Abstract
1H magnetic resonance spectroscopy has demonstrated alterations in several neurometabolites in methamphetamine (METH)-dependent individuals in brain regions implicated in addiction. Yet it is unclear whether these neurochemicals return to homeostatic levels after an individual abstains from drug use, a difficult question to address due to high recidivism and poor study retention in human subjects. We thus utilized a nonhuman primate model of addiction to explore the effects of long-term drug exposure and withdrawal on brain neurochemistry. Ten rhesus macaque monkeys on an active METH self-administration protocol (average use 4.6±0.8 years, average daily intake between 0.4-1.2 mg/kg) and 10 age- and sex-matched drug-naïve controls (CONT) served as subjects. Concentrations of several neurochemicals were evaluated at several time points following withdrawal from drug availability (10 monkeys at 1 week and 1 and 3 months, and 6 monkeys at 6 and 12 months; CONT examined at one time point). At one week following METH withdrawal, we found increases in myo-inositol in anterior cingulate cortex in the METH group relative to CONT. These alterations showed a linear pattern of decreased levels (‘normalization’) by one year of abstinence. We also found decreases in glutamine and Glx (composed mainly of glutamate and glutamine) in the caudate-putamen of the same animals at early withdrawal that showed a similar linear pattern of increasing concentration by one year. These results demonstrate that despite protracted, long-term use, neurochemical changes seen following long term drug administration do not persist following prolonged abstinence, suggesting therapeutic effects of long-term withdrawal from drug use.
Keywords: Abstinence, glutamate, glutamine, magnetic resonance spectroscopy, methamphetamine, rhesus monkey
Introduction
Methamphetamine (METH) is a highly reinforcing, widely-abused psychostimulant drug with a neurotoxic potential that was first identified preclinically several decades ago (Seiden et al, 1975; Gibb and Kogan, 1979; Hotchkiss et al, 1979). This neurotoxicity is largely constrained to striatal monoaminergic terminals, and is marked by decreased dopamine and serotonin content, transmitter precursors, transporter sites and metabolites (Seiden et al, 1975; Wagner et al, 1979; Axt and Molliver, 1991; Preston et al, 1985). Data from human METH users recapitulate these preclinical findings of monoamine terminal loss (Wilson et al, 1996; McCann et al, 1998; Sekine et al, 2006; Volkow et al 2001b) and have been extended to include structural abnormalities in the striatum (Chang et al., 2005). However, the damage appears to extend beyond the basal ganglia to include widespread areas of cortex. These abnormalities, detected by magnetic resonance imaging (MRI) or positron emission tomography (PET), include decreases in regional glucose metabolism in orbitofrontal cortex (Volkow et al 2001a; London et al 2005; Kim et al 2006) and decreases in the size of such brain regions as cingulate, limbic, paralimbic, and dorsolateral prefrontal cortices (Thompson et al., 2004).
METH use has also been associated with long-lasting changes in brain neurochemistry. A small but growing literature using proton magnetic resonance spectroscopy (1H-MRS) indicates that concentrations of N-acetylaspartate (a marker of neuronal integrity) and creatine are decreased in the brains of METH users, with concomitant increases in myo-inositol (a marker of glial injury) and choline (Ernst et al, 2000, 2008; Sekine et al 2002; Nordahl et al, 2002, 2005; Salo et al, 2007; Sung et al 2007). Mirroring the preclinical neurotoxic METH literature, these neurochemical changes have been documented not only in basal ganglia, but also in frontal cortical white and grey matter regions.
In addition to the above neurometabolites, several amino acids (including some that can serve as neurotransmitters; glutamate, glutamine, and γ-amino-butyric acid (GABA)) can also be identified using MRS. Detection of alterations in glutamate and glutamine would be of particular importance in METH toxicity in view of the role that glutamatergic transmission plays in the neural adaptations associated with drug addiction (Reissner and Kalivas, 2010). However, such measurements have traditionally been difficult because of the overlapping resonances of glutamate and glutamine (Licata and Renshaw, 2010). To circumvent this problem, most 1H-MRS studies report a “Glx” value, which represents a combination of mainly these two chemicals as well as GABA and other metabolites. Using this measure, Ernst and Chang (2008) suggested that glutamatergic system function may be impacted by METH, and that some form of neuroadaptation occurs following prolonged abstinence. However the interpretation of their results is hampered not only by the inability to parse glutamate from glutamine's contributions to the signal, but also by the lack of controlled, repeated within-subject measurements that would allow for conclusions concerning time-dependent neurochemical alterations. More recently, Sailasuta and colleagues (2010a) reported a significant excess of glutamate in frontal white matter of abstinent METH-dependent subjects.
Although there is little disagreement that METH exposure shifts the balance of normal neurochemistry and neuronal function, it is unclear whether these changes recover, in whole or part, following withdrawal from chronic drug use. Reports suggest abstinence-associated recovery of the hypofrontality and the decrease in dopamine transporter content in stimulant-dependent individuals (Wang et al., 2004; Volkow et al 2001b, 2004). A handful of MRS studies have reported what appear to be normalized neurometabolite levels in the brains of abstinent METH users (Nordahl et al., 2005, Sung et al., 2007, Ernst and Chang, 2008, Salo et al., 2011). The paucity of data is further aggravated by the lack of corresponding studies with animal models of METH exposure, critical studies that would afford elements of the longitudinal trajectory often unavailable from studies with humans.
We capitalized on the opportunity to evaluate a population of rhesus monkeys with long histories of METH self-administration (between 1 and 8 years) that were about to be retired. Using non-invasive 1H-MRS, the aim of the current prospective, longitudinal study was two-fold: 1) to determine whether neurometabolite alterations observed in human METH abusers are replicable in a nonhuman primate model of METH abuse; and (2) to assess whether these patterns rectify following a period of abstinence of up to one year. We evaluated concentrations of several metabolites (including glutamate, glutamine, creatine, N-acetylaspartate, choline, and myo-inositol) in the anterior cingulated cortex and in the caudate-putamen, regions previously identified as sensitive to the neurotoxic potential of chronic METH intake. We hypothesized that like humans, rhesus monkeys would have significant neurochemical alterations following protracted exposure to METH as well as long-term drug withdrawal. More specifically, we predicted measurable decreases in glutamate and glutamine and N-acetylaspartate and increases in myo-inositol and choline after protracted exposure and we expected the above alterations to normalize upon drug withdrawal. To the best of our knowledge, this is the first longitudinal study of its kind in animals using a nonhuman primate model of psychostimulant abuse.
