Summary
Examination of ciliary ultrastructure remains the cornerstone diagnostic test for primary ciliary dyskinesia (PCD), a disease of abnormal ciliary structure and/or function. Obtaining a biopsy with sufficient interpretable cilia and producing quality transmission electron micrographs (TEM) is challenging. Methods for processing tissues for optimal preservation of axonemal structures are not standardized. This study describes our experience using a standard operating procedure (SOP) for collecting nasal scrape biopsies and processing TEMs in a centralized laboratory. We enrolled patients with suspected PCD at research sites of the Genetic Disorders of Mucociliary Clearance Consortium. Biopsies were performed according to a SOP whereby curettes were used to scrape the inferior surface of the inferior turbinate, with samples placed in fixative. Specimens were shipped to a central laboratory where TEMs were prepared and blindly reviewed. 448 specimens were obtained from 107 young children (0–5 years), 189 older children (5–18 years), and 152 adults (> 18 years), and 88% were adequate for formal interpretation. The proportion of adequate specimens was higher in adults than in children. 50% of the adequate TEMs showed normal ciliary ultrastructure, 39% showed hallmark ultrastructural changes of PCD, and 11% had indeterminate findings. Among specimens without clearly normal ultrastructure, 72% had defects of the outer and/or inner dynein arms, while 7% had central apparatus defects with or without inner dynein arm defects. In summary, nasal scrape biopsies can be performed in the outpatient setting and yield interpretable samples, when performed by individuals with adequate training and experience according to an SOP.
Keywords: cilia, nasal biopsy, primary ciliary dyskinesia
Introduction
Primary ciliary dyskinesia (PCD) is a rare genetic disease characterized by abnormal ciliary structure and/or function1. The diagnosis of PCD is based on the presence of a typical clinical phenotype combined with identification of ciliary dysmotility and/or specific ciliary ultrastructural defects2,3. Even though genetic testing is useful in a subset of PCD patients (~20–25%), examination of ciliary ultrastructure remains the cornerstone diagnostic test for PCD. Yet, it is challenging to obtain an adequate biopsy specimen with a sufficient number of cilia that are technically satisfactory for accurate interpretation. Some clinical reference laboratories report that up to 80% of cilia samples submitted are unsatisfactory and inadequate for evaluation4. Further, significant expertise is required to produce high quality transmission electron micrographs (TEM) which are adequate to discriminate primary (“genetic”) versus secondary (“non-specific”) defects in ciliary ultrastructure. As a result, the diagnosis is often delayed or uncertain. Since the specialized techniques and expertise required for PCD diagnosis are not standardized or readily available, it has been suggested that patients suspected of having PCD be referred to tertiary diagnostic centers5.
Respiratory ciliated cells can be obtained either by nasal or tracheal brushings or biopsies. Most previous studies have described the frequency of detecting different ultrastructural abnormalities using these different techniques and the ability to distinguish between primary and secondary ultrastructural defects6–9. Only a few have examined the diagnostic yields and success rates of different techniques6,10. Papon and colleagues reported that TEM was more feasible in adults than in children, regardless of the biopsy site, at a large referral center in France10.
The objective of this study was to describe the North American multicenter experience of the Genetic Disorders of Mucociliary Clearance Consortium, a member of the Rare Disease Clinical Research Network. This consortium uses a standardized operating procedure (SOP) for the collection, fixation, TEM preparation, and centralized interpretation of nasal scrape biopsy specimens for the diagnosis of PCD.
Materials and Methods
Study Population
Subjects with suspected PCD were recruited for the study from around North America through contact with referring physicians, the PCD Foundation (www.pcdfoundation.org) and a national website (http://rarediseasesnetwork.epi.usf.edu/gdmcc/index.htm). These subjects were studied at one of seven clinical research sites of the Genetic Disorders of Mucociliary Clearance Consortium (see Appendix 1). At their study visits, all subjects underwent comprehensive clinical and laboratory evaluations. Other pathologic conditions such as cystic fibrosis, asthma, allergies, and immunodeficiency were excluded previously or at their study visits. Institutional Review Boards approved the protocols at each of the research sites. Informed consent was obtained from each of the subjects and/or their parents.
