in human MRI studies (Figure 3). Hemizygous deletion of the analogous 1.5 Mb region in mice disrupts proliferation of basal progenitors and interneuronal migration in the cerebral cortex (9), and leads to reduced dendritic spine density in the hippocampus (34), suggesting that these phenomena may be partially responsible for observed cortical thinning in human 22q11DS patients. Nevertheless, studies in animal models cannot tell us whether specific neuroanatomic alterations are relevant to variable psychiatric outcomes.
IVb. Physiologic Alterations and Synaptic Plasticity
Humans
Functional neuroimaging (fMRI) studies in humans with 22q11DS offer insights into how aforementioned structural changes manifest in terms of physiologic alterations. These studies have reported abnormal neural activity in tasks involving response inhibition (53) and working memory (54), specifically involving atypical parietal activation. Although differences in behavioral performance could contribute to the observed neurophysiologic differences, these findings offer preliminary evidence for atypical development of specific neural circuits critical for higher-order cognitive functions in 22q11DS.
Additionally, given that 22q11DS is associated with psychiatric disorders involving cortical dysconnectivity, the emerging field of resting state fMRI can offer important insights into the functional architecture of the resting brain in 22q11DS. Abnormalities in resting-state functional connectivity have been consistently implicated in idiopathic psychiatric illness (55); in particular, poorly synchronized ‘long distance’ connectivity in 22DS may serve as a biologically relevant intermediate phenotype for psychosis risk in 22q11DS (56). Functional variants in genes within the 22q11.2 locus have also been associated with schizophrenia risk, as well as relevant structural and functional neural connectivity defects, in the general population (57).
Mice
Intriguingly, studies in the Df(16)A+/− mouse model of 22q11DS have shown reduced hippocampal-prefrontal functional connectivity, suggesting a neuronal basis for long range connectivity defects in human 22q11DS patients (see Supplemental Material). Additionally, altered synaptic plasticity, in the form of hippocampal long-term potentiation (LTP; see Table 1), has been found in mouse models of 22q11DS, a mechanism that may underlie task-based functional MRI abnormalities evident in human 22q1 1DS patients. This abnormal LTP phenotype is thought to result from haploinsufficiency of Dgcr8, a gene important for microRNA biogenesis; microRNA restoration rescued abnormal LTP levels in Dgcr8+/− mice (Figure 3). Interestingly, levels of SERCA2 were increased in brains of patients with idiopathic schizophrenia, providing a direct link between abnormalities in LTP and psychiatric illness (58).
IVc. Neurocognition and Behavior
Humans
Patients with 22q11DS exhibit a characteristic cognitive profile involving deficits in nonverbal learning, as well as social cognition, although there is substantial variability in IQ (59–61). Consistent with the literature on youth at familial high risk for psychosis (19; 62), executive function deficits also predict risk for subsequent development of psychotic symptoms in 22q11DS (63).
Notably, sensorimotor gating deficits, indexed by impairments in pre-pulse inhibition (PPI; see Table 1) have been consistently identified as an endophenotype of disorders characterized by poor inhibitory control of attention, including ASD and schizophrenia (64). Similarly, PPI was significantly reduced in 22q11DS patients relative to sibling controls, and lower PPI was associated with subsyndromal psychotic-like symptoms (65).
Mice
Through a series of studies, Hiroi and colleagues (66; 67) selectively knocked out or overexpressed various combinations of genes within the 22q11.2 homolog region, in order to successfully pinpoint essential genes involved in PPI. Another study investigated mice carrying a multi-gene deletion (Df1+/−) that models 22q11DS, and subsequently used single-gene mutants to identify the causative genes involved in sensorimotor gating defects. Haploinsufficiency of two adjacent genes within the locus, Tbx1 and Gnb1l, was found to cause the PPI phenotype, suggesting that these genes may be key contributors to the psychiatric phenotype of 22q11DS (68). In humans, the relevance of TBX1 haploinsufficiency to the 22q11DS psychiatric presentation was further supported by the identification of a family in which the clinical manifestations of 22q11DS, including ASD, segregated with an inactivating mutation of TBX1 (68).
IVd. COMT as a model for multi-level investigation in 22q11DS
Although haploinsufficiency for multiple genes in the 22q11.2 locus may contribute to the clinical phenotype, dopaminergic (DA) dysfunction is implicated in many of the associated neuropsychiatric phenotypes (69; 70). As such, the catechol-O-methyltransferase (COMT) gene within the DGCR has been a particular focus of investigation. COMT encodes a postsynaptic enzyme that modulates prefrontal cortical DA clearance (71). An evolutionarily recent, common polymorphism at codon 158 of the COMT gene involves substitution of a methionine (Met) for valine (Val). COMT enzyme activity in postmortem DLPFC is ~40% higher in human subjects with the COMT-Val allele than those with the COMT-Met allele (72). A similar effect on enzyme activity was confirmed in lymphocytes. This SNP has been widely studied in healthy individuals, and has been linked to differences in executive functioning and the development of psychiatric illness (73; 74). A recent meta-analysis indicated a significant effect of COMT genotype on prefrontal cortical function in the general population (75).
Given that COMT is hemizygously deleted in 22q11DS patients, genetic variation in the intact chromosome may have a more profound effect on phenotypic expression than that observed in non-deleted individuals. As such, many studies have investigated allelic variability in this gene in relation to phenotypic expression at multiple levels (Figure 4). Overall, evidence for the contribution of COMT Val158Met genotype to neuropsychiatric symptomatology in 22q11DS is mixed. While Gothelf and colleagues (76) found that the COMT Low-activity (Met) variant increased risk for the development of psychotic symptoms in 22q11DS youth, other groups found no effect of COMT genotype on psychosis spectrum phenomena (77) or neuropsychological performance (78) (see -Table S1). Discrepancies may be due to small and heterogeneous samples, highlighting the need for better-powered studies.
