Abstract
Cell invasion through basement membrane is an essential part of normal development and physiology, and occurs during the pathological progression of human inflammatory diseases and cancer. F-actin-rich membrane protrusions, called invadopodia, have been hypothesized to be the “drill bits” of invasive cells, mediating invasion through the dense, highly cross-linked basement membrane matrix. Though studied in vitro for over 30 y, invadopodia function in vivo has remained elusive. We have recently discovered that invadopodia breach basement membrane during anchor cell invasion in C. elegans, a genetically and visually tractable in vivo invasion event. Further, we found that the netrin receptor DCC localizes to the initial site of basement membrane breach and directs invasion through a single gap in the matrix. In this commentary, we examine how the dynamics and structure of AC-invadopodia compare with in vitro invadopodia and how the netrin receptor guides invasion through a single basement membrane breach. We end with a discussion of our surprising result that the anchor cell pushes the basement membrane aside, instead of completely dissolving it through proteolysis, and provide some ideas for how proteases and physical displacement may work together to ensure efficient and robust invasion.
Keywords: invasion, basement membrane, netrin, invadopodia
Introduction
Basement membranes are thin, dense, cell-associated extracellular matrices that underlie all epithelia and endothelia, and surround muscle, fat, and neuronal cells.1 Basement membranes are the most ancient of extracellular matrices, appearing near the emergence of metazoans.2 All basement membranes have a similar structure composed of a common assembly of approximately 10 large, insoluble proteins. Most notable are the laminin and type IV collagen heterotrimers, which have unique self-assembly properties. Laminin is deposited and assembled first at the cell surface and provides a template for assembly of additional matrix components.3 A network of type IV collagen is added to the laminin matrix and cross-linked intermolecularly through covalent bonds, providing basement membranes their structural integrity.4,5
A number of cells acquire the unique ability to cross basement membrane barriers. Examples include cells that undergo epithelial-to-mesenchymal transitions in development, muscle precursor, and neural crest cells during their dispersal migrations, and leukocyte trafficking through the body for immune surveillance.6,7 Cell invasive behavior is also co-opted during disease progression in a wide range of inflammatory conditions, as well as in cancer metastasis.8,9 Protrusive, F-actin-rich subcellular structures that have matrix removal activity, called invadopodia, have been hypothesized to allow transformed fibroblasts and highly invasive cancer cells to penetrate basement membrane.10-12 Although originally identified in vitro in 1980, the physiological relevance of invadopodia has remained controversial. Invadopodia activity has been difficult to visualize as cell invasion often occurs deep in complex tissue environments. Further, cell invasion is often stochastic temporally and spatially, thus adding to the challenge of imaging this process in vivo.
In cell culture conditions, invadopodia generate numerous small holes under cells on matrix-coated glass.13,14 How an invasive cell is able to transform these small holes into a clear path for migration is also not known. Treatments that lead to additional invadopodia impede invasive ability in in vitro assays through reconstituted 3D matrices, suggesting that tight regulation of invadopodia formation, including a mechanism to shut off invadopodia function, may be critical to ensuring successful invasion.15
Our lab has developed genetic and microscopy-based approaches using anchor cell (AC) invasion in C. elegans to understand mechanisms underlying invasion through basement membrane. The AC is a specialized uterine cell that breaches the juxtaposed uterine and vulval basement membranes in a highly stereotyped manner during the mid-L3 larval stage (Fig. 1).16 This invasion event initiates connection between the developing uterine and vulval tissues. Through forward genetic screens, we have previously identified a number of genes required for AC invasion.17–21 The human orthologs of many of these genes are overexpressed in tumor cells and associated with invasive behavior, suggesting the mechanisms underlying cell invasion are conserved.18 One implicated gene was the netrin receptor unc-40, the deleted in colorectal cancer (DCC) ortholog, which we previously found localizes to the invasive cell membrane of the AC in contact with the basement membrane.20
