Abstract
The incidence and distribution of human rotavirus G types among children under 5 years old with acute gastroenteritis were determined over a 4-year period (1998 to 2002) by using monoclonal antibodies and reverse transcription-PCR methods. Rotavirus was detected in 1,155 (31%) of 3,760 specimens tested. Rotavirus was studied in every month of the 48-month survey period. Rotavirus activity occurred mainly (51%) in the typically cooler months in Spain (November to February). The age distribution of rotavirus-positive cases showed that 90% of patients (1,038 of 1,155) were under 2 years old. Rotavirus types were determined for 576 of 1,155 patients (50%). G1 was the main genotype detected (53%), and the second most common was G4 (24%). The G2, G9, and G3 rotavirus types were detected in 14, 6, and 2% of the cases, respectively. Dual infections were detected in only 0.6%. The seasonal distribution of genotypes showed a significant genotypic shift: whereas G4 strains predominated (57%) during the 1998 to 2000 seasons, the G1 gradually increased to account for 75% in the 2000 to 2002 seasons. In addition, the present study reports the first detection of the G9 genotype in human fecal samples in Spain. Therefore, additional types may be required for vaccine development strategies that currently target only types G1 to G4.
Group A rotavirus is the most important cause of severe gastroenteritis in young children worldwide (28). Rotavirus infections are associated with high rates of morbidity throughout the world and with high rates of mortality in developing countries, accounting for more than 800,000 infant deaths per year (4).
Rotavirus possesses a genome of 11 double-stranded RNA segment, each encoding one viral protein (19). The outer layer of rotavirus is composed of two proteins, VP7 and VP4, encoded by RNA segment 7, 8, or 9 (depending on the strain) and segment 4, respectively. These proteins elicit neutralizing antibody responses and form the basis of the current dual classification of group A rotavirus into G (standing for glycoprotein VP7) and P (standing for protease-sensitive protein VP4) serotypes. As the VP7 and VP4 genes segregate independently, various combinations of G and P types have been detected in natural isolates. At least 14 and 20 different G and P types have been identified, respectively (19). Of those, at least 10 G types and 11 P types have been found to infect humans (18, 25).
Serotyping by ELISA with anti-VP7 serotype-specific monoclonal antibodies and genotyping by reverse transcription-PCR (RT-PCR) have been widely used for typing (18, 19, 21, 32). The incidence of infection within a particular group A rotavirus type varies between geographical areas and from one season to the next (28). It is therefore necessary to ascertain the rotavirus types circulating in different communities over the course of a number of years. Globally, different surveys indicate that G1P[8], G2P([4], G3P[8]), and G4P[8] are the most common G and P types (2, 3, 8, 22, 29, 36). However, since the introduction and wider use of molecular biology-based typing methods over the last 10 years, other rotavirus types have increasingly been reported in different parts of world, such as G5 (30), G8 (16), and G9 (40) strains.
There are few data available about rotavirus type circulation in Spain (10, 12, 47). It has been estimated that rotavirus infections accounted for 25% of hospitalizations for gastroenteritis in Spain in one year (46), with a seasonal pattern of incidence during the cooler months of the year. In October 1998, the Viral Gastroenteritis Study Group carried out a pilot prospective program to undertake the surveillance and characterization of rotavirus strains causing annual epidemics of severe diarrhea in young children. The program was designed to monitor the antigenic variation of rotaviruses before release of a rotavirus vaccine in Spain. The study relied on the design, cooperation, and participation of the National Microbiology Center of Spain. We describe the frequency and temporal distribution of human group A rotavirus types among patients admitted to a Madrid children's hospital during a 4-year period.
MATERIALS AND METHODS
Patients.
Severo Ochoa Hospital is the reference sanitary hospital of Health Care Area IX in Madrid, serving a population of 350,000 inhabitants. The study population included children under 5 years old with acute gastroenteritis for whom stool cultures were requested. Acute diarrhea was defined as three or more liquid stools over a 24-h period.
Patients were seen during the rotavirus seasons of 1998 to 2002. A rotavirus season was defined as the 12-month period between 1 October of one year and 30 September of the following year. The date of sample collection, together with age, sex, and details of patients' attendance at a general practice or admission to hospital, were available in all cases. Guardians of the children were asked for permission to enroll the patients in the study.