Ciliary Biopsy and Ultrastructure Evaluation
Prior to starting the study, a training session on biopsy and fixation techniques was held for investigators from each research site. This training session included a powerpoint presentation and face-to-face instruction from a single trainer (J. Carson) as well as individual demonstrations of nasal curettage on physician volunteers and immediate feedback on sample quality using light microscopy. Nasal scrape biopsies were performed using a curettage technique according to a SOP (see Appendix 2) whereby ciliated epithelial cells were obtained from the inferior surface of the inferior nasal turbinate. The curettes (Rhino-Pro®, Arlington Scientific, Inc) were immediately immersed in a cold fixative consisting of 2% glutaraldehyde, 2% paraformaldehyde, and 0.5% tannic acid11. This fixative was prepared by thawing and mixing a vial of frozen glutaraldehyde and paraformaldehyde with a vial of frozen tannic acid just prior to the specimen collection. Tissue specimens were stored refrigerated and shipped to a centralized laboratory at the University of North Carolina Chapel Hill.
TEMs were prepared by one individual (KB) and reviewed in a blinded fashion by 3 independent observers using previously described techniques12,13. Adequacy of the samples was based on the presence of an adequate number of ciliated cells that were technically sufficient for interpretation, and most samples had ≥ 20 cross sections of cilia. Nasal scrape specimens were typically deemed inadequate for formal evaluation if too few or no ciliated cells were identified in the sample, or occasionally, if low quality TEM images were produced, generally due to poor preservation of the sample. Reviewers examined the TEMs for absence or shortening of outer and inner dynein arms and for gross abnormalities in the central apparatus (central pairs, radial spokes). Subjects were classified as having either normal ciliary structure, absent or shortened outer dynein arms (ODA) alone, absent or shortened inner dynein arms (IDA) alone, combined defects of both the outer and inner dynein arms, central apparatus abnormalities including transpositions, combined defects of the IDA and central apparatus, or indeterminate findings. Samples were reported as indeterminate when abnormalities were not sufficiently clear to indicate a “hallmark” PCD ciliary defect, as these were most likely secondary abnormalities (e.g. compound cilia, ciliary disorientation, ciliary changes in cells exhibiting cytopathic effects).
Statistical Analysis
Data were analyzed using PASW Statistics v18 (SPSS Inc., Chicago, IL, USA). We used Chi-squared analyses and Fisher’s exact test when examining differences between sites and age groups. The forced expiratory volume in 1 second (FEV1) was expressed as percent of predicted normal using reference equations.14 Differences were considered statistically significant at p<0.05.
Results
The demographic and clinical characteristics of the referral subjects are listed in Table 1. Using the SOP, the 7 sites had an overall 88% success rate (394/448 samples) in acquiring specimens adequate for formal interpretation (Figure 1). Among TEMs adequate for formal evaluation (n=394), 50% (196/394) demonstrated normal ciliary ultrastructure, 39% (155/394) had hallmark ultrastructural changes of PCD, and 11% (43/394) had indeterminate findings. The success rates in acquiring adequate specimens varied among sites (Figure 2), with site 1 acquiring a significantly greater proportion of adequate samples than the other sites (92%, p<0.03) and sites 2 and 6 acquiring significantly fewer adequate samples (73%, p<0.001 and 69%, p<0.004, respectively). Site 7 had a failure rate comparable to sites 2 and 6 (30%), but due to the low number of samples performed, this result did not reach statistical significance (p=0.058). Site 1 performed the most procedures (n= 223) and sites 6 and 7 the least (29 and 13, respectively). At site 2, there was improvement over time, evidenced by the fact that among the first 18 specimens, only 8 (44%) were adequate for formal evaluation, whereas among the final 37 specimens, 32 (86%) were adequate. There was site to site variability in the proportion of abnormal specimens among the specimens from which a diagnosis could be made (Figure 2). Sites 2 and 4 had a significantly higher proportion of abnormal specimens (71%, p<0.01 and 75%, p<0.02 respectively) and site 3 had a significantly lower proportion of abnormal specimens (6.8%, p<0.001) in comparison to the other sites.