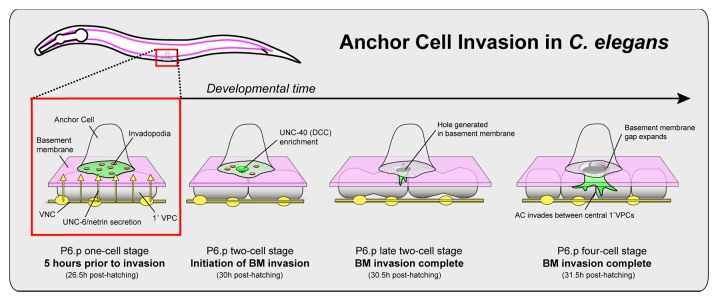
Figure 1. Anchor cell (AC) invasion in C. elegans. AC invasion occurs during the L3 larval stage and is tightly coordinated with the underlying vulval cell (P6.p) divisions. Prior to invasion (P6.p one-cell stage), the anchor cell (AC) forms a polarized invasive membrane, including F-actin-rich invadopodia (red circles). Invadopodia form and turn over until the mid L3 larval stage, when during a narrow 20 min developmental window one or more breach the basement membrane (BM; P6.P two-cell stage). The breach site becomes enriched in the netrin receptor, UNC-40 (DCC). By recruiting F-actin regulators, UNC-40 directs the formation of an invasive protrusion that extends toward the ventral nerve cord (yellow) where UNC-6 (netrin) is secreted (P6.p late two-cell stage). As the protrusion grows, the basement membrane underneath the AC is pushed aside creating a single basement membrane breach (P6.p four-cell stage).
By developing multi-dimensional time-lapse microscopy and quantitative image analysis to follow F-actin dynamics at the AC-basement membrane interface, we recently found that invadopodia form and mediate the initial basement membrane breach during AC invasion in C. elegans.22 Further, we’ve determined that the netrin receptor UNC-40 localizes to the initial breach and promotes the formation of a large invasive protrusion that guides the AC through a single expanding gap in the basement membrane (Fig. 1). Below, we highlight the findings and significance of these studies, the connection of this in vivo work to studies from in vitro tumor cell lines and discuss newly arising questions generated from these discoveries.
AC-Invadopodia Breach the Basement Membrane
Our live-cell imaging of F-actin at the invasive cell membrane of the AC revealed that prior to invasion, F-actin was organized into small (~1.0 µm) structures that turned over rapidly with a median lifetime of 45 s. Approximately 10 of these structures were present at a given time. By following their dynamics in relation to the initial basement membrane breach, we further determined that one of these structures always presaged and then occupied the initial site of basement membrane penetration (Fig. 2). Similar to invadopodia in cultured cells, we also found that these structures were dependent on the activity of the integrin matrix receptor.23 Further, the structures contained multiple components associated with invadopodia in tumor cell lines, including Rac GTPases, the Ena/VASP actin regulator, and the phospholipid PI(4,5)P2.24–26 The association of these F-actin foci with basement membrane breach, regulation by integrin, and association with known invadopodia components, indicate that these F-actin structures are in vivo invadopodia. Prior to these studies, invadopodia had only been described in metastatic cancer cell lines or transformed cells.10,14,27 Thus, more than three decades after their discovery, this work has finally put to rest doubts about the physiological relevance of invadopodia. Further, our investigations support the idea that invadopodia are a key component of a normal cell invasion program that is co-opted during cancer metastasis.
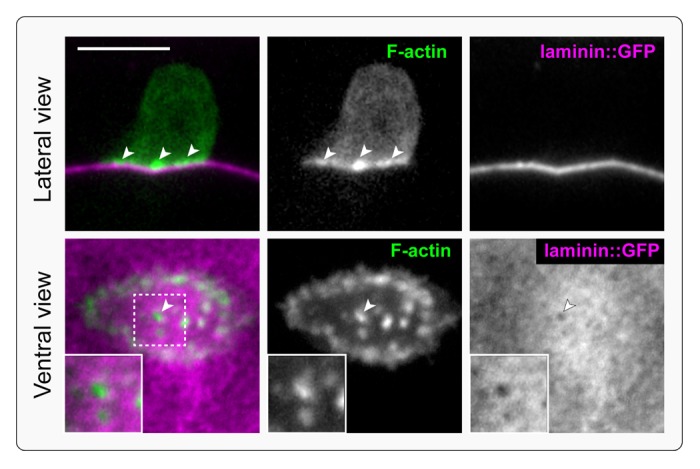
Figure 2. Invadopodia breach the basement membrane. F-actin-rich, protrusive invadopodia presage and then occupy the initial basement membrane breach. The top panel is a single lateral confocal section of the AC prior to invadopodial breach. An F-actin probe (middle panel; moeABD::mCherry) expressed in the AC shows invadopodia (arrowheads) along the AC-basement membrane interface (overlay, left, F-actin green, basement membrane magenta; basement membrane is visualized with laminin::GFP, right). The bottom panel is a ventral view of the AC showing an invadopodium breaching the basement membrane (arrowhead; magnified in inset).