Samples.
Stool specimens were collected within 24 to 48 h after admission to the hospital for all patients. Samples were obtained by direct deposition in a sterile container and were transported the same day to hospital laboratories, where they were stored at 4°C until processing. Specimens for rotavirus antigen detection were used on the day of collection. Rotavirus-positive specimens were prepared as 10% homogenates in phosphate-buffered saline (pH 7.0) and were stored at −70°C until they were typed (26). Diluted and undiluted samples were stored at −70°C until further tests were required. Duplicate samples obtained from the same patient were excluded and not counted; therefore, patients, not just stool samples, were evaluated.
Detection of rotavirus in fecal samples.
A microplate-based solid-phase sandwich enzyme immunoassay (IDEIA Rotavirus; Dako Diagnostics, Cambridge, United Kingdom) was used to detect rotavirus antigen in fecal specimens according to the manufacturer's instructions. It utilizes a polyclonal antibody to detect specific group A rotavirus proteins, particularly the internal capsid protein (VP6). Samples with ELISA cutoff values corresponding to >0.25 were considered positive. This assay is able to detect rotavirus concentrations of as low as 7.8 × 105 viral particles/ml and shows good correlation, sensitivity, and specificity in comparison with electron microscopy (99.5, 100, and 99.2%, respectively) (20). Additionally, this assay shows a good correlation with RT-PCR (48).
Rotavirus typing methods.
Rotavirus-positive specimens were confirmed and typed by ELISA and/or RT-PCR methods. To obtain a representative selection of the epidemic distribution, at least 50% of each week's rotavirus-positive samples were randomly selected for type characterization during the 4 years of the study.
Rotavirus-positive samples were G serotyped by sandwich-type enzyme immunoassay with a panel of specific monoclonal antibodies for the common (G1 to G4) group A human rotavirus according to protocol of the manufacturer (Silenus Laboratories, Hawthorn, Australia) as previously described (14, 43). This is a simple, rapid, and suitable assay for serotyping the large numbers of specimens obtained from epidemiological studies (12). Furthermore, it has been shown to be applicable to serotyping of human rotaviruses directly in stool specimens (37). The monoclonal antibodies used were RV4:2 for G1, RV5:3 for G2, RV3:1 for G3, and ST3:1 for G4. Specimens with an absorbance value of greater than 0.2 were considered positive for that serotype. The sensitivity and specificity of this method were verified by using different strains of human rotavirus that were adapted to cell culture.
Rotavirus strains that were not serotypeable by the ELISA technique were genotyped by the multiplex RT-PCR method. Viral RNA was extracted from fecal samples by with an RNAID kit (BIO101, Vista, Calif.) (11). Reverse transcription was used to synthesize the cDNA corresponding to the genomic segment encoding VP7, and the characterization of G genotypes was performed with specific oligonucleotide primers according to a previously described system (17, 40, 41). The cocktail of primers used for typing was the common primer 9con1 and the G type-specific primers G1(9T1-1), G2(9T1-2), G3(9T-3P), G4(9T-4), and G9(9T-9B). The sizes of the G type-specific PCR products were 110 bp (G9), 158 bp (G1), 244 bp (G2), 403 bp (G4), and 466 bp (G3). As positive controls the following human reference rotavirus strains Wa (G1), S2 (G2), 107E1B (G3), ST3 (G4), and 116E (G9) were used.
Statistical analysis.
Statistical analysis was performed with Epi Info software (Centers for Disease Control and Prevention, Atlanta, Ga.). Distributions of rotavirus types were compared by use of the chi-square test. All tests were two tailed, and P values of <0.05 were considered significant.
RESULTS
Study population and overall rotavirus results.
Between 1998 and 2002, a total of 3,760 stool samples from children under 5 years old with acute gastroenteritis were received for rotavirus study. Rotavirus antigen was detected in a total of 1,155 patients (31%). The mean age of the patients was 11 ± 9 months, and 57% were males and 43% were females (male/female ratio, 1.3:1). The numbers of children with rotavirus by year were 269 of 725 (37%) in 1998 to 1999, 244 of 995 (25%) in 1999 to 2000, 364 of 1,143 (32%) in 2000 to 2001, and 278 of 897 (31%) during 2001 to 2002.
