Abstract
Recently, we have reported novel and complex calcium dynamics in the RIA interneuron, which has been implicated in several navigational behaviors in C. elegans. Here, we review our findings on the compartmentalized and global calcium events in RIA and propose functional consequence as well as potential regulatory mechanisms of these intriguing calcium signals.
Keywords: compartmental calcium signal, global calcium signal, regulation of navigation, wiring diagram, acetylcholine signaling, corollary discharge
Introduction
Recently, we reported an analysis of unexpectedly complex calcium dynamics in the C. elegans RIA interneuron.1 RIA receives numerous synaptic inputs from upstream interneurons and sensory neurons, suggesting that it may integrate signals generated by multiple stimuli. These inputs are clustered together in the proximal region (?loop?) of its single unbranched axon, which extends into the ventral nerve cord first and further extends into the nerve ring.2 Within the nerve ring, RIA is innervated primarily by SMD class motor neurons, which have neuromuscular synapses with the head muscles, as well as by other motor neurons. Interspersed with these motor inputs are synapses from RIA to both the SMD and RMD motor neurons (Fig. 1).2 RIA thus receives both convergent sensory input and extensive motor feedback.
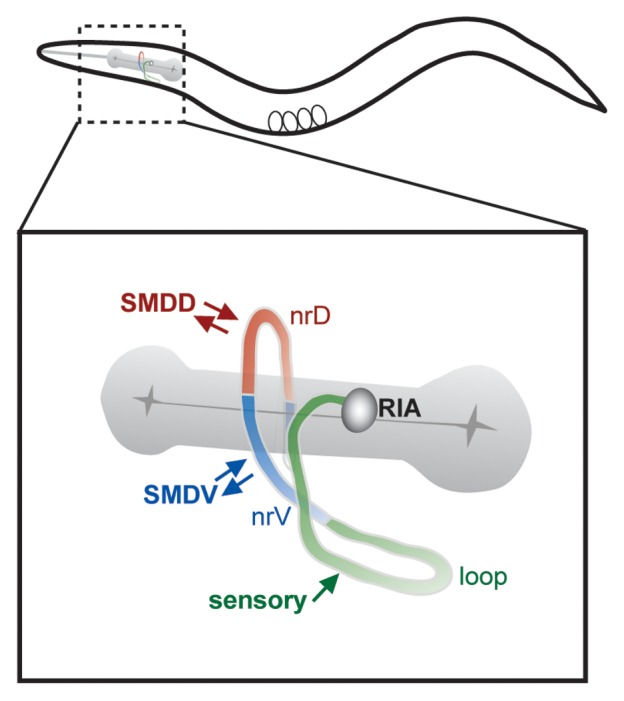
Figure 1. RIA anatomy and circuit connections. The single RIA axon can be subdivided by physiology and synaptic organization into distinct domains. Shown here are the proximal segment (loop), and the ventral (nrV) and dorsal (nrD) segments of the nerve ring. Convergent sensory input is located exclusively in the loop. Inputs from SMD motor neurons are segregated, such that SMDV innervates the nrV segment, while SMDD innervates nrD. Arrows denote synaptic inputs.
How does this subcellular synaptic organization contribute to the neuronal and circuit properties of the RIA neuron? Using the genetically encoded calcium indicator GCaMP33 expressed specifically in RIA, we have observed two classes of Ca2+ responses in the RIA axon. First, we have recorded compartmentalized Ca2+ events in either the ventral (nrV) or dorsal (nrD) segment of the RIA axon within the nerve ring. We have found that the calcium dynamics in nrV and nrD are independent of each other, are correlated with ventral and dorsal head bending, respectively, and are driven by input from SMD motor neurons. Second, we have identified synchronous events during which Ca2+ levels either rise or fall simultaneously in all three axonal domains (nrV, nrD, and loop). These synchronous events are correlated with sensory input, such that presentation of an attractive odor, isoamyl alcohol (IAA), causes synchronous suppression of RIA calcium levels, while removal of IAA causes synchronous calcium increases. Thus, the independent calcium activity in the nrD and nrV compartments represents motor feedback, while synchronized activity of RIA axon represents sensory inputs.