Interestingly, we have found that AC-invadopodia turn over rapidly, with lifetimes on average of less than a minute. These dynamics are in contrast to invadopodia behavior in vitro, where these structures can have half-lives over an hour.28 For example, studies with GFP-actin in the melanoma cell line A375mm revealed that most invadopodia persisted for 6–8 h.13 We hypothesize that these differences may reflect the lack of environmental cues and the physical constraints of the in vitro culture systems, which preclude normal regulation or formation of protrusions. Visualizing invadopodia dynamics in other invasive cell types and organisms in physiological settings would help clarify this issue.
Many additional open questions remain about AC-invadopodia. We don’t know what transcriptional program(s) specifies invadopodia, what seeds the formation of each invadopodia, what regulates the periodicity of their dynamic turnover, or what dictates the precise timing of invadopodial breach. A complete understanding of the molecular composition of AC-invadopodia would enhance our understanding of critical regulators of their function and specification. In vertebrates, over 50 proteins have been associated with invadopodia.29,30 Most of these proteins are encoded in the C. elegans genome, but three key components—the actin-nucleation-promoting factor cortactin, the Tks4/5 adaptor proteins, and membrane type matrix metalloproteinases (MT-MMPs)—are absent. These results indicate that there are differences in structural make-up of AC-invadopodia and cancer cell invadopodia, although the functional significance of this is unclear. Our imaging revealed that invadopodia began forming and turning over at least 3 h prior to initially breaching the basement membrane. Invadopodia penetrate the basement membrane during a narrow, highly stereotyped 20 min developmental window during the mid-L3 larval stage. What dictates this temporal specificity? One possible candidate is a diffusible cue secreted from the underlying vulval cells. We’ve previously shown that the underlying vulval cells help dictate the timing of invasion.16 For example, in mutant animals where the vulval cells develop precociously, the AC responds by invading early. The identity of the vulval signal remains unknown, but may provide insights into cell non-autonomous mechanisms that activate invadopodia.
The Netrin Receptor DCC Guides Invasion through the Breach
By imaging the AC after initial basement membrane breach, we found that usually only one or two invadopodia ever penetrated the basement membrane and that only one of these then rapidly transitioned into a large invasive protrusion that extended into the underlying vulval tissue. We had previously found that the netrin receptor localizes to the invasive cell membrane and regulates F-actin, but its precise role in invasion was unclear.20 We thus examined the localization of the netrin receptor UNC-40 during invasion. UNC-40 was present throughout the invasive cell membrane prior to invasion, but localized in a concentrated manner to the site of initial basement membrane breach approximately 20 min before the detection of a visible break in the basement membrane.22 At the breach we found UNC-40 recruited its F-actin regulatory effectors UNC-34 (Ena/VASP) and MIG-2 (Rac) and directed focused F-actin formation, leading to the formation of an invasive protrusion. Invasive protrusion development correlated with the cessation of invadopodia formation, likely as a result of the growing protrusion acting as a molecular sink for actin regulators that are required to create invadopodia. Consistent with this notion, loss of unc-40 led to a complete absence of invasive protrusion formation and the persistence of invadopodia. Further, many invadopodia penetrated the basement membrane in unc-40 mutant animals, leading to multiple holes in the basement membrane, reminiscent of invadopodia activity in cancer cell lines in vitro (Fig. 3). Thus, UNC-40 (netrin) activity directs the AC through a single basement membrane breach and into the vulval tissue.
