To fulfill their numerous tissue- and organ-specific roles, epithelial sheets rely on specialized architecture and specific mechanical properties, ranging from the exquisitely patterned, force-balanced sensory epithelia of the inner ear, to the highly dynamic and convoluted epithelia lining the villi and crypts of the intestinal tract. Both the morphology and mechanical characteristics of epithelial sheets are emergent properties of their constituent cells; subtle shrinking of the apical boundaries of epithelial cells can lead to larger-scale changes in packing geometry, even causing dramatic bending of epithelial sheets.1 However, the molecular and structural underpinnings for the apical constriction of epithelial cells are not fully understood. Non-muscle myosin II (NMII) has been implicated in driving a “purse string”-like contraction of the circumferential actin belt that encircles the inner surface of epithelial cells.2,3 Current models depict NMII filaments randomly distributed along the circumferential actin belt,4 but it is unclear how the activities of individual NMII filaments are integrated and coordinated to generate and transmit force across the epithelium. Understanding these processes is crucial to understanding epithelial dynamics during development, as well as the post-developmental maintenance of epithelial architecture and tensional homeostasis.
In a recent study we focused on elucidating the distribution, organization, and role of NMII isoforms within epithelial apical junctions using the organ of Corti, one of the most striking examples of mammalian epithelial patterning, as a model system.5 Seeking to investigate the effects of NMII inhibition on apical junctions, we treated explant cultures of the organ of Corti with the NMII-specific inhibitor blebbistatin.6 This induced changes in the epithelial apical surface at both a cellular and tissue level. Morphometric quantification showed an increase in junctional-length, a corresponding increase in apical cell-surface area, and a deformation of apical cell shape across the tissue, resulting in an overall expansion of the epithelial sheet. Strikingly, the changes were completely reversed upon washout of the blebbistatin. Our observations suggested that apical perimeters of epithelial cells are dynamically maintained under tension by NMII within the circumferential junctional actomyosin belt.
With data supporting a role for NMII in influencing epithelial apical junctions, we next determined the pattern of distribution of each NMII paralog, NMIIA, IIB, and IIC, along the cell perimeter. Immunofluorescence of NMIIB and NMIIC showed a striking distribution as regularly spaced puncta associated with perijunctional actin. Measurements of relative fluorescence intensity revealed low actin density at NMII fluorescence puncta and higher actin density between them, resembling the sarcomeric striations in myofibrils. This finding prompted us to test for the presence of another hallmark component of muscle sarcomeres, the actin cross-linker α-actinin. Immunofluorescence confirmed that α-actinin1 is in fact present along the junctional line in a periodic distribution, alternating with NMII and coinciding with actin, just like in sarcomeres.
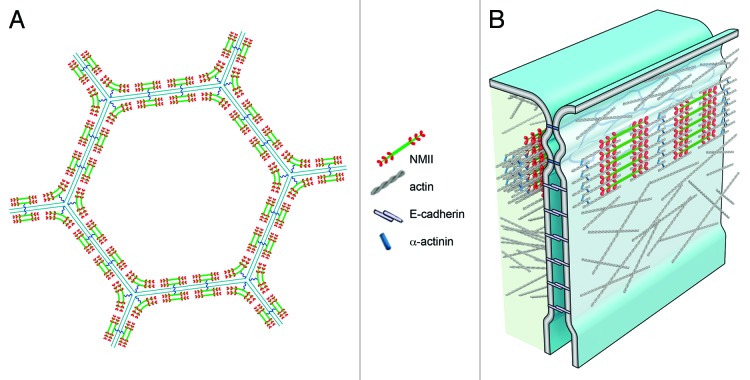
Another essential feature of sarcomeric organization is the orientation of bipolar myosin filaments parallel to actin filaments. To determine the polarity and orientation of NMII filaments along the apical perimeter of epithelial cells, we exogenously expressed, in organ of Corti cultures, NMIIC tagged with different fluorophores at the N terminus and C terminus. We also labeled tissue from an NMIIC–GFP transgenic mouse (GFP-tag at the C terminus), with antibody against the NMIIC N terminus. These experiments confirmed that bipolar NMII filaments organize as arrays within small regular sarcomeric units assembled end-to-end to form a continuous belt along the cellular perimeter, as depicted in Figure 1A. Like muscle sarcomeres, the NMII sarcomeres at the perijunctional actin belt are also contractile, increasing in length, or “relaxing,” on treatment with blebbistatin, and shortening, or “contracting,” when blebbistatin was removed. Remarkably, the changes in sarcomere length concomitantly influence apical junctional length and surface area, and, ultimately, the geometry of the entire epithelium.
Figure 1. (A) Illustration of a top-down view of an epithelial cell with 6 neighboring cells, showing NMII arranged along the cell periphery in a sarcomeric belt. Sarcomeres in adjacent cells pair precisely across the junctional line, likely via a putative molecular tether (blue wavy line). Sarcomeres at the tricellular junction “bend” in a manner that suggests that the mechanical tethering occurs at the midpoint of the sarcomere. (B) Diagram illustrating a cross-section through 2 adjacent epithelial cells, demonstrating the location of the NMII sarcomeric belt at the interface of the tight and adherens junction components of the apical junctional complex.
Notably, NMII sarcomeres along the perimeter of one cell were paired with sarcomeres in the adjacent cell. This registry was maintained regardless of the contractile state of the sarcomeres, suggesting that they are mechanically coupled across the junctional line. We propose that this coupling depends on a yet-to-be-identified transmembrane junctional component that integrates the sarcomeres into a transcellular network, along which forces are propagated across the apical epithelial surface. Interestingly, contrary to current views that associate NMII with the adherens junction,4 we showed that the NMII sarcomeric belt overlaps with both the tight and adherens junctions, as illustrated in Figure 1B. This observation, in addition to the alignment of the sarcomeres across the junctional line, also infers the interaction of NMII with other junctional components. Finally, we extended our investigation to the mucosal epithelia of the gastrointestinal tract and confirmed the presence of the sarcomeric belt along apical junctions, and the registry of the sarcomeres across the junctional line, suggesting that this transcellular sarcomeric network may be a universal epithelial feature.
Further studies will be needed to identify the key molecular players involved in regulating this novel sarcomeric network, and to determine its relationship to tight- and adherens junctions, to dissect the intricacies of its roles in development, tensional homeostasis, and diseases associated with defects in NMII paralogs.7,8
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/26229
References
- 1.Sawyer JM, et al. Dev Biol. 2010;341:5–19. doi: 10.1016/j.ydbio.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertet C, et al. Nature. 2004;429:667–71. doi: 10.1038/nature02590. [DOI] [PubMed] [Google Scholar]
- 3.Florian P, et al. J Physiol. 2002;545:485–99. doi: 10.1113/jphysiol.2002.031161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu K, Cheney RE. Myosins in cell junctions. BioArchitecture. 2012;2 doi: 10.4161/bioa.21791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebrahim S, et al. Curr Biol. 2013;23:731–6. doi: 10.1016/j.cub.2013.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Straight AF, et al. Science. 2003;299:1743–7. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 7.Lalwani AK, et al. Am J Hum Genet. 2000;67:1121–8. doi: 10.1016/s0002-9297(07)62942-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xia ZK, et al. Dis Esophagus. 2012;25:427–36. doi: 10.1111/j.1442-2050.2011.01261.x. [DOI] [PubMed] [Google Scholar]