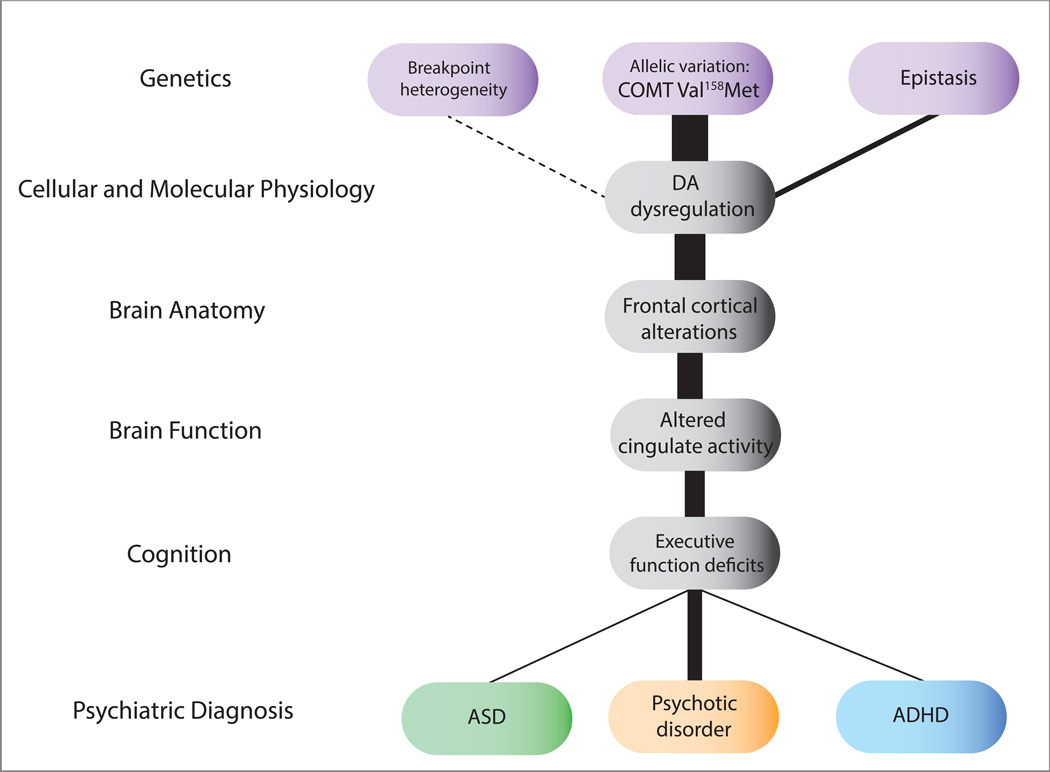
Figure 4. Endophenotypes Relevant to Neuropsychiatric Disorders in 22q11DS.
Levels illustrated here indicate how a known genetic etiology can inform pathophysiologic mechanisms relevant to neuropsychiatric phenotypes, using COMT Val158Met genotype as a particular example of how allelic variation in the intact chromosome may contribute to variable phenotypes. Potential sources of genetic variation are indicated in purple, intermediate sources of variability (e.g., endophenotypes) are indicated in gray, and varying phenotypic manifestations are indicated in the bottom row. The line thickness indicates the strength of associations between levels based on existing literature. Increased dopamine (DA) levels were found in patients with 22q11DS, and Met hemizygotes in particular show lower striatal binding potential as compared to Val hemizygotes (82). Studies of structural neuroanatomy have found that Met hemizygotes have smaller frontal lobe volume as compared to Val hemizygotes, which may be associated with inefficient breakdown of DA. One functional neuroimaging study found significantly increased cingulate activity during a Go/NoGo task in Met hemizygotes as compared to Val hemizygotes, implying that the Met subgroup of 22q11DS recruits additional cingulate activation for tasks that require attention and inhibition (53). Several studies have reported significant associations between cognition and COMT genotype such that Met hemizygotes shower better executive functioning (79), although not consistently (53; 81) (see Table S1 for details).
Variation in this SNP has more consistently been linked to differences in brain structure and cognition in 22q11DS patients (79–81). Using single photo emission computed tomography (SPECT) and a selective radiolabeled D2 receptor antagonist, Boot and colleagues found that Met-hemizygous 22q11DS patients had significantly lower mean striatal Binding Potential (BPND) compared to Val hemizygotes, and presumably, higher levels of synaptic DA, thus providing initial evidence for a functional impact of allelic variation of genes within the 22q11.2 region (82; 83). Mouse models have helped to elucidate the direct effects of COMT depletion. For example, although baseline DA levels appear normal in Comt-deficient mice (84), DA clearance from the extracellular space is twofold slower (71) , suggesting that COMT hemizygosity may influence DA function primarily under conditions of increased DA release, such as times of increased stress.
IVe. Epistatic Interactions: COMT and PRODH
Epistasis is known to occur in at least one set of genes within the 22q11.2 locus, COMT and PRODH (85). PRODH encodes an enzyme that converts proline to glutamate in mitochondria, dysfunction of which has been linked to the development of psychiatric illness (86). Prodh-deficient mice show Comt upregulation in prefrontal cortex, perhaps as a feedback mechanism to increase DA transmission; moreover, brain function was most disrupted in mice having both increased proline and decreased Comt activity (87). As a result of the interactive role of these two gene products, it is likely that these genes participate in an epistatic relationship at the level of transcription and behavior.
Working memory appears mostly intact in mouse models with hemizygous deletion of specific genes in the 1.5 Mb deletion region (i.e., NoGo receptor, Comt (88) and Prodh (42); however, interfering pharmacologically with the epistatic interaction between Comt and Prodh unmasks an underlying dopamine dysfunction and reveals working memory deficits in Prodh mutant mice (42). Inhibition of Comt has also been shown to exacerbate other behaviors influenced by cortical DA, e.g. sensitivity to amphetamine and PPI (42).