Methods
Subjects
Ten rhesus macaque (Macaca mulatta) monkeys (9.5 ± 0.8 years; 9 males, 1 female) with long histories of METH self-administration (METH group) and 10 age- and sex-matched animals (10.6 ± 0.2 years; 9 males, 1 female) living in a separate colony, but with no history of drug exposure (CONT group) were used in this study. Animals in the CONT group were part of a longitudinal aging study, and were individually housed at the National Institutes of Health (NIH) Nonhuman Primate colony in Poolesville, MD. Several days prior to MRI scanning, the animals in this CONT group were transported to the National Institute on Drug Abuse (NIDA)- Intramural Research program (IRP) in Baltimore in a climate-controlled truck to allow time to acclimate to their new housing conditions, and were returned to the Poolesville colony following scanning. Monkeys in both groups were individually housed in a humidity- and temperature-controlled room with a 12-hour light-dark cycle, and maintained on a standard diet of monkey biscuits and a variety of fresh fruits and vegetables with ad lib water. All subjects had visual, olfactory, and auditory contact with other monkeys throughout the study. Four subjects in the METH group were euthanized following the 3-month abstinence time point in order to perform various neurochemical analyses (results in preparation). Consequently, at months 6 and 12, the METH group consists of only six subjects (see Study time line, below).
METH self-administration (SA) procedures
A full description of the SA training procedures, apparatus, and behavioral results for monkeys in the METH group has been reported elsewhere (Schindler et al, 2011). Briefly, all monkeys had indwelling catheters surgically implanted into either the femoral or the jugular vein and were trained to respond for intravenous METH using standard operant training procedures beginning with food, then drug reinforcement. Following initial response training, animals were trained to respond for either 5 or 10 μg/kg/injection of methamphetamine hydrochloride (Sigma, St. Louis, MO, expressed as salt) dissolved in sterile saline at a volume of 0.5ml/injection and delivered at a rate of 3 ml/min. Dose utilized was chosen based on individual monkeys' stability of responding. An FR10 schedule of delivery was used, and monkeys were given access to METH three to five days per week for up to three hours per session. By the time the 1H-MRS studies were initiated, the experimental rhesus monkey group had extensive histories of METH SA, ranging from 1 to 8.6 years (average = 4.6 +0.8 years), with an average daily drug intake of 0.67 +0.07 mg/kg METH. As these monkeys served as subjects in other studies, they all had periodic exposure to potential therapeutic compounds in addition to METH SA, and two monkeys (Da and Sa) were also exposed to cocaine SA prior to METH (see supplementary Table S1 for complete drug histories of all subjects). Animal maintenance and all phases of this study were conducted in accordance with the NIH Guidelines for Using Animals in Intramural Research, and were approved by the Animal Care and Use Committee of the NIDA-IRP.
Study time line
1H-MRS measures were obtained at one week and 1, 3, 6, and 12 months following the last METH SA session. Measures were also taken at 24-hours (1 day) post-METH withdrawal. Throughout the course of the several-year original SA study, various withdrawal periods had been implemented, ranging from 2 to 4 (but never as many as 7) days in length. It was therefore decided that taking MRS measures at one-day post-withdrawal may have been too similar to what the monkeys were exposed to throughout their lifetime of use, and could be confounded by previous acute withdrawal periods; these data were therefore not analyzed.
MR scan procedures
Animals were initially anesthetized with Ketamine (10 mg/kg IM), intubated and catheter lines were placed into both the right and left saphenous veins. A continuous infusion of propofol (0.4 – 0.6 mg/kg/min) was started to maintain anesthesia while the animal was transported to the imaging laboratory at which time the propofol infusion was stopped. The monkey was then moved to the scanner bed, connected to an isoflurane delivery system and prepared for imaging. Isoflurane maintenance concentrations ranged between 1.5 and 2.5%, which was titrated on a continual basis according to physiological measures of depth of anesthesia. Surface coils (Nova Medical, Inc., Wilmington, MA, USA) were placed on each side of the head and secured to a custom head holder. The head of the animal was surrounded with a light layer of cotton padding and was secured with a thin layer of adhesive tape (Durapore, 3M) to minimize motion. Body temperature was maintained with a heating pad placed on the chest (37°C), and core temperature was monitored at the beginning and at the end of the scans with the use of a rectal temperature probe (Barnant, Barrington, IL). Systolic, diastolic, and mean arterial blood pressure were determined indirectly using a neonatal cuff positioned on the upper left arm, and heart rate and blood oxygen level (determined by pulse oximetry) were monitored continuously (Invivo monitor, model 3150M, Invivo Corp, Orlando, FL). Respiration rate and expired end tidal carbon dioxide were measured by sampling from the endotracheal tube. Animals breathed spontaneously and approximate inspiratory air volumes were recorded from the anesthesia delivery system (Hallowell, PA, USA). Supplemental fluids were delivered via one of the intravenous lines. Several imaging protocols were implemented in addition to the MRS, and the total scan session lasted between two and four hours. Upon completion of MR scanning, the intravenous lines were removed and the animals disconnected from the anesthesia. Once postural support and normal breathing were confirmed, the animals were returned to their home cages.