Table 1.
Clinical characteristics of referral subjects
| Clinical observation | Age 0–5 n (%) | Age 5–18 n (%) | Age>18 n (%) |
|---|---|---|---|
| Number of subjects a | 105 | 188 | 151 |
| Male gender | 60 (57%) | 98 (52%) | 48 (32%) |
| Caucasian ethnicity | 84 (80%) | 146(78%) | 127 (84%) |
| Situs status | |||
| Situs solitus | 60 (57%) | 129(69%) | 113 (75%) |
| Situs inversus | 31 (30%) | 45 (24%) | 32 (21%) |
| Situs ambiguous | 14 (13%) | 14(7%) | 6 (4%) |
| Otitis media | 86 (82%) | 165(88%) | 105 (70%) |
| Chronic sinusitis | 48 (46%) | 146(78%) | 119 (79%) |
| Bronchiectasis | 10 (10%) | 70 (37%) | 117 (77%) |
| FEV1 % predicted, mean (±SD)b | - c | 87% (±24%)d | 66% (±24%)e |
448 nasal scrapes were performed on a total of 444 subjects
FEV1. forced expiratory volume in 1 sec, SD, standard deviation, predicted values based on equations of Hankinson et al
FEV1 data is not available for subjects under 5 years of age
n = 162
n = 139
Figure 1.
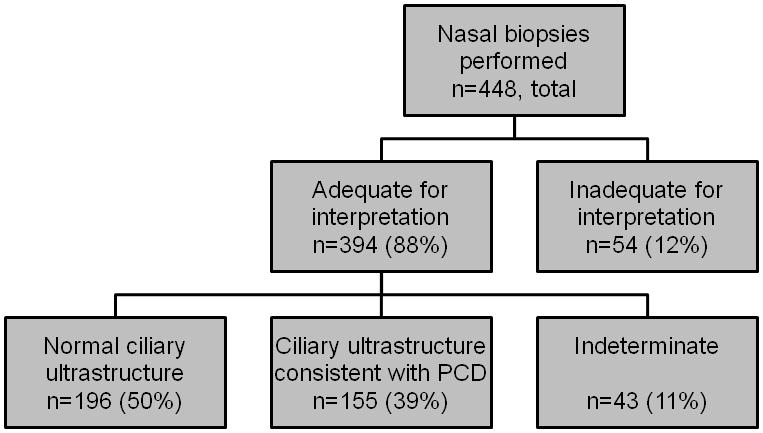
Distribution of ciliary biopsy results from all sites. 88% of all biopsies were adequate for formal interpretation.
Figure 2.
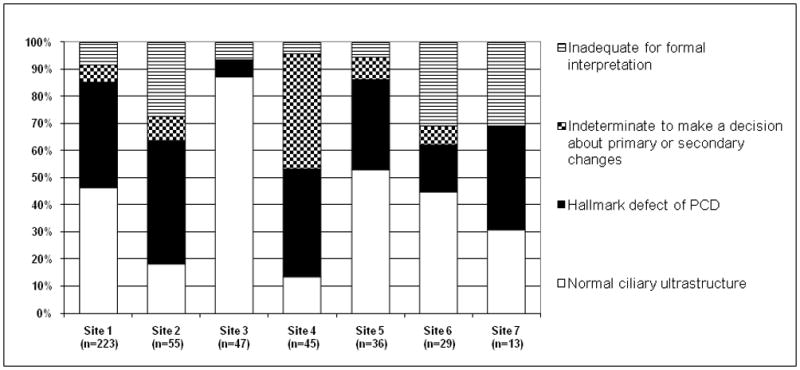
Distribution of ciliary biopsy results by site. All sites had 69% of samples that were adequate for formal interpretation.
Subject age factored into the likelihood of success. The success rate in acquiring specimens adequate for formal evaluation was higher in adults (93%) than in subjects under 18 years (86%, p<0.02, Figure 3). The success rate was similar between specimens from children under 5 years of age (85%) versus specimens from children aged 5 to 18 years (86%). The age distribution at sites with lower success rates was similar to the distribution at other study sites.