Most 22q11DS patients are haploinsufficient for both PRODH and COMT, and thus may be unable to compensate for loss of PRODH (and subsequent increase in proline levels) by means of COMT up-regulation. 22q11DS patients who carry the low-activity COMT Met allele are more likely to have elevated serum proline levels and perform poorly on eye tracking tasks (89; 90). These individuals may be less able to overcome dopaminergic dysregulation,thus placing them at greatest risk for psychotic symptom development.
Epistatic interactions between other genes within the intact chromosome, and/or with known transcription factors outside of the microdeletion region such as FGF1 (33), may also impact gene expression, and thus may offer clues about risk or resilience to various psychopathological phenotypes.
V. Biological Mechanisms of Psychotic Symptom Development
Studies in idiopathic schizophrenia have demonstrated a decline in cognitive abilities and other changes in behavior, as well as changes in brain morphology, which precede the onset of overt psychotic symptomology (91–93), and thus may have utility as predictive biomarkers. Importantly, individuals with 22q11DS and schizophrenia do not differ from patients with idiopathic schizophrenia in terms of core clinical symptoms, including age at onset and course of illness (94), but may differ with regard to auxiliary features such as medication response; however, there is little empirical evidence for this to date. Further, neurocognitive and neuroanatomic features studied to date in 22q11DS overlap with those observed in idiopathic schizophrenia (94– 96), although identified cognitive deficits appear more severe in 22q11DS-associated schizophrenia (97). Although the mechanisms underlying the development of psychotic symptoms in 22q11DS are not well understood at present, a central component of the neuropathology underlying emergence of these symptoms during adolescence is a process of neuronal volume reduction, resulting in reduced cortical connectivity. A key advantage of studying a major mutational model like 22q11DS is that it can be diagnosed in utero, allowing for identification of at-risk individuals long before symptom onset; identification of predictive biomarkers early in life may ultimately lead to the development of novel treatment targets.
Va. Environmental influences
While the availability of a well-characterized genetic model presents an ideal opportunity to investigate genetic contributions to psychopathology, the emergence of psychopathology in 22q11DS is likely modulated by environmental factors. Factors such as perinatal infection, urban environment, cannabis use, and stressful life events are all known to increase risk for schizophrenia in the general population (98; 99). 22q11DS presents a highly sensitized background for the development of psychosis, and thus offers a valuable model in which to investigate role of stress in precipitating symptom onset (100), and/or exacerbations over time.
VI. Moving Forward
Collectively, animal studies and candidate gene studies in humans implicate more than one gene within the 22q11.2 locus in the associated neurobehavioral phenotypes, suggesting an oligogenic basis. Evidence for the relevance of some of these genes to neuropsychiatric disorders in non-22q11DS individuals (see Table S1) suggests that common variants within the 22q11.2 locus may contribute to broader disease risk.
Novel methods in functional genomics and systems biology can shed light on how genetic makeup in 22q11DS can translate into varying clinical phenotypes. For instance, next generation sequencing can now pinpoint, at a single base-pair level, the precise locations of deletion breakpoints. Advances in stem cell technology, such as the generation of induced pluripotent stem cells (iPSCs), offer incredible promise for modeling in vivo neuronal development. Specifically, fibroblasts and other tissues from human patients with 22q11DS can be reprogrammed and regenerated into neural progenitors and neurons, and investigated for properties of neuronal cytoarchitecture, electrophysiology, and synaptic transmission (57). Research using these in vitro models can lead to development of novel therapeutic agents, and could ultimately even prevent psychosis onset in both 22q11DS and in the broader population.
Finally, large-scale, prospective studies are warranted, paralleling those of behaviorally defined clinical high-risk studies (101), in order to determine clinical and neurobiological predictors of psychosis, as well as the role of environmental factors in contributing to psychosis risk. This is the first, critical step in developing targeted inte rventions that can be applied early in the course of illness, leading to improved outcomes
Supplementary Material
Acknowledgments
We wish to thank Carolyn Chow, Therese Vesagas and Maria Jalbrzikowski for their helpful contributions to the manuscript.
This manuscript was partially supported by grants from the National Institute of Mental Health: RO1 MH085953 (CEB), 5 T32 MH082719-04 (CM) and NIH/NICHD grant #P50-HD-055784 (Pilot Project Grant to CEB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: All authors report no biomedical financial interests or other potential conflicts of interest.