Temporal distribution of rotavirus and incidence by age.
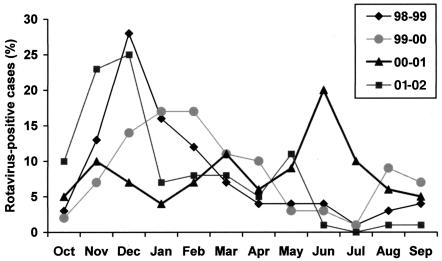
Rotavirus was detected in every month of the 48 months studied. The peak months of rotavirus activity are shown in Fig. 1. The monthly distribution varied little from season to season, with rotavirus activity occurring mainly (51%) in the typically cooler months in Spain (November to February). However, during 2000 to 2001 the variations were more marked and the circulation of rotavirus was irregular, with an epidemic peak (39%) in warm months (May to July). No statistical differences were detected in the rotavirus serotypes or ages of the subjects between this outbreak and previous or subsequent outbreaks (P > 0.05).
FIG. 1.
Seasonal peaks of rotavirus-positive reports in children under 5 years with gastroenteritis in Madrid, Spain, from October 1998 to September 2002 (n = 1,155).
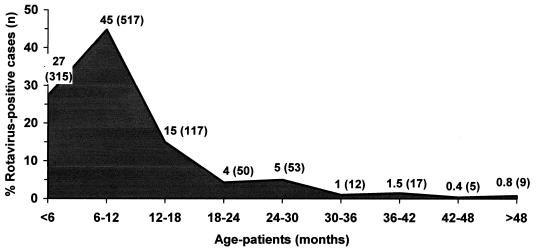
The age distribution of rotavirus-positive cases in 1998 to 2002 is shown in Fig. 2. A total of 90% (1,038 of 1,155) of the patients were under 2 years old (P < 0.05), and differences were not observed from season to season (P > 0.05).
FIG. 2.
Age distribution of patients with rotavirus gastroenteritis detected in Madrid, Spain, during 1998 to 2002 (n = 1,155).
Distribution of G types.
Rotavirus types were determined for 576 of 1,155 patients (50%), corresponding to 141 of 269 (52%) in 1998 to 1999, 86 of 244 (35%) in 1999 to 2000, 200 of 364 (55%) in 2000 to 2001, and 149 of 278 (52%) in 2001 to 2002. The rotavirus types circulating in Madrid from 1998 to 2002 are shown in Table 1.
TABLE 1.
Distribution and frequency of rotavirus G types over 4 years (1998 to 2002) in Madrid, Spaina
| Rotavirus type | No. (%) of strains typed in the following rotavirus seasons:
|
||||
|---|---|---|---|---|---|
| 1998-1999 | 1999-2000 | 2000-2001 | 2001-2002 | Total | |
| G1 | 25 (18) | 23 (27) | 141 (71) | 117 (79) | 306 (53) |
| G2 | 2 (1) | 8 (9) | 47 (24) | 25 (17) | 82 (14) |
| G3 | 1 (1) | 10 (12) | 0 (0) | 0 (0) | 11 (2) |
| G4 | 97 (69) | 34 (40) | 5 (3) | 2 (1) | 138 (24) |
| G9 | 15 (11) | 11 (13) | 6 (3) | 3 (2) | 35 (6) |
| G1 and G2 | 0 (0) | 0 (0) | 0 (0) | 2 (1) | 2 (0.3) |
| G1 and G4 | 1 (1) | 0 (0) | 1 (1) | 0 (0) | 2 (0.3) |
| Total | 141 | 86 | 200 | 149 | 576 |
The G types of at least 50% of the rotavirus-positive samples obtained each season were determined.
Overall, G1 was the main type detected, being responsible for 53% of the rotavirus infections. G4 was the second most common and was responsible for 24% of the infections. The G2, G9, and G3 rotaviruses types were detected in 14, 6, and 2% of the infections, respectively. Mixed G types reflecting dual infections were detected in only 0.6% of the samples (two cases were G1 and G2, and two cases were G1 and G4).