MR imaging/spectroscopy
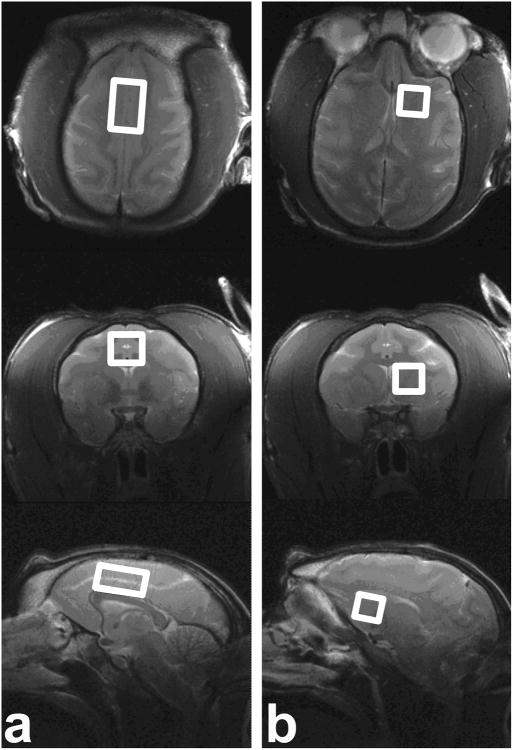
The MR scans were performed on a Siemens Allegra 3T scanner using a NOVA birdcage coil for RF transmitting and a two-piece surface coil for receiving (Nova Medical, Inc., Wilmington, MA, USA). At each abstinence time point, a single-voxel short-echo PRESS sequence was employed to acquire spectral data sequentially from each of the two ROIs of interest: the anterior cingulate cortex (ACC) (18×12×10 mm3) and the caudate-putamen (10×11×10 mm3), as shown in Fig. 1. Care was taken to ensure consistent placement of voxels for the single-voxel 1H-MRS data acquisition. Before 1H-MRS scans, three high-resolution T2-weighted images were acquired in three views in the following order: sagittal, axial, and coronal. The scan volume of T2-weighted sagittal images was placed on the Localizer images with the central slice put on the midline of the brain. The scan volume of T2-weighted axial (or transverse) images was placed on the T2-weighted sagittal images with slices parallel to the anterior commissure–posterior commissure line and perpendicular to the interhemispheric fissure (using the Siemens' “perpendicular to slice” option). The scan volume of T2-weighted coronal images was placed on the T2-weighted sagittal images with slices perpendicular to the sagittal and axial slices prescribed above. Following the above procedure, the ACC and caudate-putamen voxels were placed on the acquired T2-weighted images. For the ACC voxel, the sagittal image on the midline of the brain was chosen as the reference slice, and the voxel was placed immediately superior and parallel to the genu-rostral body-anterior midbody section of the corpus callosum. The anterior end of the voxel did not exceed the most anterior margin of the genu of the corpus callosum. On the other planes (i.e., axial and coronal), the voxel was adjusted to straddle the midline of the monkey brain. The caudate-putamen voxel was placed on the left hemisphere, parallel to the midline of the axial and coronal images. On the coronal images, the voxel was adjusted to cover as much of the caudate and putamen as possible, and to avoid the cortical regions as well as the CSF along the midline of the brain; the superior end of the voxel did not exceed the most superior part of the corpus callosum on the coronal images. After the adjustment on the coronal view, the voxel was confirmed on the sagittal images. The voxel was adjusted so that it was parallel to the genu-rostral body-anterior midbody section of the corpus callosum, and was then slightly angled in the superior-posterior direction in order to cover as much as possible the caudate and putamen. The anterior end of the voxel did not exceed the most anterior margin of the genu of the corpus callosum. After the adjustment on the above two planes, the voxel was then verified to be parallel to the midline of the brain on the axial images.
Fig. 1. Voxel ROI localization encompassing (a) ACC (18×12×10 mm3) and (b) caudate-putamen (10×11×10mm3) represented on high-resolution T2-weighted images.
The PRESS sequence parameters were TR/TE = 2000/30 ms, bandwidth = 2 kHz, sampling points = 2048, repetition = 600, 16-step phase cycling, and total time = 20 min. Unsuppressed water signal was acquired with 4 TRs following the water-suppressed 1H-MRS scan. Manual shimming was performed to further improve the magnetic field homogeneity after the automatic shimming process. The raw spectral data received on individual coil elements were combined using a weighting function obtained from the singular value decomposition (SVD)-based estimation method (Sandgren at al., 2005). Spectral quantification was performed using LCModel (version 6.2)(Provencher, 1993). Unsuppressed water signal was used as the scaling reference and also for eddy current correction. In the LCModel analysis, the basis set of model spectra of metabolites was provided by the vendor, which was made specifically for the short-echo PRESS sequence on the Siemens Allegra 3T scanner. The basis set included the following metabolites: alanine (Ala), aspartate (Asp), creatine (Cre), γ-aminobutyric acid (GABA), glucose (Glc), glutamine (Gln), glutamate (Glu), glycerophosphocholine (GPC), phosphocholine (PCh), lactate (Lac), myo-inositol (mI), N-acetylaspartate (NAA), N-Acetylaspartylglutamate (NAAG), and taurine (Tau). Total choline-containing compounds (tCho, GPC+PCh), NAANAAG (NAA+NAAG), and Glx were also estimated by the LCModel. A subset of basis set of macromolecules (MM) and lipids were incorporated into the LCModel analysis to take into account the impact of MM/lipids on spectral fitting.
LCModel reports estimated metabolite concentrations in institutional unit (i.u.), using the unsuppressed water signal as the scaling reference. LCModel also reports the ratio of metabolite concentration to the concentration of Cre. It is usually assumed that brain tissue (gray matter and white matter) contains MRS-detectable metabolites while CSF does not, so the metabolite concentration estimations scaled by the unsuppressed water signal are biased by the percentages of CSF in the voxel. However, there is no need to correct for CSF in the metabolite/Cre ratio as the effect deriving from CSF is automatically canceled by the ratio. Because there were no differences in the Cre level either within or across groups in either brain area and there were no linear patterns existing across time for the Cre level either (see Results section), we chose to normalize all remaining measured metabolites to creatine to control for differences in tissue and CSF fractions within and across individual brains. All reported results are based on these metabolite/Cre ratios. LCModel also reports the Cramer-Rao Lower Bound (CRLB) for each estimated metabolite concentration, which reflects the reliability with which the concentrations were estimated in the spectral fitting. Only metabolite concentration estimations with a CRLB of less than 20% were included in the final data analysis, except for glutamine (CRLB < 30%). These metabolites and their CRLB values were: Cre+PCr, tCho and mI (4-5%), NAA and NAANAAG (3-4 %), Glu and Glx (8-9%) and 11-20% for most Gln (although some values were less than 30%).