How are these two different types of calcium dynamics established in the same axon? We have shown that the independent calcium signals in the nrV and nrD compartments are dependent on acetylcholine release from SMD motor neurons presynaptic to RIA and the GAR-3 muscarinic acetylcholine receptor that acts in RIA, likely via mobilization of internal Ca2+ stores, to generate the local calcium activity. Synchronous events, which are initiated by the sensory input to the proximal loop region, require glutamatergic neurotransmission; the removal of synaptic signals from SMD motor neurons did not compromise the production of RIA synchronized calcium activity. These two activity patterns appeared to be additive within the nerve ring compartments. Finally, we show that selective elimination of the compartmentalized calcium dynamics while sparing the synchronized activity alters locomotion, causing exaggerated head bending and a higher amplitude gait. This result suggests that RIA is part of a negative feedback circuit that restricts head bending amplitude.
We propose that the surprising physiological complexity that we observed in RIA has important implications for understanding neural circuits and behavior in C. elegans, and is instructive in understanding the flexible nature of connections in the nervous system. First, the encoding properties of RIA suggest a model for C. elegans sensorimotor behaviors that require detection of the spatial distribution of a stimulus. Second, if this kind of mechanism is widespread in the C. elegans nervous system, it has important implications for the development of computational models based on the connectome and suggests that we should consider more carefully the subcellular distribution of synapses when working with the wiring diagram. In the following sections, we will further explain these two concepts.
Self-monitoring via Corollary Discharge and its Role in Spatial Behaviors
Animals monitor their own behavior via proprioceptive feedback and sensory input. However, to allow real-time compensation or integration of ongoing behavior with changes in sensory stimulus, the nervous system monitors its own motor commands as they happen, a category of mechanism referred to as efference copy or corollary discharge (CD).4 CD normally consists of a copy of motor commands routed back to sensory pathways. In our circuit, the cholinergic output from SMDs serves two functions: it activates either dorsal or ventral head muscles, and in parallel it induces local calcium increases in either the dorsal or ventral compartment of the RIA axon (i.e. nrD or nrV). Our results also suggest that mutations that eliminate motor-driven compartmental activity may result in enhanced sensory-evoked synchronized activity.1 Thus, we propose that the motor neuron-driven signal into RIA resembles corollary discharge and allows the monitoring of head motor commands.
Several C. elegans behaviors indicate that the nematode is able to integrate changes in sensory input with head movement in order to respond to the spatial distribution of a stimulus (Fig. 2). The first described of these is isothermal tracking, in which animals track a preferred temperature within a gradient. This behavior requires that animals continually bias their gait to follow the contour of the isotherm. A requirement during isothermal tracking was one of the first functions discovered for RIA.5-7 More recently, a chemotactic behavior was described in which animals steer in a favorable direction within a stimulus gradient, termed the weathervane strategy.8 Again, this requires smooth gait-biasing in response to stimulus changes across individual head sweeps. Finally, Albrecht et al.9 recently reported robust directionally biased turning away from unfavorable conditions when encountering a sharp stimulus boundary at an oblique angle.