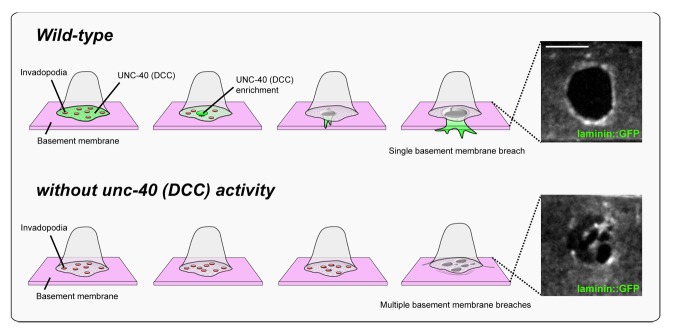
Figure 3. UNC-40 (DCC) focuses AC invasion through a single basement membrane breach. In wild-type animals, invadopodia (red circles) form and turn over until one breaches the basement membrane. UNC-40 (DCC, green) localizes to the breach site and directs the formation of a cellular protrusion, which guides invasion through a single large basement membrane breach into the vulval tissue (ventral view of laminin::GFP, right). As the protrusion grows, new invadopodia cease to form, thus inhibiting additional breaches. In the absence of the UNC-40 netrin receptor, the AC fails to build an invasive protrusion and invadopodia continue to form (bottom). Multiple breaching events occur resulting in numerous holes in the basement membrane (laminin::GFP, right).
Given that stable clusters of UNC-40 (DCC) and invadopodia presaged sites of visible basement membrane breach, the UNC-40 receptor may selectively seed invadopodia with a greater capacity to penetrate basement membrane. Alternatively, UNC-40 might detect and cluster at initial sites of basement membrane penetration that are below visible resolution, setting off expansion of basement membrane gaps at these sites. Consistent with this later possibility, UNC-40 progressively enriched at visible basement membrane breaches, indicating it can specifically target to basement membrane openings. Further, we found that UNC-40 activity accelerated visible basement membrane gap opening, likely by promoting recurrent F-actin polymerization, but was not required for basement membrane penetration.
In many developmental events, UNC-40 is thought to be polarized by localized or gradients of UNC-6 (netrin).31 While UNC-6 (netrin) was required to activate UNC-40 to form the invasive protrusion in the AC, surprisingly we found that the trafficking of UNC-40 to the breach was independent of UNC-6. This is not the first time a role for UNC-40 has been identified independent of UNC-6 (netrin). For example, localization and activity of UNC-40 in muscle arm extension does not depend upon UNC-6 (netrin).32 Further, elongation of the AVM axon along the anterior-posterior axis and the posterior migration of the QL neuroblast require UNC-40 (DCC) signaling without UNC-6.33 It’s unknown in these instances how UNC-40 signals or is localized without UNC-6. One notable observation from our work in the AC is that while UNC-6 is not required to localize UNC-40 to the breach, UNC-6 is critical to activate UNC-40 so that focused F-actin is generated at the site of breach to form a large protrusion. This observation indicates that UNC-40 localization and activation are separable processes. Interestingly, netrin proteins are homologous to domains VI and V at the N-termini of laminin β and γ chains.31 It is thus tempting to speculate that proteolytic digestion of laminin during breaching might liberate the N-termini of laminin chains, which could act as a signal to localize the netrin receptor UNC-40 (DCC) to the breach.
Interestingly, in approximately 50% of our observations, we found that more than one AC-invadopodium breached the basement membrane (sometimes as many as four). UNC-40 localized to all of these breaches and yet inevitably only one of these developed into a protrusion. These observations indicate a competition between successful breaching sites. This might be similar to mechanisms that ensure singularity in polarization in yeast and neuronal cells, where competition for a limiting reagent, coupled with positive feedback guarantees that one bud or one axon forms, respectively.34,35 It will be fascinating to determine the nature of competitive mechanism between multiple breaching sites. One possibility is that there is a positive feedback mechanism that involves the receptor itself, as UNC-40 levels increase at the site of breach.