Figure 3.
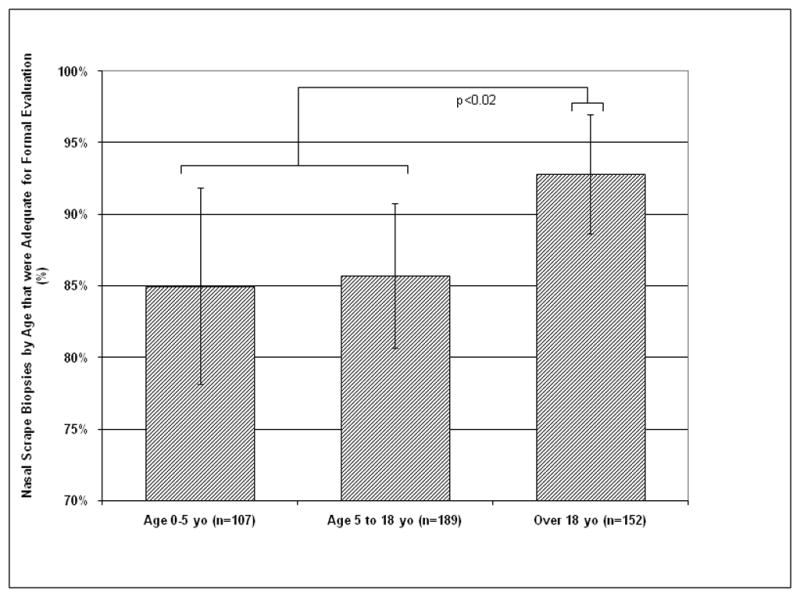
Percentage of nasal scrape biopsies by age that was adequate for formal interpretation. 85% of biopsies in infants and toddlers were adequate, but there was a higher success rate in those over 18 versus those under 18 (p <0.02).
The distribution of TEM-based diagnoses among the interpretable nasal scrape biopsies is shown in Figure 4. Among specimens without clearly normal ultrastructural findings (198/394), 42% displayed ODA defects, 12% showed IDA defects, and 18% showed combined ODA and IDA defects. Isolated central apparatus defects were rare (0.5%), but 6% of specimens showed combined central apparatus and IDA defects. No definitive diagnosis could be made from the remainder of the abnormal “indeterminate” specimens.
Figure 4.
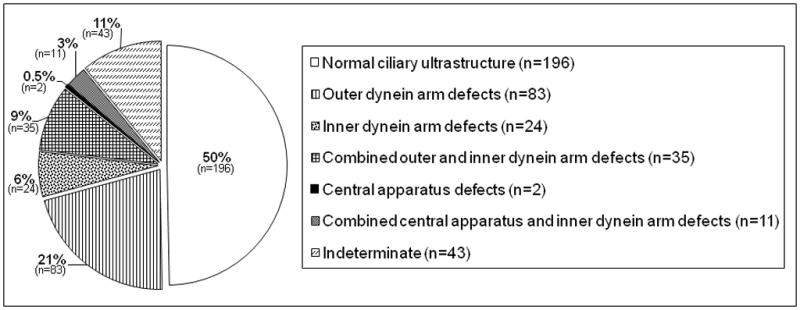
Distribution of TEM diagnoses among interpretable nasal scrape biopsies. ODA defects were the most common abnormality. Note the low frequency of central apparatus defects.
Discussion
In this multicenter study, we report a high success rate in obtaining nasal scrape biopsy specimens adequate for formal interpretation of ciliary ultrastructure. Experience appeared to be a factor in success rates, as the sites performing the highest volume of biopsies had the highest proportion of adequate specimens and the two sites performing the lowest volume had the lowest proportions of adequate specimens. Though differences were modest, nasal scrape biopsies were more successful in adults than in children. In this cohort of subjects with suspected or possible PCD, half had normal ciliary ultrastructure. Among those with ultrastructural abnormalities consistent with PCD, 72% involved a truncation or absence of outer and/or inner dynein arms while 7% involved central apparatus defects in isolation or in combination with IDA defects.