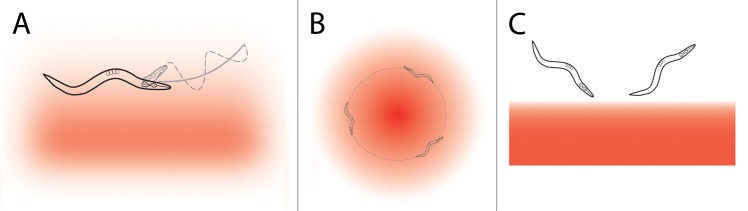
Figure 2. Behaviors expected to require spatial detection of stimuli. (A) Klinotaxis is the gradual biasing of the animals? gait in a preferred direction within a stimulus gradient (red).8 (B) During isothermal tracking, animals select a preferred temperature in a radial or linear (not shown) temperature gradient and continuously bias their gait to remain within a narrow stimulus band.5-7 (C) When animals encounter an aversive boundary (red) at an oblique angle, they perform a short reversal and then reliably turn away from the boundary.9
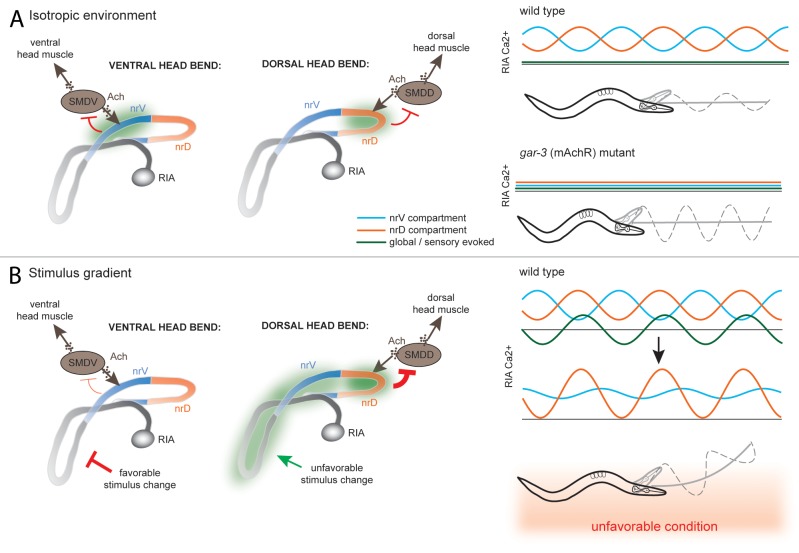
RIA?s simultaneous representation of both sensory stimuli and head bending makes it an attractive candidate for involvement in these behaviors. Our results show that, under isotropic conditions, the independent calcium activity in the nrV and nrD compartments of RIA functions to limit head bending amplitude. Meanwhile, in an animal crawling perpendicular to a stimulus gradient, RIA will be globally suppressed or activated in phase with head swings. This introduces asymmetry into the negative feedback to head motor neurons via modification of compartmental activity in nrV or nrD, such that bends in the unfavorable direction are more strongly inhibited relative to bends in the favorable direction, resulting in a curved trajectory (Fig. 3).
Figure 3. Model for RIA function in gradient navigation. (A) In an isotropic environment, RIA monitors head movement via input from SMD class motor neurons and mediates negative feedback, limiting head deflection via compartmentalized Ca2+ signals of nrV and nrD that correlate with head bending. In gar-3 mutants, compartmentalized signaling is lost, leading to an increase in gait amplitude. (B) When perpendicular to a gradient, sensory changes across head swings cause global Ca2+ changes that are in phase with head bends in the unfavorable direction and antiphase with the favorable direction. This increases Ca2+ signaling and damps head bending in the unfavorable direction and extends it in the favorable direction. This asymmetry leads to curvature of locomotion in the favorable direction.
One prediction of this model is that asymmetrically inhibiting or activating RIA in an isotropic environment should lead to smooth turning. Data supporting this prediction were recently reported by Kocabas et al.,10 who used Channelrhodopsin to selectively activate the AIY interneuron when the animal bent in one direction but not the other. AIY is activated by attractant presentation,11 directly innervates RIA, and is required for mediating the global suppression of RIA evoked by an attractive stimulus, suggesting an inhibitory synapse (MH, unpublished observation). Asymmetric stimulation of AIY, which would mimic a spatial gradient of attractant across each head swing, would thus asymmetrically inhibit RIA and extend head bends in the preferred direction. A second prediction is that RIA should be required for spatial orientation behaviors, where navigation is guided by the spatial patterns of sensory cues. Future experiments will test these strong predictions.
Topographic Organization of Synapses and Circuit Flexibility
In a recent review, it was noted that the high level of connectivity in the C. elegans wiring diagram poses a strong challenge to predict information flow within this connectome. In addition to all possible anatomical connections, the neuromodulatory signals regulate the activity of given pathways.12 We propose that the properties of RIA provide a coding mechanism in the anatomical organization of synapses in the nerve ring. This organization may provide a spatial logic for modulatory control of specific pathways. Our results suggest that locally generated signals may facilitate, potentiate, or inhibit synaptic transmission in specific segments of the nerve ring on different time scales. This dynamic and spatial control of synaptic activity could be a mechanism for generating multiple functional subnetworks from a large set of anatomical connections.