The Invasive Protrusion Displaces Basement Membrane
Based on the presence of type IV collagen degradation products and the expression of proteases near sites of invasion in vivo and in vitro, it has been proposed that basement membrane is degraded and dissolved during invasion.19,36–40 These observations led to extensive clinical trials to target matrix metalloproteinases (MMPs) in a wide range of metastatic cancers. These clinical trials were unfortunately unsuccessful, but the reasons were unclear.38 By utilizing a photo-convertible form of laminin and type IV collagen and optically highlighting the basement membrane that the AC crossed during invasion, we surprisingly found that the basement was physically displaced by the invasive protrusion. These observations implicate the contribution of a protease-independent mechanism for basement membrane removal. Quantification of displaced basement membrane was difficult to precisely determine; however, leaving open the possibility of proteases also being a factor in successful invasion. We speculate that the physical pressure from the enlarging protrusion provides the mechanical force to push against basement membrane and widen the gap during protrusion growth. This would account for the 2-fold greater rate of basement membrane removal in wild-type animals with an invasive protrusion. Notably, however, basement membrane was still displaced in unc-40 mutants, albeit at a reduced rate, indicating that another mechanism for physical displacement acts in parallel to the growing protrusion.
The role of proteases during basement membrane invasion has been controversial. While apparently required for invasion in in vitro and ex vivo invasion assays,7,37 an essential role for proteases has not been demonstrated in vivo despite extensive knockout experiments of MMPs and other protease families in mouse genetic models.7,41 Importantly, our observations do not rule out the involvement of proteases in AC invasion. In fact, we’ve previously found that the AC expresses zmp-1, a matrix metalloproteinase.19 Although loss of zmp-1 has no apparent phenotype, it might function redundantly with other proteases. The C. elegans genome encodes nearly 300 genes with predicted protease or protease inhibitor domains,42 making the identification of proteases required for AC invasion a daunting task. Profiling the gene expression of the AC using single cell isolation techniques43 will likely narrow this list. Combined knockdown of key protease gene families, such as the MMP’s, ADAMS, and cathepsins, may also provide insight. Further, sensitized screening combined with analysis of basement membrane dynamics may also reveal roles for proteases. Given the expression of zmp-1 in the AC during invasion, it seems plausible that proteases and physical forces cooperatively mediate basement membrane invasion, as they appear to do during the movement of migratory fibroblasts and cancer cells through the less dense, fibrillar type I collagen-rich interstitial matrix.44
Perspectives
Our real-time analysis of AC invasion has established the physiological relevance of invadopodia in basement membrane invasion and suggests that invadopodia are conserved structures that are utilized by invasive cells to breach basement membranes in both normal developmental events as well as diseases such as cancer. Further, we have discovered an UNC-40 (DCC)-mediated mechanism that guides invasive protrusion formation through a single basement membrane breach. The netrin-1 ligand is highly expressed in metastatic cancers45–47 and stimulates invasion in many tumor cell lines assayed in vitro and ex vivo.47–50 These results suggest that the vertebrate DCC receptor may play a conserved role in guiding invasion. Many outstanding questions regarding the specification, formation, and regulation of invadopodia, as well as the role of proteases and physical forces in removing and remodeling basement membrane during invasion remain unanswered. With its rapid forward genetics and amenability to high-resolution real-time microscopy, we expect C. elegans AC invasion will help answer many of these questions and provide important novel insights into the fundamental and fascinating way that cells breach and traverse basement membrane barriers.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank L. Lohmer for insightful comments on this manuscript. This work was supported by a graduate research fellowship from the NSF to MAM, The Pew Scholars Program in the Biomedical Sciences, and NIH Grants GM079320 and GM100083 to DRS.