The temporal distribution showed that prevalence of rotavirus types differed from year to year. G4 was the most frequent rotavirus type (57%) during 1998 to 2000, whereas G1 was less common (21%). The number of G4 type strains dropped to 2% during 2000 to 2002, while G1 types increased to 75%. Also, the G9 type was mainly detected (26 of 35 cases) during 1998 to 2000, whereas the G2 type was predominantly detected (72 of 82 cases) during 2000 to 2002. The G3 type was almost the only one detected (10 of 11 cases) during 1999 to 2000.
The distribution of rotavirus types by age of the patients does not show data of significant interest, due to all types being more frequent in patients under 2 years old. The distribution of rotavirus types by month also does not show any interesting patterns (data not shown).
DISCUSSION
In this report we describe the results of a survey in Spain to determine the importance and epidemiological features of rotavirus infections in a specific geographic area (Madrid) and well-defined target population (children under 5 years old). We considered that the true rotavirus prevalence could be higher than that estimated here (31%), due to the fact that it represents only moderate or severe cases among Spanish children with diarrhea requiring medical attention.
In Spain, rotavirus infections are not subject to specific surveillance. The main indicator for this type of infection is the information derived from the Microbiological Information System, which consists of a series of laboratories that voluntarily report cases (46). However, only limited information can be obtained from this source. As a result of this situation, it has become necessary to obtain objective information on the epidemiology and impact of this infection prior to the introduction of universal vaccination, which is expected mainly to protect against severe diarrhea and to reduce the cost of rotavirus infections (34).
The typical pattern of regular epidemics that appear in cooler months in other regions of the world (5, 13) is similar to that observed in our region. However, significant changes were observed over the 4-year study. In the period from 2000 to 2001, seasonality was not very marked and there were peaks of activity in warmer months. These variations in the timing of peak activity have been also reported in other studies (1, 12, 13, 42) and recall the pattern observed in less-developed countries with a tropical climate, in which seasonality of rotavirus infection is unclear or nonexistent (5, 13).
To our knowledge, there are insufficient data available on rotavirus types circulating in Spain. In a previous survey that we conducted from 1996 to 1997 in Madrid, we found a clear predominance of type G1 (68%) over G4 (29%) (12, 47). Others have reported that the main strains detected in Guipuzcoa (north of Spain) from 1989 to 1995 were also G1 (57%) and G4 (32%) serotypes. In this 4-year study, the G1 type was the most prevalent (53%), followed by the G4 type (24%). These results emphasize the high frequency of the G1 and G4 types circulating in European countries (3, 9, 26, 29, 33, 35, 36).
Although the G1 type was the most prevalent rotavirus type in Madrid during the sampling period, we have detected an important genotypic shift from type G4 to G1 over the 4 years of study. From 1998 to 2000 the G1 and G4 types cocirculated at high levels but G4 strains predominated (69 and 40%, respectively), while G1 strains were less frequent (18 and 27%, respectively). In contrast, from 2000 to 2002 the predominant rotavirus type was G1 (71 and 79%, respectively), and the G4 type was detected in very few cases (3 and 1%, respectively). Studies on the distribution of rotavirus types in several countries have identified important regional variations and temporal changes in G and P types (30, 39, 44). The present study, in agreement with others (6, 10, 35), shows that major shifts in the predominant rotavirus types can occur. This could reflect the dynamics of viral circulation in our region.
When two different rotaviruses of the same group coinfect one cell, the genomic fragments can undergo reassortments (24). These reassortants may be responsible for epidemics or outbreaks of gastroenteritis within a population, as has been demonstrated by sequence analysis of the VP1 and VP7 genes (49). On the basis of these findings, it is possible to speculate that the shift of the predominant genotype from G1 to G4 may be a consequence of reassortments occurring during the course of mixed infections of G1 plus G4 under the selective pressure of neutralizing antibodies or may be the result of progressive antigenic variation due to selection of viral strains by immunological determinants (10). However, this hypothesis remains speculative in the absence of supporting evidence.