Literature Cited
- 1.Edelmann L, Pandita RK, Spiteri E, Funke B, Goldberg R, Palanisamy N, et al. A common molecular basis for rearrangement disorders on chromosome 22q11. Hum Mol Genet. 1999;8:1157–1167. doi: 10.1093/hmg/8.7.1157. [DOI] [PubMed] [Google Scholar]
- 2.Drew LJ, Crabtree GW, Markx S, Stark KL, Chaverneff F, Bin Xu, et al. The 22q11.2 microdeletion: Fifteen years of insights into the genetic and neural complexity of psychiatric disorders. International Journal of Developmental Neuroscience. 2011;29:259–281. doi: 10.1016/j.ijdevneu.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy KC. Schizophrenia and velo-cardio-facial syndrome. Lancet. 2002;359:426–430. doi: 10.1016/S0140-6736(02)07604-3. [DOI] [PubMed] [Google Scholar]
- 4.Bassett AS, Chow EWC, Weksberg R. Chromosomal Abnormalities and Schizophrenia. Am J Med Genet. 2000;97:45–51. doi: 10.1002/(sici)1096-8628(200021)97:1<45::aid-ajmg6>3.0.co;2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green T, Gothelf D, Glaser B, Debbané M, Frisch A, Kotler M, et al. Psychiatric Disorders and Intellectual Functioning Throughout Development in Velocardiofacial (22q11.2 Deletion) Syndrome. JAAC. 2009;48:1060–1068. doi: 10.1097/CHI.0b013e3181b76683. [DOI] [PubMed] [Google Scholar]
- 6.Karayiorgou M, Simon TJ, Gogos JA. 22q11.2 microdeletions: linking DNA structural variation to brain dysfunction and schizophrenia. Nature Reviews Neuroscience. 2010;11:402–416. doi: 10.1038/nrn2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bassett AS, Chow EWC, Husted J, Weksberg R, Caluseriu O, Webb GD, Gatzoulis MA. Clinical features of 78 adults with 22q11 deletion syndrome. Am J Med Genet. 2005;138A:307–313. doi: 10.1002/ajmg.a.30984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vorstman J. Expression of autism spectrum and schizophrenia in patients with a 22q11.2 deletion. Schizophrenia Research. 2012:1–5. doi: 10.1016/j.schres.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Meechan DW, Tucker ES, Maynard TM, LaMantia A-S. Diminished dosage of 22q11 genes disrupts neurogenesis and cortical development in a mouse model of 22q11 deletion/DiGeorge syndrome. Proceedings of the National Academy of Sciences. 2009;106:16434–16445. doi: 10.1073/pnas.0905696106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Momma K. Cardiovascular anomalies associated with chromosome 22q11.2 deletion syndrome. Am J Cardiol. 2010;105:1617–1624. doi: 10.1016/j.amjcard.2010.01.333. [DOI] [PubMed] [Google Scholar]
- 11.Briegel W, Cohen M. Chromosome 22q11 deletion syndrome and its relevance for child and adolescent psychiatry. An overview of etiology, physical symptoms, aspects of child development and psychiatric disorders. Z Kinder Jugendpsychiatr Psychother. 2004;32:107–115. doi: 10.1024/1422-4917.32.2.107. [DOI] [PubMed] [Google Scholar]
- 12.Arinami T. Analyses of the associations between the genes of 22q11 deletion syndrome and schizophrenia. J Hum Genet. 2006;51:1037–1045. doi: 10.1007/s10038-006-0058-5. [DOI] [PubMed] [Google Scholar]
- 13.Murphy KC, Jones LA, Owen MJ. High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry. 1999;56:940–945. doi: 10.1001/archpsyc.56.10.940. [DOI] [PubMed] [Google Scholar]
- 14.Baker K, Vorstman J. Is there a core neuropsychiatric phenotype in 22q11.2 deletion syndrome? Current Opinion in Neurology. 2012;25:131–137. doi: 10.1097/WCO.0b013e328352dd58. [DOI] [PubMed] [Google Scholar]
- 15.Sebat J, Levy DL, McCarthy SE. Rare structural variants in schizophrenia: one disorder, multiple mutations; one mutation, multiple disorders. Trends in Genetics. 2009;25:528–535. doi: 10.1016/j.tig.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antshel KM, Hendricks K, Shprintzen R, Fremont W, Higgins AM, Faraone SV, Kates WR. The Longitudinal Course of Attention Deficit/Hyperactivity Disorder in Velo-Cardio-Facial Syndrome. J Pediatr. 2013 doi: 10.1016/j.jpeds.2012.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Debbané M, Glaser B, David MK, Feinstein C, Eliez S. Psychotic symptoms in children and adolescents with 22q11.2 deletion syndrome: Neuropsychological and behavioral implications. Schizophrenia Research. 2006;84:187–193. doi: 10.1016/j.schres.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 18.Vorstman J, Morcus ME, Duijff SN, Klaassen PW, Boer JAH, Beemer FA, et al. The 22q11.2 Deletion in Children: High Rate of Autistic Disorders and Early Onset of Psychotic Symptoms. J Am Acad Child Adolesc Psychiatry. 2006;45:1104–1113. doi: 10.1097/01.chi.0000228131.56956.c1. [DOI] [PubMed] [Google Scholar]
- 19.Whyte M-C, Brett C, Harrison LK, Byrne M, Miller P, Lawrie SM, Johnstone EC. Neuropsychological performance over time in people at high risk of developing schizophrenia and controls. Biological Psychiatry. 2006;59:730–739. doi: 10.1016/j.biopsych.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 20.Reichenberg A, Caspi A, Harrington H, Houts R, Keefe RSE, Murray RM, et al. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. Am J Psychiatry. 2010;167:160–169. doi: 10.1176/appi.ajp.2009.09040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kates WR, Antshel KM, Faraone SV, Fremont WP, Higgins AM, Shprintzen RJ, et al. Neuroanatomic Predictors to Prodromal Psychosis in Velocardiofacial Syndrome (22q11.2 Deletion Syndrome): A Longitudinal Study. Biological Psychiatry. 2011;69:945–952. doi: 10.1016/j.biopsych.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gothelf D, Feinstein C, Thompson T, Gu E, Penniman L, Van Stone E, et al. Risk factors for the emergence of psychotic disorders in adolescents with 22q11.2 deletion syndrome. Am J Psychiatry. 2007;164:663–669. doi: 10.1176/ajp.2007.164.4.663. [DOI] [PubMed] [Google Scholar]