Statistical analysis
Statistical analyses were performed using SAS (SAS Institute, Inc., Cary, NC), and all data are expressed as mean ± SEM. Time course data for the METH group for each metabolite were subjected to a Mixed Model test of fixed effects (to account for missing variables at the later time points of withdrawal) and a test of linear contrasts. Differences between the METH one week post-withdrawal and CONT group metabolite values were assessed with independent-samples t-tests. Significant results are reported at p<0.05.
Results
In order to justify the use of Cre as a baseline reference for the results, we performed three statistical tests to ensure that the Cre level (from the LCModel, in institutional unit) was invariant both across and within groups. Because of the inherent drop-out in the design of our study (4 monkeys were not assayed at the final two time points), we tested for a within-group effect across time points using a Mixed-Model Test of Fixed Effects. Mixed procedure analysis revealed no differences in the Cre level in either brain region (ACC or caudate-putamen) across the five time points (F(1,17)=0.06, p=0.80 for ACC; F(1,20)=0.49, p=0.49 for caudate-putamen) in the METH group. We then conducted a linear regression to test whether significant linear patterns existed across time. We found no significant linear trend for the METH group across the 5 time points for either region (F(1,19)=0.62, p=0.44 for ACC; F(1,21)=0.10, p=0.75 for caudate-putamen). Finally, there were no differences in the Cre level between the CONT and METH groups at the earliest withdrawal time point of 1 week ([t(13)=0.17, p=0.862] for ACC; [t(16)=1.03, p=0.319] for caudate-putamen). For this reason, as described in the Methods, we chose to use Cre as a reference on which to base all of the results for the rest of our measured metabolites.
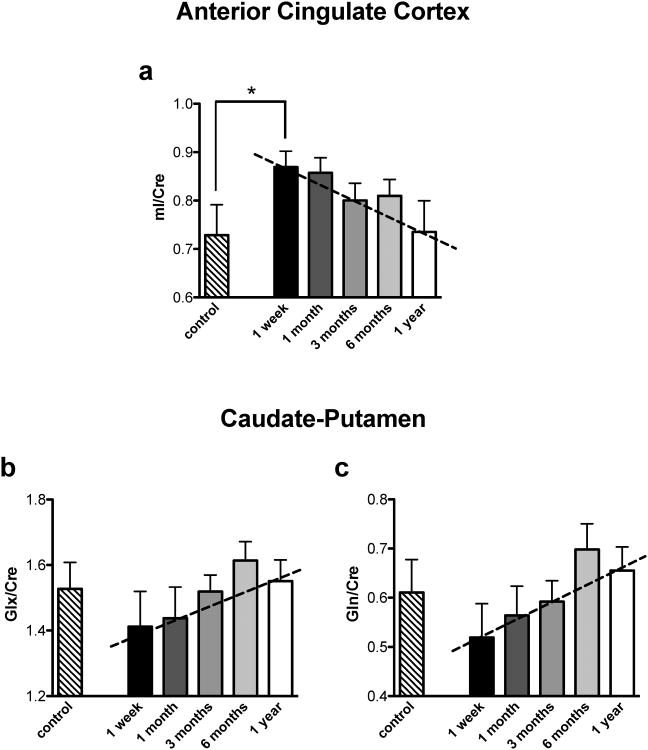
The Mixed Model test revealed no significant differences for either the ACC or caudate-putamen between time points (1 week and 1, 3, 6, and 12 months) for the Cre-normalized metabolite measures in the METH group (NAA, tCho, mI, Glx, Glu, and Gln). A linear regression test revealed a significant effect for mI/Cre [F(1,19)=7.51, p<0.01] in the ACC, as well as a significant linear pattern for Gln/Cre [F(1,21)=8.84, p<0.01] and Glx/Cre [F(1,21)=4.05, p<0.05] in the caudate-putamen (See Fig. 3). In addition, independent-sample t-test found a difference in ACC mI/Cre between the CONT and the earliest time point of withdrawal (1 week) in the METH group [t(13)=-2.06, p<0.05] (see Fig. 3a). Similar analyses (Student's t-test to explore differences between CONT and the first time point of METH withdrawal) revealed no differences for either Gln/Cre or Glx/Cre in the striatum ([t(16)=0.95, p=0.358]; and [t(16)=0.88, p=0.394], respectively).
Fig. 3.
Metabolite levels relative to creatine that showed significant linear contrasts in (a) ACC and (b, c) caudate-putamen in the CONT and the METH group calculated across withdrawal time (from one week to one year). Dashed lines represent a significant linear relationship between the 5 time points in the METH group.
* Indicates significant difference between CONT and METH at one week of withdrawal, p<0.05.
Discussion
To the best of our knowledge, this is the first report utilizing 1H-MRS to explore neurometabolite concentrations in a well-validated, psychostimulant self-administration model in nonhuman primates. An important aspect of our study was in the use of a noninvasive technique that afforded the ability to observe neurometabolite concentrations at carefully defined, well-controlled time points following cessation of drug SA. We conducted MRS evaluations at one week and one, three, six and twelve months of withdrawal, and found metabolite alterations at very early stages of withdrawal (i.e. one week) that returned towards levels seen in an age- and gender-matched control group after a one year withdrawal period. Specifically, within one year following METH withdrawal, we found a linear pattern of apparent normalization in concentrations of mI in ACC (a significant time-dependent reduction towards control monkey values) and glutamine and Glx in the caudate-putamen (significant time-dependent increase towards control monkey values) (Figs. 3B and 3C), suggestive of at least a partial reversibility of these apparent drug exposure-induced alterations. These findings are in general agreement with several studies in METH-dependent humans reporting drug withdrawal-associated normalization of metabolite concentrations including choline (Nordahl et al., 2005), NAA (Sung et al., 2007, Salo et al., 2011), and Glx (Ernst and Chang, 2008).
Investigations of drug-associated changes to the glutamatergic system in humans are scarce; glutamate is difficult to measure in vivo with 1H-MRS due to its resonance overlapping with other metabolites such as GABA and glutamine. This is especially true at low magnetic field strengths, and it is only at higher magnetic field strengths (such as 7 T and 9.4 T) that glutamate resonances can be readily separated from other metabolites (Yang et al, 2008). For this reason, a “Glx” measure, representing combined concentration of mainly glutamate and glutamine, is traditionally reported because the quantification of Glx is more reliable than individual glutamate or glutamine.