This is one of the largest reported series of biopsies to evaluate ciliary ultrastructure. When compared with other large studies, we achieved a high success rate in obtaining specimens adequate for formal evaluation 7,10,15. Two of the prior series were performed at a single center and the third series did not standardize the collection procedures among contributing sites. In the series reported by Papon and colleagues10, almost two thirds of pediatric specimens were bronchial biopsies in contrast to our study in which all specimens were obtained from nasal epithelium. The Papon group found that age was more of a factor than biopsy location (upper versus lower airway) in determining specimen adequacy, with lower rates of adequacy among children from both nasal scrapes and tracheal biopsies when compared to adults. While we similarly found a higher success rate in adults in comparison to children, the differences were modest and we still had an approximate 85% success rate in children.
We attribute our high rate of specimens adequate for formal interpretation to the establishment of strict procedural guidelines and a training session before starting the study. Care was taken to standardize the biopsy, fixation, and transport of specimens. The vast majority of these samples were collected without anesthesia in outpatient clinics. All samples were interpreted at a single site, by consensus opinion of three blinded observers, minimizing differential error at the interpretation step. Despite the standardization of procedures, experience still played a role in the success rates as the sites performing the highest volume of biopsies had the highest proportion of interpretable specimens. The most common reason for an inadequate specimen was a sample with too few or no ciliated cells identified. Direct feedback to centers with higher proportions of inadequate samples improved subsequent sample quality. The French experience highlights the fact that a large portion of nasal specimens inadequate for formal evaluation lack sufficient numbers of cilia, either due to the low density of cilia, the lack of epithelial tissue acquired, or squamous metaplasia10.
Our experience has implications for clinical practice involving PCD testing. First, we showed that it is possible to obtain nasal scrape biopsies adequate for formal interpretation in outpatient clinics, saving time and money, and avoiding the sedation risk associated with bronchial biopsies. We also showed that even among our specialized centers, additional training may be needed to improve the success rate at low volume sites. It is important to note that a normal TEM, in the absence of ciliary function studies, does not exclude PCD as a diagnostic consideration as evidenced most strongly by disease-causing mutations in the DNAH11 gene16–18. Despite 50% of our cohort having normal ciliary ultrastructure, some of these individuals have a classic clinical phenotype and low nasal nitric oxide levels, and may in fact have disease causing mutations in DNAH11 or in genes yet to be determined2,3,18.
This study has some limitations. While describing differences in success rates based on site or subject age, we note that the study was not designed or powered to evaluate specific predictors of success, but rather to describe our experience. The study was also not designed to demonstrate the highest yield biopsy location in the airway. We used the inferior surface of the inferior nasal turbinate for its ease of access in the outpatient setting coupled with its high percentage of ciliated epithelial cells19. Additionally, by using three investigators at a single site to evaluate all specimens, we may have introduced observer bias in interpretation of specimens although the blinded review should have minimized these biases. While the use of a single site to interpret specimens was appropriate for methodologic reasons in this clinical research study, this is not feasible for patients being seen clinically throughout the country. This study may also have some ascertainment bias in that approximately one half of the samples came from a single site. Further, this study did not compare the effects of different fixatives on TEM quality. Therefore, we cannot comment on whether the combination of glutaraldehyde, paraformaldehyde and tannic acid is any better than standard glutaraldehyde fixative. And finally, our consortium did not universally perform ciliary motion analysis as part of our diagnostic approach, 20 precluding us from determining how assessment of ciliary function aids in the diagnosis of PCD.
Despite these limitations, by using a standardized approach for procuring and processing nasal epithelium, we were able to offer a precise TEM-based diagnosis in the majority of subjects with abnormal electron microscopy. Until more comprehensive genetic testing becomes available, TEM currently is and will remain the cornerstone diagnostic test for PCD for at least the next few years. In summary, nasal scrape biopsies can be performed in the outpatient setting and generally yield interpretable samples, when performed by individuals with adequate training and experience according to an SOP.
Supplementary Material
Appendix 1. Participating clinical research sites in the Genetic Disorders of Mucociliary Clearance Consortium include the University of North Carolina at Chapel Hill, University of Colorado, National Institute of Allergy and Infectious Diseases, Washington University in St. Louis, Stanford University, University of Washington, and University of Toronto.