This surveillance study was also marked by the appearance of G9 rotaviruses during all periods of study, especially for the first two seasons. These strains were responsible for 11 and 13% of rotavirus strains analyzed during the 1998 to 1999 and 1999 to 2000 seasons, respectively. G9 rotaviruses have been also reported in Australia (38), India (27), Bangladesh (44), Malawi (16), the United States (40), Brazil (41), and the United Kingdom (15). To our knowledge, this is the first report of the G9 serotype in clinical fecal samples in Spain. The rapid emergence of G9 as a major infecting genotype has important implications for rotavirus vaccine strategies. Current candidate vaccines target only infections with serotypes G1 to G4 infections. Ongoing further surveillance is needed to obtain a clearer picture of the importance of the G9 rotaviruses.
Rotavirus type G2 was also very frequent from 2000 to 2002, whereas rotavirus type G3 appeared only sporadically. During 1999 to 2000 a coexistence of several rotavirus types was detected, which suggests rapid spread of virus strains across the country.
In this study we did not test for the possible presence of serotype G5 in fecal samples, because up to now this type has been rare among humans, having been found only in Brazilian (23, 31) and Argentinean (7) children. In Spain, G5 rotavirus strains have not been detected in human clinical samples. Locally, in Barcelona, Villena et al. (45) detected only three G5 strains (2%) in 104 sewage samples. One of these samples was associated with a P[8] type, while the other two could not be associated with any P type but were closely related to porcine strains by phylogenetic analysis. Further studies would need to include G5 strains in the surveillance of rotavirus-positive patients in order to know if it is a cause of viral infection among Spanish children.
Our results indicate that the epidemiology of rotavirus types in Spain is variable, although it is similar to that observed in other industrialized countries. These data illustrate some important differences in the distribution of serotypes in Spain and map the occurrence of new serotypes such as G9, which has emerged worldwide in the last few years. The factors that determine the identity of rotavirus strains in circulation among the human population, as well as their well-known temporal and periodic variations, deserve more intense investigations that will allow them to be clarified and better understood. Ongoing surveillance of seasonal rotavirus serotype patterns is needed in particular to monitor the spread of new or emerging serotypes like G9 and G5. Such information could influence the strategy for development of new generations of rotavirus vaccines and could show whether the requirements in Spain differ from those in other parts of the world. Data from continued comprehensive etiological studies of genetic and antigenic variations in rotaviruses that cause severe disease in young children will serve as baseline data for the study of the effect of vaccination on the incidence of severe rotavirus disease and on the emergence of new strains.
Acknowledgments
We thank V. Montero and A. Alonso (Centro Nacional de Microbiología, Instituto Salud Carlos III, Madrid, Spain) for technical assistance, J. Villar for critical reading of the manuscript, R. Glass (Centers for Disease Control and Prevention, Atlanta, Ga.) for support and advice, and C. Domingo (Instituto Salud Carlos III, Madrid, Spain) for continual support and advice.
REFERENCES
- 1.Anonymous. 1996. Rotavirus infections in humans. Commun. Dis. Rep. 6:85. [Google Scholar]
- 2.Arguelles, M. H., G. A. Villegas, A. Castello, A. Abrami, P. D. Ghiringhelli, L. Semorile, and G. Glikmann. 2000. VP7 and VP4 genotyping of human group A rotavirus in Buenos Aires, Argentina. J. Clin. Microbiol. 38:252-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arista, S., E. Vizzi, D. Ferraro, A. Cascio, and R. Di Stefano. 1997. Distribution of VP7 serotypes and VP4 genotypes among rotavirus strains recovered from Italian children with diarrhea. Arch. Virol. 142:2065-2071. [DOI] [PubMed] [Google Scholar]
- 4.Bern, C., and R. I. Glass. 1994. Impact of diarrhoeal diseases worldwide, p. 1-26. In A. Z. Kapikian (ed.), Viral infections of the gastrointestinal tract, 2nd ed. Marcel Dekker Inc., New York, N.Y.
- 5.Bishop, R. F. 1994. Natural history of humans rotavirus infections, p. 131-167. In A. Z. Kapikian (ed.), Viral infections of the gastrointestinal tract, 2nd ed. Marcel Dekker Inc., New York, N.Y.