The few published data on MRS-measured glutamate in METH-addicted individuals are not consistent. Ernst and Chang (2008) reported Glx reductions in frontal grey matter, whereas Sailasuta and colleagues report enhanced glutamate in frontal cortex of abstinent METH-dependent subjects, relative to controls (Sailasuta et al, 2010a). More recently, our group employed a TE-averaged PRESS method, an essential J-resolved spectroscopy technique, with an improved quantification approach, to measure in vivo glutamate concentration, and we reported lowered glutamate level in rostral ACC in cocaine-abusing compared with healthy control individuals (Yang et al, 2009). Although we do not report frontal cortex changes in any marker of glutamate function, the direction of changes found in the current study (glutamine and Glx reductions) in the caudate-putamen is in agreement with that reported in Ernst and Chang (2008).
Glutamate (and more recently, glutamate homeostasis) has increasingly been the focus of preclinical studies investigating neuronal changes that occur in the addicted brain, particularly following cocaine exposure (for review, see Reissner and Kalivas, 2010). An historic literature demonstrates soundly that acute moderate-to-high METH exposure in rodents increases extracellular concentrations of glutamate in several brain regions implicated in drug abuse, including frontal cortex, ventral tegmental area, hippocampus, and nucleus accumbens (Nash and Yamamoto, 1992), an effect that is believed to underlie the ensuing neurotoxicity seen following these administration regimens (Stephens and Yamamoto, 1994; Sonsalla et al., 1998). Extra-synaptic glutamate concentrations are decreased in the nucleus accumbens of cocaine-exposed rats (Baker et al 2003), and more recently, Schwendt et al (2012) found that extended METH access decreased surface and total levels of mGluR2/3 receptors in the striatum of rats (a decrease which was reversed with extinction), suggesting that even in the absence of frank neurotoxic cell terminal destruction, glutamatergic systems are impacted by METH exposure.
Notably, the focus of these glutamate irregularities has centered on whether, and to what degree, METH is neurotoxic to dopamine systems in humans (for review, see Marshall and O'Dell 2012). Although in the current study we did not assay neurotoxicity per se, it doesn't seem likely that the doses at which these animals self-administered METH (0.4-1.2 mg/kg daily on intermittent days) were neurotoxic, given that a typical neurotoxic regimen in nonhuman primates generally requires high, chronic METH dosing ranging from 4 to 10 mg/kg METH within a single day (Woolverton et al, 1989, Hashimoto et al, 2007). Our data would therefore suggest long-term changes in striatal glutamatergic system function (specifically, to glutamine and Glx) that may occur as a result of more modest, non-neurotoxic METH exposure. Nevertheless, the increased mI concentration in ACC we found could reflect neuronal toxicity.
Although its exact significance has yet to be determined, mI is widely regarded as a marker of glial activity and proliferation, which is consistently found to be increased in not only several substance abuse disorders (Licata and Renshaw, 2010a), but also in neurodegenerative disorders (Griffith et al., 2009; Sturrock et al., 2010) and brain trauma (Chen et al 2003). With an identified role in maintaining osmolarity and as a substrate for phosphoinositide second-messenger systems, mI alterations may also reflect abnormalities in membrane metabolism and intracellular messenger signaling. Increased concentrations of mI have been widely reported in Alzheimer's disease, diabetes mellitus, hypoxia, hyperosmolar states, and in the neonatal brain, as well as in multiple sclerosis, suggesting that mI may represent a marker of demyelination (Stork and Renshaw, 2005). Despite uncertainty surrounding the precise role of mI, it is interesting that at a preclinical level, we found what has been reported clinically: METH significantly increases the mI level.
To the best of our knowledge, only one report exists of MRS-measured Glx levels following METH exposure. Ernst and Chang (2008) reported a progressive increase in glutamate levels in chronic human METH users following withdrawal. In that report, however, the between-subject variation in abstinence duration and the lack of repeated measurements in most individuals lead to difficulties interpreting the effect of METH withdrawal. In fact, results suggested an increase over normal Glx levels after one year of METH withdrawal. Our longitudinal, tightly controlled within-subject measurements in monkeys help to clarify the results of Ernst and Chang, showing that glutamatergic systems are significantly depleted after one week of withdrawal from METH, changes which progressively return toward control levels within one year.
Collectively, our results in both the ACC and striatum could be understood in light of general glial dysfunction following METH exposure. Approximately 80% of glucose oxidation is accomplished by glial cell homeostatic regulation of the glutamate-glutamine cycle. Glia serve to produce the major clearance pathway for glutamate from the synaptic space, the density of which is decreased in frontal cortex in major depressive disorder (Cotter et al., 2001), and compromised in schizophrenia (Rothermundt et al., 2004). In fact, in a novel protocol designed to explore glial function in the frontal cortex, Sailasuta and colleagues (2010b) found that [13C] acetate, which labels tricarboxylate acid (TCA) cycle rate in glia, is significantly decreased in the brains of abstinent METH-dependent subjects. Coupled together, these findings of decreased glial function converge to indicate that in addition to the several known types of damage that METH incurs on the brain, it may also produce damage to glia.
An oft-reported finding in METH abusers is that of significant NAA decreases in frontal grey matter or white matter and basal ganglia concentrations of, and significant increases in frontal grey matter choline (Cho; Ernst et al 2000; Nordahl et al 2002, 2005; Salo et al 2007). To our surprise, we did not see significant alterations in levels of NAA or Cho in either of the two regions assayed in our study. Although we saw a slight decrease in ACC NAA and a slight increase in caudate-putamen Cho at the initial stages of withdrawal, the differences did not reach significance. This finding of non-significant differences in these two measures may be due to insufficient power, given the small cohort of animals used in this investigation. This and several other limitations of this study are given consideration in the following section.
Limitations
These data should be considered in light of a number of potential limitations of the study. These include the fact that this prospective study was not designed a priori to investigate metabolite differences incurred as a result of METH SA. Consequently, we are not able to report individual animal baseline MRS levels prior to the METH SA sessions. Thus the usage of the term ‘normalization’ should be interpreted with caution as it is a between-group measure, and a within-subject comparison of the effects of METH would have lent more strength to the argument that METH alters and withdrawal reverses metabolite levels in key brain regions implicated in addiction.