- 23.Butcher NJ, Chow EWC, Costain G, Karas D, Ho A, Bassett AS. Functional outcomes of adults with 22q11.2 deletion syndrome. Genet Med. 2012;14:836–843. doi: 10.1038/gim.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krahn LE, Maraganore DM, Michels VV. Childhood-onset schizophrenia associated with parkinsonism in a patient with a microdeletion of chromosome 22. Mayo Clin Proc. 1998;73:956–959. doi: 10.4065/73.10.956. [DOI] [PubMed] [Google Scholar]
- 25.Zaleski C, Bassett AS, Tam K, Shugar AL, Chow EWC, McPherson E. The co-occurrence of early onset Parkinson disease and 22q11.2 deletion syndrome. Am J Med Genet. 2009;149A:525–528. doi: 10.1002/ajmg.a.32650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urban AE, Korbel JO, Selzer R, Richmond T, Hacker A, Popescu GV, et al. High-resolution mapping of DNA copy alterations in human chromosome 22 using high-density tiling oligonucleotide arrays. Proc Natl Acad Sci USA. 2006;103:4534–4539. doi: 10.1073/pnas.0511340103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlson C, Sirotkin H, Pandita R, Goldberg R, McKie J, Wadey R, et al. Molecular definition of 22q11 deletions in 151 velo-cardio-facial syndrome patients. Am J Hum Genet. 1997;61:620–629. doi: 10.1086/515508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karayiorgou M, Morris MA, Morrow B, Shprintzen RJ, Goldberg R, Borrow J, et al. Schizophrenia susceptibility associated with interstitial deletions of chromosome 22q11. Proc Natl Acad Sci USA. 1995;92:7612–7616. doi: 10.1073/pnas.92.17.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Digilio MC, Angioni A, De Santis M, Lombardo A, Giannotti A, Dallapiccola B, Marino B. Spectrum of clinical variability in familial deletion 22q11.2: from full manifestation to extremely mild clinical anomalies. Clinical Genetics. 2003;63:308–313. doi: 10.1034/j.1399-0004.2003.00049.x. [DOI] [PubMed] [Google Scholar]
- 30.Carvalho CMB, Zhang F, Lupski JR. Colloquium Paper: Genomic disorders: A window into human gene and genome evolution. Proceedings of the National Academy of Sciences. 2010;107:1765–1771. doi: 10.1073/pnas.0906222107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaikh TH, Kurahashi H, Saitta SC, O'Hare AM, Hu P, Roe BA, et al. Chromosome 22-specific low copy repeats and the 22q11.2 deletion syndrome: genomic organization and deletion endpoint analysis. Hum Mol Genet. 2000;9:489–501. doi: 10.1093/hmg/9.4.489. [DOI] [PubMed] [Google Scholar]
- 32.Sachidanandam R, Weissman D, Schmidt SC, Kakol JM, Stein LD, Marth G, et al. A map of human genome sequence variation containing 1.42 million single nucleotide polymorphisms. Nature. 2001;409:928–933. doi: 10.1038/35057149. [DOI] [PubMed] [Google Scholar]
- 33.Cordell HJ. Epistasis: what it means, what it doesn't mean, and statistical methods to detect it in humans. Hum Mol Genet. 2002;11:2463–2468. doi: 10.1093/hmg/11.20.2463. [DOI] [PubMed] [Google Scholar]
- 34.Stark KL, Xu B, Bagchi A, Lai W-S, Liu H, Hsu R, et al. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat Genet. 2008;40:751–760. doi: 10.1038/ng.138. [DOI] [PubMed] [Google Scholar]
- 35.Hornstein E, Shomron N. Canalization of development by microRNAs. Nat Genet. 2006;(38 Suppl):S20–S24. doi: 10.1038/ng1803. [DOI] [PubMed] [Google Scholar]
- 36.Eliez S, Antonarakis SE, Morris MA, Dahoun SP, Reiss AL. Parental origin of the deletion 22q11.2 and brain development in velocardiofacial syndrome: a preliminary study. Arch Gen Psychiatry. 2001;58:64–68. doi: 10.1001/archpsyc.58.1.64. [DOI] [PubMed] [Google Scholar]
- 37.Glaser B, Mumme DL, Blasey C, Morris MA, Dahoun SP, Antonarakis SE, et al. Language skills in children with velocardiofacial syndrome (deletion 22q11.2) J Pediatr. 2002;140:753–758. doi: 10.1067/mpd.2002.124774. [DOI] [PubMed] [Google Scholar]
- 38.Bassett AS, Marshall CR, Lionel AC, Chow EWC, Scherer SW. Copy number variations and risk for schizophrenia in 22q11.2 deletion syndrome. Hum Mol Genet. 2008;17:4045–4053. doi: 10.1093/hmg/ddn307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riley D, Wiznitzer M, Schwartz S, Zinn AB. A 13-year-old boy with cognitive impairment, retinoblastoma, and Wilson disease. Neurology. 2001;57:141–143. doi: 10.1212/wnl.57.1.141. [DOI] [PubMed] [Google Scholar]
- 40.Ikegawa S, Ohashi H, Hosoda F, Fukushima Y, Ohki M, Nakamura Y. Pseudoachondroplasia with de novo deletion [del(11)(q21q22.2)] Am J Med Genet. 1998;77:356–359. doi: 10.1002/(sici)1096-8628(19980605)77:5<356::aid-ajmg3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 41.Liburd N, Ghosh M, Riazuddin S, Naz S, Khan S, Ahmed Z, et al. Novel mutations of MYO15A associated with profound deafness in consanguineous families and moderately severe hearing loss in a patient with Smith-Magenis syndrome. Hum Genet. 2001;109:535–541. doi: 10.1007/s004390100604. [DOI] [PubMed] [Google Scholar]
- 42.Paterlini M, Zakharenko SS, Lai W-S, Qin J, Zhang H, Mukai J, et al. Transcriptional and behavioral interaction between 22q11.2 orthologs modulates schizophrenia-related phenotypes in mice. Nat Neurosci. 2005;8:1586–1594. doi: 10.1038/nn1562. [DOI] [PubMed] [Google Scholar]