Glossary
Abbreviations:
- AC
anchor cell
- DCC
deleted in colorectal cancer
Footnotes
Previously published online: www.landesbioscience.com/journals/worm/article/26169
References
- 1.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–33. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 2.Hynes RO. The evolution of metazoan extracellular matrix. J Cell Biol. 2012;196:671–9. doi: 10.1083/jcb.201109041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hohenester E, Yurchenco PD. Laminins in basement membrane assembly. Cell Adh Migr. 2013;7:56–63. doi: 10.4161/cam.21831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khoshnoodi J, Pedchenko V, Hudson BG. Mammalian collagen IV. Microsc Res Tech. 2008;71:357–70. doi: 10.1002/jemt.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pöschl E, Schlötzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131:1619–28. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]
- 6.Hagedorn EJ, Sherwood DR. Cell invasion through basement membrane: the anchor cell breaches the barrier. Curr Opin Cell Biol. 2011;23:589–96. doi: 10.1016/j.ceb.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rowe RG, Weiss SJ. Breaching the basement membrane: who, when and how? Trends Cell Biol. 2008;18:560–74. doi: 10.1016/j.tcb.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Linder S. Invadosomes at a glance. J Cell Sci. 2009;122:3009–13. doi: 10.1242/jcs.032631. [DOI] [PubMed] [Google Scholar]
- 9.Engelhardt B, Ransohoff RM. Capture, crawl, cross: the T cell code to breach the blood-brain barriers. Trends Immunol. 2012;33:579–89. doi: 10.1016/j.it.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Chen WT. Proteolytic activity of specialized surface protrusions formed at rosette contact sites of transformed cells. J Exp Zool. 1989;251:167–85. doi: 10.1002/jez.1402510206. [DOI] [PubMed] [Google Scholar]
- 11.David-Pfeuty T, Singer SJ. Altered distributions of the cytoskeletal proteins vinculin and alpha-actinin in cultured fibroblasts transformed by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1980;77:6687–91. doi: 10.1073/pnas.77.11.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy DA, Courtneidge SA. The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol. 2011;12:413–26. doi: 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li A, Dawson JC, Forero-Vargas M, Spence HJ, Yu X, König I, Anderson K, Machesky LM. The actin-bundling protein fascin stabilizes actin in invadopodia and potentiates protrusive invasion. Curr Biol. 2010;20:339–45. doi: 10.1016/j.cub.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linder S, Wiesner C, Himmel M. Degrading devices: invadosomes in proteolytic cell invasion. Annu Rev Cell Dev Biol. 2011;27:185–211. doi: 10.1146/annurev-cellbio-092910-154216. [DOI] [PubMed] [Google Scholar]
- 15.Chan KT, Cortesio CL, Huttenlocher A. FAK alters invadopodia and focal adhesion composition and dynamics to regulate breast cancer invasion. J Cell Biol. 2009;185:357–70. doi: 10.1083/jcb.200809110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sherwood DR, Sternberg PW. Anchor cell invasion into the vulval epithelium in C. elegans. Dev Cell. 2003;5:21–31. doi: 10.1016/S1534-5807(03)00168-0. [DOI] [PubMed] [Google Scholar]
- 17.Hagedorn EJ, Yashiro H, Ziel JW, Ihara S, Wang Z, Sherwood DR. Integrin acts upstream of netrin signaling to regulate formation of the anchor cell’s invasive membrane in C. elegans. Dev Cell. 2009;17:187–98. doi: 10.1016/j.devcel.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matus DQ, Li XY, Durbin S, Agarwal D, Chi Q, Weiss SJ, Sherwood DR. In vivo identification of regulators of cell invasion across basement membranes. Sci Signal. 2010;3:ra35. doi: 10.1126/scisignal.2000654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sherwood DR, Butler JA, Kramer JM, Sternberg PW. FOS-1 promotes basement-membrane removal during anchor-cell invasion in C. elegans. Cell. 2005;121:951–62. doi: 10.1016/j.cell.