An additional limitation is the fact that not all monkeys contributing to the data at the forefront of the study were available for the later assessments. Specifically, data from 10 monkeys were obtained at withdrawal time points of 1 week, 1 month, and 3 months. As described in the Methods (Subjects), following the 3-month withdrawal assessment, four monkeys were eliminated from the study in a random selection for post-mortem histological assessment. Consequently, the 6- and 12-month withdrawal time points include data from only 6 animals in the METH group, an attrition which may have impacted the power of our statistical assessments.
Additionally, our monkeys were not exposed to METH exclusively; each of them had a history of acute exposure to various potential therapeutic test compounds (see supplementary Table S1 for full disclosure). The test compounds, always delivered systemically and just prior to a SA session, were aimed at either monoamine or cannabinoid systems. It is not possible to say with certainty whether treatment with some of these compounds attenuated some of the more deleterious effects that METH might have otherwise engendered, or independently altered metabolite levels. In fact, pre-treatment with at least one of these compounds (GBR-12909) has been shown to have a neuroprotective effect on dopaminergic terminals in the rat striatum when given just prior to a neurotoxic METH regimen (Eisch and Marshall, 1998). Despite this possible confound, the incorporation of these low doses of various test compounds yields an interesting aspect to this study in that these animals may provide a more veridical model of human poly-drug use.
Conclusion
Using proton MR spectroscopy in a cohort of rhesus monkeys with many years of METH SA, we found increases in myo-inositol in the anterior cingulate cortex and decreases in Glx and glutamine in the caudate-putamen. These changes reverted towards levels seen in a matched group of control animals within one year, suggesting recovery following METH abstinence. These findings add to the literature suggesting that chronic METH administration results in an untoward impact on brain neurochemistry, and point towards drug-induced glial dysfunction in METH-addicted individuals. Our data also provide further evidence to a growing body of literature suggesting that despite prolonged use, withdrawal from chronic stimulant drug use may induce at least partial recovery of brain neurochemistry (and perhaps function). Future studies should address key questions concerning predisposition in a similar model of drug use.
Supplementary Material
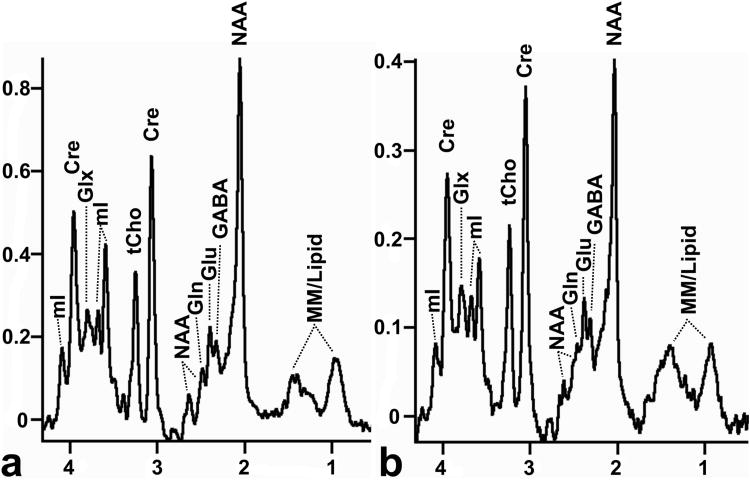
Fig. 2.
Representative spectra from (a) the ACC ROI (18×12×10 mm3) and (b) the caudate-putamen ROI (10×11×10mm3). Data are taken from the same monkey.
Abbreviations: tCho, choline-containing compounds; Cre, creatine; NAA, N-acetylaspartate; Glu, glutamate; Gln, glutamine; mI, myo-inositol; Glx, mainly glutamate and glutamine; GABA, γ-aminobutyric acid; MM/Lipid, macromolecules and lipids
Acknowledgments
Supported by the NIDA Intramural Research Program. Thanks to Dr. Xi Chen for help with image analysis and MRS scans, Dr. David Epstein for statistical consultation, Pradeep Kurup for help with image analysis, Dr. Julie Mattison and Dr. Peter Rapp from NIA for loaning the control group of monkeys, Dr. Lei K. Sheu from University of Pittsburgh for helpful discussion on statistical analysis, Dr. Thomas J. Ross for helpful discussions on an earlier version of this manuscript, and Ms. Eliscia Smith and Mr. William Rea for help with the scanning of the animals.
Footnotes
Authors Contribution: SY, SC, DBV, EAS, and YY were responsible for the conception and the design of the study. SY, AMB, SC, DBV, EAS, and YY wrote the paper. SY and AMB analyzed the data and SY, AMB, EAS, and YY interpreted the data. AMB, SC, DBV, and CWS handled the animals. SY, AMB, SC, and DBV performed the MRI/MRS scans. SY, AMB, SC, DBV, CWS, EAS, and YY gave final approval of the article.