Appendix 2. SOP used to perform nasal scrape biopsies.
Acknowledgments
Sources of support: This research was supported by the National Institutes of Health (U54 RR019480-05, U54 HL096458-06, R01 HL071798 and the Division of Intramural Research, NIAID), Cystic Fibrosis Foundation (OLIN08B0), and by the Clinical and Translational Science Awards Program, NIH/NCRR (UL1 RR025780, UL1RR025014).
The authors wish to thank the research coordinators at all sites for their extraordinary efforts to conduct this study, including Susan Minnix and Caroline O’Connor LaFave at the University of North Carolina, Jane Quante at Washington University, Shelley Mann at the University of Colorado, Sharon McNamara and Liz Cochrane at the University of Washington, Donna Wilkes at the University of Toronto, and Reginald Claypool and Meghan O’Connell at the National Institute of Allergy and Infectious Diseases. We wish to thank the participants and their families for their support of this study. Additional thanks to Brandie Wagner, PhD, who provided biostatistical help and advice.
Footnotes
A preliminary report on data contained in this manuscript was presented in abstract form at the 2009 American Thoracic Society International Conference.
References
- 1.Afzelius BA. A human syndrome caused by immotile cilia. Science. 1976;193(4250):317–319. doi: 10.1126/science.1084576. [DOI] [PubMed] [Google Scholar]
- 2.Leigh MW, Zariwala MA, Knowles MR. Primary ciliary dyskinesia: improving the diagnostic approach. Curr Opin Pediatr. 2009;21(3):320–325. doi: 10.1097/MOP.0b013e328329cddb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbato A, Frischer T, Kuehni CE, Snijders D, Azevedo I, Baktai G, Bartoloni L, Eber E, Escribano A, Haarman E, Hesselmar B, Hogg C, Jorissen M, Lucas J, Nielsen KG, O’Callaghan C, Omran H, Pohunek P, Strippoli MP, Bush A. Primary ciliary dyskinesia: a consensus statement on diagnostic and treatment approaches in children. Eur Respir J. 2009;34(6):1264–1276. doi: 10.1183/09031936.00176608. [DOI] [PubMed] [Google Scholar]
- 4.Mierau GW, Agostini R, Beals TF, Carlen B, Dardick I, Henderson DW, Pysher TJ, Weeks DA, Yowell RL. The role of electron microscopy in evaluating ciliary dysfunction: report of a workshop. Ultrastruct Pathol. 1992;16(1–2):245–254. doi: 10.3109/01913129209074565. [DOI] [PubMed] [Google Scholar]
- 5.O’Callaghan C, Chilvers M, Hogg C, Bush A, Lucas J. Diagnosing primary ciliary dyskinesia. Thorax. 2007;62(8):656–657. doi: 10.1136/thx.2007.083147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacCormick J, Robb I, Kovesi T, Carpenter B. Optimal biopsy techniques in the diagnosis of primary ciliary dyskinesia. J Otolaryngol. 2002;31(1):13–17. doi: 10.2310/7070.2002.19153. [DOI] [PubMed] [Google Scholar]
- 7.Pizzi S, Cazzato S, Bernardi F, Mantovani W, Cenacchi G. Clinico-pathological evaluation of ciliary dyskinesia: diagnostic role of electron microscopy. Ultrastruct Pathol. 2003;27(4):243–252. doi: 10.1080/01913120309919. [DOI] [PubMed] [Google Scholar]
- 8.Caruso G, Gelardi M, Passali GC, de Santi MM. Nasal scraping in diagnosing ciliary dyskinesia. Am J Rhinol. 2007;21(6):702–705. doi: 10.2500/ajr.2007.21.3107. [DOI] [PubMed] [Google Scholar]
- 9.Plesec TP, Ruiz A, McMahon JT, Prayson RA. Ultrastructural abnormalities of respiratory cilia: a 25-year experience. Arch Pathol Lab Med. 2008;132(11):1786–1791. doi: 10.5858/132.11.1786. [DOI] [PubMed] [Google Scholar]