- 6.Bishop, R. F., L. E. Unicomb, and G. L. Barnes. 1991. Epidemiology of rotavirus serotypes in Melbourne, Australia, from 1973 to 1989. J. Clin. Microbiol. 29:862-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bok, K., G. Palacios, K. Sijvarger, D. Matson, and J. Gomez. 2001. Emergence of G9 P[6] human rotaviruses in Argentina: phylogenetic relationships among G9 strains. J. Clin. Microbiol. 39:4020-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bon, F., C. Fromantin, S. Aho, P. Pothier, and E. Kohli. 2000. G and P genotyping of rotavirus strains circulating in France over a 3-year period: detection of G9 and P[6] strains at low frequencies. J. Clin. Microbiol. 38:1681-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bon, F., P. Fascia, M. Dauvergne, D. Tenenbaum, H. Planson, A. M. Petion, P. Pothier, and E. Kohli. 1999. Prevalence of group A rotavirus, human calicivirus, astrovirus, and adenovirus type 40 and 41 infections among children with acute gastroenteritis in Dijon, France. J. Clin. Microbiol. 37:3055-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buesa, J., C. O. de Souza, M. Asensi, C. Martinez, J. Prat, and M. T. Gil. 2000. VP7 and VP4 genotypes among rotavirus strains recovered from children with gastroenteritis over a 3-year period in Valencia, Spain. Eur. J. Epidemiol. 16:501-506. [DOI] [PubMed] [Google Scholar]
- 11.Buesa, J., J. Colomina, J. Raga, A. Villanueva, and J. Prat. 1996. Evaluation of reverse transcription and polymerase chain reaction (RT/PCR) for detection of rotaviruses: applications of the assay. Res. Virol. 147:353-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cilla, G., E. Perez-Trallero, M. C. Lopez-Lopategui, A. Gilsetas, and M. Gomariz. 2000. Incidence, seasonality and serotypes of rotavirus in Gipuzkoa (Basque Country), Spain. A 14-year study. Epidemiol. Infect. 125:677-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cook, S. M., R. I. Glass, C. W. LeBaron, and M. S. Ho. 1990. Global seasonality of rotavirus infections. Bull. W. H. O. 68:171-177. [PMC free article] [PubMed] [Google Scholar]
- 14.Coulson, B. S., L. E. Unicomb, G. A. Pitson, and R. F. Bishop. 1987. Simple and specific enzyme immunoassay using monoclonal antibodies for serotyping human rotaviruses. J. Clin. Microbiol. 25:509-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cubitt, W. D., A. D. Steele, and M. Iturriza. 1996. Characterisation of rotaviruses from children treated at a London hospital during 1996: emergence of strains G9P2A[6] and G3P2A[6]. J. Med. Virol. 61:150-154. [DOI] [PubMed] [Google Scholar]
- 16.Cunliffe, N. A., J. S. Gondwe, R. L. Broadhead, M. E. Molyneux, P. A. Woods, J. S. Bresee, R. I. Glass, J. R. Gentsch, and C. A. Hart. 1999. Rotavirus G and P types in children with acute diarrhea in Blantyre, Malawi, from1997 to 1998: predominance of novel P[6]G8 strains. J. Med. Virol. 57:308-312. [PubMed] [Google Scholar]
- 17.Das, B. K., J. R. Gentsch, H. G. Cicirello, P. A. Woods, A. Gupta, M. Ramachandran, R. Kumar, M. K. Bhan, and R. I. Glass. 1994. Characterization of rotavirus strains from newborns in New Delhi, India. J. Clin. Microbiol. 32:1820-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desselberger, U., M. Iturriza-Gómara, and J. Gray. 2001. Rotavirus epidemiology and surveillance. Novartis Symp. Ser. 238:125-147. [DOI] [PubMed] [Google Scholar]
- 19.Estes, M. 1996. Rotaviruses and their replication, p. 1625-1655. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.). Fields virology, 3rd ed., vol 2. Lippincott-Raven Publishers, Philadelphia, Pa.