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 20.Ziel JW, Hagedorn EJ, Audhya A, Sherwood DR. UNC-6 (netrin) orients the invasive membrane of the anchor cell in C. elegans. Nat Cell Biol. 2009;11:183–9. doi: 10.1038/ncb1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schindler AJ, Sherwood DR. The transcription factor HLH-2/E/Daughterless regulates anchor cell invasion across basement membrane in C. elegans. Dev Biol. 2011;357:380–91. doi: 10.1016/j.ydbio.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagedorn EJ, Ziel JW, Morrissey MA, Linden LM, Wang Z, Chi Q, Johnson SA, Sherwood DR. The netrin receptor DCC focuses invadopodia-driven basement membrane transmigration in vivo. J Cell Biol. 2013;201:903–13. doi: 10.1083/jcb.201301091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Destaing O, Planus E, Bouvard D, Oddou C, Badowski C, Bossy V, Raducanu A, Fourcade B, Albiges-Rizo C, Block MR. β1A integrin is a master regulator of invadosome organization and function. Mol Biol Cell. 2010;21:4108–19. doi: 10.1091/mbc.E10-07-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakahara H, Otani T, Sasaki T, Miura Y, Takai Y, Kogo M. Involvement of Cdc42 and Rac small G proteins in invadopodia formation of RPMI7951 cells. Genes Cells. 2003;8:1019–27. doi: 10.1111/j.1365-2443.2003.00695.x. [DOI] [PubMed] [Google Scholar]
- 25.Philippar U, Roussos ET, Oser M, Yamaguchi H, Kim HD, Giampieri S, Wang Y, Goswami S, Wyckoff JB, Lauffenburger DA, et al. A Mena invasion isoform potentiates EGF-induced carcinoma cell invasion and metastasis. Dev Cell. 2008;15:813–28. doi: 10.1016/j.devcel.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamaguchi H, Yoshida S, Muroi E, Kawamura M, Kouchi Z, Nakamura Y, Sakai R, Fukami K. Phosphatidylinositol 4,5-bisphosphate and PIP5-kinase Ialpha are required for invadopodia formation in human breast cancer cells. Cancer Sci. 2010;101:1632–8. doi: 10.1111/j.1349-7006.2010.01574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seiler C, Davuluri G, Abrams J, Byfield FJ, Janmey PA, Pack M. Smooth muscle tension induces invasive remodeling of the zebrafish intestine. PLoS Biol. 2012;10:e1001386. doi: 10.1371/journal.pbio.1001386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sibony-Benyamini H, Gil-Henn H. Invadopodia: the leading force. Eur J Cell Biol. 2012;91:896–901. doi: 10.1016/j.ejcb.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Hoshino D, Jourquin J, Emmons SW, Miller T, Goldgof M, Costello K, Tyson DR, Brown B, Lu Y, Prasad NK, et al. Network analysis of the focal adhesion to invadopodia transition identifies a PI3K-PKCα invasive signaling axis. Sci Signal. 2012;5:ra66. doi: 10.1126/scisignal.2002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klemke RL. Trespassing cancer cells: ‘fingerprinting’ invasive protrusions reveals metastatic culprits. Curr Opin Cell Biol. 2012;24:662–9. doi: 10.1016/j.ceb.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lai Wing Sun K, Correia JP, Kennedy TE. Netrins: versatile extracellular cues with diverse functions. Development. 2011;138:2153–69. doi: 10.1242/dev.044529. [DOI] [PubMed] [Google Scholar]
- 32.Alexander M, Chan KK, Byrne AB, Selman G, Lee T, Ono J, Wong E, Puckrin R, Dixon SJ, Roy PJ. An UNC-40 pathway directs postsynaptic membrane extension in Caenorhabditis elegans. Development. 2009;136:911–22. doi: 10.1242/dev.030759. [DOI] [PubMed] [Google Scholar]
- 33.Ziel JW, Sherwood DR. Roles for netrin signaling outside of axon guidance: a view from the worm. Dev Dyn. 2010;239:1296–305. doi: 10.1002/dvdy.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fivaz M, Bandara S, Inoue T, Meyer T. Robust neuronal symmetry breaking by Ras-triggered local positive feedback. Curr Biol. 2008;18:44–50. doi: 10.1016/j.cub.2007.11.051. [DOI] [PubMed] [Google Scholar]