References
- Axt KJ, Molliver ME. Immunocytochemical evidence for methamphetamine-induced serotonergic axon loss in the rat brain. Synapse. 1991;9:302–313. doi: 10.1002/syn.890090405. [DOI] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6(7):743–9. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Chang L, Cloak C, Patterson K, Grob C, Miller EN, Ernst T. Enlarged striatum in abstinent methamphetamine abusers: a possible compensatory response. Biological Psychiatry. 2005;57:967–74. doi: 10.1016/j.biopsych.2005.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced Glial Cell Density and Neuronal Size in the Anterior Cingulate Cortex in Major Depressive Disorder. Arch Gen Psychiatry. 2001;58(6):545–553. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- Chen XH, Iwata A, Nonaka M, Browne KD, Smith DH. Neurogenesis and glial proliferation persist for at least one year in the subventricular zone following brain trauma in rats. Journal of Neurotrauma. 2003;20(7):623–631. doi: 10.1089/089771503322144545. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Marshall JF. Methamphetamine neurotoxicity: dissociation of striatal dopamine terminal damage from parietal cortical cell body injury. Synapse. 1998;30(4):433–45. doi: 10.1002/(SICI)1098-2396(199812)30:4<433::AID-SYN10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Ernst T, Chang L, Leonido-Yee M, Speck O. Evidence for long-term neurotoxicity associated with methamphetamine abuse: A 1H MRS study. Neurology. 2000;54:1344–9. doi: 10.1212/wnl.54.6.1344. [DOI] [PubMed] [Google Scholar]
- Ernst T, Chang L. Adaptation of brain glutamate plus glutamine during abstinence from chronic methamphetamine use. J Neuroimmune Pharmacol. 2008;3(3):165–72. doi: 10.1007/s11481-008-9108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb JW, Kogan FJ. Influence of dopamine synthesis on methamphetamine-induced changes in striatal and adrenal tyrosine hydroxylase activity. Naunyn Schmiedebergs Arch Pharmacol. 1979;310:185–7. doi: 10.1007/BF00500283. [DOI] [PubMed] [Google Scholar]
- Griffith HR, den Hollander JA, Okonkwo OC, O'Brien T, Watts RL, Marson DC. Brain metabolism differs in Alzheimer's disease and Parkinson's disease dementia. Alzheimers Dement. 2008;4:421–427. doi: 10.1016/j.jalz.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Tsukada H, Nishiyama S, Fukumoto D, Kakiuchi T, Iyo M. Protective effects of minocycline on the reduction of dopamine transporters in the striatum after administration of methamphetamine: a positron emission tomography study in conscious monkeys. Biol Psych. 2007;61(5):577–81. doi: 10.1016/j.biopsych.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AJ, Morgan ME, Gibb JW. The long-term effects of multiple doses of methamphetamine on neostriatal tryptophan hydroxylase, tyrosine hydroxylase, choline acetyltransferase and glutamate decarboxylase activities. Life Sci. 1979;25:1373–8. doi: 10.1016/0024-3205(79)90414-4. [DOI] [PubMed] [Google Scholar]
- Hwang J, Lyoo IK, Kim SJ, Sung YH, Bae S, Cho SN, Lee HY, Lee DS, Renshaw PF. Decreased cerebral blood flow of the right anterior cingulate cortex in long-term and short-term abstinent methamphetamine users. Drug Alcohol Depend. 2006;82(2):177–81. doi: 10.1016/j.drugalcdep.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Licata SC, Renshaw PF. Neurochemistry of drug action: insights from proton magnetic resonance spectroscopic imaging and their relevance to addiction. Ann N Y Acad Sci. 2010;1187:148–71. doi: 10.1111/j.1749-6632.2009.05143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London ED, Berman SM, Voytek B, Simon SL, Mandelkern MA, Monterosso J, Thompson PM, Brody AL, Geaga JA, Hong MS, Hayashi KM, Rawson RA, Ling W. Cerebral metabolic dysfunction and impaired vigilance in recently abstinent methamphetamine abusers. Biol Psychiatry. 2005;58:770–778. doi: 10.1016/j.biopsych.2005.04.039. [DOI] [PubMed] [Google Scholar]
- Marshall JF, O'Dell SJ. Methamphetamine influences on brain and behavior: unsafe at any speed? Trends Neurosci. 2012;35(9):536–45. doi: 10.1016/j.tins.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C] WIN-35, 428. J Neurosci. 1998;18:8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren DG, Kosmatka KJ, Oakes TR, Korenke CD, Kohama SG, Matochik JA, Ingram DK, Johnson SC. A population-average MRI-based atlas collection of the rhesus macaque. Neuroimage. 2010;45(1):52–9. doi: 10.1016/j.neuroimage.2008.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CM, Frederick BB, Renshaw PF. Brain biochemistry using magnetic resonance spectroscopy: relevance to psychiatric illness in the elderly. J Geriatr Psychiatry Neurol. 1999;12(3):107–17. doi: 10.1177/089198879901200304. [DOI] [PubMed] [Google Scholar]
- Nash JF, Yamamoto BK. Methamphetamine neurotoxicity and striatal glutamate release: comparison to 3,4-methylenedioxymethamphetamine. Brain Res. 1992;581(2):237–43. doi: 10.1016/0006-8993(92)90713-j. [DOI] [PubMed] [Google Scholar]
- Nordahl TE, Salo R, Natsuaki Y, Galloway GP, Waters C, Moore CD, et al. Methamphetamine users in sustained abstinence: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2005;62:444–52. doi: 10.1001/archpsyc.62.4.444. [DOI] [PubMed] [Google Scholar]
- Nordahl TE, Salo R, Possin K, Gibson DR, Flynn N, Leamon M, Galloway GP, Pfefferbaum A, Spielman DM, Adalsteinsson E, Sullivan EV. Low N-acetyl-aspartate and high choline in the anterior cingulum of recently abstinent methamphetamine-dependent subjects: a preliminary proton MRS study. Psychiatry Res. 2002;116(1-2):43–52. doi: 10.1016/s0925-4927(02)00088-4. [DOI] [PubMed] [Google Scholar]
- Preston KL, Wagner GC, Schuster CR, Seiden LS. Long-term effects of repeated methylamphetamine administration on monoamine neurons in the rhesus monkey brain. Brain Res. 1985;338(2):243–8. doi: 10.1016/0006-8993(85)90153-2. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–9. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Reissner KJ, Kalivas PW. Using glutamate homeostasis as a target for treating addictive disorders. Behav Pharmacol. 2010;21(5-6):514–22. doi: 10.1097/FBP.0b013e32833d41b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothermundt M, Falkai P, Ponath G, Abel S, Bürkle H, Diedrich M, Hetzel G, Peters M, Siegmund A, Pedersen A, Maier W, Schramm J, Suslow T, Ohrmann P, Arolt V. Glial cell dysfunction in schizophrenia indicated by increased S100B in the CSF. Mol Psychiatry. 2004;9(10):897–9. doi: 10.1038/sj.mp.4001548. [DOI] [PubMed] [Google Scholar]