- 20.Flewett, T. H., C. F. Arias, L. F. Avendano, A. Ghafoor, M. M. Mathan, L. Mendis, K. Moe, and R. F. Bishop. 1989. Comparative evaluation of the W. H. O. and DAKOPATTS enzyme-linked immunoassay kits for rotavirus detection. Bull. W. H. O. 67:369-374. [PMC free article] [PubMed] [Google Scholar]
- 21.Gentsch, J. R., R. I. Glass, P. Woods, V. Gouvea, M. Gorziglia, J. Flores, B. K. Das, and M. K. Bhan. 1992. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J. Clin. Microbiol. 30:1365-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gentsch, J. R., P. A. Woods, M. Ramachandran, B. K. Das, J. P. Leite, A. Alfieri, R. Kumar, M. K. Bhan, and R. I. Glass. 1996. Review of G and P typing results from a global collection of rotavirus strains: implications for vaccine development. J. Infect. Dis. 1:30-36. [DOI] [PubMed] [Google Scholar]
- 23.Gouvea, V., L. de Castro, M. C. Timenetsky, H. Greenberg, and N. Santos N. 1994. Rotavirus serotype G5 associated with diarrhea in Brazilian children. J. Clin. Microbiol. 32:1408-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenberg, H. B., A. R. Kalica, R. G. Wyatt, R. W. Jones, A. Z. Kapikian, and R. M. Chanock. 1981. Rescue of noncultivatable human rotavirus by gene reassortment during mixed infection with ts mutants of a cultivatable bovine rotavirus. Proc. Natl. Acad. Sci. USA 78:420-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoshino, Y., and A. Z. Kapikian. 1996. Classification of rotavirus VP4 and VP7 serotypes. Arch. Virol. Suppl. 12:99-111. [DOI] [PubMed] [Google Scholar]
- 26.Iturriza-Gomara, M., J. Green, D. W. Brown, M. Ramsay, U. Desselberger, and J. J. Gray. 2000. Molecular epidemiology of human group A rotavirus infections in the United Kingdom between 1995 and 1998. J. Clin. Microbiol. 38:4394-4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jain, V., B. K. Das, M. K. Bhan, R. I. Glass, and J. R. Gentsch. 2001. Great diversity of group A rotavirus strains and high prevalence of mixed rotavirus infections in India. J. Clin. Microbiol. 39:3524-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kapikian, A. Z., and R. M. Chanock. 1996. Rotaviruses, p. 1657-1708. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.). Fields virology, 3rd ed., vol 2. Lippincott-Raven Publishers, Philadelphia, Pa.
- 29.Koopmans, M., and D. Brown. 1999. Seasonality and diversity of group A rotaviruses in Europe. Acta Paediatr. Suppl. 426:14-19. [DOI] [PubMed] [Google Scholar]
- 30.Leite, J. P., A. A. Alfieri, P. A. Woods, R. I. Glass, and J. R. Gentsch. 1996. Rotavirus G and P types circulating in Brazil: characterization by RT-PCR, probe hybridization, and sequence analysis. Arch. Virol. 141:2365-2374. [DOI] [PubMed] [Google Scholar]
- 31.Mascarenhas, J. D., A. C. Linhares, Y. B. Gabbay, and J. P. Leite. 2002. Detection and characterization of rotavirus G and P types from children participating in a rotavirus vaccine trial in Belem, Brazil. Mem. Inst. Oswaldo Cruz 97:113-117. [DOI] [PubMed] [Google Scholar]
- 32.Masendycz, P. J., E. A. Palombo, R. J. Gorrell, and R. F. Bishop. 1997. Comparison of enzyme immunoassay, PCR, and type-specific cDNA probe techniques for identification of group A rotavirus gene 4 types (P types). J. Clin. Microbiol. 35:3104-3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maunula, L., and C. H. van Bonsdorff. 1995. Rotavirus serotypes and electropherotypes in Finland from 1986 to 1990. Arch. Virol. 140:877-890. [DOI] [PubMed] [Google Scholar]