- 35.Howell AS, Savage NS, Johnson SA, Bose I, Wagner AW, Zyla TR, Nijhout HF, Reed MC, Goryachev AB, Lew DJ. Singularity in polarization: rewiring yeast cells to make two buds. Cell. 2009;139:731–43. doi: 10.1016/j.cell.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garbisa S, Kniska K, Tryggvason K, Foltz C, Liotta LA. Quantitation of basement membrane collagen degradation by living tumor cells in vitro. Cancer Lett. 1980;9:359–66. doi: 10.1016/0304-3835(80)90030-0. [DOI] [PubMed] [Google Scholar]
- 37.Hotary K, Li XY, Allen E, Stevens SL, Weiss SJ. A cancer cell metalloprotease triad regulates the basement membrane transmigration program. Genes Dev. 2006;20:2673–86. doi: 10.1101/gad.1451806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Overall CM, Kleifeld O. Tumour microenvironment - opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer. 2006;6:227–39. doi: 10.1038/nrc1821. [DOI] [PubMed] [Google Scholar]
- 39.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–33. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu J, Rodriguez D, Petitclerc E, Kim JJ, Hangai M, Moon YS, Davis GE, Brooks PC. Proteolytic exposure of a cryptic site within collagen type IV is required for angiogenesis and tumor growth in vivo. J Cell Biol. 2001;154:1069–79. doi: 10.1083/jcb.200103111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Madsen CD, Sahai E. Cancer dissemination--lessons from leukocytes. Dev Cell. 2010;19:13–26. doi: 10.1016/j.devcel.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 42.Ihara S, Hagedorn EJ, Morrissey MA, Chi Q, Motegi F, Kramer JM, Sherwood DR. Basement membrane sliding and targeted adhesion remodels tissue boundaries during uterine-vulval attachment in Caenorhabditis elegans. Nat Cell Biol. 2011;13:641–51. doi: 10.1038/ncb2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang S, Kuhn JR. Cell isolation and culture. WormBook. 2013;21:1–39. doi: 10.1895/wormbook.1.157.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, Stack MS, Friedl P. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007;9:893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- 45.Ramesh G, Berg A, Jayakumar C. Plasma netrin-1 is a diagnostic biomarker of human cancers. Biomarkers. 2011;16:172–80. doi: 10.3109/1354750X.2010.541564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fitamant J, Guenebeaud C, Coissieux MM, Guix C, Treilleux I, Scoazec JY, Bachelot T, Bernet A, Mehlen P. Netrin-1 expression confers a selective advantage for tumor cell survival in metastatic breast cancer. Proc Natl Acad Sci U S A. 2008;105:4850–5. doi: 10.1073/pnas.0709810105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dumartin L, Quemener C, Laklai H, Herbert J, Bicknell R, Bousquet C, Pyronnet S, Castronovo V, Schilling MK, Bikfalvi A, Hagedorn M. Netrin-1 mediates early events in pancreatic adenocarcinoma progression, acting on tumor and endothelial cells. Gastroenterology. 2010;138:1595, 606, 1606.e1-8. doi: 10.1053/j.gastro.2009.12.061. [DOI] [PubMed] [Google Scholar]
- 48.Kaufmann S, Kuphal S, Schubert T, Bosserhoff AK. Functional implication of Netrin expression in malignant melanoma. Cell Oncol. 2009;31:415–22. doi: 10.3233/CLO-2009-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodrigues S, De Wever O, Bruyneel E, Rooney RJ, Gespach C. Opposing roles of netrin-1 and the dependence receptor DCC in cancer cell invasion, tumor growth and metastasis. Oncogene. 2007;26:5615–25. doi: 10.1038/sj.onc.1210347. [DOI] [PubMed] [Google Scholar]
- 50.Shimizu A, Nakayama H, Wang P, König C, Akino T, Sandlund J, Coma S, Italiano JE, Jr., Mammoto A, Bielenberg DR, et al. Netrin-1 promotes glioblastoma cell invasiveness and angiogenesis by multiple pathways including activation of RhoA, cathepsin B, and cAMP-response element-binding protein. J Biol Chem. 2013;288:2210–22. doi: 10.1074/jbc.M112.397398. [DOI] [PMC free article] [PubMed] [Google Scholar]