- 10.Papon JF, Coste A, Roudot-Thoraval F, Boucherat M, Roger G, Tamalet A, Vojtek AM, Amselem S, Escudier E. A 20-year experience of electron microscopy in the diagnosis of primary ciliary dyskinesia. Eur Respir J. 2009 doi: 10.1183/09031936.00046209. [DOI] [PubMed] [Google Scholar]
- 11.Carson JL, Collier AM, Fernald GW, Hu SC. Microtubular discontinuities as acquired ciliary defects in airway epithelium of patients with chronic respiratory diseases. Ultrastruct Pathol. 1994;18(3):327–332. doi: 10.3109/01913129409023201. [DOI] [PubMed] [Google Scholar]
- 12.Noone PG, Leigh MW, Sannuti A, Minnix SL, Carson JL, Hazucha M, Zariwala MA, Knowles MR. Primary ciliary dyskinesia: diagnostic and phenotypic features. Am J Respir Crit Care Med. 2004;169(4):459–467. doi: 10.1164/rccm.200303-365OC. [DOI] [PubMed] [Google Scholar]
- 13.Carson JL, Hu SS, Collier AM. Computer-assisted analysis of radial symmetry in human airway epithelial cilia: assessment of congenital ciliary defects in primary ciliary dyskinesia. Ultrastruct Pathol. 2000;24(3):169–174. doi: 10.1080/01913120050132903. [DOI] [PubMed] [Google Scholar]
- 14.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 15.Carda C, Armengot M, Escribano A, Peydro A. Ultrastructural patterns of primary ciliary dyskinesia syndrome. Ultrastruct Pathol. 2005;29(1):3–8. doi: 10.1080/01913120490897538. [DOI] [PubMed] [Google Scholar]
- 16.Bartoloni L, Blouin JL, Pan Y, Gehrig C, Maiti AK, Scamuffa N, Rossier C, Jorissen M, Armengot M, Meeks M, Mitchison HM, Chung EM, Delozier-Blanchet CD, Craigen WJ, Antonarakis SE. Mutations in the DNAH11 (axonemal heavy chain dynein type 11) gene cause one form of situs inversus totalis and most likely primary ciliary dyskinesia. Proc Natl Acad Sci U S A. 2002;99(16):10282–10286. doi: 10.1073/pnas.152337699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwabe GC, Hoffmann K, Loges NT, Birker D, Rossier C, de Santi MM, Olbrich H, Fliegauf M, Failly M, Liebers U, Collura M, Gaedicke G, Mundlos S, Wahn U, Blouin JL, Niggemann B, Omran H, Antonarakis SE, Bartoloni L. Primary ciliary dyskinesia associated with normal axoneme ultrastructure is caused by DNAH11 mutations. Hum Mutat. 2008;29(2):289–298. doi: 10.1002/humu.20656. [DOI] [PubMed] [Google Scholar]
- 18.Zariwala MA, Knowles MR, Omran H. Genetic defects in ciliary structure and function. Annu Rev Physiol. 2007;69:423–450. doi: 10.1146/annurev.physiol.69.040705.141301. [DOI] [PubMed] [Google Scholar]
- 19.Knowles MR, Carson JL, Collier AM, Gatzy JT, Boucher RC. Measurements of nasal transepithelial electric potential differences in normal human subjects in vivo. Am Rev Respir Dis. 1981;124(4):484–490. doi: 10.1164/arrd.1981.124.4.484. [DOI] [PubMed] [Google Scholar]
- 20.Stannard WA, Chilvers MA, Rutman AR, Williams CD, O’Callaghan C. Diagnostic testing of patients suspected of primary ciliary dyskinesia. Am J Respir Crit Care Med. 2010;181(4):307–314. doi: 10.1164/rccm.200903-0459OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1. Participating clinical research sites in the Genetic Disorders of Mucociliary Clearance Consortium include the University of North Carolina at Chapel Hill, University of Colorado, National Institute of Allergy and Infectious Diseases, Washington University in St. Louis, Stanford University, University of Washington, and University of Toronto.
Appendix 2. SOP used to perform nasal scrape biopsies.