- 34.Midthun, K., and A. Z. Kapikian. 1996. Rotavirus vaccines: an overview. Clin. Microbiol. Rev. 9:423-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noel, J. S., G. M. Beards, and W. D. Cubitt. 1991. Epidemiological survey of human rotavirus serotypes and electropherotypes in young children admitted to two children's hospitals in northeast London from 1984 to 1990. J. Clin. Microbiol. 29:2213-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Mahony, J., B. Foley, S. Morgan, J. G. Morgan, and C. Hill. 1999. VP4 and VP7 genotyping of rotavirus samples recovered from infected children in Ireland over a 3-year period. J. Clin. Microbiol. 37:1699-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Ryan, M. L., D. O. Matson, M. K. Estes, A. V. Bartlett, and L. K. Pickering. 1990. Molecular epidemiology of rotavirus in children attending day care centers in Houston. J. Infect. Dis. 162:810-816. [DOI] [PubMed] [Google Scholar]
- 38.Palombo, E. A., P. J. Masendycz, H. C. Bugg, N. Bogdanovic-Sakran, G. L. Barnes, and R. F. Bishop. 2000. Emergence of serotype G9 human rotaviruses in Australia. J. Clin. Microbiol. 38:1305-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramachandran, M., B. K. Das, A. Vij, R. Kumar, S. S. Bhambal, N. Kesari, H. Rawat, L. Bahl, S. Thakur, P. A. Woods, R. I Glass, M. K. Bhan, and J. R. Gentsch. 1996. Unusual diversity of human rotavirus G and P genotypes in India. J. Clin. Microbiol. 34:436-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramachandran, M., J. R. Gentsch, U. D. Parashar, S. Jin, P. A. Woods, P. A., J. L. Holmes, C. D. Kirkwood, R. F. Bishop, H. B. Greenberg, S. Urasawa, G. Gerna, B. S. Coulson, K. Taniguchi, J. S. Bresee, and R. I. Glass. 1998. Detection and characterisation of novel rotavirus strains in the United States. J. Clin. Microbiol. 36:3223-3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santos, N., C. C. Soares, E. M. Volotao, M. C. Albuquerque, and Y. Hoshino. 2003. Surveillance of rotavirus strains in Rio de Janeiro, Brazil, from 1997 to 1999. J. Clin. Microbiol. 41:3399-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torok, T. J., P. E. Kilgore, M. J. Clarke, R. C. Holman, J. S. Bresee, and R. I. Glass. 1997. Visualizing geographic and temporal trends in rotavirus activity in the United States, 1991 to 1996. Pediatr. Infect. Dis. J. 16:941-946. [DOI] [PubMed] [Google Scholar]
- 43.Unicomb, L. E., B. S. Coulson, and R. F. Bishop. 1989. Experience with an enzyme immunoassay for serotyping human group A rotaviruses. J. Clin. Microbiol. 27:586-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Unicomb, L. E., G. Podder, J. R. Gentsch, P. A. Woods, K. Z. Hasan, A. S. Faruque, M. J. Albert, and R. I. Glass. 1999. Evidence of high-frequency genomic reassortment of group A rotavirus strains in Bangladesh: emergence of type G9 in 1995. J. Clin. Microbiol. 37:1885-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Villena, C., W. M. El-Senousy, F. X. Abad, R. M. Pinto, and A. Bosch. 2003. Group A rotavirus in sewage samples from Barcelona and Cairo: emergence of unusual genotypes. Appl. Environ. Microbiol. 69:3919-3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Visser, L. E., R. Cano Portero, N. J. Gay, and J. F. Martínez Navarro. 1999. Impact of rotavirus disease in Spain: an estimate of hospital admissions due to rotavirus. Acta Paediatr. Suppl. 426:72-77. [DOI] [PubMed] [Google Scholar]
- 47.Wilhelmi, I., C. Mier, E. Roman, J. Colomina, J. Prat, and A. Sanchez-Fauquier. 1999. The molecular epidemiology of the rotavirus in Spanish children. Enferm. Infecc. Microbiol. Clin. 17:509-514. [PubMed] [Google Scholar]
- 48.Wilhelmi, I., J. Colomina, D. Martin-Rodrigo, E. Roman, and A. Sanchez-Fauquier. 2001. New immunochromatographic method for rapid detection of rotaviruses in stool samples compared with standard enzyme immunoassay and latex agglutination techniques. Eur. J. Clin. Microbiol. Infect. Dis. 20:741-743. [DOI] [PubMed] [Google Scholar]
- 49.Zao, C. L., W. N. Yu, C. L. Kao, K. Taniguchi, C. Y. Lee, and C. N. Lee. 1999. Sequence analysis of VP1 and VP7 genes suggests occurrence of a reassortant of G2 rotavirus responsible for an epidemic of gastroenteritis. J. Gen. Virol. 80:1407-1415. [DOI] [PubMed] [Google Scholar]