- 43.Bird A. DNA methylation patterns and epigenetic memory. Genes & Development. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 44.Gothelf D, Schaer M, Eliez S. Genes, brain development and psychiatric phenotypes in velo-cardio-facial syndrome. Dev Disabil Res Revs. 2008;14:59–68. doi: 10.1002/ddrr.9. [DOI] [PubMed] [Google Scholar]
- 45.Tan GM, Arnone D, McIntosh AM, Ebmeier KP. Meta-analysis of magnetic resonance imaging studies in chromosome 22q11.2 deletion syndrome (velocardiofacial syndrome) Schizophrenia Research. 2009;115:173–181. doi: 10.1016/j.schres.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 46.Bearden CE, van Erp TGM, Dutton RA, Lee AD, Simon TJ, Cannon TD, et al. Alterations in Midline Cortical Thickness and Gyrification Patterns Mapped in Children with 22q11.2 Deletions. Cerebral Cortex. 2008;19:115–126. doi: 10.1093/cercor/bhn064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toyoda R, Assimacopoulos S, Wilcoxon J, Taylor A, Feldman P, Suzuki-Hirano A, et al. FGF8 acts as a classic diffusible morphogen to pattern the neocortex. Development. 2010;137:3439–3448. doi: 10.1242/dev.055392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kates WR, Burnette CP, Bessette BA, Folley BS, Strunge L, Jabs EW, Pearlson GD. Frontal and caudate alterations in velocardiofacial syndrome (deletion at chromosome 22q11.2) J Child Neurol. 2004;19:337–342. doi: 10.1177/088307380401900506. [DOI] [PubMed] [Google Scholar]
- 49.Bish JP, Nguyen V, Ding L, Ferrante S, Simon TJ. Thalamic reductions in children with chromosome 22q11.2 deletion syndrome. Neuroreport. 2004;15:1413–1415. doi: 10.1097/01.wnr.0000129855.50780.85. [DOI] [PubMed] [Google Scholar]
- 50.Antshel KM, Conchelos J, Lanzetta G, Fremont W, Kates WR. Behavior and corpus callosum morphology relationships in velocardiofacial syndrome (22q11.2 deletion syndrome) Psychiatry Res. 2005;138:235–245. doi: 10.1016/j.pscychresns.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 51.Machado AMC, Simon TJ, Nguyen V, McDonald-McGinn DM, Zackai EH, Gee JC. Corpus callosum morphology and ventricular size in chromosome 22q11.2 deletion syndrome. Brain Research. 2007;1131:197–210. doi: 10.1016/j.brainres.2006.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frank DU, Fotheringham LK, Brewer JA, Muglia LJ, Tristani-Firouzi M, Capecchi MR, Moon AM. An Fgf8 mouse mutant phenocopies human 22q11 deletion syndrome. Development. 2002;129:4591–4603. doi: 10.1242/dev.129.19.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gothelf D, Hoeft F, Hinard C, Hallmayer JF, Van Dover Stoecker J, Antonarakis SE, et al. Abnormal cortical activation during response inhibition in 22q11.2 deletion syndrome. Hum Brain Mapp. 2007;28:533–542. doi: 10.1002/hbm.20405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Azuma R, Daly EM, Campbell LE, Stevens AF, Deeley Q, Giampietro V, et al. Visuospatial working memory in children and adolescents with 22q11.2 deletion syndrome; an fMRI study. J Neurodevelop Disord. 2009;1:46–60. doi: 10.1007/s11689-009-9008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJS. Default-mode brain dysfunction in mental disorders: A systematic review. Neurosci Biobehav Rev. 2009;33:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 56.Debbané M, Lazouret M, Lagioia A, Schneider M, Van De Ville D, Eliez S. Resting-state networks in adolescents with 22q11.2 deletion syndrome: Associations with prodromal symptoms and executive functions. Schizophrenia Research. 2012;139:33–39. doi: 10.1016/j.schres.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 57.Kempf L, Nicodemus KK, Kolachana B, Vakkalanka R, Verchinski BA, Egan MF, et al. Functional Polymorphisms in PRODH Are Associated with Risk and Protection for Schizophrenia and Fronto-Striatal Structure and Function. PLoS Genet. 2008;4:e1000252. doi: 10.1371/journal.pgen.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Earls LR, Fricke RG, Yu J, Berry RB, Baldwin LT, Zakharenko SS. Age-Dependent MicroRNA Control of Synaptic Plasticity in 22q11 Deletion Syndrome and Schizophrenia. Journal of Neuroscience. 2012;32:14132–14144. doi: 10.1523/JNEUROSCI.1312-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bearden CE, Woodin MF, Wang PP, Moss E, McDonald-McGinn D, Zackai E, et al. The neurocognitive phenotype of the 22q11.2 deletion syndrome: selective deficit in visual-spatial memory. J Clin Exp Neuropsychol. 2001;23:447–464. doi: 10.1076/jcen.23.4.447.1228. [DOI] [PubMed] [Google Scholar]
- 60.Swillen A, Devriendt K, Legius E, Eyskens B, Dumoulin M, Gewillig M, Fryns JP. Intelligence and psychosocial adjustment in velocardiofacial syndrome: a study of 37 children and adolescents with VCFS. J Med Genet. 1997;34:453–458. doi: 10.1136/jmg.34.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Philip N, Bassett A. Cognitive, Behavioural and Psychiatric Phenotype in 22q11.2 Deletion Syndrome. Behav Genet. 2011;41:403–412. doi: 10.1007/s10519-011-9468-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davalos DB, Compagnon N, Heinlein S, Ross RG. Neuropsychological deficits in children associated with increased familial risk for schizophrenia. Schizophrenia Research. 2004;67:123–130. doi: 10.1016/S0920-9964(03)00187-7. [DOI] [PubMed] [Google Scholar]