- Sailasuta N, Abulseoud O, Hernandez M, Haghani P, Ross BD. Metabolic Abnormalities in Abstinent Methamphetamine Dependent Subjects. Subst Abuse. 2010a;4:9–20. doi: 10.4137/sart.s4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailasuta N, Abulseoud O, Harris KC, Ross BD. Glial dysfunction in abstinent methamphetamine abusers. J Cereb Blood Flow Metab. 2010b;30:950–60. doi: 10.1038/jcbfm.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Buonocore MH, Leamon M, Natsuaki Y, Waters C, Moore CD, Galloway GP, Nordahl TE. Extended findings of brain metabolite normalization in MA-dependent subjects across sustained abstinence: A proton MRS study. Drug Alcoh Depend. 2011;113:133–138. doi: 10.1016/j.drugalcdep.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Natsuaki Y, Leamon MH, Galloway GP, Waters C, Moore CD, Buonocore MH. Attentional control and brain metabolite levels in methamphetamine abusers. Biol Psychiatry. 2007;61(11):1272–80. doi: 10.1016/j.biopsych.2006.07.031. [DOI] [PubMed] [Google Scholar]
- Sandgren N, Stoica P. Frequency-selective analysis of multichannel magnetic resonance spectroscopy data. Conf Proc IEEE Eng Med Biol Soc. 2005;3:2371–4. doi: 10.1109/IEMBS.2005.1616943. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Gilman JP, Panlilio LV, McCann DJ, Goldberg SR. Comparison of the effects of methamphetamine, bupropion, and methylphenidate on the self-administration of methamphetamine by rhesus monkeys. Experimental and Clinical Psychopharmacology. 2011;19(1):1–10. doi: 10.1037/a0022432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwendt M, Reichel CM, See RE. Extinction-dependent alterations in corticostriatal mGluR2/3 and mGluR7 receptors following chronic methamphetamine self-administration in rats. PLoS ONE. 2012;7(3):e34299. doi: 10.1371/journal.pone.0034299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiden LS. Brain monoamines and behavior. Psychopharmacol Bull. 1975;11:60–61. [PubMed] [Google Scholar]
- Sekine Y, Minabe Y, Kawai M, Suzuki K, Iyo M, Isoda H, Ashby CR, Jr, Takei N, Mori N. Metabolite alterations in basal ganglia associated with methamphetamine-related psychiatric symptoms. A proton MRS study. Neuropsychopharmacology. 2002;27(3):453–61. doi: 10.1016/S0893-133X(02)00321-4. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Takei N, Yoshikawa E, Nakamura K, Futatsubashi M, Okada H, Minabe Y, Suzuki K, Iwata Y, Tsuchiya KJ, Tsukada H, Iyo M, Mori N. Brain serotonin transporter density and aggression in abstinent methamphetamine abusers. Arch Gen Psychiatry. 2006;63:90–100. doi: 10.1001/archpsyc.63.1.90. [DOI] [PubMed] [Google Scholar]
- Sonsalla PK, Albers DS, Zeevalk GD. Role of glutamate in neurodegeneration of dopamine neurons in several animal models of parkinsonism. Amino Acids. 1998;14(1-3):69–74. doi: 10.1007/BF01345245. [DOI] [PubMed] [Google Scholar]
- Stephans SE, Yamamoto BK. Methamphetamine-induced neurotoxicity: roles for glutamate and dopamine efflux. Synapse. 1994;17:203–9. doi: 10.1002/syn.890170310. [DOI] [PubMed] [Google Scholar]
- Stork C, Renshaw PF. Mitochondrial dysfunction in bipolar disorder: evidence from magnetic resonance spectroscopy research. Mol Psychiatry. 2005;10(10):900–19. doi: 10.1038/sj.mp.4001711. [DOI] [PubMed] [Google Scholar]
- Sturrock A, Laule C, Decolongon J, Dar Santos R, Coleman AJ, Creighton S, Bechtel N, Reilmann R, Hayden MR, Tabrizi SJ, Mackay AL, Leavitt BR. Magnetic resonance spectroscopy biomarkers in premanifest and early Huntington disease. Neurology. 2010;75:1702–1710. doi: 10.1212/WNL.0b013e3181fc27e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung YH, Cho SC, Hwang J, Kim SJ, Kim H, Bae S, Kim N, Chang KH, Daniels M, Renshaw PF, Lyoo IK. Relationship between N-acetyl-aspartate in gray and white matter of abstinent methamphetamine abusers and their history of drug abuse: a proton magnetic resonance spectroscopy study. Drug Alcohol Depend. 2007;88(1):28–35. doi: 10.1016/j.drugalcdep.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects whouse methamphetamine. J Neurosci. 2004;24(26):6028–36. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: Association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001a;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow N, Chang L, Wang GJ, Fowler J, Franceschi D, Sedler M, et al. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. Journal of Neuroscience. 2001b;21:9414–8. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GC, Ricaurte GA, Seiden LS, Schuster CR, Miller RJ, Westley J. Long-lasting depletions of striatal dopamine and loss of dopamine uptake sites following repeated administration of methamphetamine. Brain Research. 1980;181(1):151–160. doi: 10.1016/0006-8993(80)91265-2. [DOI] [PubMed] [Google Scholar]
- Wang G, Volkow N, Chang L, Miller E, Sedler M, Hitzemann R, et al. Partial recovery of brain metabolism in methamphetamine abusers after protracted abstinence. American Journal of Psychiatry. 2004;161:242–8. doi: 10.1176/appi.ajp.161.2.242. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Ricuarte GA, Forno LS, Seiden LS. Long-term effects of chronic methamphetamine administration in rhesus monkeys. Brain Res. 1989;486(1):73–8. doi: 10.1016/0006-8993(89)91279-1. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med. 1996;2(6):699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- Yang S, Hu J, Kou Z, Yang Y. Spectral simplification for resolved glutamate and glutamine measurement using a standard STEAM sequence with optimized timing parameters at 3, 4, 4.7, 7, and 9.4 Tesla. Magnetic Resonance in Medicine. 2008;59(2):236–244. doi: 10.1002/mrm.21463. [DOI] [PubMed] [Google Scholar]
- Yang S, Salmeron BJ, Ross TJ, Xi ZX, Stein EA, Yang Y. Lower glutamate levels in rostral anterior cingulate of chronic cocaine users – a 1H-MRS study using TE-averaged PRESS at 3T with an optimized quantification strategy. Psychiatry Research: Neuroimaging. 2009;174(3):171–176. doi: 10.1016/j.pscychresns.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.