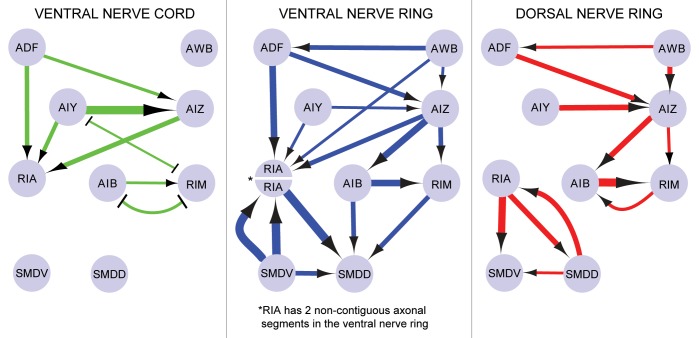
This model requires that synaptic connections be non-randomly distributed in the nerve ring. However, even non-random arrangements of synapses do not necessarily imply functional organization, because synaptic location may result from anatomical or developmental constraints. To illustrate this point, we looked at the anatomical distribution of synapses in a circuit that mediates aversive olfactory learning.13 Segmenting these locations according to the anatomical divisions we observed in RIA (near the ventral ganglia, the dorsal nerve ring, or the ventral nerve ring) produces strikingly different network topologies (Fig. 4).
Figure 4. Synaptic topography as a mechanism for network flexibility. Local modulation of synapses based on anatomical position within the nerve ring would allow functional selection of particular synaptic connections appropriate for a given behavior or state. The figure illustrates how connectivity patterns differ for different anatomical domains for a circuit involved in pathogen avoidance learning.13 Arrows denote synapses and blunt-ended lines denote gap junctions. The thickness of the arrows correlates with the number of the synapses.
Regulation of RIA Calcium Dynamics
Our results suggest that the local calcium signals generated in the nrV and nrD compartments of RIA axon depend on the signaling that activates intracellular calcium stores, compatible with the previous model proposing that C. elegans neurons are likely to be isopotential.14 It is intriguing to speculate how compartmental calcium dynamics in RIA axons, once initiated by motor neuron inputs, are regulated. At least two possible mechanisms are worth considering. First, while the wiring diagram reveals synaptic and electrical connections among all the neurons, it does not provide a detailed picture on some other properties of the worm nervous system. For example, we do not yet know to what extent the shape of the RIA axon may generate certain physical confinement and, thus, contributes to the observed local calcium dynamics. Second, it would be interesting to examine whether the subcellular distribution of mitochondria regulates RIA calcium properties, given the high calcium buffering capacity of mitochondria.15
Meanwhile, while our result demonstrates that the local calcium transients in the RIA axonal compartments require the acetylcholine signals from the SMD motor neurons, it does not completely exclude the possibility that these locally generated calcium signals propagate along the axon. However, our unpublished results argue against this possibility. Although the animal mostly alternates its head bending between dorsal and ventral directions, it occasionally generates two or more repeated head bends in either the dorsal or ventral direction; in these cases, local changes in calcium signals are observed only in the nrD or nrV compartments, respectively. It is conceptually important to note that the sensory-evoked synchronized calcium events among RIA axonal compartments suggest that these axonal domains are not electrically isolated; instead independent calcium dynamics in different axonal compartments provides one mechanism for the small nervous system to enhance its computational potential by integrating global activity with functional domains on a subcellular scale.