- 63.Antshel KM, Shprintzen R, Fremont W, Higgins AM, Faraone SV, Kates WR. Cognitive and psychiatric predictors to psychosis in velocardiofacial syndrome: a 3-year follow-up study. J Am Acad Child Adolesc Psychiatry. 2010;49:333–344. [PMC free article] [PubMed] [Google Scholar]
- 64.Baker N, Adler LE, Franks RD, Waldo M, Berry S, Nagamoto H, et al. Neurophysiological assessment of sensory gating in psychiatric inpatients: comparison between schizophrenia and other diagnoses. Biological Psychiatry. 1987;22:603–617. doi: 10.1016/0006-3223(87)90188-0. [DOI] [PubMed] [Google Scholar]
- 65.Sobin C. Lower Prepulse Inhibition in Children With the 22q11 Deletion Syndrome. Am J Psychiatry. 2005;162:1090–1099. doi: 10.1176/appi.ajp.162.6.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hiramoto T, Kang G, Suzuki G, Satoh Y, Kucherlapati R, Watanabe Y, Hiroi N. Tbx1: identification of a 22q11.2 gene as a risk factor for autism spectrum disorder in a mouse model. Hum Mol Genet. 2011;20:4775–4785. doi: 10.1093/hmg/ddr404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harper KM, Hiramoto T, Tanigaki K, Kang G, Suzuki G, Trimble W, Hiroi N. Alterations of social interaction through genetic and environmental manipulation of the 22q11.2 gene Sept5 in the mouse brain. Hum Mol Genet. 2012;21:3489–3499. doi: 10.1093/hmg/dds180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paylor R, Glaser B, Mupo A, Ataliotis P, Spencer C, Sobotka A, et al. Tbx1 haploinsufficiency is linked to behavioral disorders in mice and humans: implications for 22q11 deletion syndrome. Proc Natl Acad Sci USA. 2006;103:7729–7734. doi: 10.1073/pnas.0600206103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, Kapur S. The Nature of Dopamine Dysfunction in Schizophrenia and What This Means for Treatment. Arch Gen Psychiatry. 2012;69:776. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abdolmaleky HM, Smith CL, Zhou J-R, Thiagalingam S. Epigenetic alterations of the dopaminergic system in major psychiatric disorders. Methods Mol Biol. 2008;448:187–212. doi: 10.1007/978-1-59745-205-2_9. [DOI] [PubMed] [Google Scholar]
- 71.Yavich L, Forsberg MM, Karayiorgou M, Gogos JA, Mannistö PT. Site-specific role of catechol-O-methyltransferase in dopamine overflow within prefrontal cortex and dorsal striatum. Journal of Neuroscience. 2007;27:10196–10209. doi: 10.1523/JNEUROSCI.0665-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stein DJ, Newman TK, Savitz J, Ramesar R. Warriors versus worriers: the role of COMT gene variants. CNS Spectr. 2006;11:745–748. doi: 10.1017/s1092852900014863. [DOI] [PubMed] [Google Scholar]
- 75.Ho B-C, Wassink TH, O'Leary DS, Sheffield VC, Andreasen NC. Catechol-O-methyl transferase Val158Met gene polymorphism in schizophrenia: working memory, frontal lobe MRI morphology and frontal cerebral blood flow. Molecular Psychiatry. 2005;10:229–229. doi: 10.1038/sj.mp.4001616. [DOI] [PubMed] [Google Scholar]
- 76.Gothelf D, Eliez S, Thompson T, Hinard C, Penniman L, Feinstein C, et al. COMT genotype predicts longitudinal cognitive decline and psychosis in 22q11.2 deletion syndrome. Nat Neurosci. 2005;8:1500–1502. doi: 10.1038/nn1572. [DOI] [PubMed] [Google Scholar]
- 77.Murphy KC, Jones LA, Owen MJ. High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry. 1999;56:940–945. doi: 10.1001/archpsyc.56.10.940. [DOI] [PubMed] [Google Scholar]
- 78.Glaser B, Debbané M, Hinard C, Morris MA, Dahoun SP, Antonarakis SE, Eliez S. No evidence for an effect of COMT Val158Met genotype on executive function in patients with 22q11 deletion syndrome. Am J Psychiatry. 2006;163:537–539. doi: 10.1176/appi.ajp.163.3.537. [DOI] [PubMed] [Google Scholar]
- 79.Shashi V, Keshavan M, Howard T, Berry M, Basehore M, Lewandowski E, Kwapil T. Cognitive correlates of a functional COMT polymorphism in children with 22q11.2 deletion syndrome. Clinical Genetics. 2006;69:234–238. doi: 10.1111/j.1399-0004.2006.00569.x. [DOI] [PubMed] [Google Scholar]
- 80.Baker K, Baldeweg T, Sivagnanasundaram S, Scambler P, Skuse D. COMT Val108/158Met Modifies Mismatch Negativity and Cognitive Function in 22q11 Deletion Syndrome. Biological Psychiatry. 2005;58:23–31. doi: 10.1016/j.biopsych.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 81.Gothelf D, Eliez S, Thompson T, Hinard C, Penniman L, Feinstein C, et al. COMT genotype predicts longitudinal cognitive decline and psychosis in 22q11.2 deletion syndrome. Nat Neurosci. 2005;8:1500–1502. doi: 10.1038/nn1572. [DOI] [PubMed] [Google Scholar]
- 82.Boot E, Booij J, Abeling N, Meijer J, da Silva Alves F, Zinkstok J, et al. Dopamine metabolism in adults with 22q11 deletion syndrome, with and without schizophrenia - relationship with COMT Val108/158 Met polymorphism, gender and symptomatology. Journal of Psychopharmacology. 2011;25:888–895. doi: 10.1177/0269881111400644. [DOI] [PubMed] [Google Scholar]
- 83.Boot E, Booij J, Zinkstok JR, De Haan L, Linszen DH, Baas F, van Amelsvoort TA. Striatal D receptor binding in 22q11 deletion syndrome: an [¹²³I]IBZM SPECT study. Journal of Psychopharmacology. 2010;24:1525–1531. doi: 10.1177/0269881109104854. [DOI] [PubMed] [Google Scholar]
- 84.Huotari M, Gogos JA, Karayiorgou M, Koponen O, Forsberg M, Raasmaja A, et al. Brain catecholamine metabolism in catechol-O-methyltransferase (COMT)-deficient mice. European Journal of Neuroscience. 2002;15:246–256. doi: 10.1046/j.0953-816x.2001.01856.x. [DOI] [PubMed] [Google Scholar]
- 85.Li T, Ma X, Hu X, Wang Y, Yan C, Meng H, et al. PRODH gene is associated with executive function in schizophrenic families. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:654–657. doi: 10.1002/ajmg.b.30648. [DOI] [PubMed] [Google Scholar]
- 86.Jacquet H. PRODH mutations and hyperprolinemia in a subset of schizophrenic patients. Hum Mol Genet. 2002;11:2243–2249. doi: 10.1093/hmg/11.19.2243. [DOI] [PubMed] [Google Scholar]
- 87.Paterlini M, Zakharenko SS, Lai W-S, Qin J, Zhang H, Mukai J, et al. Transcriptional and behavioral interaction between 22q11.2 orthologs modulates schizophrenia-related phenotypes in mice. Nat Neurosci. 2005;8:1586–1594. doi: 10.1038/nn1562. [DOI] [PubMed] [Google Scholar]
- 88.Papaleo F, Crawley JN, Song J, Lipska BK, Pickel J, Weinberger DR, Chen J. Genetic dissection of the role of catechol-O-methyltransferase in cognition and stress reactivity in mice. Journal of Neuroscience. 2008;28:8709–8723. doi: 10.1523/JNEUROSCI.2077-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vorstman J, Turetsky BI, Sijmens-Morcus MEJ, de Sain MG, Dorland B, Sprong M, et al. Proline affects brain function in 22q11DS children with the low activity COMT 158 allele. Neuropsychopharmacology. 2009;34:739–746. doi: 10.1038/npp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Magnée MJCM, Lamme VAF, de Sain-van der Velden MGM, Vorstman JAS, Kemner C. Proline and COMT Status Affect Visual Connectivity in Children with 22q11.2 Deletion Syndrome. In: Yoshikawa T, editor. PLoS ONE. Vol. 6. 2011. p. e25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- 92.Giuliano AJ, Li H, Mesholam-Gately RI, Sorenson SM, Woodberry KA, Seidman LJ. Neurocognition in the psychosis risk syndrome: a quantitative and qualitative review. Curr Pharm Des. 2012;18:399–415. doi: 10.2174/138161212799316019. [DOI] [PubMed] [Google Scholar]
- 93.Jalbrzikowski M, Bearden CE. Clinical and genetic high-risk paradigms: converging paths to psychosis meet in the temporal lobes. Biological Psychiatry. 2011;69:910–911. doi: 10.1016/j.biopsych.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bassett AS, Chow EWC, AbdelMalik P, Gheorghiu M, Husted J, Weksberg R. The schizophrenia phenotype in 22q11 deletion syndrome. Am J Psychiatry. 2003;160:1580–1586. doi: 10.1176/appi.ajp.160.9.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Duijff SN, Klaassen PWJ, de Veye HFNS, Beemer FA, Sinnema G, Vorstman JAS. Cognitive development in children with 22q11.2 deletion syndrome. The British Journal of Psychiatry. 2012;200:462–468. doi: 10.1192/bjp.bp.111.097139. [DOI] [PubMed] [Google Scholar]
- 96.Chow EWC, Watson M, Young DA, Bassett AS. Neurocognitive profile in 22q11 deletion syndrome and schizophrenia. Schizophrenia Research. 2006;87:270–278. doi: 10.1016/j.schres.2006.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van Amelsvoort T, Henry J, Morris R, Owen M, Linszen D, Murphy K, Murphy D. Cognitive deficits associated with schizophrenia in velo-cardio-facial syndrome. Schizophrenia Research. 2004;70:223–232. doi: 10.1016/j.schres.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 98.Geddes JR, Lawrie SM. Obstetric complications and schizophrenia: a meta-analysis. The British Journal of Psychiatry. 1995;167:786–793. doi: 10.1192/bjp.167.6.786. [DOI] [PubMed] [Google Scholar]
- 99.Schlosser DA, Pearson R, Perez VB, Loewy RL. Environmental Risk and Protective Factors and Their Influence on the Emergence of Psychosis. Adolesc Psychiatry (Hilversum) 2012;2:163–171. doi: 10.2174/2210676611202020163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Beaton EA, Simon TJ. How might stress contribute to increased risk for schizophrenia in children with chromosome 22q11.2 deletion syndrome? J Neurodevelop Disord. 2011;3:68–75. doi: 10.1007/s11689-010-9069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Seidman LJ, Giuliano AJ, Meyer EC, Addington J, Cadenhead KS, Cannon TD, et al. Neuropsychology of the prodrome to psychosis in the NAPLS consortium: relationship to family history and conversion to psychosis. Arch Gen Psychiatry. 2010;67:578–588. doi: 10.1001/archgenpsychiatry.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shaikh TH, O'Connor RJ, Pierpont ME, McGrath J, Hacker AM, Nimmakayalu M, et al. Low copy repeats mediate distal chromosome 22q11.2 deletions: Sequence analysis predicts breakpoint mechanisms. Genome Research. 2007;17:482–491. doi: 10.1101/gr.5986507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Niklasson L, Rasmussen P, Oskarsdóttir S, Gillberg C. Autism, ADHD, mental retardation and behavior problems in 100 individuals with 22q11 deletion syndrome. Research in Developmental Disabilities. 2009;30:763–773. doi: 10.1016/j.ridd.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 104.Fung WLA, McEvilly R, Fong J, Silversides C, Chow E, Bassett A. Elevated Prevalence of Generalized Anxiety Disorder in Adults With 22q11.2 Deletion Syndrome. Am J Psychiatry. 2010;167:997–998. doi: 10.1176/appi.ajp.2010.09101463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fenelon K, Mukai J, Xu B, Hsu P-K, Drew LJ, Karayiorgou M, et al. Deficiency of Dgcr8, a gene disrupted by the 22q11.2 microdeletion, results in altered short-term plasticity in the prefrontal cortex. Proceedings of the National Academy of Sciences. 2011;108:4447–4452. doi: 10.1073/pnas.1101219108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.