Perspectives on the Wiring Diagram
It is known that synapses are dynamically modulated. Our observations of RIA suggest that synaptic topography, particularly within the nerve ring, may be an organizing principle in determining which pathways are activated or suppressed during a particular behavior. When researchers employ the wiring diagram to generate testable hypotheses about circuit function, it is usually necessary to arbitrarily ignore most synapses due to the overly abundant connectivity.12 It is possible that many or most connections are functionally silent during a given behavior or in response to a particular stimulus. We propose that spatially restricted modulatory signaling in the nerve ring plays a role in selecting specific sub-circuits from a large set of possible circuits. To illustrate this point hypothetically, we can consider the connections between a set of neurons that regulate learned pathogen avoidance13 (Fig. 4). By considering only those synapses in the dorsal nerve ring, ventral nerve ring, or within the ventral nerve cord, strikingly different patterns of connectivity between the neurons are revealed.
Identifying the site and mode of action of modulatory signals is a major challenge. The primary means of monitoring neuronal activity?calcium imaging and recording membrane potential?do not capture many of the intracellular signaling pathways employed by modulators. In the case of RIA, intracellular calcium stores mediate some form of inhibitory feedback to motor neurons, presumably in the absence of changes in membrane potential. While whole-cell recordings of calcium and voltage are essential for understanding network dynamics, tools that measure local signaling pathways or allow the visualization of transmitter release at the synaptic level may be required to tease apart the finer-scaled mechanisms that sculpt functional circuits from an abundance of connections.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/worm/article/25546
References
- 1.Hendricks M, Ha H, Maffey N, Zhang Y. Compartmentalized calcium dynamics in a C. elegans interneuron encode head movement. Nature. 2012;487:99–103. doi: 10.1038/nature11081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 3.Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–81. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crapse TB, Sommer MA. Corollary discharge across the animal kingdom. Nat Rev Neurosci. 2008;9:587–600. doi: 10.1038/nrn2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hedgecock EM, Russell RL. Normal and mutant thermotaxis in the nematode Caenorhabditis elegans. Proc Natl Acad Sci USA. 1975;72:4061–5. doi: 10.1073/pnas.72.10.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mori I, Ohshima Y. Neural regulation of thermotaxis in Caenorhabditis elegans. Nature. 1995;376:344–8. doi: 10.1038/376344a0. [DOI] [PubMed] [Google Scholar]
- 7.Luo L, Clark DA, Biron D, Mahadevan L, Samuel AD. Sensorimotor control during isothermal tracking in Caenorhabditis elegans. J Exp Biol. 2006;209:4652–62. doi: 10.1242/jeb.02590. [DOI] [PubMed] [Google Scholar]
- 8.Iino Y, Yoshida K. Parallel use of two behavioral mechanisms for chemotaxis in Caenorhabditis elegans. J Neurosci. 2009;29:5370–80. doi: 10.1523/JNEUROSCI.3633-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albrecht DR, Bargmann CI. High-content behavioral analysis of Caenorhabditis elegans in precise spatiotemporal chemical environments. Nat Methods. 2011;8:599–605. doi: 10.1038/nmeth.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kocabas A, Shen C-H, Guo ZV, Ramanathan S. Controlling interneuron activity in Caenorhabditis elegans to evoke chemotactic behaviour. Nature. 2012;490:273–7. doi: 10.1038/nature11431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chalasani SH, Chronis N, Tsunozaki M, Gray JM, Ramot D, Goodman MB, et al. Dissecting a circuit for olfactory behaviour in Caenorhabditis elegans. Nature. 2007;450:63–70. doi: 10.1038/nature06292. [DOI] [PubMed] [Google Scholar]
- 12.Bargmann CI. Beyond the connectome: how neuromodulators shape neural circuits. Bioessays. 2012;34:458–65. doi: 10.1002/bies.201100185. [DOI] [PubMed] [Google Scholar]
- 13.Ha HI, Hendricks M, Shen Y, Gabel CV, Fang-Yen C, Qin Y, et al. Functional organization of a neural network for aversive olfactory learning in Caenorhabditis elegans. Neuron. 2010;68:1173–86. doi: 10.1016/j.neuron.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodman MB, Hall DH, Avery L, Lockery SR. Active currents regulate sensitivity and dynamic range in C. elegans neurons. Neuron. 1998;20:763–72. doi: 10.1016/S0896-6273(00)81014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol. 2012;13:566–78. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]